Seasonal Variations in the Foraging Strategies of Plateau Pikas (Ochotona curzoniae)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Plant Sample Collection and Pretreatment
2.3. Animal Sample Collection and Pretreatment
2.4. Stable Isotope Determination
2.5. Calculation of Stable Isotope Trophic Discrimination Factors
2.6. Screening of Potential Food Sources for Plateau Pika
2.7. Estimating the Tood Contribution of the Plateau Pika
2.8. Ecological Niche Width and Overlap Calculations
2.9. Statistical Analysis
3. Results
3.1. Stable Isotope Trophic Discrimination Factors of Different Organs in Plateau Pikas
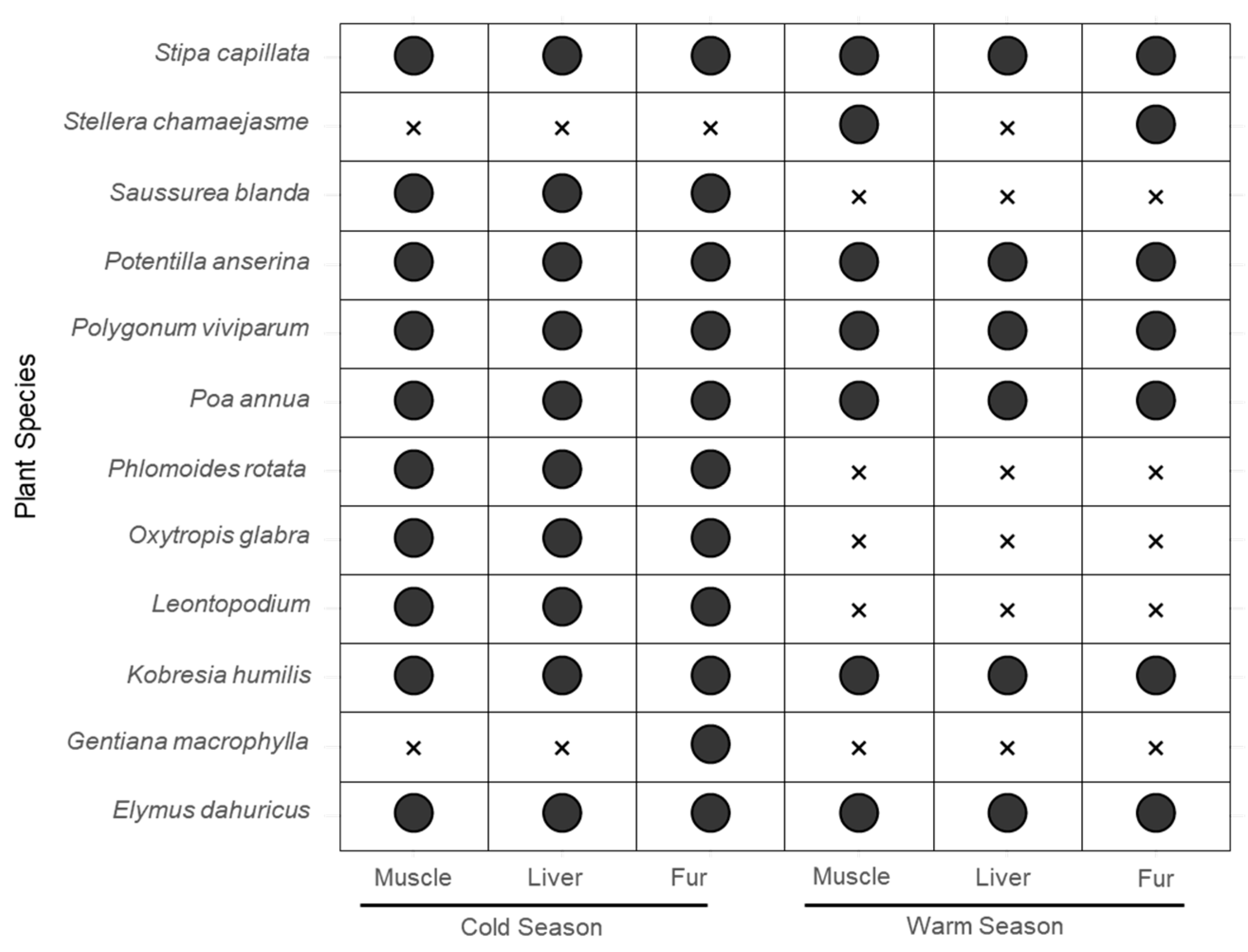
3.2. Screening of Potential Food Sources of Plateau Pika in Cold and Warm Seasons
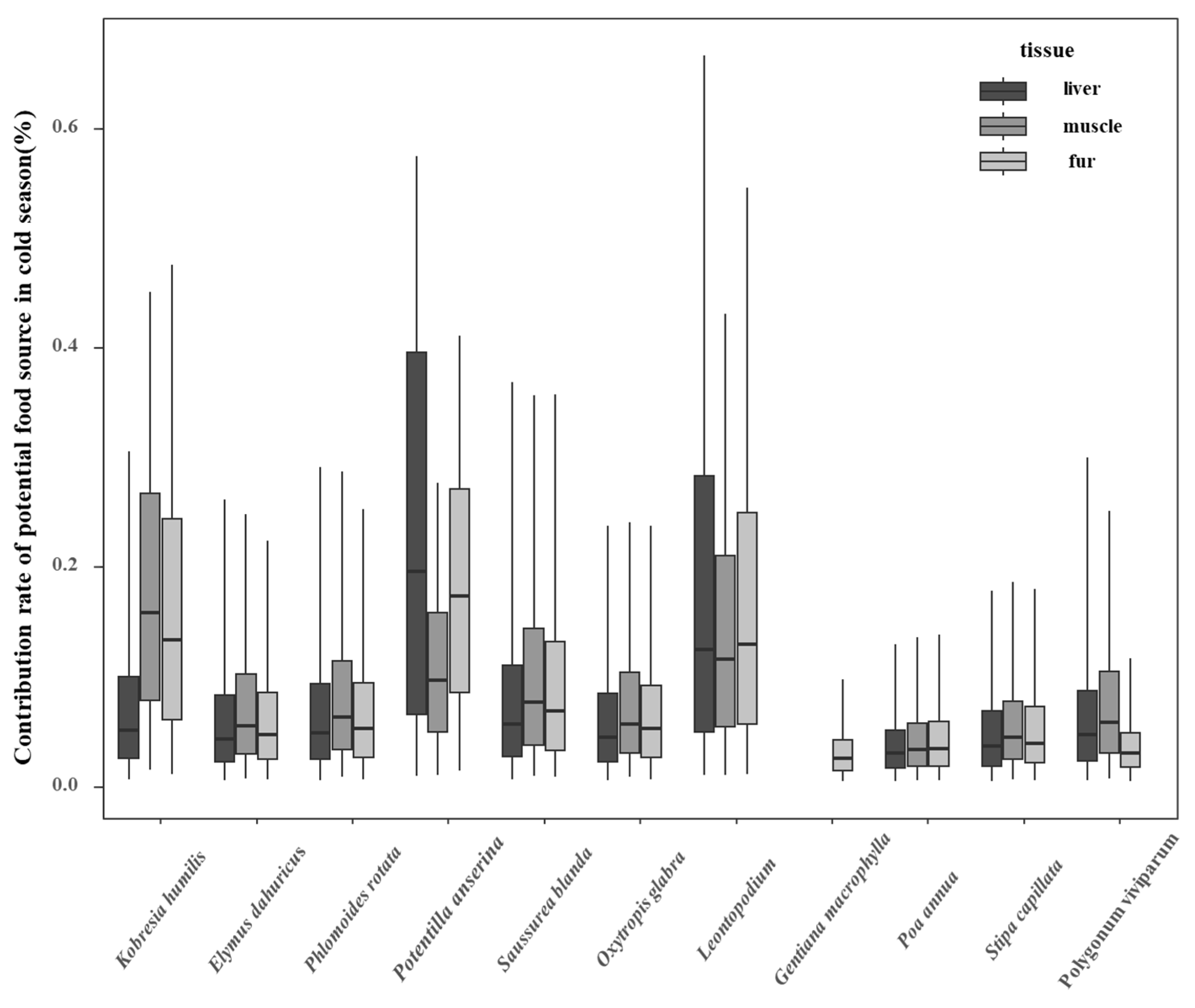
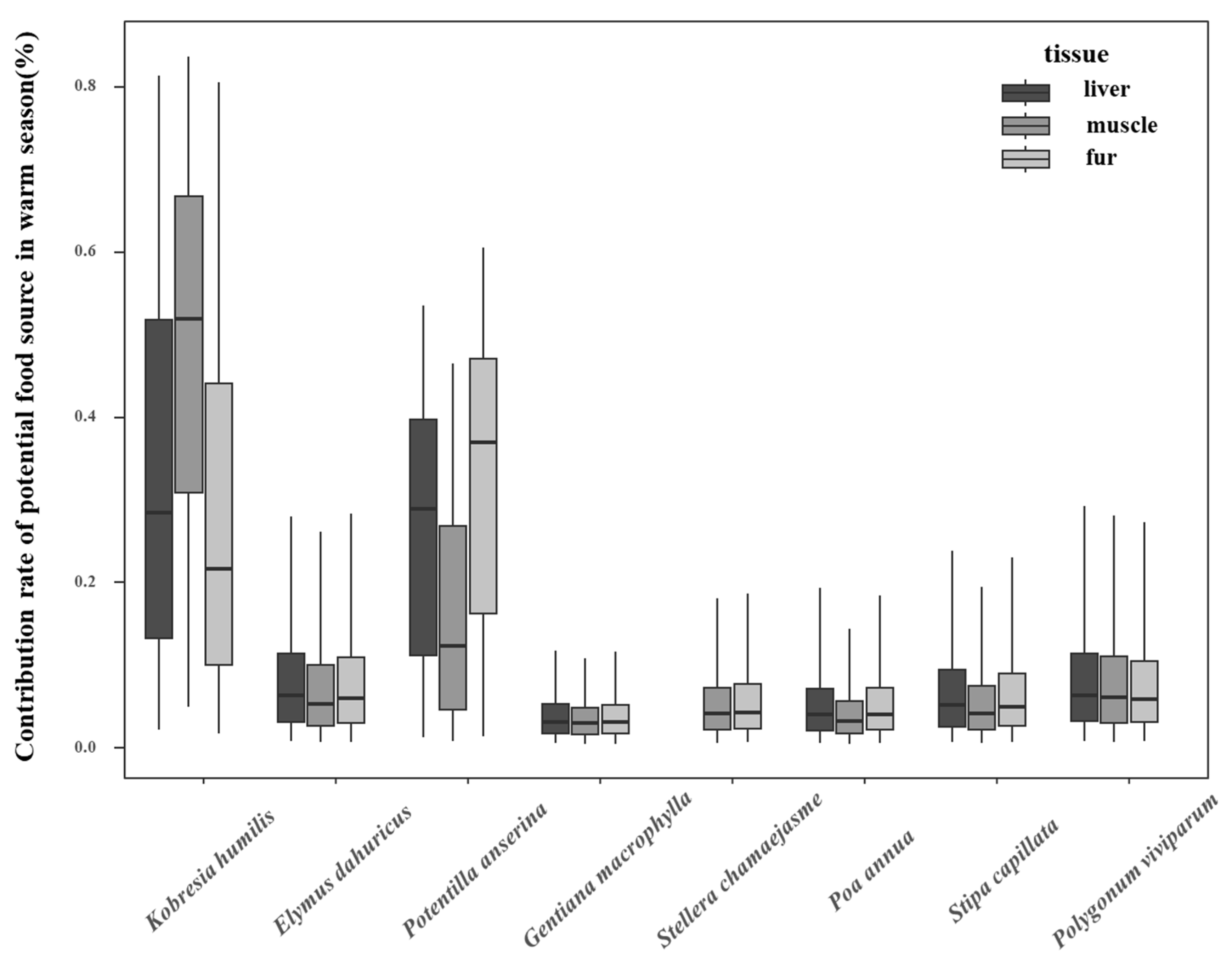
3.3. Contribution of Potential Food Sources to Plateau Pikas in Cold and Warm Seasons
3.4. Niche Width and Overlap of Different Organs of Plateau Pika in Cold and Warm Seasons
4. Discussion
4.1. Determination of Stable Isotope Nutrition Discrimination Factors in Various Organs of Plateau Pika
4.2. Changes in Feeding Habits of Plateau Pika with Time Scale
4.3. Trophic Niche Characteristics of Plateau Pika at Different Time Scales
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Renagül, E. Microscopy Histological Analysis of Diet and Trophic Niche of Four Sympatric Small Mammals in Altun Mountain National Nature Reserve. Master’s Thesis, Xinjiang Agricultural University, Urumqi, China, 2015. [Google Scholar]
- Chen, G.; Wu, Z.; Gu, B.H.; Liu, D.Y.; Li, X.; Wang, Y. Isotopic niche overlap of two planktivorous fish in southern China. Limnology 2011, 12, 151–155. [Google Scholar] [CrossRef]
- Yue, C.; Guo, Q.W.; Zhang, Z.R.; Li, X.; Man, D.H.; Yuan, S.; Fu, H.P.; Wu, X.D.; Jin, G.; Liu, J.W.; et al. Trophic niche of Brandt’ s voles (Lasiopodomys brandtii) and their interspecific relationships with other common rodents in a typical steppe, Inner Mongolia. J. Vet. Sci. 2020, 40, 424–434. [Google Scholar]
- Zhen, R.Q.; Bao, Y.X. Study methods and procedures for ungulate food habits. Acta Ecol. Sin. 2004, 24, 1532–1539. [Google Scholar]
- Robb, G.N.; Harrison, A.; Woodborne, S.; Bennett, N.C. Diet composition of two common mole-rat populations in arid and mesic environments in South Africa as determined by stable isotope analysis. J. Zool. 2016, 300, 257–264. [Google Scholar] [CrossRef]
- Andreychev, A.V. Daily and seasonal feeding activity of the greater mole-rat (Spalax microphtalmus, Rodentia, Spalacidae). Biol. Bull. 2019, 46, 1172–1181. [Google Scholar] [CrossRef]
- Li, H.D.; Su, X.M. The studies on the food web structures and trophic relationships in Guangxi Dongfang Cave by means of stable carbon and nitrogen isotopes. Acta Ecol. Sin. 2012, 32, 3497–3504. [Google Scholar]
- Pedro, P.I.; Ramos, J.A.; Neves, V.C.; Paiva, V.H. Past and present trophic position and decadal changes in diet of Yellow-legged Gull in the Azores Archipelago, NE Atlantic. Eur. J. Wildl. Res. 2013, 59, 833–845. [Google Scholar] [CrossRef]
- Li, Y.M.; Huang, Y.H.; Liu, C.W. Application of carbon and nitrogen stable isotope technique in animal feeding analysis. J. Guangdong Ocean. Univ. 2007, 27, 99–103. [Google Scholar]
- Guo, B.L.; Wei, Y.M.; Pan, J.R. Study on the Changing Law of Stable Carbon lsotope Composition in Cattle Tail Hair. Sci. Agric. Sin. 2008, 41, 2105–2111. [Google Scholar]
- Wang, N. On the function and position of ecological protection on Qinghai—Tibet Plateau. Theor. Platf. Tibet. 2020, 6, 27–30. [Google Scholar]
- Bu, H.Y.; Du, G.Z.; Chen, X.L.; Xu, X.L.; Liu, K.; Wen, S.J. Community-wide germination strategies in an alpine meadow on the eastern Qinghai-Tibet plateau: Phylogenetic and life-history correlates. Plant Ecol. 2008, 195, 87–98. [Google Scholar] [CrossRef]
- Ding, M.M.; Zhang, D.C. lnvestigation and Protection Strategy of Degraded Alpine Meadows in Southeast Tibet. For. Sci. Technol. 2022, 593, 54–56. [Google Scholar]
- Yang, D.; Yue, F.Z.; Li, X.; Xu, Z.T.; Wang, Z.P.; Cheng, T.T.; Pang, X.P.; Qin, S.Y.; Guan, Z.H.; Feng, Z.H.; et al. Current Research Status and Hot Spots of Grassland Rodent Pests Based on CiteSpace. Acta Agrestia Sin. 2022, 30, 670–681. [Google Scholar]
- Pan, D.F. Interactions and Mechanisms Among Herbivores in an Alpine Meadow of Qinghai-Tibetan Plateau. Ph.D. Thesis, Northeast Normal University, Jilin, China, 2020. [Google Scholar]
- Wang, Z.P. A Study on Nutritional Niche of Plateau Zokor and White Yak in the East of Qilian Mountain Based on Stable Isotope Techniques. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2018. [Google Scholar]
- Renagül, E.; Li, Y.; Zhang, Y.; Shi, L. Microscopic histological analysis of diet of Ochotona curzoniae in the Altun Mountain National Nature Reserve. Chin. J. Vector Biol. Control 2015, 26, 164–167. [Google Scholar]
- Wei, W.R.; Zhang, W.G. Architecture Characteristics of Burrow System of Plateau Pika, Ochotona curzoniae. Pak. J. Zool. 2018, 50, 311–316. [Google Scholar] [CrossRef]
- La, M.C. A Study on Limiting Factors for Vegetation Restoration in the Bald Patch Damaged by Plateau Pika and Evaluation of Vegetation Restoration Measures in Alpine Meadow. Master’s Thesis, Gansu Agricultural University, Lanzhou, China, 2023. [Google Scholar]
- Guo, B.L.; Wei, Y.M.; Pan, J.R.; Li, Y.; Zhang, F.S. Study on the Change of Stable Carbon lsotope Composition in Cattle Tissues. Sci. Agric. Sin. 2006, 39, 1885–1890. [Google Scholar]
- Schmidt, O.; Quilter, J.M.; Bahar, B.; Moloney, A.P.; Scrimgeour, C.M.; Begley, I.S.; Monahan, F.J. Inferring the origin and dietary history of beef from C, N and S stable isotope ratio analysis. Food Chem. 2005, 91, 545–549. [Google Scholar] [CrossRef]
- Healy, K.; Guillerme, T.; Kelly Seán, B.A.; Richard, I.; Stuart, B.; Andrew, L.J. SIDER: An R package for predicting trophic discrimination factors of consumers based on their ecology and phylogenetic relatedness. Ecography 2017, 41, 1393–1400. [Google Scholar] [CrossRef]
- Andre, C.; Manuela, G.F.; Julie, C.M.; Francisco, R. Searching for the true diet of marine predators: Incorporating bayesian priors into stable isotope mixing models. PLoS ONE 2014, 9, e92665. [Google Scholar]
- Sun, F.D.; Zhang, F.F.; Huo, F.; Chen, D.W.; Zhou, S.; Tang, M.K.; Yang, T.Y. Seasonal Changes of Flavonoids and Polyphenols Contents of Plants in Zoige AlpineGrassland of Ruoergai and Their Effects on Feeding Habits of Plateau Pika. J. Sichuan Agric. Univ. 2023, 41, 925–934. [Google Scholar]
- Kang, Y.K.; Zhang, D.G.; Guo, J.Y.; Wang, H.F.; Yang, Y.B.; Su, J.H. Food habits and its seasonal changes of plateau pika (Ochotona curzniae) in Gannan meadow. J. Gansu Agric. Univ. 2019, 54, 132–138. [Google Scholar]
- Andrew, C.P.; Donald, L.P.; Stuart, B.; Brice, X.S.; Eric, J.W.; Jonathan, W.M.; Andrew, L.J.; Jonathan, G.; David, J.K.; Richard, I. 2013. Bayesian stable isotope mixing models. Environmetrics 2019, 24, 387–399. [Google Scholar]
- Krebs, C.J. Ecology: The experimental analysis of distribution and abundance. Research 1972, 48, 133–148. [Google Scholar]
- Ogden, L.J.E.; Hobson, K.A.; Lank, D.B.; Martínez, d.R.C. Blood isotopic (δ13C and δ15N) turnover and diet-tissue fractionation factors in captivedunlin (Calidris alpina pacifica). Auk 2004, 121, 170–177. [Google Scholar] [CrossRef]
- Martínez, D.R.C.; Nathan, W.; Scott, A.C.; Leonard, Z.G. Isotopic ecology ten years after a call for more laboratory experiments. Biol. Rev. 2009, 84, 91–111. [Google Scholar] [CrossRef] [PubMed]
- Caut, S.; Angulo, E.; Courchamp, F. Variation in discrimination factors (Δ15N and Δ13C): The effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol. 2009, 46, 443–453. [Google Scholar] [CrossRef]
- Wang, X. Application of Stable Isotope Technique in Research Feeding Habits of Rodents in Desert Regin. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2017. [Google Scholar]
- Robb, G.N.; Stephan, W.; Bennett, N.C. Subterranean Sympatry: An Investigation into Diet Using Stable Isotope Analysis. PLoS ONE 2012, 7, e48572. [Google Scholar] [CrossRef]
- Darimont, C.T.; Reimchen, T.E. Intra-hair stable isotope analysis implies seasonal shift to salmon in gray wolf diet. Can. J. Zool. 2002, 80, 1638–1642. [Google Scholar] [CrossRef]
- Cui, X.F.; Xie, J.X.; Zhang, S.D.; Lin, G.H.; Zhang, T.Z.; Su, J.P. Relationship between overwintering preference and nutritional content of the foods of plateau zokor (Eospalax baileyi). Acta Theriol. Sin. 2014, 34, 340–347. [Google Scholar]
- Cai, X.C.; Bao, D.E.H.; Zhou, R.; Ma, Y.J.; Dong, K.C.; Hua, L.M. Comparison of the Gastrointestinal Tract Morphology between Plateau Pika and Plateau Zokor. Acta Agrestia Sin. 2022, 30, 1237–1245. [Google Scholar]
- Thompson, D.R.; Lilliendahl, K.; Solmundsson, J.; Furness, R.W.; Waldron, S.; Phillips, R.A. Trophic Relationships among Six Species of Icelandic Seabirds as Determined through Stable Isotope Analysis. Condor 1999, 101, 898–903. [Google Scholar] [CrossRef]
- Chen, G.k. Study on Food Traceability and Trophic Niches of Major Rodents in Alax Desert Area. Master’s Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2023. [Google Scholar]
- Wang, F.; Ju, R.T.; Li, Y.Z.; Du, Y.Z. Niche concept and its application in insect ecology. Chin. J. Ecol. 2006, 10, 1280–1284. [Google Scholar]
- Ranganath, H.A. Darwin’s finches: A goldmine for evolutionary biologists. J. Genet. 2018, 97, 807–809. [Google Scholar] [CrossRef] [PubMed]
| Item | Cold Season | Warm Season | ||
|---|---|---|---|---|
| Female (n = 6) | Male (n = 6) | Female (n = 5) | Male (n = 7) | |
| Body Mass (g) | 132.1 ± 6.2 | 143.9 ± 13.8 | 139.0 ± 14.6 | 148.0 ± 13.1 |
| Body Length (cm) | 17.4 ± 0.3 | 18.1 ± 0.7 | 15.4 ± 1.0 | 17.0 ± 0.9 |
| Hind Foot Length (cm) | 2.7 ± 0.5 | 2.9 ± 0.5 | 2.7 ± 0.3 | 3.1 ± 0.3 |
| Organ | Δδ13C | SD13C | Δδ15N | SD15N |
|---|---|---|---|---|
| Muscle | 2.126 | 0.862 | 3.354 | 0.522 |
| Liver | 2.148 | 0.865 | 3.428 | 0.526 |
| Fur | 2.957 | 0.911 | 3.391 | 0.504 |
| Organs of Plants and Animals | Niche Breadth | Cold Season | Warm Season | |||||
|---|---|---|---|---|---|---|---|---|
| Muscle | Liver | Fur | Muscle | Liver | Fur | |||
| Cold season | Muscle | 2.879 | 1 | |||||
| Liver | 2.585 | 0.844 | 1 | |||||
| Fur | 3.246 | 0.951 | 0.952 | 1 | ||||
| Warm season | Muscle | 2.034 | 0.718 | 0.411 | 0.635 | 1 | ||
| Liver | 2.032 | 0.758 | 0.716 | 0.803 | 0.852 | 1 | ||
| Fur | 3.478 | 0.684 | 0.782 | 0.802 | 0.707 | 0.971 | 1 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, L.; Cai, X.; Gan, R.; Zhang, J.; Dong, R.; Dong, K.; Hua, L.; Zhou, R. Seasonal Variations in the Foraging Strategies of Plateau Pikas (Ochotona curzoniae). Animals 2025, 15, 902. https://doi.org/10.3390/ani15070902
Dong L, Cai X, Gan R, Zhang J, Dong R, Dong K, Hua L, Zhou R. Seasonal Variations in the Foraging Strategies of Plateau Pikas (Ochotona curzoniae). Animals. 2025; 15(7):902. https://doi.org/10.3390/ani15070902
Chicago/Turabian StyleDong, Longming, Xincheng Cai, Ruixun Gan, Jing Zhang, Rui Dong, Kechi Dong, Limin Hua, and Rui Zhou. 2025. "Seasonal Variations in the Foraging Strategies of Plateau Pikas (Ochotona curzoniae)" Animals 15, no. 7: 902. https://doi.org/10.3390/ani15070902
APA StyleDong, L., Cai, X., Gan, R., Zhang, J., Dong, R., Dong, K., Hua, L., & Zhou, R. (2025). Seasonal Variations in the Foraging Strategies of Plateau Pikas (Ochotona curzoniae). Animals, 15(7), 902. https://doi.org/10.3390/ani15070902





