Emergence of Novel Anaplasma Species in the Mediterranean Area
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Statement
2.2. Study Area and Design
2.3. DNA Extraction and PCR Strategies
2.4. Purification, Sequencing and Phylogenetic Analyses
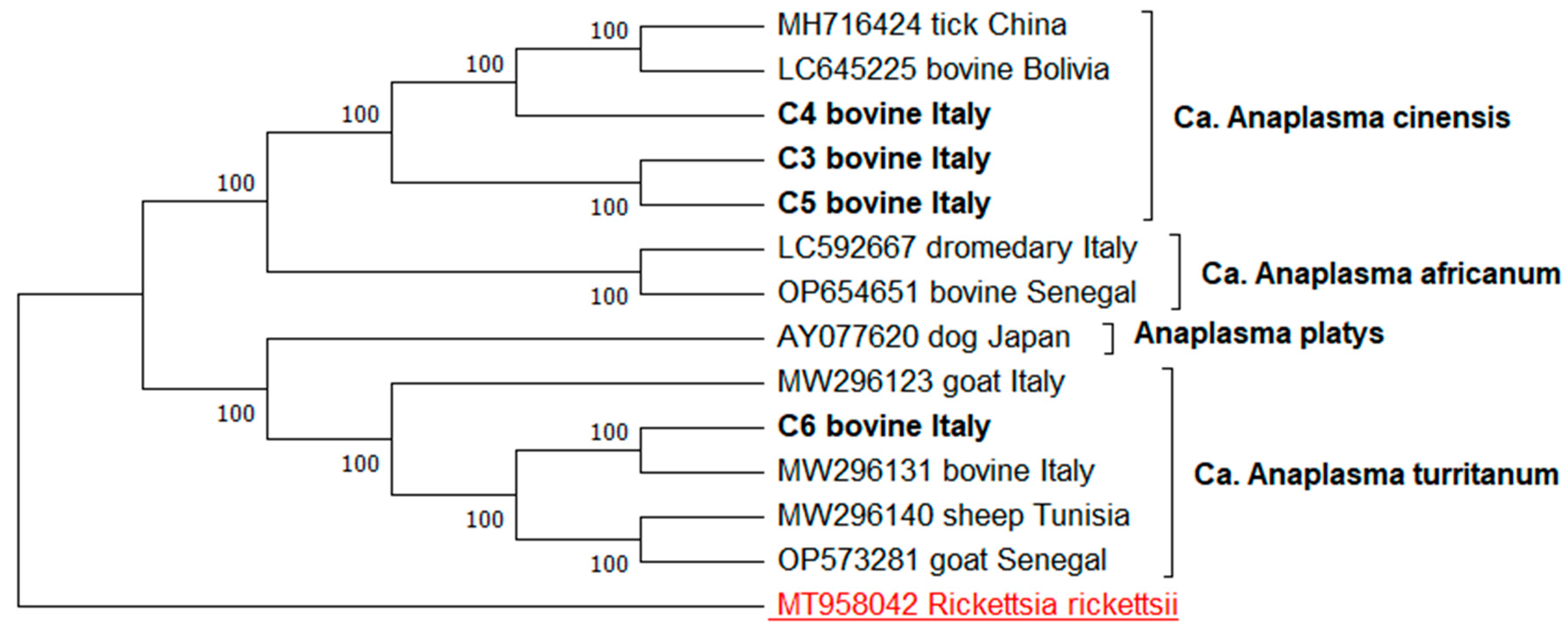
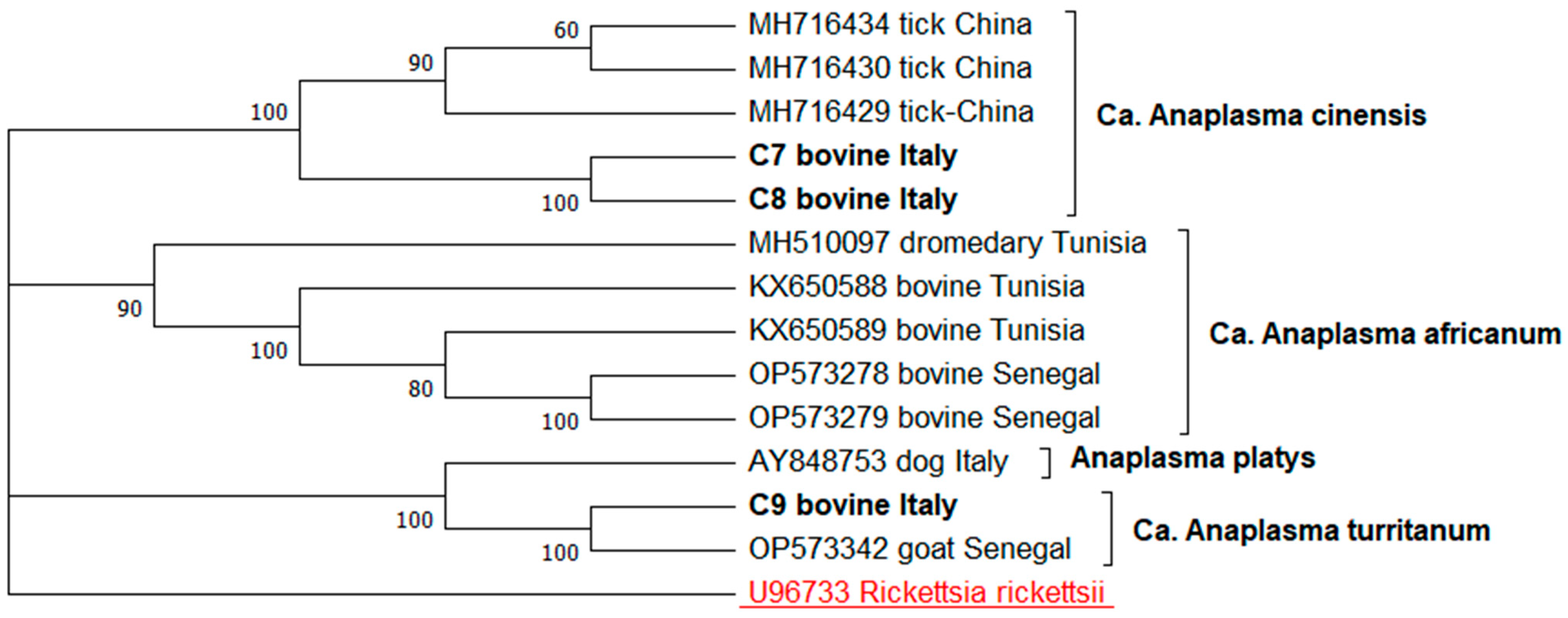
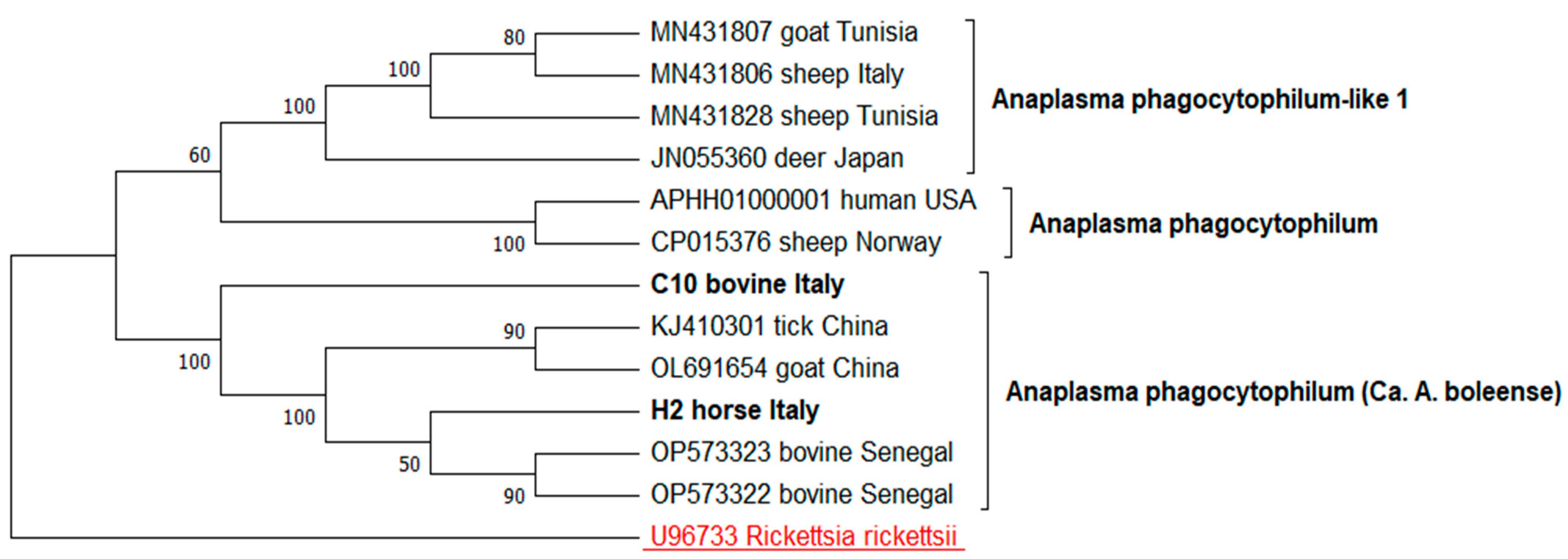
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Atif, F.A. Alpha proteobacteria of genus Anaplasma (Rickettsiales: Anaplasmataceae): Epidemiology and characteristics of Anaplasma species related to veterinary and public health importance. Parasitology 2016, 143, 659–685. [Google Scholar] [CrossRef] [PubMed]
- Karpathy, S.E.; Kingry, L.; Pritt, B.S.; Berry, J.C.; Chilton, N.B.; Dergousoff, S.J.; Cortinas, R.; Sheldon, S.W.; Oatman, S.; Anacker, M.; et al. Anaplasma bovis—Like infections in humans, United States, 2015–2017. Emerg. Infect. Dis. 2023, 29, 1904–1907. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Li, F.; Liao, Y.; Shen, J.-J.; Xu, J.-M.; Chen, Y.-Z.; Li, J.-H.; Holmes, E.C.; Zhang, Y.-Z. Epidemiology and Diversity of Rickettsiales Bacteria in Humans and Animals in Jiangsu and Jiangxi Provinces, China. Sci. Rep. 2019, 9, 13176. [Google Scholar] [CrossRef]
- Li, H.; Zheng, Y.-C.; Ma, L.; Jia, N.; Jiang, B.-G.; Jiang, R.-R.; Huo, Q.-B.; Wang, Y.-W.; Liu, H.-B.; Chu, Y.-L.; et al. Human infection with a novel tick-borne Anaplasma species in China: A surveillance study. Lancet Infect. Dis. 2015, 15, 663–670. [Google Scholar] [CrossRef]
- Bakken, J.S.; Dumler, J.S.; Chen, S.M.; Eckman, M.R.; Van Etta, L.L.; Walker, D.H. Human granulocytic ehrlichiosis in the upper midwest United States. A New Species Emerging? JAMA 1994, 272, 212–218. [Google Scholar] [CrossRef]
- Chen, S.M.; Dumler, J.S.; Bakken, J.S.; Walker, D.H. Identification of a granulocytotropic Ehrlichia species as the etiologic agent of human disease. J. Clin. Microbiol. 1994, 32, 589–595. [Google Scholar] [CrossRef]
- Rar, V.; Tkachev, S.; Tikunova, N. Genetic diversity of Anaplasma bacteria: Twenty years later. Infect. Genet. Evol. 2021, 91, 104833. [Google Scholar] [CrossRef] [PubMed]
- Dumler, J.S.; Barbet, A.F.; Bekker, C.P.; Dasch, G.A.; Palmer, G.H.; Ray, S.C.; Rikihisa, Y.; Rurangirwa, F.R. Reorganization of genera in the families Rickettsiaceae and Anaplasmataceae in the order Rickettsiales: Unification of some species of Ehrlichia with Anaplasma, Cowdria with Ehrlichia and Ehrlichia with Neorickettsia, descriptions of six new species combinations and designation of Ehrlichia equi and ‘HGE agent’ as subjective synonyms of Ehrlichia phagocytophila. Int. J. Syst. Evol. Microbiol. 2001, 51, 2145–2165. [Google Scholar]
- Tate, C.M.; Howerth, E.W.; Mead, D.G.; Dugan, V.G.; Luttrell, M.P.; Sahora, A.I.; Munderloh, U.G.; Davidson, W.R.; Yabsley, M.J. Anaplasma odocoilei sp. nov. (family Anaplasmataceae) from white-tailed deer (Odocoileus virginianus). Ticks Tick-Borne Dis. 2013, 4, 110–119. [Google Scholar] [CrossRef]
- Guo, W.-P.; Tian, J.-H.; Lin, X.-D.; Ni, X.-B.; Chen, X.-P.; Liao, Y.; Yang, S.-Y.; Dumler, J.S.; Holmes, E.C.; Zhang, Y.-Z. Extensive genetic diversity of Rickettsiales bacteria in multiple mosquito species. Sci. Rep. 2016, 6, 38770. [Google Scholar] [CrossRef]
- Kang, Y.-J.; Diao, X.-N.; Zhao, G.-Y.; Chen, M.-H.; Xiong, Y.; Shi, M.; Fu, W.-M.; Guo, Y.-J.; Pan, B.; Chen, X.-P.; et al. Extensive diversity of Rickettsiales bacteria in two species of ticks from China and the evolution of the Rickettsiales. BMC Evol. Biol. 2014, 14, 167. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Yang, J.; Guan, G.; Liu, Z.; Luo, J.; Song, M. Molecular investigation of Anaplasma species in sheep from Heilongjiang province, Northeast China identified four Anaplasma species and a novel genotype of Anaplasma capra. Parasitol. Int. 2020, 76, 102072. [Google Scholar]
- Liu, Z.; Ma, M.; Wang, Z.; Wang, J.; Peng, Y.; Li, Y.; Guan, G.; Luo, J.; Yin, H. Molecular survey and genetic identification of Anaplasma species in goats from central and southern China. Appl. Environ. Microbiol. 2012, 78, 464–470. [Google Scholar] [PubMed]
- Altay, K.; Erol, U.; Sahin, O.F. Anaplasma capra: A new emerging tick-borne zoonotic pathogen. Vet. Res. Commun. 2024, 48, 1329–1340. [Google Scholar]
- Zobba, R.; Schianchi, E.; Ben Said, M.; Belkahia, H.; Messadi, L.; Piredda, R.; Pittau, M.; Alberti, A. GltA typing of Anaplasma strains related to A. platys: Taxonomical and one health implications. Ticks Tick-Borne Dis. 2022, 13, 101850. [Google Scholar]
- Rymaszewska, A.; Grenda, S. Bacteria of the genus Anaplasma—Characteristics of Anaplasma and their vectors: A review. Veterinární Medicína 2008, 53, 573–584. [Google Scholar]
- Aubry, P.; Geale, D.W. A review of bovine anaplasmosis. Transbound. Emerg. Dis. 2011, 58, 1–30. [Google Scholar]
- Potgieter, F. Bovine anaplasmosis. In Infectious Diseases of Livestock, 2nd ed.; Coetzer, J.A.W., Tustin, R.C., Eds.; Oxford University Press: Cape Town, South Africa, 2004; pp. 594–616. [Google Scholar]
- Battilani, M.; De Arcangeli, S.; Balboni, A.; Dondi, F. Genetic diversity and molecular epidemiology of Anaplasma. Infect. Genet. Evol. 2017, 49, 195–211. [Google Scholar]
- Shabana, I.I.; Alhadlag, N.M.; Zaraket, H. Diagnostic tools of caprine and ovine anaplasmosis: A direct comparative study. BMC Vet. Res. 2018, 14, 165. [Google Scholar]
- Silaghi, C.; Santos, A.S.; Gomes, J.; Christova, I.; Matei, I.A.; Walder, G.; Domingos, A.; Bell-Sakyi, L.; Sprong, H.; Von Loewenich, F.D.; et al. Guidelines for the direct detection of Anaplasma spp. in diagnosis and epidemiological studies. Vector-Borne Zoonotic Dis. 2017, 17, 12–22. [Google Scholar]
- Pan, L.; Zhang, L.; Wang, G.; Liu, Q.; Yu, Y.; Wang, S.; Yu, H.; He, J. Rapid, simple, and sensitive detection of Anaplasma phagocytophilum by loop-mediated isothermal amplification of the msp2 gene. J. Clin. Microbiol. 2011, 49, 4117–4120. [Google Scholar] [PubMed]
- McGuire, T.C.; Davis, W.C.; Brassfield, A.L.; McElwain, T.F.; Palmer, G.H. Identification of Anaplasma marginale long-term carrier cattle by detection of serum antibody to isolated MSP-3. J. Clin. Microbiol. 1991, 29, 788–793. [Google Scholar]
- Von Fricken, M.E.; Lkhagvatseren, S.; Boldbaatar, B.; Nymadawa, P.; Weppelmann, T.A.; Baigalmaa, B.-O.; Anderson, B.D.; Reller, M.E.; Lantos, P.M.; Gray, G.C. Estimated seroprevalence of Anaplasma spp. and spotted fever group Rickettsia exposure among herders and livestock in Mongolia. Acta Trop. 2018, 177, 179–185. [Google Scholar]
- Yoo, J.; Chung, J.-H.; Kim, C.-M.; Yun, N.R.; Kim, D.-M. Asymptomatic-anaplasmosis confirmation using genetic and serological tests and possible coinfection with spotted fever group Rickettsia: A case report. BMC Infect. Dis. 2020, 20, 458. [Google Scholar]
- Alberti, A.; Zobba, R.; Chessa, B.; Addis, M.F.; Sparagano, O.; Pinna Parpaglia, M.L.; Cubeddu, T.; Pintori, G.; Pittau, M. Equine and canine Anaplasma phagocytophilum strains isolated on the island of Sardinia (Italy) are phylogenetically related to pathogenic strains from the United States. Appl. Environ. Microbiol. 2005, 71, 6418–6422. [Google Scholar]
- Zobba, R.; Anfossi, A.G.; Pinna Parpaglia, M.L.; Dore, G.M.; Chessa, B.; Spezzigu, A.; Rocca, S.; Visco, S.; Pittau, M.; Alberti, A. Molecular investigation and phylogeny of Anaplasma spp. in mediterranean ruminants reveal the presence of neutrophil-tropic strains closely related to Anaplasma platys. Appl. Environ. Microbiol. 2014, 80, 271–280. [Google Scholar]
- Zobba, R.; Ben Said, M.; Belkahia, H.; Pittau, M.; Cacciotto, C.; Pinna Parpaglia, M.L.; Messadi, L.; Alberti, A. Molecular epidemiology of Anaplasma spp. related to A. phagocytophilum in mediterranean small ruminants. Acta Trop. 2020, 202, 105286. [Google Scholar]
- Chisu, V.; Zobba, R.; Lecis, R.; Sotgiu, F.; Masala, G.; Foxi, C.; Pisu, D.; Alberti, A. GroEL Typing and phylogeny of Anaplasma species in ticks from domestic and wild vertebrates. Ticks Tick-Borne Dis. 2018, 9, 31–36. [Google Scholar]
- Chisu, V.; Loi, F.; Foxi, C.; Chessa, G.; Masu, G.; Rolesu, S.; Masala, G. Coexistence of tick-borne pathogens in ticks collected from their hosts in Sardinia: An update. Acta Parasitol. 2020, 65, 999–1004. [Google Scholar]
- Guccione, C.; Colomba, C.; Tolomeo, M.; Trizzino, M.; Iaria, C.; Cascio, A. Rickettsiales in Italy. Pathogens 2021, 10, 181. [Google Scholar] [CrossRef]
- Kolbert, C. Detection of the agent of human granulocytic ehrlichiosis by PCR. In PCR Protocols for Emerging Infectious Diseases; Persing, D.H., Ed.; ASM Press: Washington, DC, USA, 1996; pp. 106–111. [Google Scholar]
- Guo, W.P.; Zhang, B.; Wang, Y.H.; Xu, G.; Wang, X.; Ni, X.; Zhou, E.M. Molecular identification and characterization of Anaplasma capra and Anaplasma platys-like in Rhipicephalus microplus in Ankang, Northwest China. BMC Infect. Dis. 2019, 19, 434. [Google Scholar]
- Ybañez, A.P.; Matsumoto, K.; Kishimoto, T.; Inokuma, H. Molecular analyses of a potentially novel Anaplasma species closely related to Anaplasma phagocytophilum detected in sika deer (Cervus nippon yesoensis) in Japan. Vet. Microbiol. 2012, 157, 232–236. [Google Scholar] [PubMed]
- Kumar, S.; Stecher, G.; Suleski, M.; Sanderford, M.; Sharma, S.; Tamura, K. MEGA12: Molecular Evolutionary Genetic Analysis version 12 for adaptive and green computing. Mol. Biol. Evol. 2024, 41, 263. [Google Scholar]
- Torina, A.; Alongi, A.; Naranjo, V.; Estrada-Peña, A.; Vicente, J.; Scimeca, S.; Marino, A.M.F.; Salina, F.; Caracappa, S.; De La Fuente, J. Prevalence and genotypes of Anaplasma species and habitat suitability for ticks in a mediterranean ecosystem. Appl. Environ. Microbiol. 2008, 74, 7578–7584. [Google Scholar]
- Ben Said, M.; Belkahia, H.; Karaoud, M.; Bousrih, M.; Yahiaoui, M.; Daaloul-Jedidi, M.; Messadi, L. First molecular survey of Anaplasma bovis in small ruminants from Tunisia. Vet. Microbiol. 2015, 179, 322–326. [Google Scholar]
- Chisu, V.; Dei Giudici, S.; Foxi, C.; Chessa, G.; Peralta, F.; Sini, V.; Masala, G. Anaplasma Species in ticks infesting mammals of Sardinia, Italy. Animals 2023, 13, 1332. [Google Scholar] [CrossRef]
- Saratsis, A.; Ligda, P.; Aal, F.; Jelicic, M.; Polgar, J.; De Vries, M.; Mastranestasis, I.; Musella, V.; Rinaldi, L.; Jongejan, F.; et al. The scenario of ticks and tick-borne pathogens of sheep on a mediterranean island. Microorganisms 2022, 10, 1551. [Google Scholar] [CrossRef]
- Zobba, R.; Murgia, C.; Dahmani, M.; Mediannikov, O.; Davoust, B.; Piredda, R.; Schianchi, E.; Scagliarini, A.; Pittau, M.; Alberti, A. Emergence of Anaplasma species related to A. phagocytophilum and A. platys in Senegal. Int. J. Mol. Sci. 2022, 24, 35. [Google Scholar] [CrossRef]
- Kolo, A. Anaplasma species in Africa—A century of discovery: A review on molecular epidemiology, genetic diversity, and control. Pathogens 2023, 12, 702. [Google Scholar] [CrossRef]
| Pathogens | Gene Target | PCR Assay | Gene Primers | Primer Sequence (5′ to 3′) | Amplicon Size (bp) | References |
|---|---|---|---|---|---|---|
| Anaplasma spp. | 16S rRNA | PCR | HGE–521F HGE–790R | TGTAGGCGGTTCGGTAAGTTAAAG | 293 bp | [32] |
| CTTAACGCGTTAGCTACAACACAG | ||||||
| A. platys | gltA | Nested PCR | Pglt-F | ATGAWAGAAAAWGCTGTTTT | 660 bp | [33] |
| Pglt-R1 | TCATGRTCTGCATGCATKATG | |||||
| Pglt-R2 | CATGCATKATGAARATMGCAT | |||||
| Pglt-L-F1 | ATGAWAGAAAAWGCTGTTTT | 610 bp | ||||
| Pglt-L-F2 | TCATGRTCTGCATGCATKATG | |||||
| Pglt-L-R | CATGCATKATGAARATMGCAT | |||||
| GroEL | Nested PCR | Pgro_F_F | GATGCWCATCCYATSGCMATG | 1195 bp | ||
| Pgro_F_R1 | CGTGMTSGCTATAGCGMAART | |||||
| Pgro_F_R2 | TCAYACCATTGDGAYRCCCAT | |||||
| A. phagocytophilum | GroEL | Nested PCR | EphplGroEL(569)F | ATGGTATGCAGTTTGATCGC | 573 bp | [26] |
| EphplGroEL(1193)R | TCTACTCTGTCTTTGCGTTC | |||||
| EphGroEL(1142)R | TTGAGTACAGCAACACCACCGGAA | |||||
| A. phagocytophilum-like 1 | GroEL | Nested PCR | APGRSP41F3 | GAATCTAGCTATGTTGCATGATAATGT | 1446 bp | [34] |
| APSPGR1697R1 | CAGCATAAACACGCACTACGAA | |||||
| APSPGR234F2 | CGTAGTAGGACTTTCCGGTTTTTG | |||||
| APSPGR1680R2 | TGCACAGCATAAACACGCACTA | |||||
| A. phagocytophilum-like 2 | GroEL | Nested PCR | APHAGOVAR2GROEL_F | TACTCTAGAAGACGCGGTAG | 793 bp | [28] |
| APHAGOVAR2GROEL_R1 | ACGAACATTCTTAGCAGTCC | |||||
| APHAGOVAR2GROEL_R2 | CTTCTATCACCAAATCCTGG |
| Target Gene | Sequence Type * | Host Sources | Locality | BlastN | GenBank Accession Number (s) |
|---|---|---|---|---|---|
| 16S rRNA | HCS1 | Horse, Cattle, Swine | Aglientu, Arzachena, Baunei, Birori, Chiaramonti, Fordongianus, Laerru, Orgosolo, Santu LussurgiuSedini, Villanova Monteleone | 100% A. capra/A. ovis/A. centrale/A. marginale group | PQ579998–PQ580007 |
| C1 | Cattle | Anela, Chiaramonti, Milis, Oristano, Monti | 100% A. phagocytophilum/A. platys/Ca. A. cinensis group | PQ580008–PQ580009 | |
| C2 | Cattle | Orgosolo, Santa Giusta, Villacidro, Laerru, Chiaramonti | 100% A. phagocytophilum/A. platys/Ca. A. cinensis group | PQ580010–PQ580013 | |
| H1 | Horse | Santa Giusta | 98.78% A. phagocytophilum/A. platys/Ca. A. cinensis group | PQ580014 | |
| gltA | C3 | Cattle | Orgosolo | 99.56% Ca. A. cinensis | PQ609681–PQ609682 |
| C4 | Cattle | Orgosolo | 99.47% Ca. A. cinensis | PQ609683 | |
| C5 | Cattle | Orgosolo | 99.47% Ca. A. cinensis | PQ609684 | |
| C6 | Cattle | Illorai | 99.47% Ca. A. turritanum | PQ609685 | |
| GroEL | C7 | Cattle | Orgosolo | 99.32% Ca A. cinensis | PQ588703–PQ588704 |
| C8 | Cattle | Orgosolo | 99.22% Ca. A. cinensis | PQ588705 | |
| C9 | Cattle | Milis | 99–100% Ca. A. turritanum | PQ588706 | |
| GroEL | C10 | Cattle | Bortigiadas | 97.74% Anaplasma sp | PQ588707 |
| H2 | Horse | Santa Giusta | 99.47% Anaplasma sp. | PQ588708 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chisu, V.; Zobba, R.; Masala, G.; Chessa, G.; Giua, L.; Bianco, P.; Cacciotto, C.; Bazzoni, E.; Alberti, A. Emergence of Novel Anaplasma Species in the Mediterranean Area. Animals 2025, 15, 1029. https://doi.org/10.3390/ani15071029
Chisu V, Zobba R, Masala G, Chessa G, Giua L, Bianco P, Cacciotto C, Bazzoni E, Alberti A. Emergence of Novel Anaplasma Species in the Mediterranean Area. Animals. 2025; 15(7):1029. https://doi.org/10.3390/ani15071029
Chicago/Turabian StyleChisu, Valentina, Rosanna Zobba, Giovanna Masala, Giovanna Chessa, Laura Giua, Piera Bianco, Carla Cacciotto, Emanuela Bazzoni, and Alberto Alberti. 2025. "Emergence of Novel Anaplasma Species in the Mediterranean Area" Animals 15, no. 7: 1029. https://doi.org/10.3390/ani15071029
APA StyleChisu, V., Zobba, R., Masala, G., Chessa, G., Giua, L., Bianco, P., Cacciotto, C., Bazzoni, E., & Alberti, A. (2025). Emergence of Novel Anaplasma Species in the Mediterranean Area. Animals, 15(7), 1029. https://doi.org/10.3390/ani15071029










