Association of Stress and Inflammatory Diseases with Serum Ferritin and Iron Concentrations in Neonatal Calves
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals, Housing, and Feeding
2.2. Experimental Procedures
2.3. Blood Parameters
2.4. Evaluation of Health Status
2.5. Statistics and Data Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| WBC | White blood cell |
| TP | Total protein |
| IL1β | Interleukin 1β |
| IL6 | Interleukin 6 |
| SP | Substance P |
| FRAP | Ferric-reducing ability of plasma |
| AUC | Area under the curve |
| ROS | Reactive oxygen species |
| ANOVA | Analysis of variance |
| SAA | Serum amyloid A |
| SIRS | Systemic inflammatory response syndrome |
References
- Allan, J.; Plate, P.; van Winden, S. The effect of iron dextran injection on daily weight gain and haemoglobin values in whole milk fed calves. Animals 2020, 10, 853. [Google Scholar] [CrossRef]
- Mohri, M.; Poorsina, S.; Sedaghat, R. Effects of parenteral supply of iron on RBC parameters, performance, and health in neonatal dairy calves. Biol. Trace Elem. Res. 2010, 136, 33–39. [Google Scholar] [CrossRef]
- Khaleghnia, N.; Mohri, M.; Seifi, H.A. The effects of parenteral iron administration on thyroid hormones, hematology, oxidative stress characteristics, performance, and health in neonatal Holstein calves. Biol. Trace Elem. Res. 2021, 199, 1823–1832. [Google Scholar] [CrossRef]
- Baydar, E.; Dabak, M. Serum iron as an indicator of acute inflammation in cattle. J. Dairy Sci. 2014, 97, 222–228. [Google Scholar] [CrossRef]
- Tsukano, K.; Suzuki, K. Serum iron concentration is a useful biomarker for assessing the level of inflammation that causes systemic symptoms in bovine acute mastitis similar to plasma haptoglobin. J. Vet. Med. Sci. 2020, 82, 1440–1444. [Google Scholar] [CrossRef]
- Humann-Ziehank, E. Neuer Blick auf ein altes Element—Eisen, hepcidin und Entzündung [New insights on a long-known element—Iron, hepcidin and inflammation]. Tierarztl. Prax. Ausg. G Grosstiere/Nutztiere 2020, 48, 183–190. [Google Scholar] [CrossRef]
- Kupczyński, R.; Bednarski, M.; Śpitalniak, K.; Pogoda-Sewerniak, K. Effects of protein-iron complex concentrate supplementation on iron metabolism, oxidative and immune status in preweaning calves. Int. J. Mol. Sci. 2017, 18, 1501. [Google Scholar] [CrossRef]
- Rajabian, F.; Mohri, M.; Heidarpour, M. Relationships between oxidative stress, haematology and iron profile in anaemic and non-anaemic calves. Vet. Rec. 2017, 181, 265. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef]
- Ganz, S.; Failing, K.; Hassan, A.A.; Bülte, M.; Wehrend, A. Influence of first colostrum pasteurization on serum immunoglobulin G, iron, and activity of gamma-glutamyltransferase in newborn dairy calves. Vet. World 2021, 14, 2267–2272. [Google Scholar] [CrossRef]
- Mann, G.R.; Duncan, S.E.; Knowlton, K.F.; Dietrich, A.D.; O’Keefe, S.F. Effects of mineral content of bovine drinking water: Does iron content affect milk quality? J. Dairy Sci. 2013, 96, 7478–7489. [Google Scholar] [CrossRef]
- Moretti, D.B.; Santos, C.B.; Alencar, S.M.; Machado-Neto, R. Colostrum from primiparous Holstein cows shows higher antioxidant activity than colostrum of multiparous ones. J. Dairy Res. 2020, 87, 356–359. [Google Scholar] [CrossRef]
- Jegasothy, H.; Weerakkody, R.; Selby-Pham, S.; Bennett, L.E. In vitro heme and non-heme iron capture from hemoglobin, myoglobin and ferritin by bovine lactoferrin and implications for suppression of reactive oxygen species in vivo. Biometals 2014, 27, 1371–1382. [Google Scholar] [CrossRef]
- Cuisiniere, T.; Calvé, A.; Fragoso, G.; Oliero, M.; Hajjar, R.; Gonzalez, E.; Santos, M.M. Oral iron supplementation after antibiotic exposure induces a deleterious recovery of the gut microbiota. BMC Microbiol. 2021, 21, 259. [Google Scholar] [CrossRef]
- Welchman, D.D.; Whelehan, O.P.; Webster, A.J. Haematology of veal calves reared in different husbandry systems and the assessment of iron deficiency. Vet. Rec. 1988, 123, 505–510. [Google Scholar] [CrossRef]
- Bus, J.D.; Stockhofe, N.; Webb, L.E. Invited review: Abomasal damage in veal calves. J. Dairy Sci. 2019, 102, 943–960. [Google Scholar] [CrossRef]
- McGillivray, S.R.; Searcy, G.P.; Hirsch, V.M. Serum iron, total iron binding capacity, plasma copper and hemoglobin types in anemic and poikilocytic calves. Can. J. Comp. Med. 1985, 49, 286–290. [Google Scholar]
- Bezeau, L.M.; Clark, R.D. Effect of injectable iron-dextran on dairy calves and lambs. Can. J. Comp. Med. Vet. Sci. 1965, 29, 283–285. [Google Scholar]
- Joerling, J.; Doll, K. Monitoring of iron deficiency in calves by determination of serum ferritin in comparison with serum iron: A preliminary study. Open Vet. J. 2019, 9, 177–184. [Google Scholar] [CrossRef]
- Kanno, Y.; Ohtsuka, H.; Yoshikawa, Y.; Watanabe, K.; Orino, K. Measurement of ferritin and anti-ferritin autoantibodies in serum and colostrum of Holstein and Japanese Black cows. Anim. Sci. J. 2013, 84, 556–561. [Google Scholar] [CrossRef]
- Daru, J.; Colman, K.; Stanworth, S.J.; de La Salle, B.; Wood, E.M.; Pasricha, S.-R. Serum ferritin as an indicator of iron status: What do we need to know? Am. J. Clin. Nutr. 2017, 106 (Suppl. 6), 1634S–1639S. [Google Scholar] [CrossRef]
- Kolb, E.; Kolb, E. Neuere Erkenntnisse zur Struktur und zur Funktion des Laktoferrins und des ferritins [Recent knowledge of the structure and function of lactoferrin and ferritin]. Z. Gesamte Inn. Med. 1989, 44, 345–350. [Google Scholar]
- Wang, W.; Knovich, M.A.; Coffman, L.G.; Torti, F.M.; Torti, S.V. Serum ferritin: Past, present and future. Biochim. Biophys. Acta (BBA) 2010, 1800, 760–769. [Google Scholar] [CrossRef]
- Miyata, Y.; Furugouri, K.; Shijimaya, K. Developmental changes in serum ferritin concentration of dairy calves. J. Dairy Sci. 1984, 67, 1256–1263. [Google Scholar] [CrossRef]
- Furugouri, K.; Miyata, Y.; Shijimaya, K. Ferritin in blood serum of dairy cows. J. Dairy Sci. 1982, 65, 1529–1534. [Google Scholar] [CrossRef]
- Daru, J.; Allotey, J.; Peña-Rosas, J.P.; Khan, K.S. Serum ferritin thresholds for the diagnosis of iron deficiency in pregnancy: A systematic review. Transfus. Med. 2017, 27, 167–174. [Google Scholar] [CrossRef]
- Schnell, S.A.; Ohtsuka, H.; Kakinuma, S.; Yoshikawa, Y.; Watanabe, K.; Orino, K. Iron and ferritin levels in the serum and milk of bovine leukemia virus-infected dairy cows. Front. Vet. Sci. 2015, 2, 12. [Google Scholar] [CrossRef]
- Ueda, N.; Takasawa, K. Impact of inflammation on ferritin, hepcidin and the management of iron deficiency anemia in chronic kidney disease. Nutrients 2018, 10, 1173. [Google Scholar] [CrossRef]
- Sickinger, M.; Joerling, J.; Büttner, K.; Roth, J.; Wehrend, A. Influence of an iron dextran injection in various diseases on hematological blood parameters, including serum ferritin, neonatal dairy calves. BMC Vet. Res. 2024, 20, 379. [Google Scholar] [CrossRef]
- Donohue, J.; Sgoutas, D. Improved radioimmunoassay of plasm cortisol. Clin. Chem. 1975, 21, 770–773. [Google Scholar] [CrossRef]
- Gelaw, Y.; Woldu, B.; Melku, M. The role of reticulocyte hemoglobin content for diagnosis of iron deficiency and iron deficiency anemia, and monitoring of iron therapy: A literature review. Clin. Lab. 2019, 65, 2211–2218. [Google Scholar] [CrossRef]
- Hansen, S.L.; Trakooljul, N.; Liu, H.C.; Hicks, J.A.; Ashwell, M.S.; Spears, J.W. Proteins involved in iron metabolism in beef cattle are affected by copper deficiency in combination with high dietary manganese, but not by copper deficiency alone. J. Anim. Sci. 2010, 88, 275–283. [Google Scholar] [CrossRef]
- Suchdev, P.S.; Williams, A.M.; Mei, Z.; Flores-Ayala, R.; Pasricha, S.-R.; Rogers, L.M.; Namaste, S.M. Assessment of iron status in settings of inflammation: Challenges and potential approaches. Am. J. Clin. Nutr. 2017, 106 (Suppl. 6), 1626S–1633S. [Google Scholar] [CrossRef]
- Chao, K.C.; Chang, C.C.; Chiou, H.Y.; Chang, J.S. Serum ferritin is inversely correlated with testosterone in boys and young male adolescents: A cross-sectional study in Taiwan. PLoS ONE 2015, 10, e0144238. [Google Scholar] [CrossRef]
- Qian, Y.; Yin, C.; Chen, Y.; Zhang, S.; Jiang, L.; Wang, F.; Zhao, M.; Liu, S. Estrogen contributes to regulating iron metabolism through governing ferroportin signaling via an estrogen response element. Cell Signal. 2015, 27, 934–942. [Google Scholar] [CrossRef]
- Landinger, A.; Zablotski, Y.; Knubben-Schweizer, G.; Tschoner, T. Comparison of plasma substance P concentrations in the blood of healthy male and female German Simmental calves. BMC Vet. Res. 2024, 20, 226. [Google Scholar] [CrossRef]
- Dunn, S.E.; Perry, W.A.; Klein, S.L. Mechanisms and consequences of sex differences in immune responses. Nat. Rev. Nephrol. 2024, 20, 37–55. [Google Scholar] [CrossRef]
- Cabello, G. Plasma cortisol and aldosterone levels in healthy and diarrhoeic calves. Br. Vet. J. 1980, 136, 160–167. [Google Scholar] [CrossRef]
- Brückmann, A.; Höck, C.; Linke, K.; Hennies, M.; Schallenberger, E. Alterations of growth hormone, cortisol, luteinizing hormone, and insulin concentrations in early-postnatal calves affected with diarrhea. Domest. Anim. Endocrinol. 2000, 18, 187–197. [Google Scholar] [CrossRef]
- Masmeijer, C.; Deprez, P.; van Leenen, K.; de Cremer, L.; Cox, E.; Devriendt, B.; Pardon, B. Arrival cortisol measurement in veal calves and its association with body weight, protein fractions, animal health and performance. Prev. Vet. Med. 2021, 187, 105251. [Google Scholar] [CrossRef]
- Córdova, A.; Mielgo-Ayuso, J.; Fernandez-Lazaro, C.I.; Caballero-García, A.; Roche, E.; Fernández-Lázaro, D. Effect of iron supplementation on the modulation of iron metabolism, muscle damage biomarkers and cortisol in professional cyclists. Nutrients 2019, 11, 500. [Google Scholar] [CrossRef]
- Santinello, M.; Lora, I.; Villot, C.; Cozzi, G.; Penasa, M.; Chevaux, E.; Martin, B.; Guerra, A.; Righi, F.; De Marchi, M. Metabolic profile of Charolais young bulls transported over long-distance. Prev. Vet. Med. 2024, 231, 106296. [Google Scholar] [CrossRef]
- Mohri, M.; Sharifi, K.; Eidi, S. Hematology and serum biochemistry of Holstein dairy calves: Age related changes and comparison with blood composition in adults. Res. Vet. Sci. 2007, 83, 30–39. [Google Scholar] [CrossRef]
- Panousis, N.; Siachos, N.; Kitkas, G.; Kalaitzakis, E.; Kritsepi-Konstantinou, M.; Valergakis, G.E. Hematology reference intervals for neonatal Holstein calves. Res. Vet. Sci. 2018, 118, 1–10. [Google Scholar] [CrossRef]
- Manosalva, C.; Quiroga, J.; Hidalgo, A.I.; Alarcón, P.; Anseoleaga, N.; Hidalgo, M.A.; Burgos, R.A. Role of lactate in inflammatory processes: Friend or foe. Front. Immunol. 2021, 12, 808799. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, S.; Li, Q.; Wu, Y.; Jia, X.; Feng, W.; Li, Z.; Shi, Y.; Hou, Q.; Ma, J.; et al. Lactate modulates iron metabolism by binding soluble adenylyl cyclase. Cell Metab. 2023, 35, 1597–1612.e6. [Google Scholar] [CrossRef]
- Rodriguez, A.R.; Herzberg, D.E.; Werner, M.P.; Müller, H.Y.; Bustamante, H.A. Plasma concentration of norepinephrine, β-endorphin, and substance P in lame dairy cows. J. Vet. Res. 2018, 62, 193–197. [Google Scholar] [CrossRef]
- Zhu, Z.; Bhatia, M. Inflammation and organ injury the role of substance P and its receptors. Int. J. Mol. Sci. 2023, 24, 6140. [Google Scholar] [CrossRef]
- Abultdinova, A.; Jakupov, I.; Roth, J.; Failing, K.; Wehrend, A.; Sickinger, M. Association of bovine uterine involution disturbances with serum neuropeptide concentrations. Vet. World 2020, 13, 1854–1857. [Google Scholar] [CrossRef]
- Tschoner, T.; Feist, M. Substance P concentrations in the blood plasma and serum of adult cattle and calves during different painful procedures and conditions—A systematic review. BMC Vet. Res. 2022, 18, 232. [Google Scholar] [CrossRef]
- Tsukano, K.; Shimamori, T.; Fukuda, T.; Nishi, Y.; Otsuka, M.; Kitade, Y.; Suzuki, K. Serum iron concentration as a marker of inflammation in young cows that underwent dehorning operation. J. Vet. Med. Sci. 2019, 81, 626–628. [Google Scholar] [CrossRef]
- Ganz, T.; Nemeth, E. Hypoferremia of inflammation: Innate host defense against infections. Blood Cells Mol. Dis. 2024, 104, 102777. [Google Scholar] [CrossRef] [PubMed]
- Saco, Y.; Bassols, A. Acute phase proteins in cattle and swine: A review. Vet. Clin. Pathol. 2023, 52 (Suppl. 1), 50–63. [Google Scholar] [CrossRef] [PubMed]
- Pirkkalainen, H.; Talvio, I.; Kujala-Wirth, M.; Soveri, T.; Orro, T. Acute phase response of sole ulcer, white line disease and digital dermatitis in dairy cows. Vet. Anim. Sci. 2022, 17, 100253. [Google Scholar] [CrossRef]
- Trefz, F.M.; Balmer, M.; Peters, L.M.; Bruckmaier, R.M.; Meylan, M. Association of results of the glutaraldehyde coagulation test with plasma acute phase protein concentrations and hematologic findings in hospitalized cows. Front. Vet. Sci. 2024, 11, 1404809. [Google Scholar] [CrossRef]
- Smith, B.I.; Kauffold, J.; Sherman, L. Serum haptoglobin concentrations in dairy cattle with lameness due to claw disorders. Vet. J. 2010, 186, 162–165. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.F.; Windeyer, M.C.; Duffield, T.F.; Haley, D.B.; Pearl, D.L.; Waalderbos, K.M.; Leslie, K.E. Associations of serum haptoglobin in newborn dairy calves with health, growth, and mortality up to 4 months of age. J. Dairy Sci. 2014, 97, 7844–7855. [Google Scholar] [CrossRef]
- Jaramillo, C.; Renaud, D.L.; Arroyo, L.G.; Kenney, D.G.; Gamsjaeger, L.; Gomez, D.E. Serum haptoglobin concentration and liver enzyme activity as indicators of systemic inflammatory response syndrome and survival of sick calves. J. Vet. Intern. Med. 2022, 36, 812–819. [Google Scholar] [CrossRef]
- Choi, K.-S.; Kang, J.-H.; Cho, H.-C.; Yu, D.-H.; Park, J. Changes in serum protein electrophoresis profiles and acute phase proteins in calves with diarrhea. Can. J. Vet. Res. 2021, 85, 45–50. [Google Scholar]
- Ider, M.; Maden, M. Biomarkers of infectious pneumonia in naturally infected calves. Am. J. Vet. Res. 2022, 83, ajvr.21.10.0172. [Google Scholar] [CrossRef]
- Worwood, M. Serum ferritin. CRC Crit. Rev. Clin. Lab. Sci. 1979, 10, 171–204. [Google Scholar] [CrossRef] [PubMed]
- Slaats, J.; ten Oever, J.; van de Veerdonk, F.L.; Netea, M.G. IL-1β/IL-6/CRP and IL-18/ferritin: Distinct inflammatory programs in infections. PLoS Pathog. 2016, 12, e1005973. [Google Scholar] [CrossRef]
- DePalma, R.G.; Hayes, V.W.; O’Leary, T.J. Optimal serum ferritin level range: Iron status measure and inflammatory biomarker. Metallomics 2021, 13, mfab030. [Google Scholar] [CrossRef] [PubMed]
- Albera, E.; Kankofer, M. The comparison of antioxidative/oxidative profile in blood, colostrum and milk of early post-partum cows and their newborns. Reprod. Domest. Anim. 2011, 46, 763–769. [Google Scholar] [CrossRef]
- Fu, Z.L.; Yang, Y.; Ma, L.; Malmuthuge, N.; Guan, L.L.; Bu, D.P. Dynamics of oxidative stress and immune responses in neonatal calves during diarrhea. J. Dairy Sci. 2024, 107, 1286–1298. [Google Scholar] [CrossRef]
- Nemeth, E.; Ganz, T. Hepcidin-ferroportin interaction controls systemic iron homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef]
- Wolff, F.; Gentelet, M.; Gulbis, B.; Cotton, F. Hepcidin on 24-hour urine collection: Preanalytical aspects and correlation with ferritin levels. Scand. J. Clin. Lab. Investig. 2016, 76, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Taheri, N.; Roshandel, G.; Mojerloo, M.; Hadad, M.; Mirkarimi, H.; Nejad, R.K.; Joshaghani, H.R. Comparison of serum levels of hepcidin and pro-hepcidin in hemodialysis patients and healthy subjects. Saudi J. Kidney Dis. Transpl. 2015, 26, 34–38. [Google Scholar] [CrossRef]
- Galesloot, T.E.; Vermeulen, S.H.; Geurts-Moespot, A.J.; Klaver, S.M.; Kroot, J.J.; van Tienoven, D.; Wetzels, J.F.M.; Kiemeney, L.A.L.M.; Sweep, F.C.; den Heijer, M.; et al. Serum hepcidin: Reference ranges and biochemical correlates in the general population. Blood 2011, 117, e218–e225. [Google Scholar] [CrossRef]
- Blake, M.J.; Castro, L.; Leeder, J.S.; Kearns, G.L. Ontogeny of drug metabolizing enzymes in the neonate. Semin. Fetal Neonatal Med. 2005, 10, 123–138. [Google Scholar] [CrossRef]

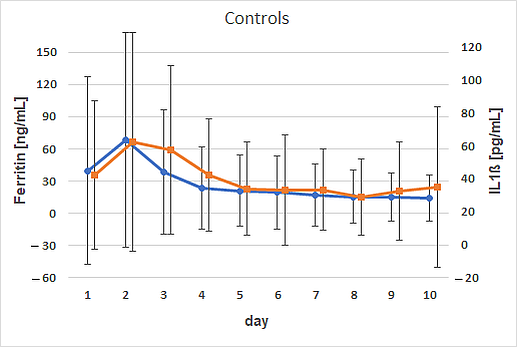

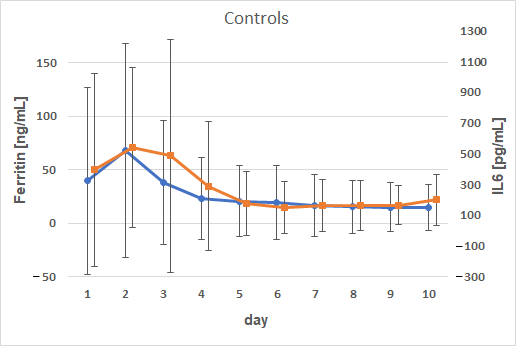
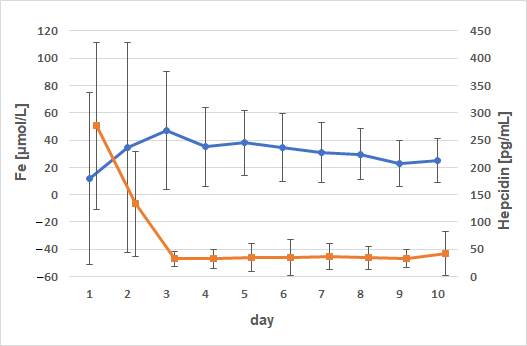
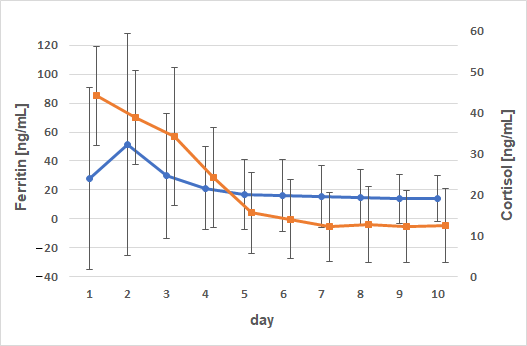

| Variable | Grouping by Sex or Treatment | p-Value | Spearman Correlation Coefficient |
|---|---|---|---|
| Fe | Treatment: Yes | 0.0024 | −0.64 |
| Fe | Sex: Female | 0.0021 | −0.65 |
| Hepcidin | Treatment: No | <0.0001 | −0.83 |
| Hepcidin | Sex: Female | 0.0016 | −0.66 |
| Hepcidin | Sex: Male | 0.0046 | −0.61 |
| Lactate | Treatment: Yes | 0.0386 | −0.47 |
| IL1β | Sex: Male | 0.0427 | −0.46 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sickinger, M.; Jörling, J.; Büttner, K.; Roth, J.; Wehrend, A. Association of Stress and Inflammatory Diseases with Serum Ferritin and Iron Concentrations in Neonatal Calves. Animals 2025, 15, 1021. https://doi.org/10.3390/ani15071021
Sickinger M, Jörling J, Büttner K, Roth J, Wehrend A. Association of Stress and Inflammatory Diseases with Serum Ferritin and Iron Concentrations in Neonatal Calves. Animals. 2025; 15(7):1021. https://doi.org/10.3390/ani15071021
Chicago/Turabian StyleSickinger, Marlene, Jessica Jörling, Kathrin Büttner, Joachim Roth, and Axel Wehrend. 2025. "Association of Stress and Inflammatory Diseases with Serum Ferritin and Iron Concentrations in Neonatal Calves" Animals 15, no. 7: 1021. https://doi.org/10.3390/ani15071021
APA StyleSickinger, M., Jörling, J., Büttner, K., Roth, J., & Wehrend, A. (2025). Association of Stress and Inflammatory Diseases with Serum Ferritin and Iron Concentrations in Neonatal Calves. Animals, 15(7), 1021. https://doi.org/10.3390/ani15071021






