CTNNB1 and CDH1 Regulate Trophoblast Cell Adhesion and Junction Formation in Yak Placental Tissue at Different Gestational Stages
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Preparation and Collection
2.2. Hematoxylin–Eosin (H&E) Staining
2.3. Immunofluorescence (IF) Staining
2.4. DIA Sequence and Bioinformatics Analysis
2.5. Immunohistochemistry (IHC) Staining
2.6. RNA Isolation, cDNA Synthesis, and Quantitative PCR Assays
2.7. Western Blotting
2.8. Statistical Analysis
3. Results
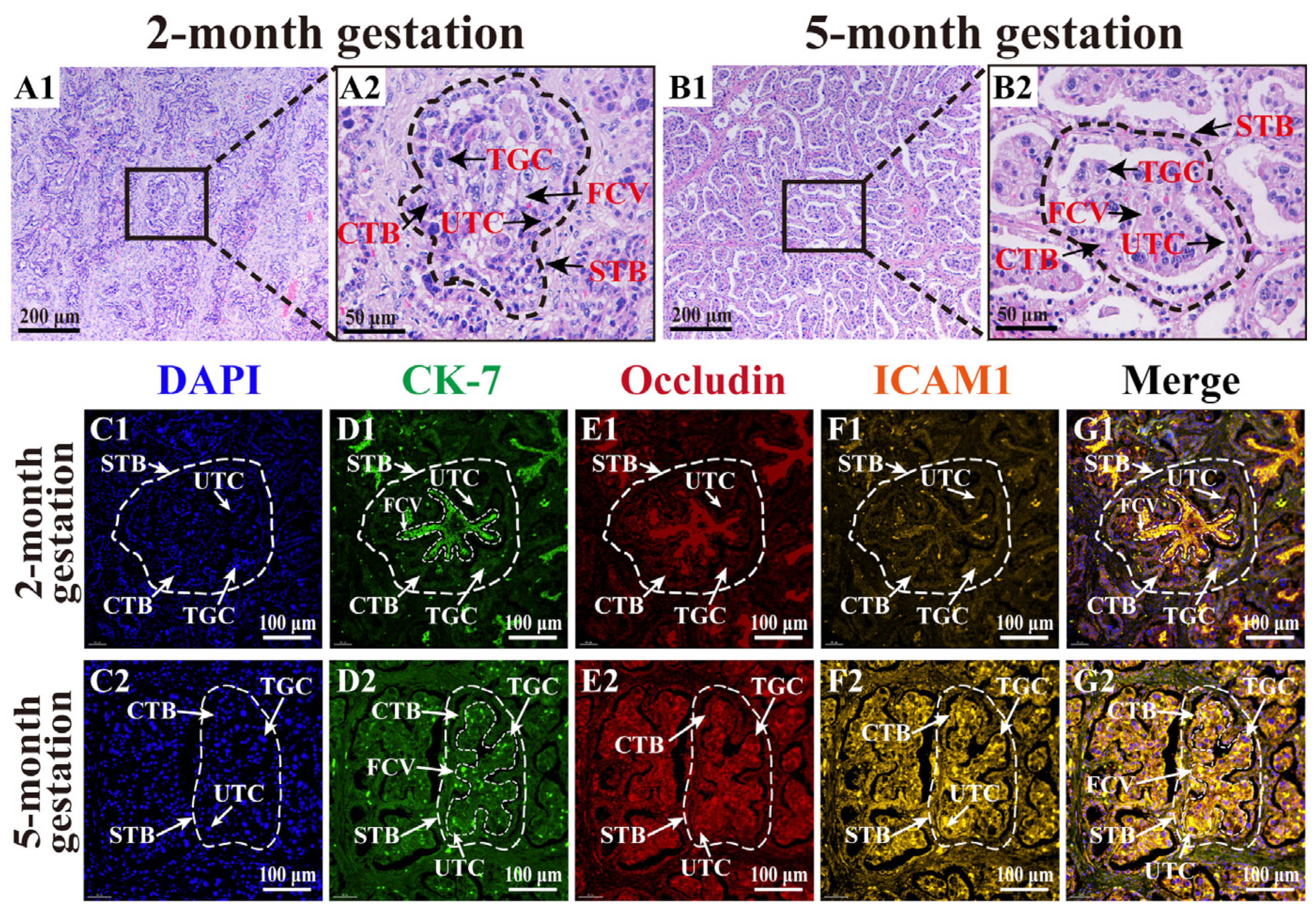
3.1. Morphology of TCs in Yak Placenta at Different Gestation Stages
3.2. Proteome Analysis and Protein Identification and Annotation Results
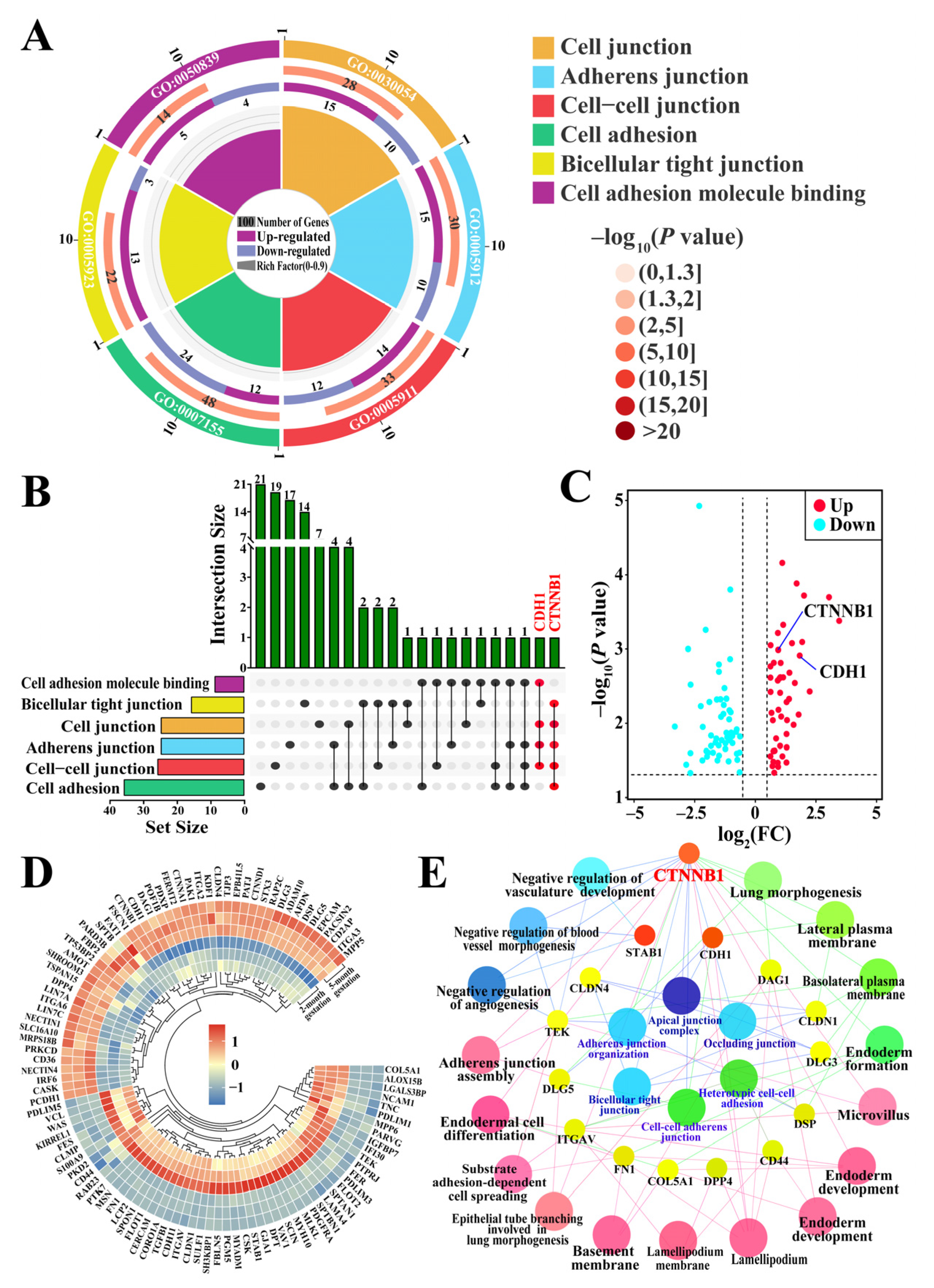
3.3. Identification of Candidate DEPs Related to Cell Adhesion and Junctions from GO Terms
3.4. Identification of Pathways and Candidate DEPs Related to CTNNB1 and CDH1
3.5. Distribution and Co-Localization Analysis of CTNNB1 and CDH1 Proteins in Yak Placental Tissues
3.6. CTNNB1 and CDH1 mRNA and Protein Expression Patterns in Yak Placental Tissues
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Q.; Wang, Q.; Gong, J.; Du, J.; Zhang, Y.; Zhao, X.J.G. Yak igf2 promotes fibroblast proliferation via suppression of igf1r and pi3kcg expression. Genes 2018, 9, 169. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.; Zhao, S.; Yin, M.; Wu, F.; Li, J.; Zhang, G.; Wu, X.; Bao, P.; Xiong, L.; Song, W.; et al. Differential proteomics of placentas reveals metabolic disturbance and oxidative damage participate yak spontaneous miscarriage during late pregnancy. BMC Vet. Res. 2022, 18, 248. [Google Scholar] [CrossRef] [PubMed]
- Ner-Kluza, J.; Wawrzykowski, J.; Franczyk, M.; Siberring, J.; Kankofer, M. Identification of protein patterns in bovine placenta at early-mid pregnancy–Pilot studies. Rapid Commun. Mass Spectrom. 2019, 33, 1084–1090. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.A.; Fraile-Martínez, O.; García-Montero, C.; Sáez, M.A.; Álvarez-Mon, M.A.; Torres-Carranza, D.; Álvarez-Mon, M.; Bujan, J.; García-Honduvilla, N.; Bravo, C.J.C. The pivotal role of the placenta in normal and pathological pregnancies: A focus on preeclampsia, fetal growth restriction, and maternal chronic venous disease. Cells 2022, 11, 568. [Google Scholar] [CrossRef]
- Tanner, A.R.; Kennedy, V.C.; Lynch, C.S.; Hord, T.K.; Winger, Q.A.; Rozance, P.J.; Anthony, R.V. In vivo investigation of ruminant placenta function and physiology—A review. J. Anim. Sci. 2022, 100, skac045. [Google Scholar] [CrossRef]
- Igwebuike, U. Trophoblast cells of ruminant placentas—A minireview. Anim. Reprod. Sci. 2006, 93, 185–198. [Google Scholar] [CrossRef]
- Watson, E.D.; Hughes, M.; Simmons, D.G.; Natale, D.R.; Sutherland, A.E.; Cross, J.C. Cell–cell adhesion defects in Mrj mutant trophoblast cells are associated with failure to pattern the chorion during early placental development. Dev. Dyn. 2011, 240, 2505–2519. [Google Scholar] [CrossRef]
- Cao, C.; Dai, Y.; Wang, Z.; Zhao, G.; Duan, H.; Zhu, X.; Wang, J.; Zheng, M.; Weng, Q.; Wang, L. The role of junctional adhesion molecule-C in trophoblast differentiation and function during normal pregnancy and preeclampsia. Placenta 2022, 118, 55–65. [Google Scholar] [CrossRef]
- Zhou, H.; Zhao, C.; Wang, P.; Yang, W.; Zhu, H.; Zhang, S. Regulators involved in trophoblast syncytialization in the placenta of intrauterine growth restriction. Front. Endocrinol. 2023, 14, 1107182. [Google Scholar] [CrossRef]
- Gumina, D.L.; Su, E.J. Mechanistic insights into the development of severe fetal growth restriction. Clin. Sci. 2023, 137, 679–695. [Google Scholar] [CrossRef]
- Luan, X.; Zhai, J.; Li, S.; Du, Y. Downregulation of FHL2 suppressed trophoblast migration, invasion and epithelial–mesenchymal transition in recurrent miscarriage. Reprod. BioMedicine Online 2024, 48, 103342. [Google Scholar] [CrossRef] [PubMed]
- Adu-Gyamfi, E.A.; Czika, A.; Gorleku, P.N.; Ullah, A.; Panhwar, Z.; Ruan, L.-L.; Ding, Y.-B.; Wang, Y.-X. The involvement of cell adhesion molecules, tight junctions, and gap junctions in human placentation. Reprod. Sci. 2021, 28, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Bridger, P.S.; Haupt, S.; Leiser, R.; Johnson, G.A.; Burghardt, R.C.; Tinneberg, H.-R.; Pfarrer, C. Integrin activation in bovine placentomes and in caruncular epithelial cells isolated from pregnant cows. Biol. Reprod. 2008, 79, 274–282. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.A.; Burghardt, R.C.; Bazer, F.W.; Seo, H.; Cain, J.W. Integrins and their potential roles in mammalian pregnancy. J. Anim. Sci. Biotechnol. 2023, 14, 115. [Google Scholar] [CrossRef]
- Xie, M.; McCoski, S.R.; Johnson, S.E.; Rhoads, M.L.; Ealy, A.D. Combinatorial effects of epidermal growth factor, fibroblast growth factor 2 and insulin-like growth factor 1 on trophoblast cell proliferation and embryogenesis in cattle. Reprod. Fertil. Dev. 2017, 29, 419–430. [Google Scholar] [CrossRef]
- Lu, W.; Tu, Z.; Wang, S.; Lu, J.; Wang, Q.; Wang, W.; Wang, B.; Wang, H.; Ni, H.; Guo, Y. Spatiotemporal expression of Wnt signaling pathway components during bovine placental development. Theriogenology 2013, 80, 893–902. [Google Scholar] [CrossRef]
- Dietrich, B.; Haider, S.; Meinhardt, G.; Pollheimer, J.; Knöfler, M.J.C.; Sciences, M.L. WNT and NOTCH signaling in human trophoblast development and differentiation. Cell. Mol. Life Sci. 2022, 79, 292. [Google Scholar] [CrossRef]
- Assis Neto, A.C.; Pereira, F.; Santos, T.C.d.; Ambrosio, C.; Leiser, R.; Miglino, M. Morpho-physical recording of bovine conceptus (Bos indicus) and placenta from days 20 to 70 of pregnancy. Reprod. Domest. Anim. 2010, 45, 760–772. [Google Scholar] [CrossRef]
- Zhang, Q.; Bai, X.; Lin, T.; Wang, X.; Zhang, B.; Dai, L.; Shi, J.; Zhang, Y.; Zhao, X. HMOX1 promotes ferroptosis in mammary epithelial cells via FTH1 and is involved in the development of clinical mastitis in dairy cows. Antioxidants 2022, 11, 2221. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Q.; Zhang, Y.; Cheng, S.; Hu, J.; Ma, Y.; Zhao, X. Comprehensive analysis of microrna–messenger rna from white yak testis reveals the differentially expressed molecules involved in development and reproduction. Int. J. Mol. Sci. 2018, 19, 3083. [Google Scholar] [CrossRef]
- Zhang, Q.; Bai, X.; Shi, J.; Wang, X.; Zhang, B.; Dai, L.; Lin, T.; Gao, Y.; Zhang, Y.; Zhao, X. DIA proteomics identified the potential targets associated with angiogenesis in the mammary glands of dairy cows with hemorrhagic mastitis. Front. Vet. Sci. 2022, 9, 980963. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Zhang, Q.; Shi, J.; Bai, X.; An, X.; Zhang, B.; Zhang, Y.; Zhao, X. The distribution, expression patterns and functional analysis of NR1D1 and NR4A2 in the reproductive axis tissues of the male Tianzhu White Yak. Animals 2021, 11, 3117. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Kang, Y.; Li, L.; Zhao, G.; Sun, J.; Liu, Z. Proteome analysis of rainbow trout (Oncorhynchus mykiss) liver responses to chronic heat stress using DIA/SWATH. J. Proteom. 2021, 233, 104079. [Google Scholar] [CrossRef]
- Bindea, G.; Galon, J.; Mlecnik, B. CluePedia Cytoscape plugin: Pathway insights using integrated experimental and in silico data. Bioinformatics 2013, 29, 661–663. [Google Scholar] [CrossRef]
- Bathla, S.; Sindhu, A.; Kumar, S.; Dubey, S.K.; Pattnaik, S.; Rawat, P.; Chopra, A.; Mohanty, A.K. Quantitative proteomics revealed the putative biomarker for detection of early-stage intra-mammary gland infection in cow. J. Proteins Proteom. 2020, 11, 173–181. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Zhang, B.; Lin, T.; Bai, X.; An, X.; Dai, L.; Shi, J.; Zhang, Y.; Zhao, X.; Zhang, Q. Sulfur Amino Acid Metabolism and the Role of Endogenous Cystathionine-γ-lyase/H2S in Holstein Cows with Clinical Mastitis. Animals 2022, 12, 1451. [Google Scholar] [CrossRef]
- Sağsöz, H.; Liman, N.; Akbalık, M.E.; Alan, E.; Saruhan, B.G.; Ketani, M.A.; Erdoğan, S. Expression of cadherins and some connective tissue components in cow uterus and placenta during pregnancy. Res. Vet. Sci. 2022, 151, 64–79. [Google Scholar] [CrossRef]
- Liu, B.; Cui, Y.; Yang, B.; Fan, J.; Zhao, Z.; Yu, S. Morphometric analysis of yak placentomes during gestation. Anat. Rec. Adv. Integr. Anat. Evol. Biol. 2010, 293, 1873–1879. [Google Scholar] [CrossRef]
- Xu, T.; Liu, B.; Cui, Y.; He, J.; Fan, J.; Yu, S. A light and electron microscopy study of the yak placentome. Folia Morphol. 2019, 78, 818–826. [Google Scholar] [CrossRef]
- Haeger, J.-D.; Hambruch, N.; Pfarrer, C. Placental development and its control in cattle. Biosci. Proc. 2019, 8, 10–1530. [Google Scholar] [CrossRef]
- Huppertz, B. Traditional and new routes of trophoblast invasion and their implications for pregnancy diseases. Int. J. Mol. Sci. 2019, 21, 289. [Google Scholar] [CrossRef] [PubMed]
- Boro, P.; Kumaresan, A.; Singh, A.; Gupta, D.; Kumar, S.; Manimaran, A.; Mohanty, A.; Mohanty, T.; Pathak, R.; Attupuram, N. Expression of short chain fatty acid receptors and pro-inflammatory cytokines in utero-placental tissues is altered in cows developing retention of fetal membranes. Placenta 2014, 35, 455–460. [Google Scholar] [CrossRef]
- Aplin, J.; Jones, C.; Harris, L. Adhesion molecules in human trophoblast—A review. I. Villous trophoblast. Placenta 2009, 30, 293–298. [Google Scholar] [CrossRef]
- Medrek, C.; Landberg, G.; Andersson, T.; Leandersson, K. Wnt-5a-CKIα signaling promotes β-catenin/E-cadherin complex formation and intercellular adhesion in human breast epithelial cells. J. Biol. Chem. 2009, 284, 10968–10979. [Google Scholar] [CrossRef]
- Der, B.; Bugacov, H.; Briantseva, B.-M.; McMahon, A.P. Cadherin adhesion complexes direct cell aggregation in the epithelial transition of Wnt-induced nephron progenitor cells. Development 2024, 151, dev202303. [Google Scholar] [CrossRef]
- Nelson, W.J. Regulation of cell–cell adhesion by the cadherin–catenin complex. Biochem. Soc. Trans. 2008, 36, 149–155. [Google Scholar] [CrossRef]
- Nakano, H.; Shimada, A.; Imai, K.; Takahashi, T.; Hashizume, K. The cytoplasmic expression of E-cadherin and β-catenin in bovine trophoblasts during binucleate cell differentiation. Placenta 2005, 26, 393–401. [Google Scholar] [CrossRef]
- Sonderegger, S.; Pollheimer, J.; Knöfler, M. Wnt signalling in implantation, decidualisation and placental differentiation–review. Placenta 2010, 31, 839–847. [Google Scholar] [CrossRef]
- van der Wal, T.; Van Amerongen, R. Walking the tight wire between cell adhesion and WNT signalling: A balancing act for β-catenin. Open Biol. 2020, 10, 200267. [Google Scholar] [CrossRef]
- Ma, F.; Li, W.; Liu, C.; Li, W.; Yu, H.; Lei, B.; Ren, Y.; Li, Z.; Pang, D.; Qian, C. MiR-23a promotes TGF-β1-induced EMT and tumor metastasis in breast cancer cells by directly targeting CDH1 and activating Wnt/β-catenin signaling. Oncotarget 2017, 8, 69538. [Google Scholar] [CrossRef] [PubMed]
- Nonn, O.; Debnath, O.; Valdes, D.S.; Sallinger, K.; Secener, A.K.; Haider, S.; Fischer, C.; Tiesmeyer, S.; Nimo, J.; Kuenzer, T. Disturbed trophoblast transition links preeclampsia progression from placenta to the maternal syndrome. bioRxiv 2022. [Google Scholar] [CrossRef]
- Takahashi, H.; Takizawa, T.; Matsubara, S.; Ohkuchi, A.; Kuwata, T.; Usui, R.; Matsumoto, H.; Sato, Y.; Fujiwara, H.; Okamoto, A. Extravillous trophoblast cell invasion is promoted by the CD44–hyaluronic acid interaction. Placenta 2014, 35, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.-Y.; Lee, E.-H.; Kim, E.N.; Son, W.-C.; Kim, Y.H.; Park, S.-Y.; Kim, I.-S.; Kim, J.-E. Identifying stabilin-1 and stabilin-2 double knockouts in reproduction and placentation: A descriptive study. Int. J. Mol. Sci. 2020, 21, 7235. [Google Scholar] [CrossRef]
- Hua, Q.; Li, Z.; Zhou, Y.; Wang, Y.; Yu, Y.; Sun, L.; Ye, J.; Li, L. Single-cell RNA sequencing reveals association of aberrant placental trophoblasts and FN1 reduction in late-onset fetal growth restriction. Placenta 2024, 146, 30–41. [Google Scholar] [CrossRef]
- Frank, J.W.; Seo, H.; Burghardt, R.C.; Bayless, K.J.; Johnson, G.A. ITGAV (alpha v integrins) bind SPP1 (osteopontin) to support trophoblast cell adhesion. Reproduction 2017, 153, 695–706. [Google Scholar] [CrossRef]
- Ishiguro, H.; Wakasugi, T.; Terashita, Y.; Sakamoto, N.; Tanaka, T.; Mizoguchi, K.; Sagawa, H.; Okubo, T.; Takeyama, H. Decreased expression of CDH1 or CTNNB1 affects poor prognosis of patients with esophageal cancer. World J. Surg. Oncol. 2016, 14, 240. [Google Scholar] [CrossRef]
- Tanabe, S.; Kawabata, T.; Aoyagi, K.; Yokozaki, H.; Sasaki, H. Gene expression and pathway analysis of CTNNB1 in cancer and stem cells. World J. Stem Cells 2016, 8, 384. [Google Scholar] [CrossRef]
- Kusafuka, K.; Yamada, H.; Ishino, K.; Maeda, M.; Yamanegi, K.; Baba, S.; Ohuchi, T.; Inagaki, H.; Yamamoto, H.; Iwasaki, T. Salivary Duct Carcinoma with Rhabdoid Features—No or Aberrant Expression of E-cadherin and Genetic Changes in CDH1: Immunohistochemical and Genetic Analyses of 17 Cases. Am. J. Surg. Pathol. 2021, 45, 439–449. [Google Scholar] [CrossRef]
- Jalali, B.M.; Lukasik, K.; Witek, K.; Baclawska, A.; Skarzynski, D.J. Changes in the expression and distribution of junction and polarity proteins in the porcine endometrium during early pregnancy period. Theriogenology 2020, 142, 196–206. [Google Scholar] [CrossRef]
- Herington, J.L.; Bi, J.; Martin, J.D.; Bany, B.M. β-Catenin (CTNNB1) in the mouse uterus during decidualization and the potential role of two pathways in regulating its degradation. J. Histochem. Cytochem. 2007, 55, 963–974. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, B.; Song, C.; Zhou, B.; Zhang, J.; Dong, W.; Zhang, Y.; Zhao, X.; Zhang, Q. CTNNB1 and CDH1 Regulate Trophoblast Cell Adhesion and Junction Formation in Yak Placental Tissue at Different Gestational Stages. Animals 2025, 15, 876. https://doi.org/10.3390/ani15060876
Zhang B, Song C, Zhou B, Zhang J, Dong W, Zhang Y, Zhao X, Zhang Q. CTNNB1 and CDH1 Regulate Trophoblast Cell Adhesion and Junction Formation in Yak Placental Tissue at Different Gestational Stages. Animals. 2025; 15(6):876. https://doi.org/10.3390/ani15060876
Chicago/Turabian StyleZhang, Bohao, Chen Song, Bin Zhou, Junjun Zhang, Weitao Dong, Yong Zhang, Xingxu Zhao, and Quanwei Zhang. 2025. "CTNNB1 and CDH1 Regulate Trophoblast Cell Adhesion and Junction Formation in Yak Placental Tissue at Different Gestational Stages" Animals 15, no. 6: 876. https://doi.org/10.3390/ani15060876
APA StyleZhang, B., Song, C., Zhou, B., Zhang, J., Dong, W., Zhang, Y., Zhao, X., & Zhang, Q. (2025). CTNNB1 and CDH1 Regulate Trophoblast Cell Adhesion and Junction Formation in Yak Placental Tissue at Different Gestational Stages. Animals, 15(6), 876. https://doi.org/10.3390/ani15060876





