Evaluation of the Effectiveness of a Phytogenic Supplement (Alkaloids and Flavonoids) in the Control of Eimeria spp. in Experimentally Challenged Broiler Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Coccidia Oocysts Challenge
2.3. Oocysts Count
2.4. Intestinal Lesion Assessment
2.5. Histopathological Analysis
2.6. Performance Evaluation
2.7. Statistical Analysis
3. Results
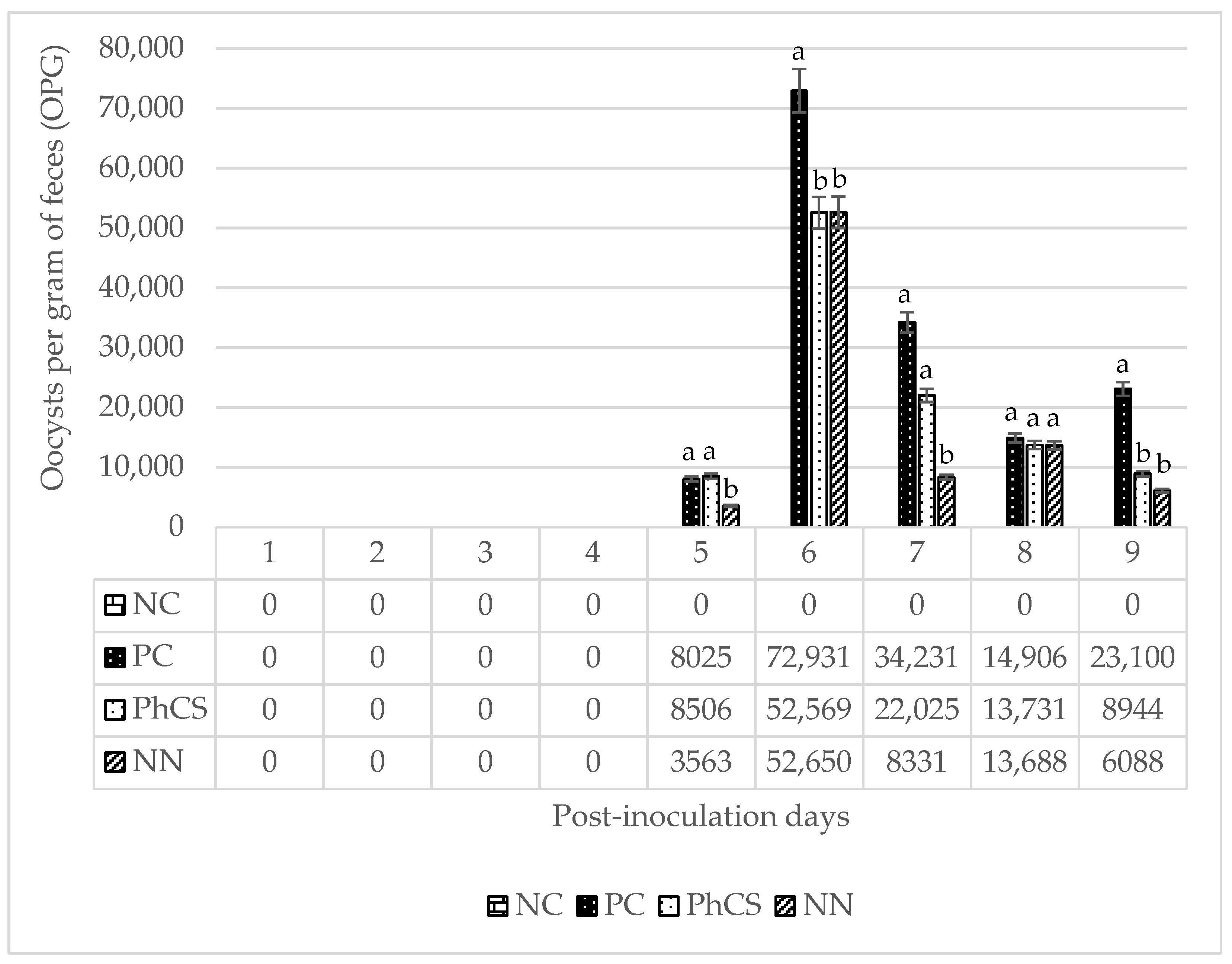
3.1. Oocyst Count
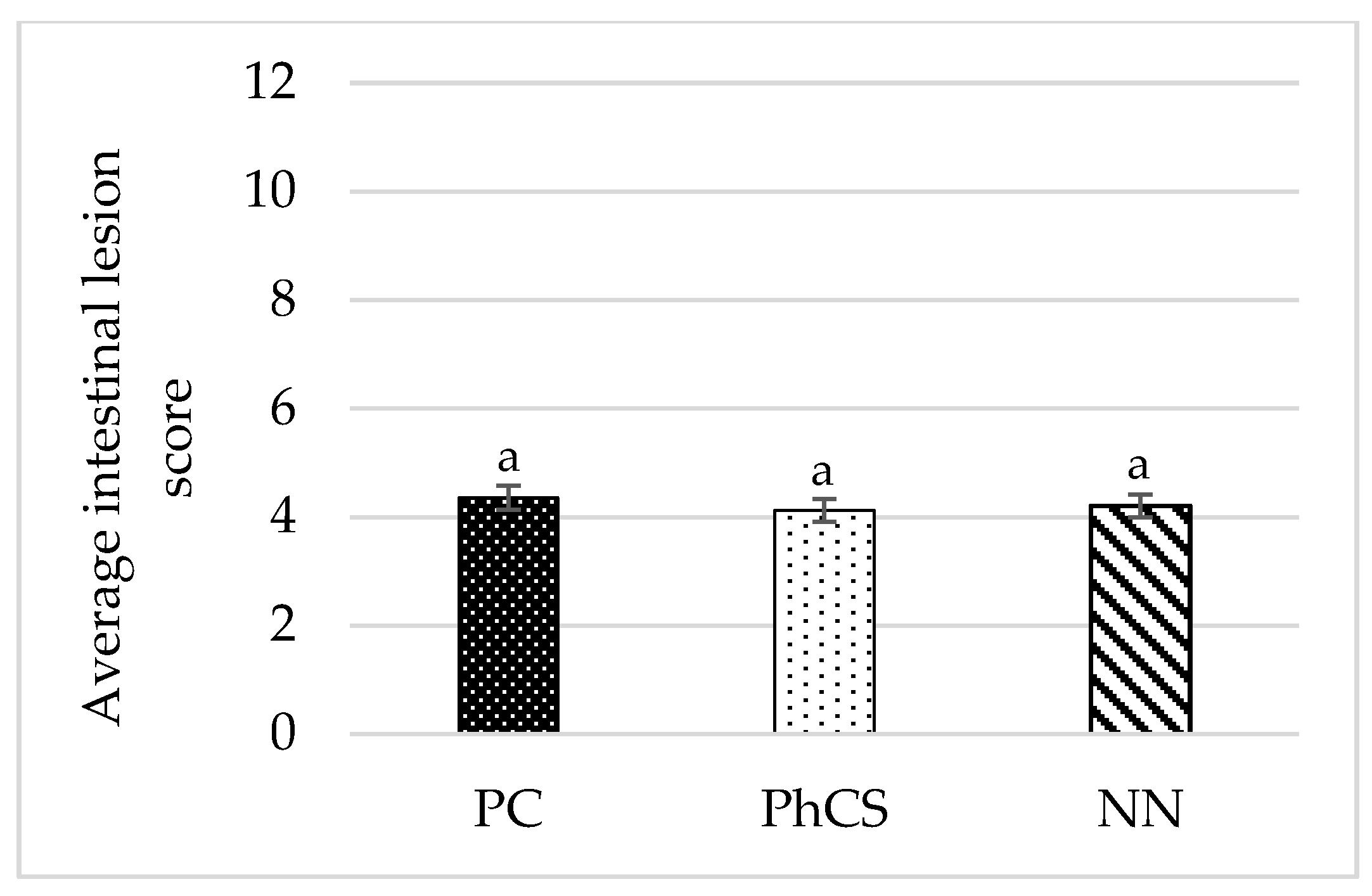
3.2. Intestinal Lesions
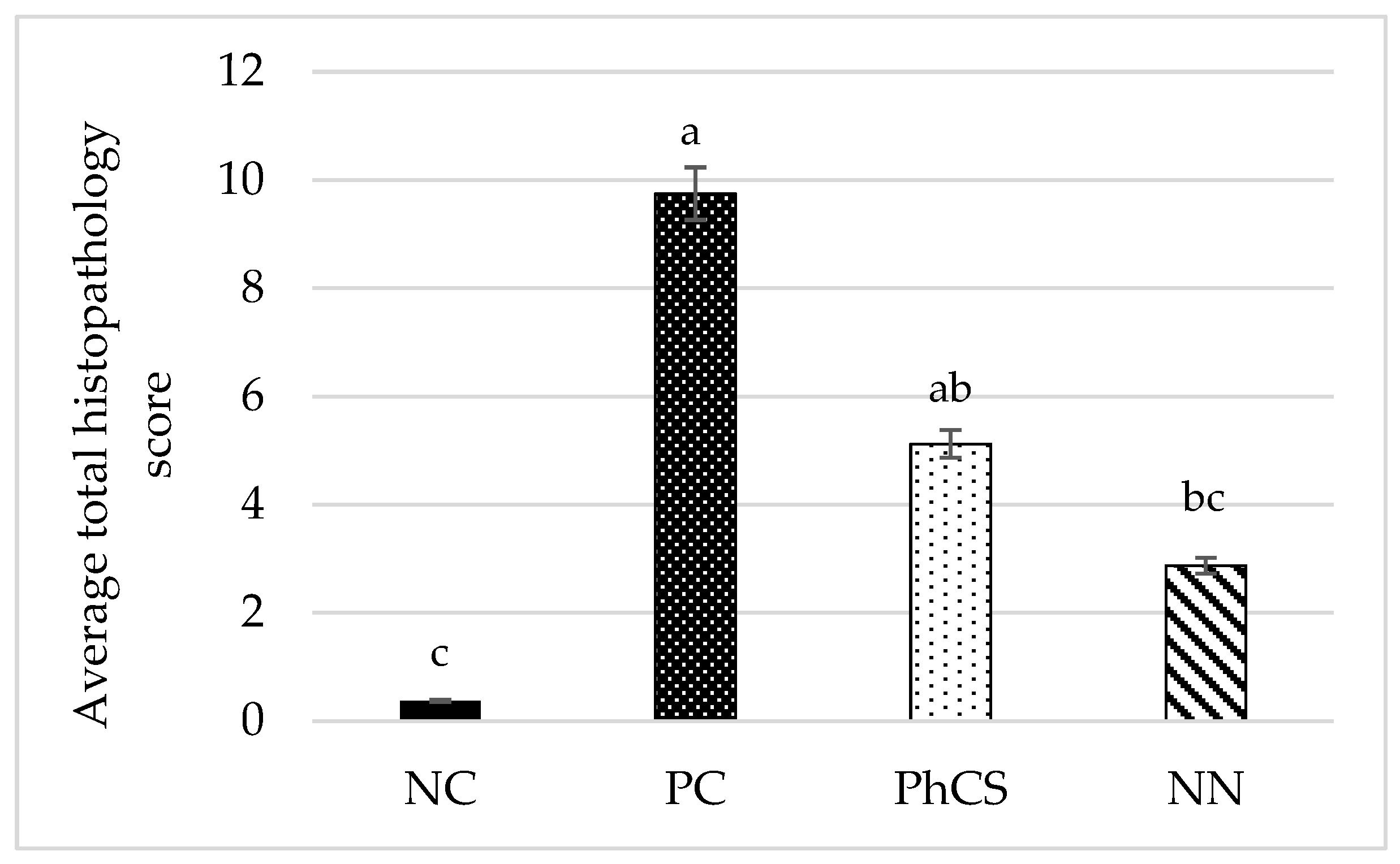
3.3. Histopathology
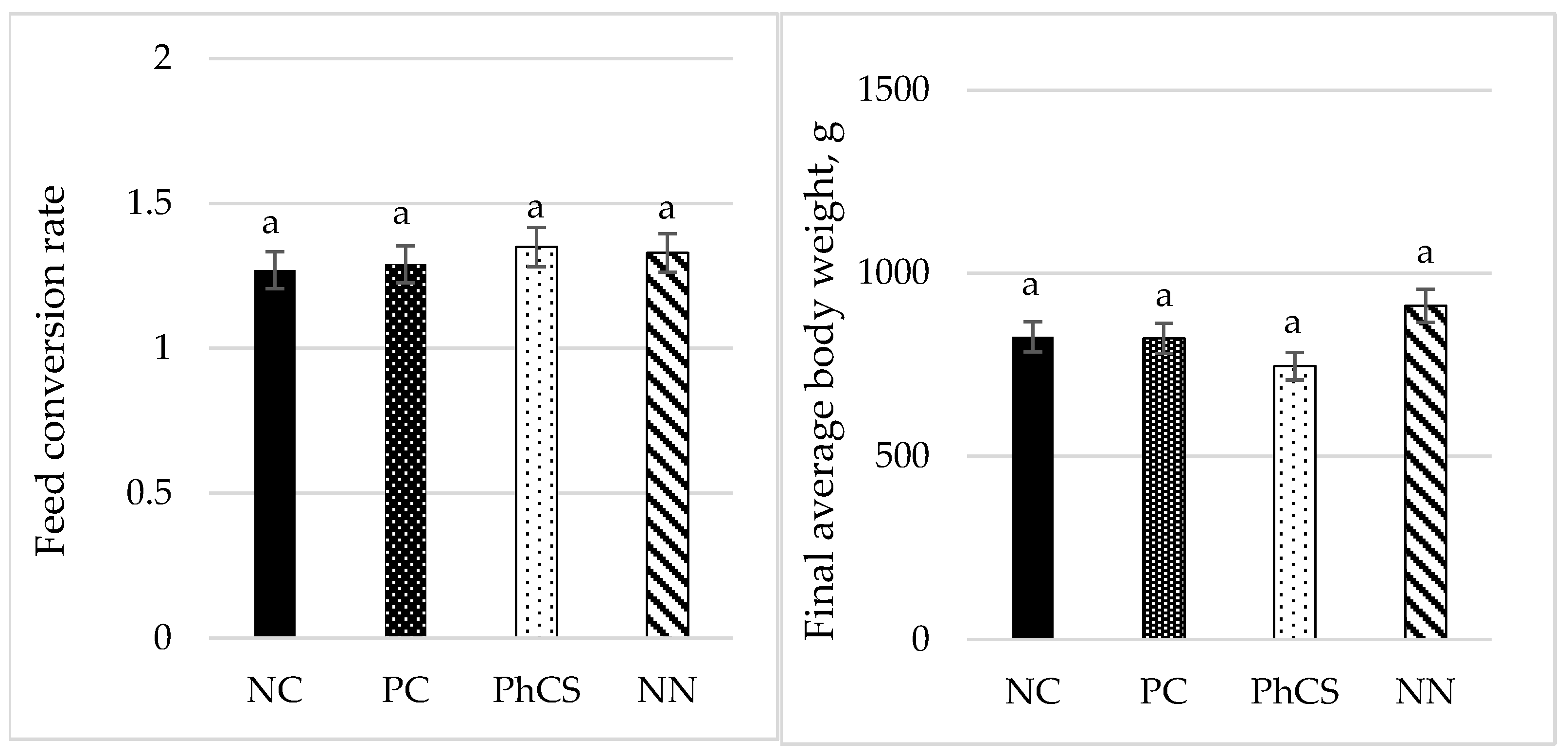
3.4. Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| NC | Negative control |
| PC | Positive control |
| PhCS | Phytogenic combination supplement |
| NN | Narasin and nicarbazin |
| PI | Post-inoculation |
| OPG | Oocyst per gram of feces |
| ADWG | Average daily weight gain |
| FCR | Feed conversion ratio |
References
- Blake, D.P.; Knox, J.; Dehaeck, B.; Huntington, B.; Rathinam, T.; Ravipati, V.; Ayoade, S.; Gilbert, W.; Adebambo, A.O.; Jatau, I.D.; et al. Re-Calculating the Cost of Coccidiosis in Chickens. Vet. Res. 2020, 51, 115. [Google Scholar] [CrossRef]
- Quiroz-Castañeda, R.E.; Dantán-González, E. Control of Avian Coccidiosis: Future and Present Natural Alternatives. Biomed. Res. Int. 2015, 2015, 430610. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M.W.; Smith, A.L.; Tomley, F.M. The Biology of Avian Eimeria with an Emphasis on Their Control by Vaccination. Adv. Parasitol. 2005, 60, 285–330. [Google Scholar] [CrossRef] [PubMed]
- Blake, D.P.; Tomley, F.M. Securing Poultry Production from the Ever-Present Eimeria Challenge. Trends Parasitol. 2014, 30, 12–19. [Google Scholar] [CrossRef] [PubMed]
- Nesse, L.L.; Bakke, A.M.; Eggen, T.; Hoel, K.; Kaldhusdal, M.; Ringø, E.; Yazdankhah, S.P.; Lock, E.-J.; Olsen, R.E.; Ørnsrud, R.; et al. The Risk of Development of Antimicrobial Resistance with the Use of Coccidiostats in Poultry Diets. Eur. J. Nutr. Food Saf. 2019, 11, 40–43. [Google Scholar] [CrossRef]
- Noack, S.; Chapman, H.D.; Selzer, P.M. Anticoccidial Drugs of the Livestock Industry. Parasitol. Res. 2019, 118, 2009–2026. [Google Scholar] [CrossRef]
- Peek, H. Resistance to Anticoccidial Drugs: Alternative Strategies to Control Coccidiosis in Broilers; University Utrecht: Utrecht, The Netherlands, 2010. [Google Scholar]
- Flores, R.A.; Nguyen, B.T.; Cammayo, P.L.T.; Võ, T.C.; Naw, H.; Kim, S.; Kim, W.H.; Na, B.K.; Min, W. Epidemiological Investigation and Drug Resistance of Eimeria Species in Korean Chicken Farms. BMC Vet. Res. 2022, 18, 277. [Google Scholar] [CrossRef]
- Arabkhazaeli, F.; Modrisanei, M.; Nabian, S.; Mansoori, B.; Madani, A. Evaluating the Resistance of Eimeria spp. Field Isolates to Anticoccidial Drugs Using Three Different Indices. Iran. J. Parasitol. 2013, 8, 234–241. [Google Scholar]
- Abbas, R.Z.; Iqbal, Z.; Blake, D.; Khan, M.N.; Saleemi, M.K. Anticoccidial Drug Resistance in Fowl Coccidia: The State of Play Revisited. Worlds Poult. Sci. J. 2011, 67, 337–350. [Google Scholar] [CrossRef]
- Avrain, L.; Humbert, F.; L’Hospitalier, R.; Sanders, P.; Vernozy-Rozand, C.; Kempf, I. Antimicrobial Resistance in Campylobacter from Broilers: Association with Production Type and Antimicrobial Use. Vet. Microbiol. 2003, 96, 267–276. [Google Scholar] [CrossRef]
- Lan, L.H.; Sun, B.B.; Zuo, B.X.Z.; Chen, X.Q.; Du, A.F. Prevalence and Drug Resistance of Avian Eimeria Species in Broiler Chicken Farms of Zhejiang Province, China. Poult. Sci. 2017, 96, 2104–2109. [Google Scholar] [CrossRef]
- Ojimelukwe, A.E.; Emedhem, D.E.; Agu, G.O.; Nduka, F.O.; Abah, A.E. Populations of Eimeria tenella Express Resistance to Commonly Used Anticoccidial Drugs in Southern Nigeria. Int. J. Vet. Sci. Med. 2018, 6, 192–200. [Google Scholar] [CrossRef] [PubMed]
- Ferdji, A.; Mimoune, N.; Amrouche, T.; Degui, D.; Temim, S.; Khelef, D. Anticoccidial Resistance in Poultry: Determination of Ionophore Sensitivity for Eimeria acervulina and Eimeria maxima Isolated from Broiler Chicken Farms in Tizi Ouzou Province (Algeria). Veterinarska Stanica 2022, 53, 261–271. [Google Scholar] [CrossRef]
- Hansson, I.; Ellström, P.; Nilsson, O.; Chaba, M.; Skarin, M.; Fernström, L.L.; Frosth, S. Differences in Genotype and Antimicrobial Resistance between Campylobacter spp. Isolated from Organic and Conventionally Produced Chickens in Sweden. Pathogens 2021, 10, 1630. [Google Scholar] [CrossRef] [PubMed]
- Nweze, N.E.; Obiwulu, I.S. Anticoccidial Effects of Ageratum conyzoides. J. Ethnopharmacol. 2009, 122, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, A.; Sani, D.; Abdullhi, S.; Jatau, I. In vitro Anticoccidial Activity of Ethanolic Leaf Extract of Citrus aurantium L. against Eimeria tenella Oocysts. Sokoto J. Vet. Sci. 2022, 20, 37–43. [Google Scholar] [CrossRef]
- Abudabos, A.E.; Alyemni, A.H.; Osman, E.; Hussein, S. Anticoccidial Effect of Some Natural Products in Experimentally Induced Eimeria spp. Infection on Carcass Quality, Intestinal Lesion and Ileal Histology in Broilers. J. Anim. Plant Sci. 2018, 28, 1–7. [Google Scholar]
- Christaki, E.; Florou-Paneri, P.; Giannenas, I.; Papazahariadou, M.; Botsoglou, N.A.; Spais, A.B. Effect of a Mixture of Herbal Extracts on Broiler Chickens Infected with Eimeria tenella. Anim. Res. 2004, 53, 137–144. [Google Scholar] [CrossRef]
- Bozkurt, M.; Selek, N.; Küçükyilmaz, K.; Eren, H.; Güven, E.; Çatli, A.U.; Çinar, M. Effects of Dietary Supplementation with a Herbal Extract on the Performance of Broilers Infected with a Mixture of Eimeria Species. Br. Poult. Sci. 2012, 53, 325–332. [Google Scholar] [CrossRef]
- Chapman, H.D.; Barta, J.R.; Blake, D.; Gruber, A.; Jenkins, M.; Smith, N.C.; Suo, X.; Tomley, F.M. Chapter Two—A Selective Review of Advances in Coccidiosis Research. In Advances in Parasitology; Rollinson, D., Ed.; Academic Press: Cambridge, MA, USA, 2013; Volume 83, pp. 93–171. [Google Scholar]
- Christaki, E.; Bonos, E.; Giannenas, I.; Florou-Paneri, P. Aromatic Plants as a Source of Bioactive Compounds. Agriculture 2012, 2, 228–243. [Google Scholar] [CrossRef]
- Cowan, M.M. Plant Products as Antimicrobial Agents. Clin. Microbiol. Rev. 1999, 12, 564–582. [Google Scholar] [CrossRef] [PubMed]
- Mohiti-Asli, M.; Ghanaatparast-Rashti, M. Dietary Oregano Essential Oil Alleviates Experimentally Induced Coccidiosis in Broilers. Prev. Vet. Med. 2015, 120, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Giannenas, I.; Florou-Paneri, P.; Papazahariadou, M.; Christaki, E.; Botsoglou, N.A.; Spais, A.B. Effect of Dietary Supplementation with Oregano Essential Oil on Performance of Broilers after Experimental Infection with Eimeria tenella. Arch. Anim. Nutr. 2003, 57, 99–106. [Google Scholar] [CrossRef]
- Chen, P.; Liu, K.; Yue, T.; Lu, Y.; Li, S.; Jian, F.; Huang, S. Plants, Plant-Derived Compounds, Probiotics, and Postbiotics as Green Agents to Fight against Poultry Coccidiosis: A Review. Anim. Res. One Health 2024, 1–21. [Google Scholar] [CrossRef]
- El-Shall, N.A.; Abd El-Hack, M.E.; Albaqami, N.M.; Khafaga, A.F.; Taha, A.E.; Swelum, A.A.; El-Saadony, M.T.; Salem, H.M.; El-Tahan, A.M.; AbuQamar, S.F.; et al. Phytochemical Control of Poultry Coccidiosis: A Review. Poult. Sci. 2022, 101, 101542. [Google Scholar] [CrossRef]
- Ahmad, R.; Yu, Y.H.; Hua, K.F.; Chen, W.J.; Zaborski, D.; Dybus, A.; Hsiao, F.S.H.; Cheng, Y.H. Management and Control of Coccidiosis in Poultry—A Review. Anim. Biosci. 2024, 37, 1–15. [Google Scholar] [CrossRef]
- Aviagen. POLLO De ENGORDE ROSS 308/308 FF: Objetivos de Rendimiento; Aviagen: Huntsville, AL, USA, 2022. [Google Scholar]
- Subcommittee on Poultry Nutrition, National Research Council (N.R.C.). Nutrient Requirements of Poultry, 9th rev. ed.; National Academy Press: Washington, DC, USA, 1994; ISBN 0-309-59632-7. [Google Scholar]
- Grenier, B.; Dohnal, I.; Shanmugasundaram, R.; Eicher, S.D.; Selvaraj, R.K.; Schatzmayr, G.; Applegate, T.J. Susceptibility of Broiler Chickens to Coccidiosis When Fed Subclinical Doses of Deoxynivalenol and Fumonisins—Special Emphasis on the Immunological Response and Themycotoxin Interaction. Toxins 2016, 8, 231. [Google Scholar] [CrossRef]
- Long, P.L.; Rowell, J.G. Counting Oocysts of Chicken Coccidia. Lab. Pract. 1958, 7, 515–518. [Google Scholar]
- Vadlejch, J.; Petrtýl, M.; Zaichenko, I.; Čadková, Z.; Jankovská, I.; Langrová, I.; Moravec, M. Which McMaster Egg Counting Technique Is the Most Reliable? Parasitol. Res. 2011, 109, 1387–1394. [Google Scholar] [CrossRef]
- Vadlejch, J.; Petrtýl, M.; Lukešová, D.; Čadková, Z.; Kudrnáčová, M.; Jankovská, I.; Langrová, I. The Concentration McMaster Technique Is Suitable for Quantification of Coccidia Oocysts in Bird Droppings. Pak. Vet. J. 2013, 33, 291–295. [Google Scholar]
- Zinke, A.; Schnebel, B.; Dierschke, V.; Ryll, M. Prevalence and Intensity of Excretion of Coccidial Oocysts in Migrating Passerines on Helgoland. J. Ornithol. 2004, 145, 74–78. [Google Scholar] [CrossRef]
- Johnson, J.; Reid, W.M. Anticoccidial Drugs: Lesion Scoring Techniques in Battery and Floor-Pen Experiments with Chickens. Exp. Parasitol. 1970, 28, 30–36. [Google Scholar] [CrossRef]
- Conway, D.P.; Dayton, A.D.; Mckenzie, M.E. Comparative Testing of Anticoccidials in Broiler Chickens: The Role of Coccidial Lesion Scores. Poult. Sci. 1999, 78, 529–535. [Google Scholar] [CrossRef]
- Idris, A.B.; Bounous, D.I.; Goodwin, M.A.; Brown, J.; Krushinskie, E.A. Lack of Correlation between Microscopic Lesion Scores and Gross Lesion Scores in Commercially Grown Broilers Examined for Small Intestinal Eimeria spp. Coccidiosis. Am. Assoc. Avian Pathol. 1997, 41, 388–391. [Google Scholar] [CrossRef]
- Sultan, R.; Aslam, A.; Tipu, M.Y.; Rehman, H.U.; Anjum, A.; Krull, W.; Kumosani, T.; Shaib, H.; Barbour, E.K. Appraisal of a New Patented Method for Control of Chicken Coccidiosis. J. Appl. Anim. Res. 2019, 47, 573–581. [Google Scholar] [CrossRef]
- Nguyen, B.T.; Flores, R.A.; Cammayo, P.L.T.; Kim, S.; Kim, W.H.; Min, W. Anticoccidial Activity of Berberine against Eimeria-Infected Chickens. Korean J. Parasitol. 2021, 59, 403–408. [Google Scholar] [CrossRef]
- Allen, P.C. Anticoccidial Effects of Xanthohumol. Avian Dis. 2007, 51, 21–26. [Google Scholar] [CrossRef]
- Tanweer, A.J.; Chand, N.; Saddique, U.; Bailey, C.A.; Khan, R.U. Antiparasitic Effect of Wild Rue (Peganum harmala L.) against Experimentally Induced Coccidiosis in Broiler Chicks. Parasitol. Res. 2014, 113, 2951–2960. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.U.; Nikousefat, Z.; Tufarelli, V.; Naz, S.; Javdani, M.; Laudadio, V. Garlic (Allium sativum) Supplementation in Poultry Diets: Effect on Production and Physiology. World’s Poult. Sci. Assoc. 2012, 68, 417–424. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, L.; Lai, M.; Zhang, X.; Li, J.; Li, Z.; Yang, D.; Zhao, M.; Wang, D.; Wen, P.; et al. Histopathologic Observations in a Coccidiosis Model of Eimeria tenella. Anim. Model. Exp. Med. 2024, 7, 893–903. [Google Scholar] [CrossRef]
- Mcdougald, L.R.; Fuller, L.; Mattiello, R. A Survey of Coccidia on 43 Poultry Farms in Argentina. Avian Dis. 1997, 41, 923–929. [Google Scholar] [CrossRef] [PubMed]
- Landi-Librandi, A.P.; Caleiro Seixas Azzolini, A.E.; De Oliveira, C.A.; Lucisano-Valim, Y.M. Inhibitory Activity of Liposomal Flavonoids during Oxidative Metabolism of Human Neutrophils upon Stimulation with Immune Complexes and Phorbol Ester. Drug Deliv. 2012, 19, 177–187. [Google Scholar] [CrossRef]
- Robbins, R.J. Phenolic Acids in Foods: An Overview of Analytical Methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Erlund, I. Review of the Flavonoids Quercetin, Hesperetin, and Naringenin. Dietary Sources, Bioactivities, Bioavailability, and Epidemiology. Nutr. Res. 2004, 24, 851–874. [Google Scholar] [CrossRef]
- Chand, N.; Naz, S.; Shah, Z.; Khan, S.; Ali Shah, A.; Ullah Khan, R. Growth Performance and Immune Status of Broilers Fed Graded Levels of Albizia lebbeck Seeds. Park. J. Zool. 2014, 46, 574–577. [Google Scholar]
- Yan, Y.; Li, X.; Zhang, C.; Lv, L.; Gao, B.; Li, M. Research Progress on Antibacterial Activities and Mechanisms of Natural Alkaloids: A Review. Antibiotics 2021, 10, 318. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Huang, H.B.; Pan, T.X.; Wang, N.; Shi, C.W.; Zhang, B.; Wang, C.F.; Yang, G.L. Sanguinarine Induces Apoptosis in Eimeria tenella Sporozoites via the Generation of Reactive Oxygen Species. Poult. Sci. 2022, 101, 101771. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Huang, K.; Bai, Y.; Feng, X.; Gong, L.; Wei, C.; Huang, H.; Zhang, H. Dietary Supplementation with Berberine Improves Growth Performance and Modulates the Composition and Function of Cecal Microbiota in Yellow-Feathered Broilers. Poult. Sci. 2021, 100, 1034–1048. [Google Scholar] [CrossRef]
- Quinteros, J.A.; Scott, P.C.; Wilson, T.B.; Anwar, A.M.; Scott, T.; Muralidharan, C.; Van, T.T.H.; Moore, R.J. Isoquinoline Alkaloids Induce Partial Protection of Laying Hens from the Impact of Campylobacter hepaticus (Spotty Liver Disease) Challenge. Poult. Sci. 2021, 100, 101423. [Google Scholar] [CrossRef]
- Othman, L.; Sleiman, A.; Abdel-Massih, R.M. Antimicrobial Activity of Polyphenols and Alkaloids in Middle Eastern Plants. Front. Microbiol. 2019, 10, 911. [Google Scholar] [CrossRef]
- Fetterer, R.H.; Allen, P.C. Eimeria acervulina Infection Elevates Plasma and Muscle 3-Methylhistidine Levels in Chickens. J. Parasitol. 2000, 86, 783–791. [Google Scholar] [CrossRef] [PubMed]
- MSD Salud Animal. FICHA TÉCNICA. COCCIVAC® D2 Suspensión Oral; MSD Salud Animal, Intervet Chile: Santiago, Chile, 2021. [Google Scholar]
- Mathis, G.F.; Newman, L.J.; Fitz-Coy, S.; Lumpkins, B.; Charette, R.; Fuller, L. Comparison of Breeder/Layer Coccidiosis Vaccines: Part 1 -Precocity and Pathogenicity. J. Appl. Poult. Res. 2018, 27, 33–37. [Google Scholar] [CrossRef]
- Catalá-Gregori, P.; Mallet, S.; Travel, A.; Orengo, J.; Lessire, M. Efficiency of a Prebiotic and a Plant Extract Alone or in Combination on Broiler Performance and Intestinal Physiology. Can. J. Anim. Sci. 2008, 88, 623–629. [Google Scholar] [CrossRef]
| Experimental Group | Abbreviation | Coccidial Challenge | Supplementation |
|---|---|---|---|
| Negative control | NC | No | No |
| Positive control | PC | Yes | No |
| Phytogenic compound supplement | PhCS | Yes | Eimex® at 1 kg/ton of feed, for 23 days |
| Anticoccidial | NN | Yes | Narasin and nicarbazin at 0.6 kg/ton of feed, for 23 days |
| Experimental Group | Coccidia Score | Inflammation Score | Average Total Histopathology Score |
|---|---|---|---|
| NC | 0 | 0.375 | 0.375 |
| PC | 8 | 1.750 | 9.75 |
| PhCS | 3 | 2.125 | 5.125 |
| NN | 1.875 | 1 | 2.875 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hascoët, A.-S.; Torres-Celpa, P.; Riquelme-Neira, R.; Hidalgo-Olate, H. Evaluation of the Effectiveness of a Phytogenic Supplement (Alkaloids and Flavonoids) in the Control of Eimeria spp. in Experimentally Challenged Broiler Chickens. Animals 2025, 15, 847. https://doi.org/10.3390/ani15060847
Hascoët A-S, Torres-Celpa P, Riquelme-Neira R, Hidalgo-Olate H. Evaluation of the Effectiveness of a Phytogenic Supplement (Alkaloids and Flavonoids) in the Control of Eimeria spp. in Experimentally Challenged Broiler Chickens. Animals. 2025; 15(6):847. https://doi.org/10.3390/ani15060847
Chicago/Turabian StyleHascoët, Anne-Sophie, Paulina Torres-Celpa, Roberto Riquelme-Neira, and Héctor Hidalgo-Olate. 2025. "Evaluation of the Effectiveness of a Phytogenic Supplement (Alkaloids and Flavonoids) in the Control of Eimeria spp. in Experimentally Challenged Broiler Chickens" Animals 15, no. 6: 847. https://doi.org/10.3390/ani15060847
APA StyleHascoët, A.-S., Torres-Celpa, P., Riquelme-Neira, R., & Hidalgo-Olate, H. (2025). Evaluation of the Effectiveness of a Phytogenic Supplement (Alkaloids and Flavonoids) in the Control of Eimeria spp. in Experimentally Challenged Broiler Chickens. Animals, 15(6), 847. https://doi.org/10.3390/ani15060847








