The Cryoprotectant Effects of Safflower Polysaccharides on the Quality of Frozen–Thawed Boar Sperm
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Approval
2.2. Chemicals and Sources
2.3. Sperm Collection, Cryopreservation, and Thawing
2.4. Determination of Sperm Quality and Kinetic Parameters
2.5. Determination of Sperm Acrosome Integrity and Plasma Membrane Integrity
2.6. Determination of Sperm Mitochondrial Activity and DNA Integrity
2.7. Determination of Sperm Antioxidant Capacity
2.8. Statistical Analysis
3. Results
3.1. Effects of SPS on Boar Sperm Quality and Kinetic Parameters
3.2. Effects of SPS on Acrosome Integrity and Plasma Membrane Integrity of Boar Sperm
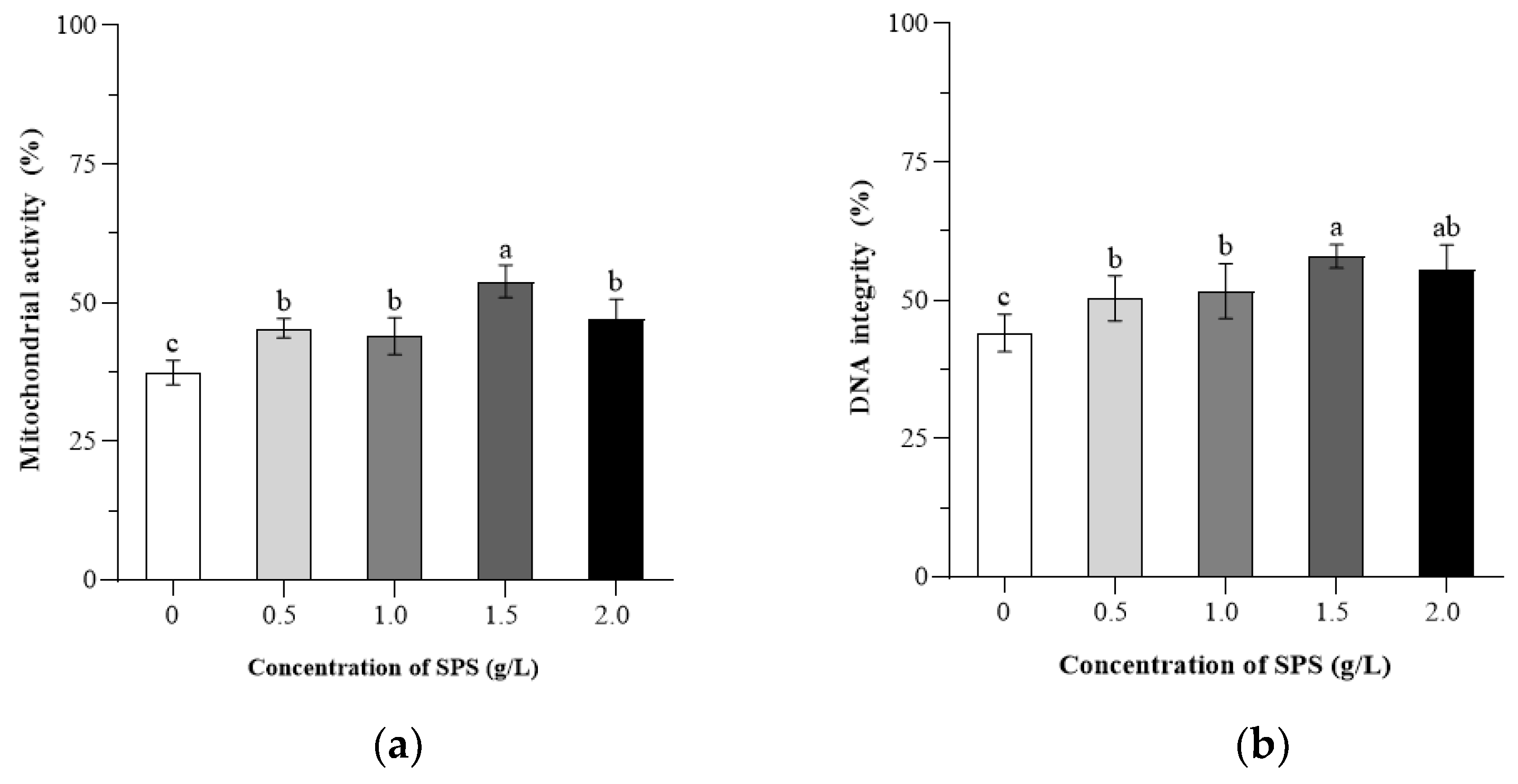
3.3. Effects of SPS on Mitochondrial Activity and DNA Integrity of Boar Sperm
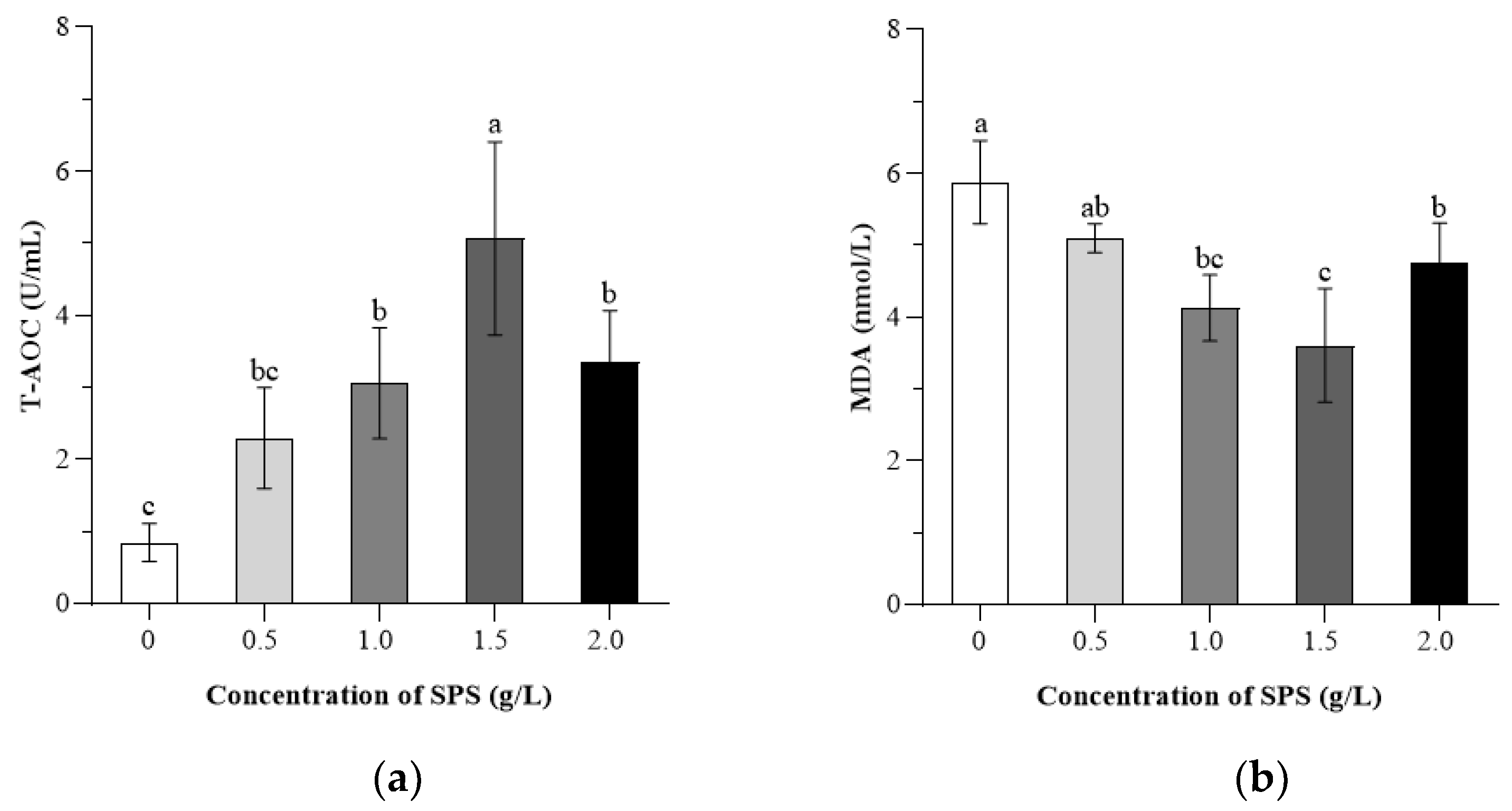
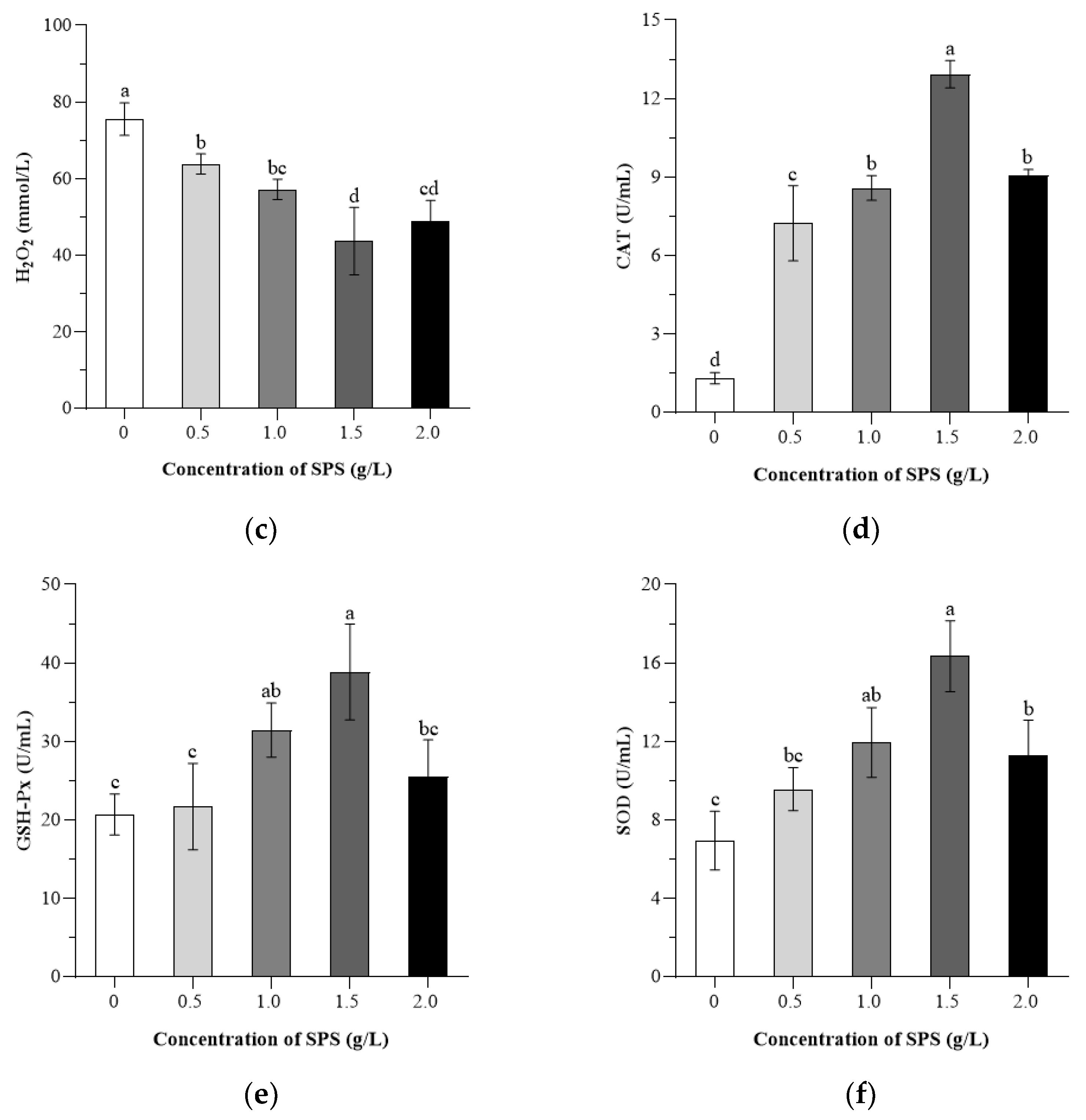
3.4. Effects of SPS on the Antioxidant Capacity of Boar Sperm
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roca, J.; Hernández, M.; Carvajal, G.; Vázquez, J.M.; Martínez, E.A. Factors Influencing Boar Sperm Cryosurvival. J. Anim. Sci. 2006, 84, 2692–2699. [Google Scholar] [CrossRef] [PubMed]
- Waberski, D.; Riesenbeck, A.; Schulze, M.; Weitze, K.F.; Johnson, L. Application of Preserved Boar sperm for Artificial Insemination: Past, Present and Future Challenges. Theriogenology 2019, 137, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Basioura, A.; Tsakmakidis, I.A.; Martinez, E.A.; Roca, J.; Li, J.; Molina, M.F.; Theodoridis, A.; Boscos, C.M.; Parrilla, I. Effect of Astaxanthin in Extenders on Sperm Quality and Functional Variables of Frozen-Thawed Boar sperm. Anim. Reprod. Sci. 2020, 218, 106478. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, E.; García-Casado, P.; Castaño, C.; Toledano-Díaz, A.; Bóveda, P.; Santiago-Moreno, J. Sperm Response to in Vitro Stress Conditions in Wild and Domestic Species Measured by Functional Variables and ROS Production. Front. Vet. Sci. 2021, 8, 650946. [Google Scholar] [CrossRef]
- Betarelli, R.P.; Rocco, M.; Yeste, M.; Fernández-Novell, J.M.; Placci, A.; Azevedo Pereira, B.; Castillo-Martín, M.; Estrada, E.; Peña, A.; Zangeronimo, M.G.; et al. The Achievement of Boar Sperm in Vitro Capacitation is Related to an Increase of Disrupted Disulphide Bonds and Intracellular Reactive Oxygen Species levels. Andrology 2018, 6, 781–797. [Google Scholar] [CrossRef]
- Pezo, F.; Yeste, M.; Zambrano, F.; Uribe, P.; Risopatrón, J.; Sánchez, R. Antioxidants and Their Effect on the Oxidative/Nitrosative Stress of Frozen-Thawed Boar Sperm. Cryobiology 2021, 98, 5–11. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Hu, Z.; Zhang, G.; Wen, F.; Xian, M.; Guo, S.; Zhang, G.; Zhang, X.; Hu, J. Supplementation of Epimedium polysaccharide (EPS) improves goat semen characteristics following cryopreservation. Anim. Reprod. Sci. 2025, 272, 107654. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, K.; Gao, J.; Xu, J.; Wu, C.; He, M.; Zhang, S.; Zhang, D.; Dai, J.; Sun, L. Effect of Poria cocos Mushroom Polysaccharides (PCPs) on the Quality and DNA Methylation of Cryopreserved Shanghai White Pig Spermatozoa. Cells 2023, 12, 1456. [Google Scholar] [CrossRef]
- Shen, T.; Jiang, Z.L.; Liu, H.; Li, Q.W. Effect of Salvia miltiorrhiza Polysaccharides on Boar Spermatozoa During Freezing-Thawing. Anim. Reprod. Sci. 2015, 159, 25–30. [Google Scholar] [CrossRef]
- Yang, S.M.; Wang, T.; Wen, D.G.; Hou, J.Q.; Li, H.B. Protective Effect of Rhodiola rosea Polysaccharides on Cryopreserved Boar Sperm. Carbohydr. Polym. 2016, 135, 44–47. [Google Scholar] [CrossRef]
- Fu, J.; Yang, Q.; Li, Y.; Li, P.; Wang, L.; Li, X. A mechanism by which Astragalus polysaccharide protects against ROS toxicity through inhibiting the protein dephosphorylation of boar sperm preserved at 4 °C. J Cell. Physiol. 2018, 233, 5267–5280. [Google Scholar] [CrossRef] [PubMed]
- Qin, Y.; Fan, K.; Yimamu, A.; Zhan, P.; Lv, L.; Li, G.; Liu, J.; Hu, Z.; Yan, X.; Hu, X.; et al. Integrated Genetic Diversity and Multi-Omics Analysis of Colour Formation in Safflower. Int. J. Mol. Sci. 2025, 26, 647. [Google Scholar] [CrossRef] [PubMed]
- Li, J.X.; Xu, D.Q.; Cui, D.X.; Fu, R.J.; Niu, Z.C.; Liu, W.J.; Tang, Y.P. Exploring the structure-activity relationship of Safflower polysaccharides: From the structural characteristics to biological function and therapeutic applications. J. Ethnopharmacol. 2025, 339, 119131. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.F. The Effects of Carthamus tinctorius Polysaccharides on the Immunity Function and Antioxidant Function of H22 Tumor-Bearing Mice. J. North Pharm. 2016, 13, 111–112. [Google Scholar]
- Zou, Y.F.; Ren, A.N.; Yao, M.M.; Gu, X.H. Effect of Macroporous Adsorptive Resins on Decoloration Technology of Carthamus tinctorius Polysaccharide. China Pharm. 2011, 22, 1380–1382. [Google Scholar]
- Hu, Y. Structural Analysis and Antioxidant Activities of Polysaccharide Isolated from Carthamus tinctorius L. Master’s Thesis, Shihezi University, Shihezi, China, 2020. [Google Scholar]
- Li, J.; Wang, H.; Guo, M.; Li, T.; Zhang, H.; Zhang, Q.; Wang, Q.; Song, Y.; Feng, H.; Wei, G. Exogenous Spermidine Effectively Improves the Quality of Cryopreserved Boar Sperm. Anim. Sci. J. 2023, 94, 13859. [Google Scholar] [CrossRef]
- Álvarez-Rodríguez, M.; Nieto-Cristobal, H.; de Mercado, E. Bovine serum albumin inclusion in the thawing extender improves boar sperm membrane and acrosomal integrity. Reprod. Domest. Anim. 2024, 59 (Suppl. S3), e14592. [Google Scholar] [CrossRef]
- Tvrdá, E.; Greifová, H.; Mackovich, A.; Hashim, F.; Lukáč, N. Curcumin Offers Antioxidant Protection to Cryopreserved Bovine Semen. Czech J. Anim. Sci. 2018, 63, 247–255. [Google Scholar] [CrossRef]
- Takeshima, M.; Gotoh, A. Establishment of a rapid, cost-effective, and accurate method for assessing insect sperm viability. J. Insect. Physiol. 2024, 158, 104682. [Google Scholar] [CrossRef]
- Zuchowicz, N.; Daly, J.; Bouwmeester, J.; Lager, C.; Henley, E.M.; Nuñez Lendo, C.I.; Hagedorn, M. Assessing coral sperm motility. Sci. Rep. 2021, 11, 61. [Google Scholar] [CrossRef]
- Menkveld, R. Clinical significance of the low normal sperm morphology value as proposed in the fifth edition of the WHO Laboratory Manual for the Examination and Processing of Human Semen. Asian J Androl. 2010, 12, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Fan, X.; Zeng, Y.; Wang, L.; Zhu, Z.; Li, R.; Tian, X.; Wang, Y.; Lin, Y.; Wu, D.; et al. Resveratrol Protects Boar Sperm in Vitro Via its Antioxidant Capacity. Zygote 2020, 28, 417–424. [Google Scholar] [CrossRef]
- Kou, Z.; Hu, B.; Li, Y.; Cai, R.; Gao, L.; Chu, G.; Yang, G.; Pang, W. Boar Seminal Plasma Improves Sperm Quality by Enhancing its Antioxidant Capacity During Liquid Storage at 17 °C. Zygote 2022, 30, 695–703. [Google Scholar] [CrossRef] [PubMed]
- Prochowska, S.; Eberhardt, M.; Smalec, B.; Niżański, W. In search of freezability predictors for feline spermatozoa-osmotic challenge tests and markers of sperm membrane structure. Anim Reprod Sci. 2024, 268, 107563. [Google Scholar] [CrossRef] [PubMed]
- Ammar, O.; Mehdi, M.; Muratori, M. Teratozoospermia: Its Association with Sperm DNA Defects, Apoptotic Alterations, and Oxidative Stress. Andrology 2020, 8, 1095–1106. [Google Scholar] [CrossRef]
- Nur Karakus, F.; Bulgurcuoglu Kuran, S.; Solakoglu, S. Effect of Curcumin on Sperm Parameters after the Cryopreservation. Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 267, 161–166. [Google Scholar] [CrossRef]
- Shi, L.; Zhang, Y.; Huang, X.; Shi, M.; Sun, D.; Zhang, Y.; Li, W.; Jin, T.; Feng, J.; Xing, J.; et al. Effects of Mitoquinone (MitoQ) Supplementation During Boar sperm Cryopreservation on Sperm Quality, Antioxidant Status and Mitochondrial Proteomics. Anim. Reprod. Sci. 2022, 247, 107099. [Google Scholar] [CrossRef]
- Wang, M.; Wu, S.; Yang, B.; Ye, M.; Tan, J.; Zan, L.; Yang, W. Grape Seed Proanthocyanidins Improve the Quality of Fresh and Cryopreserved Semen in Bulls. Animals 2023, 13, 2781. [Google Scholar] [CrossRef]
- Funahashi, H. Methods for Improving in Vitro and in Vivo Boar Sperm Fertility. Reprod. Domest. Anim. 2015, 50, 40–47. [Google Scholar] [CrossRef]
- Wu, X.; Cai, X.; Ai, J.; Zhang, C.; Liu, N.; Gao, W. Extraction, Structures, Bioactivities and Structure-Function Analysis of the Polysaccharides from Safflower (Carthamus tinctorius L.). Front. Pharmacol. 2021, 12, 767947. [Google Scholar] [CrossRef]
- Cui, D.; Zhao, D.; Wang, B.; Liu, B.; Yang, L.; Xie, H.; Wang, Z.; Cheng, L.; Qiu, X.; Ma, Z.; et al. Safflower (Carthamus tinctorius L.) Polysaccharide Attenuates Cellular Apoptosis in Steroid-Induced Avascular Necrosis of Femoral Head by Targeting Caspase-3-Dependent Signaling Pathway. Int. J. Biol. Macromol. 2018, 116, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Lewandowska, E.; Węsierski, D.; Mazur-Milecka, M.; Liss, J.; Jezierska, A. Ensembling noisy segmentation masks of blurred sperm images. Comput. Biol. Med. 2023, 166, 107520. [Google Scholar] [CrossRef] [PubMed]
- Van de Hoek, M.; Rickard, J.P.; de Graaf, S.P. Motility Assessment of Ram Spermatozoa. Biology 2022, 11, 1715. [Google Scholar] [CrossRef]
- Hirano, Y.; Shibahara, H.; Obara, H.; Suzuki, T.; Takamizawa, S.; Yamaguchi, C.; Tsunoda, H.; Sato, I. Relationships between Sperm Motility Characteristics Assessed by the Computer-Aided Sperm Analysis (CASA) and Fertilization Rates in Vitro. J. Assist. Reprod. Genet. 2001, 18, 213–218. [Google Scholar] [CrossRef]
- Liu, D.Y.; Clarke, G.N.; Baker, H.W. Relationship between Sperm Motility Assessed with the Hamilton-Thorn Motility Analyzer and Fertilization Rates in Vitro. J. Androl. 1991, 12, 231–239. [Google Scholar] [CrossRef]
- Muvhali, P.T.; Bonato, M.; Malecki, I.A.; Cloete, S.W.P. Mass Sperm Motility Is Correlated to Sperm Motility as Measured by Computer-Aided Sperm Analysis (CASA) Technology in Farmed Ostriches. Animals 2022, 12, 1104. [Google Scholar] [CrossRef]
- Byrne, C.J.; Fair, S.; English, A.M.; Holden, S.A.; Dick, J.R.; Lonergan, P.; Kenny, D.A. Dietary Polyunsaturated Fatty Acid Supplementation of Young Post-Pubertal Dairy Bulls Alters the Fatty Acid Composition of Seminal Plasma and Spermatozoa but has no Effect on sperm Volume or Sperm Quality. Theriogenology 2017, 90, 289–300. [Google Scholar] [CrossRef]
- Nasiri, K.; Akbari, A.; Nimrouzi, M.; Ruyvaran, M.; Mohamadian, A. Safflower Seed Oil Improves Steroidogenesis and Spermatogenesis in Rats with Type II Diabetes Mellitus by Modulating the Genes Expression Involved in Steroidogenesis, Inflammation and Oxidative Stress. J. Ethnopharmacol. 2021, 275, 114139. [Google Scholar] [CrossRef]
- Ritagliati, C.; Baro Graf, C.; Stival, C.; Krapf, D. Regulation mechanisms and implications of sperm membrane hyperpolarization. Mech. Dev. 2018, 154, 33–43. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Q.; Min, L.; Cao, H.; Adetunji, A.O.; Zhou, K.; Zhu, Z. Sperm Pyrroloquinoline Quinone Improved Boar Sperm Quality via Maintaining Mitochondrial Function During Cryopreservation. Antioxidants 2025, 14, 102. [Google Scholar] [CrossRef]
- Almubarak, A.; Kim, E.; Yu, I.J.; Park, H.; Jeon, Y. The Effect of κ-Carrageenan on Porcine Sperm Cryo-Survival. Animals 2024, 14, 1387. [Google Scholar] [CrossRef] [PubMed]
- Pinart, E.; Yeste, M.; Bonet, S. Acrosin Activity is a Good Predictor of Boar Sperm Freezability. Theriogenology 2015, 83, 1525–1533. [Google Scholar] [CrossRef] [PubMed]
- Ded, L.; Dostalova, P.; Zatecka, E.; Dorosh, A.; Komrskova, K.; Peknicova, J. Fluorescent Analysis of Boar Sperm Capacitation Process in vitro. Reprod. Biol. Endocrinol. 2019, 17, 109. [Google Scholar] [CrossRef]
- Alkmin, D.V.; Martinez-Alborcia, M.J.; Parrilla, I.; Vazquez, J.M.; Martinez, E.A.; Roca, J. The Nuclear DNA Longevity in Cryopreserved Boar Spermatozoa Assessed Using the Sperm-Sus-Halomax. Theriogenology 2013, 79, 1294–1300. [Google Scholar] [CrossRef]
- Kuchakulla, M.; Narasimman, M.; Khodamoradi, K.; Khosravizadeh, Z.; Ramasamy, R. How Defective Spermatogenesis Affects Sperm DNA Integrity. Andrologia 2021, 53, e13615. [Google Scholar] [CrossRef]
- Woelders, H.; Matthijs, A.; Engel, B. Effects of Trehalose and Sucrose, Osmolality of the Freezing Medium, and Cooling Rate on Viability and Intactness of Bull Sperm after Freezing and Thawing. Cryobiology 1997, 35, 93–105. [Google Scholar] [CrossRef]
- Namula, Z.; Kodama, R.; Tanihara, F.; Morita, Y.; Sato, Y.; Wittayarat, M.; Taniguchi, M.; Otoi, T. Effects of Skim-Milk Supplementation on the Quality and Penetrating Ability of Boar sperm after Long-Term Preservation at 15°C. Acta Vet. Hung. 2014, 62, 106–116. [Google Scholar] [CrossRef]
- Gómez-Fernández, J.; Gómez-Izquierdo, E.; Tomás, C.; Mocé, E.; de Mercado, E. Is Sperm Freezability Related to the Post-Thaw Lipid Peroxidation and the Formation of Reactive Oxygen Species in Boars? Reprod. Domest. Anim. 2013, 48, 177–182. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, C.; Zhang, H.; Duan, Y. Review of Isolation, Structural Properties, Chain Conformation, and Bioactivities of Psyllium Polysaccharides. Int. J. Biol. Macromol. 2019, 139, 409–420. [Google Scholar] [CrossRef]
- Prestera, T.; Talalay, P.; Alam, J.; Ahn, Y.I.; Lee, P.J.; Choi, A.M. Parallel Induction of Heme Oxygenase-1 and Chemoprotective Phase 2 Enzymes by Electrophiles and Antioxidants: Regulation by Upstream Antioxidant-Responsive Elements (ARE). Mol. Med. 1995, 1, 827–837. [Google Scholar] [CrossRef]
- Lin, D.; Xu, C.J.; Liu, Y.; Zhou, Y.; Xiong, S.L.; Wu, H.C.; Deng, J.; Yi, Y.W.; Qiao, M.F.; Xiao, H.; et al. Chemical Structures and Antioxidant Activities of Polysaccharides from Carthamus tinctorius L. Polymers 2022, 14, 3510. [Google Scholar] [CrossRef] [PubMed]
- Weng, X.G.; Cai, M.M.; Zhang, Y.T.; Liu, Y.; Gao, Z.L.; Song, J.; Liu, Z.H. Effect of Astragalus Polysaccharide Addition to Thawed Boar Sperm on in Vitro Fertilization and Embryo Development. Theriogenology 2018, 121, 21–26. [Google Scholar] [CrossRef] [PubMed]
- Zangishhi, N.; Hajarian, H.; Karamishabankareh, H.; Soltani, L. The effect of different concentrations of laminarin on the quality of cryopreserved ram semen. Cryo Lett. 2024, 45, 60–68. [Google Scholar] [CrossRef]

| Groups | Items | ||
|---|---|---|---|
| Viability (%) | Motility (%) | Abnormality Rate (%) | |
| 0 g/L SPS | 72.33 ± 0.33 d | 62.65 ± 0.73 c | 22.52 ± 0.85 a |
| 0.5 g/L SPS | 73.33 ± 0.04 c | 64.53 ± 1.03 c | 18.26 ± 0.91 b |
| 1.0 g/L SPS | 73.77 ± 0.07 c | 69.27 ± 1.47 b | 14.87 ± 0.09 c |
| 1.5 g/L SPS | 78.00 ± 0.37 a | 73.92 ± 2.04 a | 12.46 ± 0.13 d |
| 2.0 g/L SPS | 76.83 ± 0.30 b | 70.70 ± 2.20 b | 12.81 ± 1.06 d |
| Groups | Items | |||
|---|---|---|---|---|
| VAP (μm/s) | VSL (μm/s) | VCL (μm/s) | BCF (Hz) | |
| 0 g/L SPS | 18.22 ± 4.31 c | 37.95 ± 1.57 c | 26.69 ± 7.28 c | 4.26 ± 0.83 d |
| 0.5 g/L SPS | 21.39 ± 5.73 bc | 42.82 ± 1.25 b | 34.98 ± 6.82 bc | 8.57 ± 0.57 c |
| 1.0 g/L SPS | 25.31 ± 3.38 bc | 43.58 ± 0.98 b | 43.76 ± 6.48 b | 11.47 ± 1.21 b |
| 1.5 g/L SPS | 35.35 ± 4.93 a | 49.77 ± 3.36 a | 55.22 ± 1.79 a | 15.64 ± 1.24 a |
| 2.0 g/L SPS | 28.08 ± 1.38 ab | 48.91 ± 2.42 a | 54.03 ± 3.61 a | 12.99 ± 0.14 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Dong, Y.; Wang, H.; Zhang, Q.; Guo, Q.; Li, Y. The Cryoprotectant Effects of Safflower Polysaccharides on the Quality of Frozen–Thawed Boar Sperm. Animals 2025, 15, 843. https://doi.org/10.3390/ani15060843
Li J, Dong Y, Wang H, Zhang Q, Guo Q, Li Y. The Cryoprotectant Effects of Safflower Polysaccharides on the Quality of Frozen–Thawed Boar Sperm. Animals. 2025; 15(6):843. https://doi.org/10.3390/ani15060843
Chicago/Turabian StyleLi, Jingchun, Yingying Dong, Hechuan Wang, Qun Zhang, Qing Guo, and Yanbing Li. 2025. "The Cryoprotectant Effects of Safflower Polysaccharides on the Quality of Frozen–Thawed Boar Sperm" Animals 15, no. 6: 843. https://doi.org/10.3390/ani15060843
APA StyleLi, J., Dong, Y., Wang, H., Zhang, Q., Guo, Q., & Li, Y. (2025). The Cryoprotectant Effects of Safflower Polysaccharides on the Quality of Frozen–Thawed Boar Sperm. Animals, 15(6), 843. https://doi.org/10.3390/ani15060843




