Simple Summary
Feeding restriction refers to artificially controlling the amount of feed consumed by animals so that the quality of the nutrients consumed is lower than the animal’s demand level, which can effectively improve the body’s immune function and disease resistance. Feed restriction has been used to improve body health in growing rabbits. In addition, meat rabbits are monogastric herbivores with developed caeca and a strong ability to decompose and digest nutrients. However, the effects of feed restriction on the caecum microbiota and metabolites in rabbits remain unclear. Thus, in the present study, we investigated the effects of feed restriction on the growth performance, nutrient utilisation, lipid metabolism, antioxidant activity, caecal microbiota, and metabolites of rabbits. Our research results provide a theoretical reference for the development of healthy feeding restriction standards for meat rabbits.
Abstract
The main objective of this research was to observe the effects of feed restriction on caecum microbiota and metabolites in rabbits. Forty-eight male 8-week-old rabbits with similar body weights (1872.11 ± 180.85 g) were randomly assigned to two treatments according to completely randomized design: (1) the control group received ad libitum access to feed (AL), and (2) the treatment received 80% of the feed consumed by the control (FR). The results showed that FR did not differ (p > 0.05) for average daily weight gain or feed conversion ratio between the two groups. FR treatment led to a significant increase (p < 0.05) in acid detergent fibre apparent faecal digestibility, nitrogen digestibility and retention, and gross energy digestibility and retention. The FR treatment showed significantly (p < 0.05) lower blood triglycerides, creatinine, high-density lipoprotein cholesterol, malondialdehyde, and hydroxyl free radicals but significantly (p < 0.05) greater total antioxidant capacity and superoxide dismutase. The FR group presented greater (p < 0.05) Firmicutes and Ruminococcus abundances but a lower (p < 0.05) Akkermansiaceae abundance in the caecal content. Moreover, 222 differentiated metabolites were identified, and beta-alanine metabolism was the top enriched pathway. Collectively, FR can improve nutrient utilisation, lipid metabolism, antioxidant activity, caecum microbiota, and metabolites in rabbits.
1. Introduction
Mild feed restriction (FR) is beneficial for the health of animals, as it can improve digestive system health and reduce the incidence of animal epidemics [1,2]. For example, Halai et al. [3] reported that FR could improve health in male mice by increasing the mean lifespan and influencing biochemical parameters. Similarly, rats fed a 30% restriction diet showed regulated disordered gut microbiota and inhibited metabolic endotoxaemia and inflammation [4]. Similar findings have also been found in rabbits. Gidenne et al. [5] showed that 80% FR did not affect carcass traits, whereas it reduced the feed conversion ratio (FCR) and the mortality of young rabbits. In addition, Martignon et al. [6] reported that 75% FR significantly increased nutrient digestibility and decreased mortality in weaned rabbits. Thus, FR may improve body health by regulating antioxidant capacity, lipid metabolism, and immune function in animals [7,8].
FR has been used to improve the performance of rabbits, pigs, lambs, chickens, and turkey [9]. Previous studies have shown that FR affects the gastrointestinal tract microbiota and metabolites [10,11]. FR can significantly modulate the microbiota of the caecum and increase immune and antioxidant functions in rats [4]. Artdita et al. [12] showed that 80% FR could decrease excessive fat without affecting the egg production rate and upregulate lipid, carbohydrate, amino acid, and nucleic acid pathways in the caecum in late-stage laying hens, as shown by a Kyoto Encyclopedia of Genes and Genomes (KEGG) assay. In addition, Makovicky et al. [13] indicated that FR could increase mean villus height and the length of the small intestine in weaned Hyplus rabbits. Similarly, FR increased digestible area in the small and large intestines (consisting of villus height and crypt depth) in growing rabbits [14]. Hence, FR might affect the gut microbiota and metabolism and may be a healthy dietary strategy for improving animal health [15,16].
The rabbit caecum represents a structurally highly developed organ where feed can be fermented, which is crucial for digestion and nutrient absorption [17]. Previous studies with rabbits have indicated that ad libitum feeding might have a negative effect on digestive disorders and that FR preserves digestive health and thus reduces mortality and morbidity rates [18,19,20]. Furthermore, 80% FR has been widely applied in growing rabbit breeding systems to improve body health [5,21]. For example, Crespo et al. [22] found that 80% FR did not affect the mortality and growth rate but improved nitrogen and energy utilization in New Zealand × Californian crossbred hybrid rabbits. Moreover, our previous study did show that 80% FR could enhance muscle antioxidant activity and meat quality in growing rabbits [23]. However, previous studies have mainly investigated the effects of FR on the growth performance, meat quality, mortality, and apparent faecal digestibility [6,22,23]; the effects of FR on the caecal microbiota and metabolites in rabbits remain unclear. We hypothesize that mild FR enhances nutrient utilisation, improves antioxidant activity, and alters caecal microbiota composition by increasing beneficial bacteria and modifying metabolic pathways in growing rabbits. Accordingly, this study aimed to investigate the effects of FR on the growth performance, nutrient utilisation, lipid metabolism, antioxidant activity, and caecal microbiota and metabolites of rabbits.
2. Materials and Methods
2.1. Animals and Experimental Design
All animal procedures were reviewed and approved by the Guizhou University Animal Ethics Committee (No. EAE-GZU-2024-E028). In general, meat rabbits are slaughtered at age 3–4 months, and our previous study showed that 80% FR resulted in improved meat quality in rabbits [23]. Therefore, forty-eight male, 8-week-old pure New Zealand white rabbits with similar body weights (1872.11 ± 180.85 g; mean ± standard deviation) were randomly assigned to two equal treatment groups in a completely randomized design. The control group received ad libitum access to feed (AL), and the treatment group received 80% of the feed consumed by the control (FR). The restriction programme was applied by giving a daily meal starting at 8 weeks. Before the start of the experimental period, a pretest was conducted to calculate the feed intake of 24 randomly fed rabbits. This provided the feed consumption for both groups during the restricted feeding period. Moreover, daily feed intake was calculated, and the amount of rabbit feed was adjusted weekly. The diets were fed in equal amounts twice daily at 08:30 and 17:30. The feeding trial lasted for 7 weeks and included a 1-week pretrial period and a 6-week formal period. The feed of the rabbits in the AL group was gradually reduced to 80% over 4 d, and the rabbits were allowed to acclimate to 80% for 3 d before beginning the experimental period. Each treatment consisted of 12 replicates (n = 12) with 2 rabbits each (2 rabbits per cage). The rabbits were placed in pairs in cages (160 × 70 × 195 cm). All rabbits had free access to water during the whole feeding period. The experimental diet was manufactured and pelleted with a 4 mm millstone by a granule presser at one time (Jixiang Animal Husbandry Machinery Co., Ltd., Zhengzhou, China) using one batch of raw materials. The nutritional requirements were followed according to the Chinese standard (NY/T 4049-2021) [24], and the ingredients of the basal diet are shown in Table 1.

Table 1.
The ingredient of basal diet.
The dry matter (DM; method 934.01), crude protein (CP; method 988.05), and ash (method 942.05) contents were analysed according to the AOAC [25]. The neutral detergent fibre (NDF) and acid detergent fibre (ADF) contents were analysed according to the methods of Van Soest et al. [26]. The gross energy (GE) was analysed using an adiabatic oxygen bomb calorimeter (WGR-WR3, Changsha Bente Instrument Co., Ltd., Changsha, China). The chemical composition of the basal diet is shown in Table 2.

Table 2.
The chemical composition of basal diet.
2.2. Growth Performance and Digestibility of Feed Nutrients
The feed weight was measured every day to monitor the dry matter intake (DMI). The animal body weight was weighed on the first day (initial weight) and last day (final weight), and the average daily weight gain (ADG) was calculated. The FCR was calculated by dividing the DMI by the ADG. In addition, no rabbits died throughout the entire experimental period.
The digestion and metabolism experiment was carried out at 100–105-day-old rabbits. Briefly, faecal and urine samples were collected during the last 5 d using the total faecal and urinary collection method. The faeces was divided into two parts: the first part was stored directly in a −20 °C refrigerator, and the second part was added to 20% H2SO4 to determine the N content. At the end of the experiment, the faecal sample was dried under vacuum at 60 °C for 72 h, ground, and passed through a 1 mm sieve for chemical composition analysis. The urine was added to 20% H2SO4 to maintain the pH at lower than 3 and kept at −20 °C for N and GE analysis.
2.3. Plasma Parameters
The plasma samples were collected from all tested rabbits on the last day of the experiment. Before slaughter, blood was collected from the heart using a vacuum blood collection tube (Kangweishi Medical Technology Co., Ltd., Shijiazhuang, China). Then, the blood was centrifuged for 10 min at 4000× g (TD4, Shanghai Lu Xiangyi Centrifuge Instrument Co., Ltd., Shanghai, China), and the plasma was stored at −80 °C for later testing. The following plasma parameters were detected: (1) biochemical parameters: glucose (Glu), total protein (TP), and albumin (Alb); (2) lipid metabolism parameters: triglyceride (TG), creatinine (Cr), total cholesterol (TCH), low-density lipoprotein cholesterol (LDL-C), and high-density lipoprotein cholesterol (HDL-C); and (3) antioxidant activity parameters: total antioxidant capacity (T-AOC), superoxide dismutase (SOD), glutathione peroxidase (GSH-Px), catalase (CAT), 2,2-diphenyl-1-picrylhydrazyl (DPPH), free radical scavenging capacity (DPPH scavenging activity), malondialdehyde (MDA), hydroxyl free radical (·OH), and superoxide anion (O2). All kits were obtained from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China).
2.4. Caecal Microbiota Analysis
After the slaughter, 24 caecum samples were immediately collected from each group (2 rabbits/cage), and the caecum samples from 2 rabbits per cage were pooled. Then, the microbiome and metabolome were analysed. The DNA was extracted from the caecal samples by the E.Z.N.A.® soil DNA Kit (Omega Bio-tek, Norcross, GA, USA). The DNA extract was examined on 1% agarose gel, and the DNA concentration and purity were detected using a NanoDrop 2000 UV–vis spectrophotometer. The hypervariable region V3–V4 of the bacterial 16S rRNA gene was amplified using the primer pair 338F (5′-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5′-GGACTACHVGGGTWTCTAAT-3′) by an ABI GeneAmp® 9700 PCR thermocycler (Foster City, CA, USA).
Purified amplicons were pooled in equimolar amounts and paired-end sequenced on an Illumina MiSeq PE300 platform/NovaSeq PE250 platform (Illumina, San Diego, CA, USA). The raw 16S rRNA gene sequencing reads were demultiplexed, quality filtered by fastp version 0.20.0, and merged by FLASH version 1.2.7. Operational taxonomic unit (OTU) with 97% similarity cut-off was clustered by the UPARSE version 7.1, and chimeric sequence was identified and removed. The taxonomy of each OTU representative sequence was analysed using the RDP Classifier version 2.2 against the 16S rRNA database (e.g., SILVA v138) with a confidence threshold of 0.7. The raw reads were deposited into the NCBI Sequence Read Archive (SRA) database (Accession Number: PRJNA1222279).
2.5. Caecal Metabolomic Analysis
A total of 12 caecum samples (2 rabbits per cage were pooled) were analysed for their metabolome. A 100 g caecal sample was added to 400 μL of solution (acetonitrile/methanol = 1:1 (v:v)) containing 0.02 mg/mL internal standard (L-2-chlorophenylalanine) to extract metabolites. The sample was mixed by vortexing for 30 s and then sonicated at 5 °C for 30 min with 40 kHz. Next, the supernatant was removed after centrifugation at 13,000× g for 15 min at 4 °C and then was blown dry under nitrogen. The sample was then resolubilized with 100 µL of solution (acetonitrile/water = 1:1) and extracted by low-temperature ultrasonication for 5 min at 5 °C with 40 kHz. The supernatant was collected after centrifugation at 13,000× g for 10 min at 4 °C for liquid chromatography–mass spectrometry (LC-MS/MS) analysis.
The data matrix obtained by searching the database was uploaded to the Majorbio cloud platform (https://cloud.majorbio.com, accessed on 25 October 2023). The orthogonal least partial squares–discriminant analysis (OPLS-DA) was analysed by the R package “ropls” (version 1.6.2), and the stability of the model was evaluated by the 7-cycle interactive validation. Differentially abundant metabolites were mapped to their biochemical pathways by metabolic enrichment and pathway analysis based on the KEGG database (http://www.genome.jp/kegg/, accessed on 25 October 2024).
2.6. Statistical Analysis
One cage (2 rabbits per cage) was considered an experimental unit in this study. The sample sizes were analysed by Statistical Analysis System 9.1.3 (SAS Institute, Cary, NC, USA); twelve replicates (n = 12) were shown to obtain a power of 0.80 and a 0.05 significance level, according to our previous study [23]. Data on growth performance, nutrient digestibility, and plasma parameters were analysed by Student’s t-test using SAS 9.1.3 software. The abundances of the microbiota were analysed by the Kruskal–Wallis H test. The metabolites with variable importance in the projection (VIP) > 1 and p < 0.05 were detected to be significantly differentially abundant metabolites obtained by the OPLS-DA model and the p-value generated by the Student’s t-test.
3. Results
3.1. Dry Matter Intake, Growth Performance, and Apparent Faecal Digestibility
The DMI value in the FR treatment group was significantly lower (p < 0.05) than that in the AL treatment (Table 3).

Table 3.
Effect of feed restriction on dry matter intake, growth performance, and apparent faecal digestibility of growing rabbits.
In contrast, no significant differences (p > 0.05) were observed for the initial weight, final weight, ADG, or FCR values between the two treatments. The apparent faecal digestibilities of DM, NDF, and ash did not differ (p > 0.05) between the two treatments. In addition, compared with the AL treatment, the FR treatment led to a significant increase (p < 0.05) in ADF apparent faecal digestibility (increased 12.44%).
3.2. Nitrogen Utilisation
Compared with the AL group, the FR group presented lower (p < 0.05) levels of N intake, N excretion in faeces, N excretion in urine, and total N excretion (Table 4). However, FR treatment showed significantly (p < 0.05) higher N digestibility and N retention contents relative to the AL treatment.

Table 4.
Effect of feed restriction on nitrogen utilisation of growing rabbits.
3.3. Energy Utilisation
Compared with the AL group, the FR treatment group presented lower (p < 0.05) concentrations of GE intake, GE excretion in faeces, GE excretion in urine, and total GE excretion (Table 5). In contrast, the FR treatment had significantly (p < 0.05) higher GE digestibility and GE retention levels compared to the AL group.

Table 5.
Effect of feed restriction on energy utilisation of growing rabbits.
3.4. Plasma Biochemical, Lipid Metabolism, and Antioxidant Activity
There were no significant differences (p > 0.05) detected for the plasma Glu, TP, Alb, TCH, LDL-C, GSH-Px, CAT, and DPPH scavenging activities or O2· concentrations between the two groups (Table 6). The FR treatment showed significantly (p < 0.05) lower values of TG, Cr, HDL-C, MDA, and ·OH in plasma compared with the AL group. In contrast, the FR treatment had significantly (p < 0.05) higher T-AOC and SOD activities relative to the AL group.

Table 6.
Effect of feed restriction on blood biochemical, lipid metabolism, and antioxidant activity parameters in rabbit.
3.5. Caecal Microbiota
A total of 1,590,531 raw reads were determined in this study among the 24 samples. A total of 1,514,566 effective tags were identified by eliminating low-quality sequences. The effective number of bases was 618,406,083 bp, and the average length was 408 nt from 24 samples. In terms of α diversity, there was no (p > 0.05) significant difference in the Sobs, Shannon, Simpson, Ace, or Chao1 index between the AL and FR treatments (Figure 1).
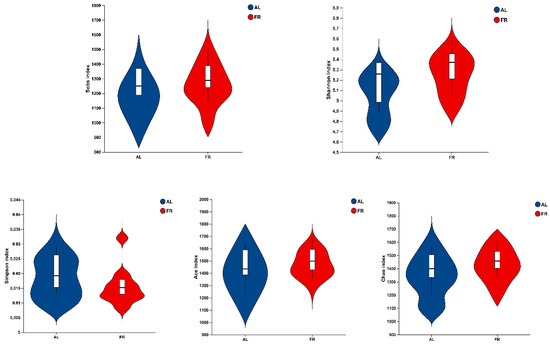
Figure 1.
Analysis of α diversity index in caecal content between AL and FR treatments in rabbits. AL, rabbit was fed ad libitum diet; FR, rabbit was fed 80% ad libitum diet.
For the OTU cluster analysis, a total of 4709 OTUs were obtained, which were classified into 12 phyla, 20 classes, 45 orders, 77 families, 171 genera, and 349 species. Specifically, 2376 OTUs were shared between the two groups (Figure 2A), 1098 were exclusive to the AL group, and 1268 were exclusive to the FR group. Principal component analysis showed that the caecal microbiota was different between the two treatments (Figure 2B).
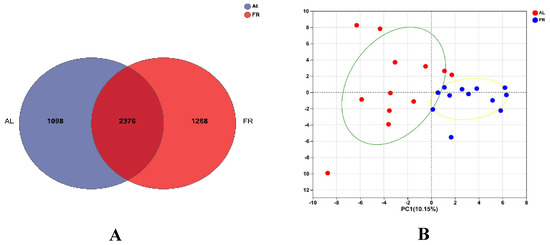
Figure 2.
Effect of FR on the caecal bacterial communities in rabbit. (A) Comparison of Venn diagram among the two treatments; (B) principal component analysis of bacterial communities. AL, rabbit was fed ad libitum diet; FR, rabbit was fed 80% ad libitum diet.
At the phylum level, the FR group presented greater (p < 0.05) Firmicutes abundance in the caecal content but lower (p < 0.05) Verrucomicrobiota and Actinobacteriota abundances than the AL group did (Figure 3A). At the family level, the FR treatment led to a greater (p < 0.05) abundance in the caecal content of norank_o__Clostridia_vadinBB60_group and lower (p < 0.05) abundances of Akkermansiaceae, Erysipelotrichaceae, Eggerthellaceae, and Atopobiaceae compared with the AL group (Figure 3B). At the genus level, FR treatment resulted in greater (p < 0.05) abundances in the caecal content of norank_f__norank_o__Clostridia_vadinBB60_group, V9D2013_group, Ruminococcus, Eubacterium_siraeum_group, norank_f__Oscillospiraceae, norank_f__Barnesiellaceae, and Eubacterium_ruminantium_group but lower (p < 0.05) abundances of Akkermansia, Subdoligranulum, and Family_XIII_AD3011_group compared with the AL group (Figure 3C).
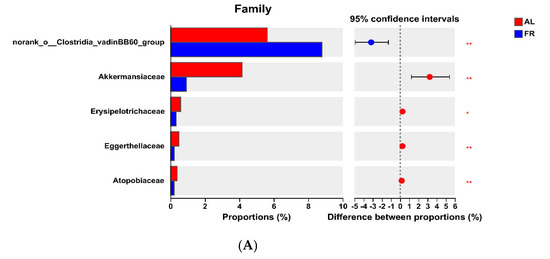
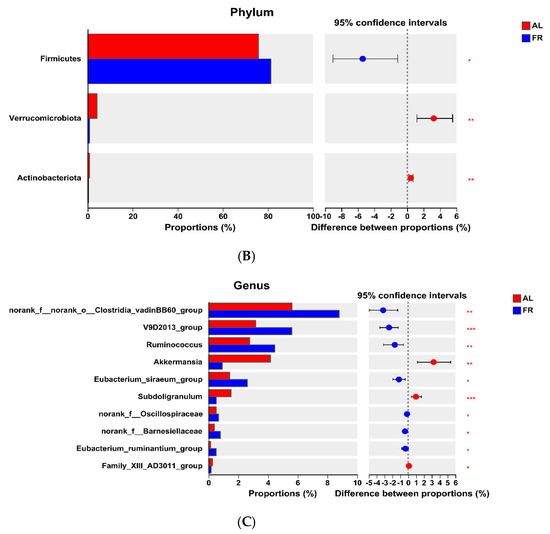
Figure 3.
Significant differences of caecal microbiota analysis. (A) Phylum level. (B) Family level. (C) Genus level. * p < 0.05, ** p < 0.01, and *** p < 0.001 mean the significant difference between groups. AL, rabbit was fed ad libitum diet; FR, rabbit was fed 80% ad libitum diet.
3.6. Caecal Metabolome
The metabolome of the caecal content samples was determined using LC-MS/MS, and 2114 metabolites were detected in the two groups (Figure 4A).
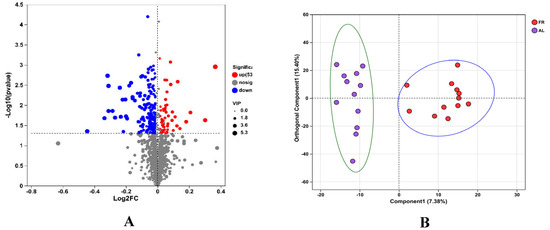
Figure 4.
Differential caecal metabolites in this study. (A) Volcano plot. (B) Orthogonal projections to latent structure discrimination analysis. AL, rabbit was fed ad libitum diet; FR, rabbit was fed 80% ad libitum diet.
With respect to the fold change (FC) and VIP of metabolites in the FR or AL groups, 222 differentiated metabolites were identified (positive and negative ions). Among the 222 metabolites, 53 metabolites were classified as upregulated, and 169 were downregulated (Figure 4A).
The OPLS-DA results showed that the metabolites of the FR treatment could be completely separated from those of the AL group, indicating that FR treatment could change the caecal metabolites (Figure 4B).
To further identify the target metabolites modulated by FR, metabolic pathway analysis of these 222 differentially abundant metabolites revealed enrichment of pathways, the top five of which included beta-alanine metabolism (pathway id map00410), glycine, serine and threonine metabolism (pathway id map00260), arginine and proline metabolism (pathway id map00330), histidine metabolism (pathway id map00340), and lysine degradation (pathway id map00310; Figure 5).
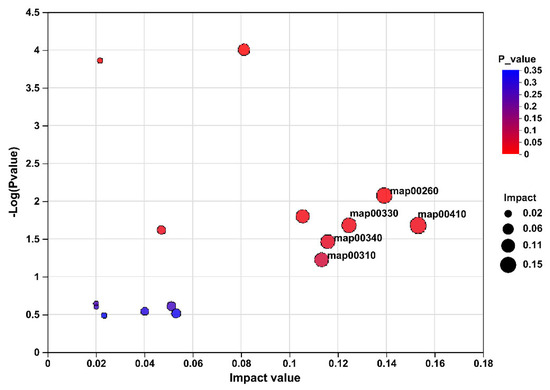
Figure 5.
The pathway analysis of caecal content differentially expressed metabolites based on the comparison of rabbits in AL and FR. map00410, beta-alanine metabolism; map00260, glycine, serine, and threonine metabolism; map00330, arginine and proline metabolism; map00340, histidine metabolism; map00310, lysine degradation. AL, rabbit was fed ad libitum diet; FR, rabbit was fed 80% ad libitum diet.
4. Discussion
The effect of FR on growth performance relies on the severity and duration of FR and the age of the growing rabbits [27]. The restriction of feed intake and feeding time for meat rabbits is related to improvements in the health status, digestion, and feed conversion rate of growing rabbits [28,29]. Zhuang et al. [30] reported that 85% FR did not affect the final weight, ADG, or FCR of growing weanling rabbits. FR might improve the small intestine and caecum health and affect the caecum contents and thus might contribute to the animals’ growth [9]. In addition, FR reduced the pathogenic microbiota population and improved the beneficial microbiota population in the caecal content of growing rabbits [31,32]. In the present study, we found that FR did not show different growth performance parameters, perhaps because FR promoted nutrient utilisation and regulated caecal microbiota. This phenomenon was also validated in terms of nutrient digestibility, nitrogen, and energy utilisation (Table 3, Table 4 and Table 5). Consistent with our findings, Romero et al. [33] indicated that 85% FR did not affect the growth performance or FCR values of growing rabbits.
The feed utilisation rate is an indicator that reflects the degree to which feed is decomposed and absorbed in the digestive tract [34]. Nutrias that received 75% FR showed improvements in the length of the caecum and the small intestine [9]. Martignon et al. [6] suggested that FR increased organic matter, GE, CP, NDF, ADF, and hemicellulose digestibilities in New Zealand white × Californian rabbits. Thus, we found that FR increased nutrient digestibility and utilisation in growing rabbits, possibly because FR promotes caecal development and thus improves the digestion and utilisation efficiency of nutrients. Another possible reason might be that FR prolongs feed retention in the gastrointestinal tract, stimulates digestive enzymes, and improves mucosal absorption in feed-restricted rabbits [21]. Consistent with our observations, Martignon et al. [6] reported that FR increased CP, GE, and fibre digestibilities in growing rabbits. Similarly, Gidenne et al. [35] reported that FR improved the N balance, with 40% less total (faeces + urine) N excretion in restricted rabbits. Combes et al. [31] reported that the microbiota is a beneficial source of digestive health biomarkers. Thus, another possible reason might be that FR increases the abundances in the caecal content of some digestive microbiota, such as Firmicutes and Ruminococcus (Figure 3), in rabbits.
FR can improve lipid metabolism and promote the bodily health of rabbits [36]. This might be because FR can significantly alter the liver lipid deposition-related gene expression and subsequently regulate lipid metabolism in animals [37]. For example, restricted feeding improved deranged lipid profiles (TCH, TG, HDL-C, and LDL-C) in rats [38]. In addition, Liu et al. [39] reported that calorie restriction decreased blood TCH, TG, LDL-C, and HDL-C concentrations in castrated male pigs. Similarly, in the present study, FR resulted in reduced plasma TG, Cr, and HDL-C values in rabbits, suggesting its anti-hyperlipidaemic effect. This was probably because FR downregulates key lipogenic genes (e.g., sterol regulatory element-binding transcription factor 1 and peroxisome proliferator-activated receptor) in animals [40]. Consistent with our findings, Chen et al. [41] reported that FR decreased blood TCH and HDL-C values in broiler chickens.
The T-AOC can reflect the body’s compensation level for external environmental factors and free radical metabolism in animals [42]. SOD is an important antioxidant enzyme that can eliminate the superoxide anion radicals generated during biological oxidation processes and enhance antioxidant capacity in animals [43]. MDA is an oxidative end product, and the level of MDA in the body reflects the degree of peroxidation damage in animals [44]. A previous study showed that FR can increase innate immunity, which might enhance antioxidant activity in animals [45]. For example, Chen et al. [41] reported that FR increased the serum SOD content and reduced the MDA value in broiler chickens. Thus, we found that FR enhanced T-AOC and SOD activities but decreased the MDA and ·OH contents, possibly because FR reduced mitochondrial free radical production and alleviated oxidative damage in animals [46]. Consistent with our findings, Lu et al. [23] reported that FR might increase antioxidant potential in growing rabbits by increasing the muscle SOD activity and DPPH free radical scavenging activity and decreasing the MDA content. Similarly, Zhuang et al. [30] reported that 70% FR increased SOD and reduced MDA levels in the jejunum of New Zealand rabbits at 70 d.
The caecum of meat rabbits is highly developed and contains many beneficial microorganisms that can fully decompose the partially digested chyme in the small intestine and maintain a normal intestinal microbiota, thus maintaining the health of the body [47]. A previous study revealed that 80% FR can improve lipid metabolism and meat quality by altering the structure of the caecal microbial community in animals [48]. In addition, the caecum of meat rabbits contains abundant microorganisms, which can secrete cellulases to degrade plant cellulose in the caecum [49]. Specifically, Firmicutes, an important component of the gut microbiota, are involved in the digestion and absorption of feed and in the degradation of cellulose [50]. Therefore, the current results showed that FR increased caecal Firmicutes abundance in growing rabbits. This finding corresponds with the apparent ADF faecal digestibility in rabbits. This may be related to FR increasing the fibrinolytic activity (cellulase and xylanase activities) in rabbits [6].
FR might participate in stabilizing the intestinal microbial balance and thus inhibit pathogenic bacteria development in fattening rabbits [27]. Hence, FR can alter the diversity of the gut microbiota and reduce intestinal inflammatory responses in animals [51]. Zhuang et al. [30] demonstrated that FR improved immune function by regulating the secretion of interferon-γ, interleukin-10, and tumour necrosis factor-α. Notably, Akkermansia is involved in inflammation and anti-inflammatory effects, and colitis resulted from increased Akkermansiaceae abundance in mice [52]. In the present study, we found that FR increased caecal Akkermansia abundance, perhaps because FR resulted in increased antioxidant activity (e.g., T-AOC and SOD activities) and reduced oxidation products (e.g., MDA and ·OH) in rabbits.
Bacteria of the Ruminococcaceae family break down substances such as cellulose, proteins, and lipids, releasing short-chain fatty acids, amino acids, and lipid metabolites [53]. These metabolites not only provide energy and nutrients for intestinal epithelial cells but also increase the acidity of the intestinal wall, inhibit the growth of harmful bacteria, and maintain the balance of the intestinal microbiota [54]. Specifically, FR not only modulates the overall structure of the gut microbiota but also selectively enriches anti-inflammatory bacteria (e.g., Oscillibacter, Allobaculum, and Lachnospiraceae_NK4A136_group) and decreases the abundances of proinflammatory pathogenic bacteria (e.g., Bifidobacterium, Bacteroides, and Lachnoclostridium) [4]. Thus, FR might regulate the gut microbiota balance and then improve immune response in rabbits [27]. Additionally, Ruminococcus is a genus of bacteria that produces abundant propionic and butyric acids and can participate in feed digestion and maintain intestinal barrier function [55]. The results of this study revealed that FR increased the abundance of Ruminococcus in the caecal content, indicating that FR might improve nutrient digestibility, reduce the inflammatory response, and improve antioxidant activity in rabbits. This observation suggested that FR improved nutrient utilisation and plasma antioxidant activity in rabbits. Consistent with our results, Combes et al. [31] reported that FR regulated caecal microbiota contents, especially those of dominant genera belonging to the Ruminococcaceae family, in young rabbits.
β-alanine is a type of β-amino acid that exists in nature and does not participate in protein synthesis [56]; it is a metabolite of uracil and cytosine [57]. On the one hand, the function of β-alanine in animals is achieved mainly through the synthesis of carnosine, which is an endogenous active peptide that has the ability to scavenge oxidative free radicals and enhance antioxidant properties [58,59]. On the other hand, β-alanine can alleviate oxidative stress, inhibit fat oxidation, and thus enhance the body’s antioxidant capacity [60]. For example, β-alanine has strong antioxidant activity, which can increase the activity of SOD in muscle tissue and serum, reduce the MDA content, and increase the antioxidant capacity of muscles in animals [61]. Similarly, dietary supplementation with β-alanine could increase GSH-Px activity and reduce the MDA content in broiler chickens [62,63]. In this study, FR was found to enrich β-alanine metabolism in rabbits, possibly by enhancing antioxidant function (Table 6). In brief, FR can regulate microorganisms and metabolites in the caecal contents of rabbits, thereby increasing their antioxidant capacity.
5. Conclusions
In conclusion, FR increased the apparent faecal digestibility of acid detergent fibre, nitrogen digestibility, nitrogen retention, gross energy digestibility, and gross energy retention values. FR increased the total antioxidant capacity and superoxide dismutase activities and reduced the triglyceride, creatinine, high-density lipoprotein cholesterol, malondialdehyde, and hydroxyl free radical contents. FR increased Firmicutes and Ruminococcus abundances in the caecal content but decreased Akkermansiaceae abundance. FR simultaneously changed the structure of caecal metabolites in growing rabbits. These results indicated that FR can enhance nutrient utilisation, improve antioxidant activity, and alter caecal microbiota composition by increasing beneficial bacteria and modifying metabolic pathways in growing rabbits. However, we only tested 80% FR in this study, and further studies are needed to determine the impact of different levels of FR on bodily health in rabbits. Another potential limitation was that we did not compare other intestinal microbiota and metabolites in rabbits.
Author Contributions
Q.L., data curation, project administration, writing—original draft, and writing—review and editing; J.Q., S.X., R.C., X.W., and Y.X., resources, investigation, and writing—review and editing; Y.B., C.G., and P.L., writing—review and editing; D.Z., resources, writing—original draft, and writing—review and editing; X.T., writing—original draft, writing—review and editing, data curation, software, and funding acquisition. All authors have read and agreed to the published version of the manuscript.
Funding
This research was partly funded by the Basic Research Program (Natural Science) Youth Guidance Project of Guizhou Province (project no. Qiankehe Foundation [2024] Youth 106), the Youth Science and Technology Talent Development Project of Guizhou Province (project no. Qianjiaoji [2024] 33), and the Basic Research Project of Guizhou University (project no. 2023-16).
Institutional Review Board Statement
The animal procedures were reviewed and approved by the Guizhou University Animal Ethics Committee (No. EAE-GZU-2024-E028).
Informed Consent Statement
Not applicable.
Data Availability Statement
The caecal microbiota datasets presented can be found in online repositories, and the BioProject ID is PRJNA1222279.
Conflicts of Interest
All authors declare that they have no conflicts of interest for this article.
References
- Van Harten, S.; Cardoso, L.A. Feed restriction and genetic selection on the expression and activity of metabolism regulatory enzymes in rabbits. Animal 2010, 4, 1873–1883. [Google Scholar] [CrossRef] [PubMed]
- Knudsen, C.; Combes, S.; Briens, C.; Duperray, J.; Rebours, G.; Salaun, J.M.; Travel, A.; Weissman, D.; Gidenne, T.; Oswald, I.P. Quantitative feed restriction rather than caloric restriction modulates the immune response of growing rabbits. J. Nutr. 2015, 145, 483–489. [Google Scholar] [CrossRef] [PubMed]
- Halai, S.F. The Effects of Dietary Free Radical Inhibitors and Feed Restriction on Age Associated Biochemical Parameter and Lifespan of AKR Male Mice. Ph.D. Thesis, University of Tennessee, Knoxville, TN, USA, 1972. [Google Scholar]
- Zhang, L.; Zhang, T.; Sun, J.; Huang, Y.; Liu, T.; Ye, Z.; Hu, J.; Zhang, G.; Chen, H.; Ye, Z.; et al. Calorie restriction ameliorates hyperglycemia, modulates the disordered gut microbiota, and mitigates metabolic endotoxemia and inflammation in type 2 diabetic rats. J. Endocrinol. Investig. 2023, 46, 699–711. [Google Scholar] [CrossRef] [PubMed]
- Gidenne, T.; Combes, S.; Feugier, A.; Jehl, N.; Arveux, P.; Boisot, P.; Briens, C.; Corrent, E.; Fortune, H.; Montessuy, S.; et al. Feed restriction strategy in the growing rabbit. 2. Impact on digestive health, growth and carcass characteristics. Animal 2009, 3, 509–515. [Google Scholar] [CrossRef]
- Martignon, M.; Burel, C.; Cauquil, L.; Combes, S.; Gidenne, T. Impact of feed restriction and fragmented feed distribution on performance, intake behaviour and digestion of the growing rabbit. Animal 2021, 15, 100270. [Google Scholar] [CrossRef]
- Ebeid, T.A.; Tůmová, E.; Al-Homidan, I.H.; Ketta, M.; Chodová, D. Recent advances in the role of feed restriction in poultry productivity: Part I—performance, gut development, microbiota and immune response. World Poultry Sci. J. 2022, 78, 971–988. [Google Scholar] [CrossRef]
- Xu, C.; Liu, W.B.; Remø, S.C.; Wang, B.K.; Shi, H.J.; Zhang, L.; Liu, J.D.; Li, X.F. Feeding restriction alleviates high carbohydrate diet-induced oxidative stress and inflammation of Megalobrama amblycephala by activating the AMPK-SIRT1 pathway. Fish Shellfish Immunol. 2019, 92, 637–648. [Google Scholar] [CrossRef]
- Tmová, E.; Volek, Z.; Chodová, D.; Skivanová, V.; Němeek, T.; Ketta, M. Effect of quantitative feed restriction on the performance, organ development and cecal activity of growing nutrias (Myocastor coypus). Anim. Feed Sci. Technol. 2021, 280, 115077. [Google Scholar] [CrossRef]
- Hou, L.; Wang, L.; Qiu, Y.; Xiong, Y.; Xiao, H.; Yi, H.; Wen, X.; Lin, Z.; Wang, Z.; Yang, X.; et al. Effects of protein restriction and subsequent realimentation on body composition, gut microbiota and metabolite profiles in weaned piglets. Animals 2021, 11, 686. [Google Scholar] [CrossRef]
- Fondevila, G.; Archs, J.L.; Cámara, L.; de Juan, A.F.; Mateos, G.G. The length of the feed restriction period affects eating behavior, growth performance, and the development of the proximal part of the gastrointestinal tract of young broilers. Poult. Sci. 2020, 99, 1010–1018. [Google Scholar] [CrossRef]
- Artdita, C.A.; Zhuang, Y.R.; Liu, T.Y.; Cheng, C.Y.; Hsiao, F.S.; Lin, Y.Y. The effect of feeding restriction on the microbiota and metabolome response in late-phase laying hens. Animals 2021, 11, 3043. [Google Scholar] [CrossRef] [PubMed]
- Makovicky, P.; Tumova, E.; Volek, Z.; Makovicky, P.; Vodicka, P. Histological aspects of the small intestine under variable feed restriction: The effects of short and intense restriction on a growing rabbit model. Exp. Ther. Med. 2014, 8, 1623–1627. [Google Scholar] [CrossRef] [PubMed]
- Tůmová, E.; Volek, Z.; Chodová, D.; Härtlová, H.; Makovický, P.; Svobodová, J.; Ebeid, T.A.; Uhlířová, L. The effect of 1-week feed restriction on performance, digestibility of nutrients and digestive system development in the growing rabbit. Animal 2016, 10, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ye, Y.; Xu, H.; Xie, Z.; Wang, L.; Sun, Y.; Yang, H.; Hu, D.; Mao, Y. Time-restricted feeding reduces the detrimental effects of a high-fat diet, possibly by modulating the circadian rhythm of hepatic lipid metabolism and gut microbiota. Front. Nutr. 2020, 7, 596285. [Google Scholar] [CrossRef]
- Schmidt, N.S.; Lorentz, A. Dietary restrictions modulate the gut microbiota: Implications for health and disease. Nutr. Res. 2021, 89, 10–22. [Google Scholar] [CrossRef]
- Snipes, R.L. Anatomy of the rabbit cecum. Anat. Embryol. 1978, 155, 57–80. [Google Scholar] [CrossRef]
- Abdel-Wareth, A.A.; Kehraus, S.; Ali, A.H.; Ismail, Z.S.; Südekum, K.H. Effects of temporary intensive feed restriction on performance, nutrient digestibility and carcass criteria of growing male Californian rabbits. Arch. Anim. Nutr. 2015, 69, 69–78. [Google Scholar] [CrossRef]
- Gidenne, T.; Combes, S.; Fortun-Lamothe, L. Feed intake limitation strategies for the growing rabbit: Effect on feeding behaviour, welfare, performance, digestive physiology and health: A review. Animal 2012, 6, 1407–1419. [Google Scholar] [CrossRef]
- Dalmau, A.; Abdel-Khalek, A.M.; Ramon, J.; Piles, M.; Sanchez, J.P.; Velarde, A.; Rafel, O. Comparison of behaviour, performance and mortality in restricted and ad libitum-fed growing rabbits. Animal 2015, 9, 1172–1180. [Google Scholar] [CrossRef]
- Gidenne, T.; Feugier, A. Feed restriction strategy in the growing rabbit. 1. Impact on digestion, rate of passage and microbial activity. Animal 2009, 3, 501–508. [Google Scholar] [CrossRef]
- Crespo, R.; Alfonso, C.; del Barrio, A.S.; Garcia-Ruiz, A.I.; Marco, M.; Nicodemus, N. Effect of feed restriction on performance, carcass yield and nitrogen and energy balance in growing rabbits. Livest. Sci. 2020, 241, 104278. [Google Scholar] [CrossRef]
- Lu, Q.; Qin, J.X.; Xie, S.L.; Chen, R.; Xu, Y.Q.; Wang, X.; Zhou, D.; Tian, X.Z. Effects of feed restriction on the slaughter performance, antioxidant activity, and meat quality of rabbits. Food Sci. Anim. Prod. 2024, 2, 9240080. [Google Scholar] [CrossRef]
- Chinese Standard NY/T 4049-2021; Nutrient Requirement of Meat Rabbit. The Standard Press of PR China: Beijing, China, 2021. (In Chinese)
- AOAC. Official Methods of Analysis, 18th ed.; Association of Official Analtical Chemists: Arlington, MA, USA, 2005. [Google Scholar]
- Van Soest, P.V.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Ebeid, T.A.; Al-Homidan, I.H.; Saleh, A.A.; Barakat, H.A. Physiological and immunological aspects of feed restriction and its beneficial impacts in fattening rabbits’ productivity-an updated review. Trop. Anim. Health Prod. 2024, 6, 33. [Google Scholar] [CrossRef]
- Birolo, M.; Trocino, A.; Tazzoli, M.; Xiccato, G. Effect of feed restriction and feeding plans on performance, slaughter traits and body composition of growing rabbits. World Rabbit Sci. 2017, 25, 113–122. [Google Scholar] [CrossRef]
- Le Floc’h, N.; Knudsen, C.; Gidenne, T.; Montagne, L.; Merlot, E.; Zemb, O. Impact of feed restriction on health, digestion and faecal microbiota of growing pigs housed in good or poor hygiene conditions. Animal 2014, 8, 1632–1642. [Google Scholar] [CrossRef]
- Zhuang, J.; Zhou, T.; Bai, S.; Zhao, B.; Wu, X.; Chen, Y. Effects of restricted feeding on growth performance, intestinal immunity, and skeletal muscle development in New Zealand rabbits. Animals 2022, 12, 160. [Google Scholar] [CrossRef]
- Combes, S.; Massip, K.; Martin, O.; Furbeyre, H.; Cauquil, L.; Pascal, G.; Bouchez, O.; Le Floc’h, N.; Zemb, O.; Oswald, I.P.; et al. Impact of feed restriction and housing hygiene conditions on specific and inflammatory immune response, the cecal bacterial community and the survival of young rabbits. Animal 2017, 11, 854–863. [Google Scholar] [CrossRef]
- Drouilhet, L.; Achard, C.S.; Zemb, O.; Molette, C.; Gidenne, T.; Larzul, C.; Ruesche, J.; Tircazes, A.; Segura, M.; Bouchez, T.; et al. Direct and correlated responses to selection in two lines of rabbits selected for feed efficiency under ad libitum and restricted feeding: I. Production traits and gut microbiota characteristics. J. Anim. Sci. 2016, 94, 38–48. [Google Scholar] [CrossRef]
- Romero, C.; Cuesta, S.; Astillero, J.R.; Nicodemus, N.; Blas, C.D. Effect of early feed restriction on performance and health status in growing rabbits slaughtered at 2 kg live-weight. World Rabbit Sci. 2010, 18, 211–218. [Google Scholar] [CrossRef]
- Dong, L.; Li, Y.; Zhang, Y.; Zhang, Y.; Ren, J.; Zheng, J.; Diao, J.; Ni, H.; Yin, Y.; Sun, R.; et al. Effects of organic zinc on production performance, meat quality, apparent nutrient digestibility and gut microbiota of broilers fed low-protein diets. Sci. Rep. 2023, 13, 10803. [Google Scholar] [CrossRef] [PubMed]
- Gidenne, T.; Fortun-Lamothe, L.; Bannelier, C.; Molette, C.; Gilbert, H.; Chemit, M.L.; Segura, M.; Benitez, F.; Richard, F.; Garreau, H.; et al. Direct and correlated responses to selection in two lines of rabbits selected for feed efficiency under ad libitum and restricted feeding: III. digestion and excretion of nitrogen and minerals. J. Anim. Sci. 2017, 95, 1301–1312. [Google Scholar] [PubMed]
- Rebollar, P.G.; Pereda, N.; Schwarz, B.F.; Millán, P.; Lorenzo, P.L.; Nicodemus, N. Effect of feed restriction or feeding high-fibre diet during the rearing period on body composition, serum parameters and productive performance of rabbit does. Anim. Feed Sci. Technol. 2021, 163, 67–76. [Google Scholar] [CrossRef]
- Richards, M.P.; Poch, S.M.; Coon, C.N.; Rosebrough, R.W.; Ashwell, C.M.; Mcmurtry, J.P. Feed restriction significantly alters lipogenic gene expression in broiler breeder chickens. J. Nutr. 2003, 133, 707. [Google Scholar] [CrossRef]
- Aouichat, S.; Chayah, M.; Bouguerra-Aouichat, S.; Agil, A. Time-restricted feeding improves body weight gain, lipid profiles, and atherogenic indices in cafeteria-diet-fed rats: Role of browning of inguinal white adipose tissue. Nutrients 2020, 12, 2185. [Google Scholar] [CrossRef]
- Liu, J.; Wei, Y.; Di, H.; Zhong, Y.; Hua, W.; Wang, H. Calorie restriction improves serum lipid metabolism, colon metabolites and microbiota in pigs. Anim. Nutr. 2024, 1, e11. [Google Scholar] [CrossRef]
- Woodie, L.N.; Luo, Y.; Wayne, M.J.; Graff, E.C.; Ahmed, B.; O’Neill, A.M.; Greene, M.W. Restricted feeding for 9 h in the active period partially abrogates the detrimental metabolic effects of a Western diet with liquid sugar consumption in mice. Metabolism 2018, 82, 1–13. [Google Scholar] [CrossRef]
- Chen, W.; Guo, Y.M.; Huang, Y.Q.; Shi, Y.H.; Zhang, C.X.; Wang, J.W. Effect of energy restriction on growth, slaughter performance, serum biochemical parameters and Lpin2/WDTC1/mRNA expression of broilers in the later phase. J. Poult. Sci. 2012, 49, 12–19. [Google Scholar] [CrossRef]
- Halliwell, B.; Gutteridge, J.M. The definition and measurement of antioxidants in biological systems. Free Radic. Biol. Med. 1995, 18, 125–126. [Google Scholar] [CrossRef]
- Gulcin, İ. Antioxidants and antioxidant methods: An updated overview. Arch. Toxicol. 2020, 94, 651–715. [Google Scholar] [CrossRef]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef] [PubMed]
- Hangalapura, B.N.; Nieuwland, M.G.; De Vries Reilingh, G.; Buyse, J.; Van Den Brand, H.; Kemp, B.; Parmentier, H.K. Severe feed restriction enhances innate immunity but suppresses cellular immunity in chicken lines divergently selected for antibody responses. Poult. Sci. 2005, 84, 1520–1529. [Google Scholar] [CrossRef] [PubMed]
- Julian, D.; Leeuwenburgh, C. Linkage between insulin and the free radical theory of aging. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2004, 286, R20–R21. [Google Scholar] [CrossRef] [PubMed]
- Cotozzolo, E.; Cremonesi, P.; Curone, G.; Menchetti, L.; Riva, F.; Biscarini, F.; Marongiu, M.L.; Castrica, M.; Castiglioni, B.; Miraglia, D.; et al. Characterization of bacterial microbiota composition along the gastrointestinal tract in rabbits. Animals 2020, 11, 31. [Google Scholar] [CrossRef]
- Ye, J.; Jiang, S.; Cheng, Z.; Ding, F.; Fan, Q.; Lin, X.; Wang, Y.; Gou, Z. Feed restriction improves lipid metabolism by changing the structure of the cecal microbial community and enhances the meat quality and flavor of bearded chickens. Animals 2022, 12, 970. [Google Scholar] [CrossRef]
- Liu, B.; Cui, Y.; Ali, Q.; Zhu, X.; Li, D.; Ma, S.; Wang, Z.; Wang, C.; Shi, Y. Gut microbiota modulate rabbit meat quality in response to dietary fiber. Front. Nutr. 2022, 9, 849429. [Google Scholar] [CrossRef]
- Flint, H.J.; Scott, K.P.; Duncan, S.H.; Louis, P.; Forano, E. Microbial degradation of complex carbohydrates in the gut. Gut Microbes 2012, 3, 289–306. [Google Scholar] [CrossRef]
- Fan, P.; Liu, P.; Song, P.; Chen, X.; Ma, X. Moderate dietary protein restriction alters the composition of gut microbiota and improves ileal barrier function in adult pig model. Sci. Rep. 2017, 7, 43412. [Google Scholar] [CrossRef]
- Fujiki, Y.; Tanaka, T.; Yakabe, K.; Seki, N.; Akiyama, M.; Uchida, K.; Kim, Y.G. Hydrogen gas and the gut microbiota are potential biomarkers for the development of experimental colitis in mice. Gut Microbiome 2023, 5, e3. [Google Scholar] [CrossRef]
- Tian, S.; Chu, Q.; Ma, S.; Ma, H.; Song, H. Dietary fiber and its potential role in obesity: A focus on modulating the gut microbiota. J. Agric. Food Chem. 2023, 71, 14853–14869. [Google Scholar] [CrossRef]
- Yadav, S.; Jha, R. Strategies to modulate the intestinal microbiota and their effects on nutrient utilization, performance, and health of poultry. J. Anim. Sci. Biotechnol. 2019, 10, 2. [Google Scholar] [CrossRef]
- Markowiak-Kopeć, P.; Śliżewska, K. The effect of probiotics on the production of short-chain fatty acids by human intestinal microbiome. Nutrients 2020, 12, 1107. [Google Scholar] [CrossRef] [PubMed]
- Sale, C.; Saunders, B.; Harris, R.C. Effect of beta-alanine supplementation on muscle carnosine concentrations and exercise performance. Amino Acids 2010, 39, 321–333. [Google Scholar] [CrossRef] [PubMed]
- Caruso, J.; Charles, J.; Unruh, K.; Giebel, R.; Learmonth, L.; Potter, W. Ergogenic effects of β-alanine and carnosine: Proposed future research to quantify their efficacy. Nutrients 2012, 4, 585–601. [Google Scholar] [CrossRef]
- Smith-Ryan, A.E.; Fukuda, D.H.; Stout, J.R.; Kendall, K.L. The influence of β-alanine supplementation on markers of exercise-induced oxidative stress. Appl. Physiol. Nutr. Metab. 2014, 39, 38–46. [Google Scholar] [CrossRef]
- Derave, W.; Everaert, I.; Beeckman, S.; Baguet, A. Muscle carnosine metabolism and beta-alanine supplementation in relation to exercise and training. Sports Med. 2010, 40, 247–263. [Google Scholar] [CrossRef]
- Smith, A.E.; Stout, J.R.; Kendall, K.L.; Fukuda, D.H.; Cramer, J.T. Exercise-induced oxidative stress: The effects of β-alanine supplementation in women. Amino Acids 2012, 43, 77–90. [Google Scholar] [CrossRef]
- Wu, H.C.; Shiau, C.Y.; Chen, H.M.; Chiou, T.K. Antioxidant activities of carnosine, anserine, some free amino acids and their combination. J. Food Drug Anal. 2003, 11, 148–153. [Google Scholar] [CrossRef]
- Mannion, A.F.; Jakeman, P.M.; Dunnett, M.; Harris, R.C.; Willan, P.L. Carnosine and anserine concentrations in the quadriceps femoris muscle of healthy humans. Eur. J. Appl. Physiol. Occup. Physiol. 1992, 64, 47–50. [Google Scholar] [CrossRef]
- Intarapichet, K.O.; Maikhunthod, B. Genotype and gender differences in carnosine extracts and antioxidant activities of chicken breast and thigh meats. Meat Sci. 2005, 71, 634–642. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).