The Influence of Different Light Day Distribution in Hy-Line W36 Laying Hens on Egg Production and Egg Quality
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics
2.2. Animals and Husbandry
2.3. Measures of Performance
2.4. Measures of Egg Quality
2.4.1. Collection and Evaluation
2.4.2. Breaking Strength and Internal Quality Parameters
2.4.3. External Egg Quality Analysis
2.5. Eggshell Ash Percentage
2.6. Statistical Analysis
3. Results
3.1. Performance Results
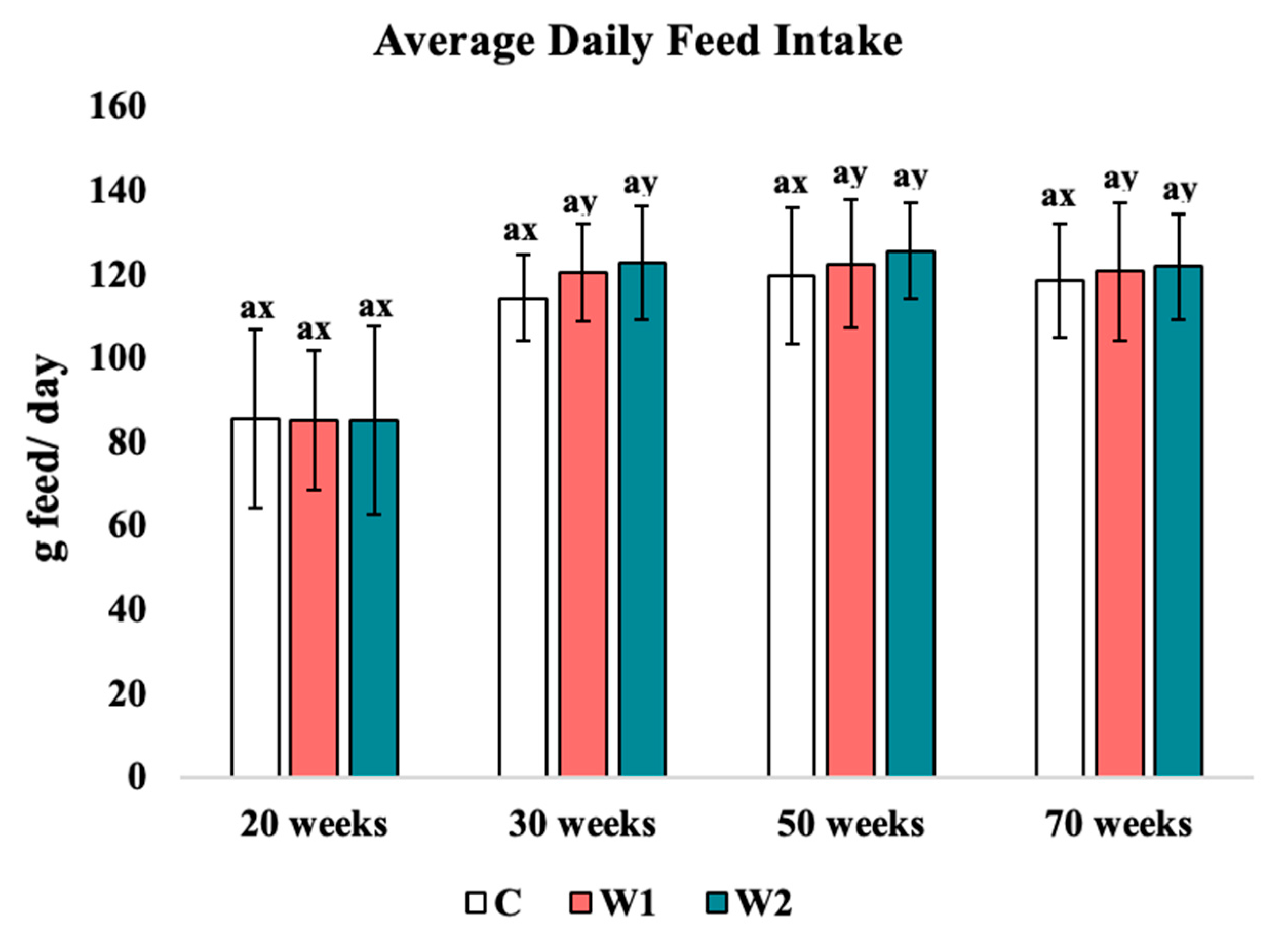
3.1.1. Average Daily Feed Intake
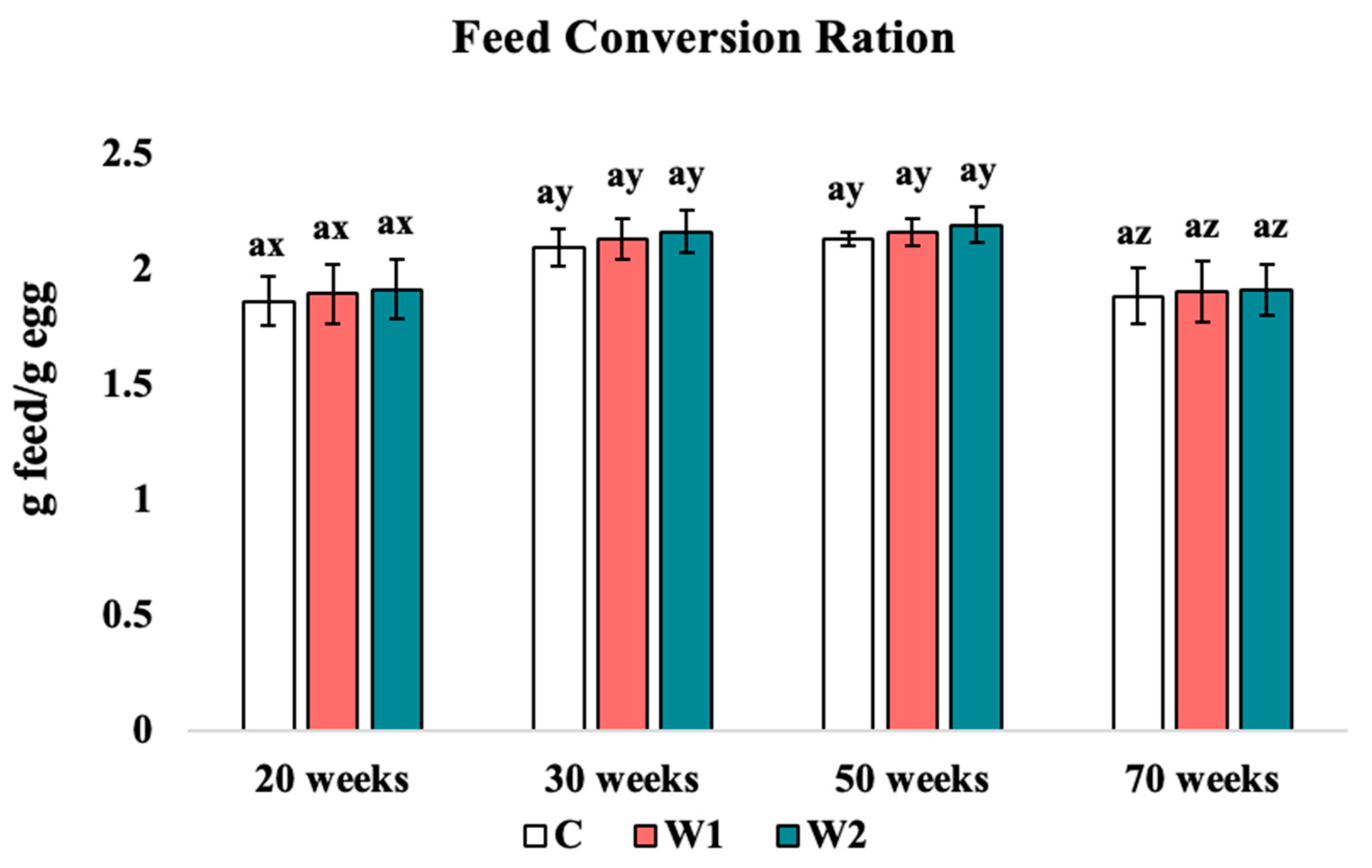
3.1.2. Feed Conversion Ratio
3.1.3. Hen/Day Egg Production
3.2. Egg Quality Results
3.2.1. Internal Egg Quality
3.2.2. External Egg Quality
4. Discussion
4.1. Average Daily Feed Intake and Feed Conversion Ratio
4.2. Hen Daily Egg Production
4.3. Internal Egg Quality
4.4. External Egg Quality
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Farsund, A.A.; Daugbjerg, C.; Langhelle, O. Food security and trade: Reconciling discourses in the Food and Agriculture Organization and the World Trade Organization. Food Secur. 2015, 7, 383–391. [Google Scholar] [CrossRef]
- Bain, M.M.; Nys, Y.; Dunn, I.C. Increasing persistency in lay and stabilising egg quality in longer laying cycles. What are the challenges? Br. Poult. Sci. 2016, 57, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Różewicz, M. Effect of Age on Egg Quality of Lakenvelder Hens Kept Under Extensive Rearing Conditions. Int. J. Poult. Ornam. Birds Sci. Technol. 2023, 4, 1–7. [Google Scholar]
- Türker, İ.; Kalebaşi, S. Effect of fluctuate lighting on performance of laying hens (Short Communication). Arch. Anim. Breed. 2009, 52, 200–204. [Google Scholar]
- Ma, H.; Li, B.; Xin, H.; Shi, Z.; Zhao, Y. Effect of Intermittent Lighting on Production Performance of Laying-Hen Parent Stocks; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2013. [Google Scholar]
- Geng, A.L.; Xu, S.F.; Zhang, Y.; Zhang, J.; Chu, Q.; Liu, H.G. Effects of photoperiod on broodiness, egg-laying and endocrine responses in native laying hens. Br. Poult. Sci. 2014, 55, 264–269. [Google Scholar] [CrossRef]
- Farghly, M.F.A.; Makled, M.N. Application of Intermittent Feeding and Flash Lighting Regimens in Broiler Chickens Management. Egypt. J. Nutr. Feed. 2015, 18, 261–276. [Google Scholar] [CrossRef]
- Kermanshahi, H.; Hadavi, A. Effect of Added Extra Calcium Carbonate into the Diets, One Hour Before Starting Dark Period on Performance and Egg Quality of Laying Hens. Int. J. Poult. Sci. 2006, 5, 946–948. [Google Scholar]
- Roberts, J.R. Factors Affecting Egg Internal Quality and Egg Shell Quality in Laying Hens. J. Poult. Sci. 2004, 41, 161–177. [Google Scholar] [CrossRef]
- Saunders-Blades, J.L.; MacIsaac, J.L.; Korver, D.R.; Anderson, D.M. The effect of calcium source and particle size on the production performance and bone quality of laying hens. Poult. Sci. 2009, 88, 338–353. [Google Scholar] [CrossRef]
- Araujo, J.A.; Silva, J.H.; Costa, F.G.; Sousa, J.M.; Givisiez, P.E.; Sakomura, N.K. Effect of the levels of calcium and particle size of limestone on laying hens. Rev. Bras. Zootec. 2011, 40, 997–1005. [Google Scholar] [CrossRef]
- Hrabia, A. Chapter 35—Reproduction in the female. In Sturkie’s Avian Physiology, 7th ed.; Scanes, C.G., Dridi, S., Eds.; Academic Press: Cambridge, MA, USA, 2022; Volume 7, pp. 941–986. [Google Scholar]
- Sinclair-Black, M.; Garcia, R.A.; Ellestad, L.E. Physiological regulation of calcium and phosphorus utilization in laying hens. Front. Physiol. 2023, 14, 1112499. [Google Scholar] [CrossRef] [PubMed]
- Korver, D.R. Calcium nutrition, bone metabolism, and eggshell quality in longer-persisting layer flocks. Proc. Aust. Poult. Sci. Symp. 2020, 31, 17–23. [Google Scholar]
- Whitehead, C.C.; Fleming, R.H. Osteoporosis in Cage Layers. Poult. Sci. 2000, 79, 1033–1041. [Google Scholar] [CrossRef]
- Whitehead, C.C. Overview of bone biology in the egg-laying hen. Poult. Sci. 2004, 83, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Aguado, E.; Pascaretti-Grizon, F.; Goyenvalle, E.; Audran, M.; Chappard, D. Bone Mass and Bone Quality Are Altered by Hypoactivity in the Chicken. PLoS ONE 2015, 10, e0116763. [Google Scholar] [CrossRef]
- Garcia-Mejia, R.A.; Sinclair-Black, M.; Blair, L.R.; Angel, R.; Jaramillo, B.; Regmi, P.; Neupane, N.; Proszkowiec-Weglarz, M.; Arbe, X.; Cavero, D.; et al. Physiological changes in the regulation of calcium and phosphorus utilization that occur after the onset of egg production in commercial laying hens. Front. Physiol. 2024, 15, 1465817. [Google Scholar] [CrossRef]
- Nasr, M.A.F.; Murrell, J.; Wilkins, L.J.; Nicol, C.J. Effect of keel fractures on egg-production parameters, mobility and behavior in individual laying hens. Anim. Welf. 2012, 21, 127–135. [Google Scholar] [CrossRef]
- Whitehead, C.C. Skeletal disorders in laying hens: The problem of osteoporosis and bone fractures. In Welfare of the Laying Hen, Proceedings of the 27th Poultry Science Symposium of the World’s Poultry Science Association (UK Branch), Bristol, UK, 6 July 2003; CABI Publishing: Wallingford, UK, 2004; pp. 259–278. [Google Scholar]
- Dunn, I.C.; Joseph, N.T.; Bain, M.; Edmond, A.; Wilson, P.W.; Milona, P.; Nys, Y.; Gautron, J.; Schmutz, M.; Preisinger, R.; et al. Polymorphisms in eggshell organic matrix genes are associated with eggshell quality measurements in pedigree Rhode Island Red hens. Anim. Genet. 2009, 40, 110–114. [Google Scholar] [CrossRef]
- Nys, Y. Laying hen nutrition: Optimising hen performance and health, bone, and eggshell quality. Achiev. Sustain. Prod. Eggs 2017, 2, 47–74. [Google Scholar]
- Arpasova, H.; Halaj, M.; Halaj, P. Eggshell quality and calcium utilization in feed of Hens in repeated laying cycles. Czech J. Anim. Sci. 2010, 55, 66–74. [Google Scholar] [CrossRef]
- Hunton, P. Research on eggshell structure and quality: An historical overview. Braz. J. Poult. Sci. 2005, 7, 67–71. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, A.; Kalin, O.; Nys, Y.; Garcia-Ruiz, J.M. Influence of the microstructure on the shell strength of eggs laid by hens of different ages. Br. Poult. Sci. 2002, 43, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.R.; Chousalkar, K. Egg quality and age of laying hens: Implications for product safety. Anim. Prod. Sci. 2013, 53, 1291–1297. [Google Scholar] [CrossRef]
- Benavides-Reyes, C.; Folegatti, E.; Dominguez-Gasca, N.; Litta, G.; Sanchez-Rodriguez, E.; Rodriguez-Navarro, A.B.; Faruk, M.U. Research Note: Changes in eggshell quality and microstructure related to hen age during a production cycle. Poult. Sci. 2021, 100, 101287. [Google Scholar] [CrossRef]
- Bain, M.M.; McDade, K.; Burchmore, R.; Law, A.; Wilson, P.W.; Schmutz, M.; Preisinger, R.; Dunn, I.C. Enhancing the egg’s natural defence against bacterial penetration by increasing cuticle deposition. Anim. Genet. 2013, 44, 661–668. [Google Scholar] [CrossRef]
- Scott, M.L.; Hull, S.J.; Mullenhoff, P.A. The Calcium Requirements of Laying Hens and Effects of Dietary Oyster Shell Upon Egg Shell Quality. Poult. Sci. 1971, 50, 1055–1063. [Google Scholar] [CrossRef]
- Newman, S.; Leeson, S. Skeletal integrity in layers at the completion of egg production. Worlds Poult. Sci. J. 1997, 53, 265–277. [Google Scholar] [CrossRef]
- Makled, M.N.; Charles, O.W. Eggshell Quality as Influenced by Sodium Bicarbonate, Calcium Source, and Photoperiod1. Poult. Sci. 1987, 66, 705–712. [Google Scholar] [CrossRef]
- Clark, A.J.; Harrison, C.; Bragg, A.J.; House, G.M.; Stephan, A.B.; Arguelles-Ramos, M.; Ali, A. Effect of Interrupting the Daily Scotophase Period on Laying Hen Performance, Bone Health, Behavior, and Welfare; Part I: Bone Health. Poultry 2024, 3, 364–382. [Google Scholar] [CrossRef]
- Johnson, A.M.; Anderson, G.; Arguelles-Ramos, M.; Ali, A.A. Effect of dietary essential oil of oregano on performance parameters, gastrointestinal traits, blood lipid profile, and antioxidant capacity of laying hens during the pullet phase. Front. Anim. Sci. 2022, 3, 1072712. [Google Scholar] [CrossRef]
- Bates, D.; Mächler, M.; Bolker, B.; Walker, S. Fitting Linear Mixed-Effects Models Using lme4. J. Stat. Soft. 2015, 67, 1. [Google Scholar] [CrossRef]
- Hothorn, T.; Bretz, F.; Westfall, P. Simultaneous inference in general parametric models. Biom. J. 2008, 50, 346–363. [Google Scholar] [CrossRef] [PubMed]
- Dunn, I.C. Long Life Layer; genetic and physiological limitations to extend the laying period. In Proceedings of the 19th European Symposium on Poultry Nutrition, Potsdam, Germany, 26–29 August 2013; Volume 19, pp. 124–129. [Google Scholar]
- Lennards, R.M.; Roland, D.A.S.R. The influence of time of dietary calcium intake on shell quality. Poult. Sci. 1981, 60, 2106–2113. [Google Scholar] [CrossRef]
- Jansen, S.; Bues, M.; Baulain, U.; Habig, C.; Halle, I.; Petow, S.; Sharifi, A.R.; Weigend, A.; Wilkens, M.R.; Weigend, S. Bone health or performance? Adaptation response of genetically divergent chicken layer lines to a nutritive calcium depletion. Animals 2020, 10, 1645. [Google Scholar] [CrossRef]
- Metwally, M.A.; Farghly, M.F.A.; Sharaqa, T.M. Effects of light regimens and vitamin d3 levels and their interactions on broilers growth performance and carcass traits. Egypt. J. Nutr. Feed. 2021, 24, 171–185. [Google Scholar] [CrossRef]
- Shen, L.; Shi, Z.X.; Li, B.M.; Wang, C.Y.; Ma, H. The effect of lighting programmes on egg production and quality of Beijing you-chicken. In Animal Production Technology. In Proceedings of the International Conference of Agricultural Engineering-CIGR-AgEng 2012: Agriculture and Engineering for a Healthier Life, Valencia, Spain, 8–12 July 2012; CIGR-EurAgEng: Cranfield, UK, 2012. [Google Scholar]
- Morris, T.R. Environmental control for layers. Worlds Poult. Sci. J. 2004, 60, 163–175. [Google Scholar] [CrossRef]
- Bryant, S.L. A case for dawn and dusk for housed livestock. Appl. Anim. Behav. Sci. 1987, 18, 379–382. [Google Scholar] [CrossRef]
- Savory, C.J. Diurnal feeding patterns in domestic fowls: A review. Appl. Anim. Ethol. 1980, 6, 71–82. [Google Scholar] [CrossRef]
- Romero, C.; Arija, I.; Viveros, A.; Chamorro, S. Productive Performance, Egg Quality and Yolk Lipid Oxidation in Laying Hens Fed Diets including Grape Pomace or Grape Extract. Animals 2022, 12, 1076. [Google Scholar] [CrossRef]
- Farghly, M. Improvement of productive and reproductive performance of Dandarawi chicken through flash light program. Egypt. J. Anim. Prod. 2014, 51, 129–144. [Google Scholar]
- Lewis, P.D.; Backhouse, D.; Gous, R.M. Constant photoperiods and sexual maturity in broiler breeder pullets. Br. Poult. Sci. 2004, 45, 557–560. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.D.; Gous, R.M. Constant and changing photoperiods in the laying period for broiler breeders allowed [corrected] normal or accelerated growth during the rearing period. Poult. Sci. 2006, 85, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Lewis, P.D.; Gous, R.M. Effect of final photoperiod and twenty-week body weight on sexual maturity and early egg production in broiler breeders. Poult. Sci. 2006, 85, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Shao, X.; Chen, Z.; Wei, C.; Lei, M.; Ying, S.; Yu, J.; Shi, Z. Induction of out-of-season egg laying by artificial photoperiod in Yangzhou geese and the associated endocrine and molecular regulation mechanisms. Anim. Reprod. Sci. 2017, 180, 127–136. [Google Scholar] [CrossRef]
- Lewis, P.D.; Gous, R.M.; Ghebremariam, W.K.; Sharp, P.J. Broiler breeders do not respond positively to photoperiodic increments given during the laying period. Br. Poult. Sci. 2007, 48, 245–252. [Google Scholar] [CrossRef]
- Lewis, P.D.; Danisman, R.; Gous, R.M. Photoperiods for broiler breeder females during the laying period. Poult. Sci. 2010, 89, 108–114. [Google Scholar] [CrossRef]
- Geng, A.L.; Zhang, Y.; Zhang, J.; Wang, H.H.; Chu, Q.; Liu, H.G. Effects of lighting pattern and photoperiod on egg production and egg quality of a native chicken under free-range condition. Poult. Sci. 2018, 97, 2378–2384. [Google Scholar] [CrossRef]
- Farghly, M.F.; Mahrose, K.M.; Rehman, Z.U.; Yu, S.; Abdelfattah, M.G.; El-Garhy, O.H. Intermittent lighting regime as a tool to enhance egg production and eggshell thickness in Rhode Island Red laying hens. Poult. Sci. 2019, 98, 2459–2465. [Google Scholar] [CrossRef]
- Curtis, P.A.; Kerth, L.K.; Anderson, K.E. Impact of strain on egg quality and composition during a single production cycle. Poult. Sci. 2005, 84, 78. [Google Scholar]
- Yuri, F.M.; Souza, C.D.; Schneider, A.F.; Gewehr, C.E. Intermittent lighting programs for layers with different photophases in the beginning of the laying phase. Ciência Rural. 2016, 46, 2012–2017. [Google Scholar] [CrossRef]
- Dikmen, B.Y.; Ipek, A.; Şahan, Ü.; Sözcü, A.; Baycan, S.C. Impact of different housing systems and age of layers on egg quality characteristics. Turk. J. Vet. Anim. Sci. 2017, 41, 77–84. [Google Scholar] [CrossRef]
- Nys, Y.; Gautron, J. Structure and formation of the eggshell. In Bioactive Egg Compounds; Springer: Berlin/Heidelberg, Germany, 2007; pp. 99–102. [Google Scholar]
- Hincke, M.T.; Nys, Y.; Gautron, J.; Mann, K.; Rodriguez-Navarro, A.B.; McKee, M.D. The eggshell: Structure, composition and mineralization. Front. Biosci. 2012, 17, 1266–1280. [Google Scholar] [CrossRef] [PubMed]
- Bell, D. Egg Shell Quality: Its Impact on Production, Processing and Marketing Economics; CABI Digital Library: Surrey, UK, 1998; pp. 447–466. [Google Scholar]
- Leeson, S.; Walker, J.P.; Summers, J.D. Performance of laying hens subjected to intermittent lighting initiated at 24 weeks of age. Poult. Sci. 1982, 61, 567–568. [Google Scholar] [CrossRef]

| Guaranteed Analysis | |
|---|---|
| Crude Protein (Min.) | 16% |
| Lysine (Min.) | 0.85% |
| Methionine (Min.) | 0.36% |
| Crude Fat (Min.) | 3% |
| Crude Fiber (Max.) | 6.5% |
| Calcium (Min.) | 4.1% |
| Calcium (Max.) | 4.4% |
| Phosphorus (Min.) | 0.6% |
| Salt (Min.) | 0.45% |
| Salt (Max.) | 0.8% |
| Parameter | Treatment | 20 Weeks | 30 Weeks | Parameter | 70 Weeks |
|---|---|---|---|---|---|
| Egg Weight (g) | C | 45.68 ± 1.89 ax | 63.58 ± 2.33 ay | 61.52 ± 2.12 ay | 61.58 ± 2.55 ay |
| W1 | 45.26 ± 1.52 ax | 62.85 ± 1.58 ay | 63.09 ± 1.01 ay | 61.16 ± 1.78 ay | |
| W2 | 44.98 ± 1.32 ax | 63.62 ± 2.52 ay | 62.58 ± 1.98 ay | 62.09 ± 1.85 ay | |
| Albumen Weight (g) | C | 26.96 ± 0.96 ax | 40.93 ± 0.85 ay | 39.61 ± 1.01 ay | 39.36 ± 0.78 ay |
| W1 | 26.69 ± 1.12 ax | 39.97 ± 0.79 ay | 40.71 ± 0.88 ay | 38.89 ± 0.78 ay | |
| W2 | 26.28 ± 1.45 ax | 40.67 ± 1.52 ay | 40.23 ± 1.02 ay | 39.74 ± 1.12 ay | |
| Yolk Weight (g) | C | 11.89 ± 3.22 ax | 14.88 ± 2.52 ax | 14.36 ± 2.52 ax | 14.96 ± 2.56 ax |
| W1 | 12.01 ± 1.23 ax | 14.96 ± 1.85 ax | 14.52 ± 1.66 ax | 14.58 ± 1.85 ax | |
| W2 | 12.23 ± 2.52 ax | 14.63 ± 1.59 ax | 14.55 ± 2.46 ax | 14.96 ± 3.06 ax | |
| Yolk Color | C | 8.09 ± 2.2x ax | 8.96 ± 1.63 ax | 8.23 ± 3.03 ax | 8.67 ± 2.35 ax |
| W1 | 8.12 ± 1.25 ax | 8.86 ± 1.22 ax | 8.52 ± 2.85 ax | 8.76 ± 1.96 ax | |
| W2 | 8.22 ± 1.96 ax | 8.63 ± 2.63 ax | 8.85 ± 2.55 ax | 8.89 ± 3.66 ax | |
| Haugh Unit (HU) | C | 75.63 ± 1.96 ax | 76.89 ± 2.52 ax | 78.58 ± 2.03 ax | 80.23 ± 2.16 ax |
| W1 | 76.52 ± 1.55 ax | 78.25 ± 3.52 ax | 76.58 ± 3.52 ax | 79.58 ± 2.88 ax | |
| W2 | 77.85 ± 2.03 ax | 77.25 ± 2.85 ax | 78.55 ± 1.69 ax | 80.99 ± 2.11 ax | |
| Damaged Eggs Ratio (%) | C | 1.03 ± 5.23 ax | 3.52 ± 1.55 ax | 7.88 ± 1.69 ay | 11.25 ± 2.69 ay |
| W1 | 2.52 ± 3.23 ax | 1.22 ± 1.89 ax | 1.99 ± 1.85 bx | 1.85 ± 2.96 bx | |
| W2 | 1.66 ± 3.52 ax | 1.52 ± 1.88 ax | 1.58 ± 1.69 bx | 2.09 ± 3.59 bx |
| Parameter | Treatment | 20 Weeks | 30 Weeks | 50 Weeks | 70 Weeks |
|---|---|---|---|---|---|
| Eggshell Weight (g) | C | 7.16 ± 0.36 ax | 7.21 ± 0.13 ax | 7.56 ± 0.16 ax | 7.57 ± 0.12 ax |
| W1 | 7.09 ± 0.46 ax | 7.99 ± 0.14 by | 7.89 ± 0.15 by | 7.90 ± 0.16 by | |
| W2 | 7.12 ± 0.31 ax | 7.96 ± 0.15 by | 7.92 ± 0.12 by | 7.91 ± 0.16 by | |
| Eggshell thickness (mm) | C | 0.46 ± 0.09 ax | 0.38 ± 0.02 ay | 0.36 ± 0.03 ay | 0.34 ± 0.04 ay |
| W1 | 0.46 ± 0.08 ax | 0.43 ± 0.03 bx | 0.42 ± 0.02 bx | 0.40 ± 0.02 bx | |
| W2 | 0.45 ± 0.05 ax | 0.44 ± 0.03 bx | 0.41 ± 0.03 bx | 0.41 ± 0.03 bx | |
| Eggshell ash% | C | 96.63 ± 0.63 ax | 94.69 ± 0.56 ay | 94.23 ± 0.26 ay | 94.06 ± 0.34 ay |
| W1 | 96.85 ± 0.99 ax | 96.62 ± 0.66 bx | 96.06 ± 0.36 bx | 95.89 ± 0.22 bx | |
| W2 | 96.06 ± 0.58 ax | 96.36 ± 0.43 bx | 95.96 ± 0.39 bx | 95.91 ± 0.29 bx | |
| Eggshell strength (N) | C | 40.36 ± 4.63 ax | 26.88 ± 2.56 ay | 24.85 ± 3.52 ay | 24.25 ± 2.59 ay |
| W1 | 41.12 ± 3.52 ax | 36.56 ± 5.63 bx | 37.63 ± 6.25 bx | 36.12 ± 3.52 bx | |
| W2 | 39.85 ± 4.25 ax | 37.89 ± 3.52 bx | 39.62 ± 5.69 bx | 39.69 ± 6.52 bx |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Clark, A.J.; Bragg, A.J.; Alqhtani, A.H.; Arguelles-Ramos, M.; Ali, A. The Influence of Different Light Day Distribution in Hy-Line W36 Laying Hens on Egg Production and Egg Quality. Animals 2025, 15, 838. https://doi.org/10.3390/ani15060838
Clark AJ, Bragg AJ, Alqhtani AH, Arguelles-Ramos M, Ali A. The Influence of Different Light Day Distribution in Hy-Line W36 Laying Hens on Egg Production and Egg Quality. Animals. 2025; 15(6):838. https://doi.org/10.3390/ani15060838
Chicago/Turabian StyleClark, Alexis J., Ari J. Bragg, Abdulmohsen Hussen Alqhtani, Mireille Arguelles-Ramos, and Ahmed Ali. 2025. "The Influence of Different Light Day Distribution in Hy-Line W36 Laying Hens on Egg Production and Egg Quality" Animals 15, no. 6: 838. https://doi.org/10.3390/ani15060838
APA StyleClark, A. J., Bragg, A. J., Alqhtani, A. H., Arguelles-Ramos, M., & Ali, A. (2025). The Influence of Different Light Day Distribution in Hy-Line W36 Laying Hens on Egg Production and Egg Quality. Animals, 15(6), 838. https://doi.org/10.3390/ani15060838






