Effects of Climate and Land Use on the Population Dynamics of the Bank Vole (Clethrionomys glareolus) in the Southernmost Part of Its Range
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area and Sampling Design
2.2. Data Analysis
2.2.1. Abundance Models
2.2.2. Occupancy Models
2.2.3. Analysis of Population Trends
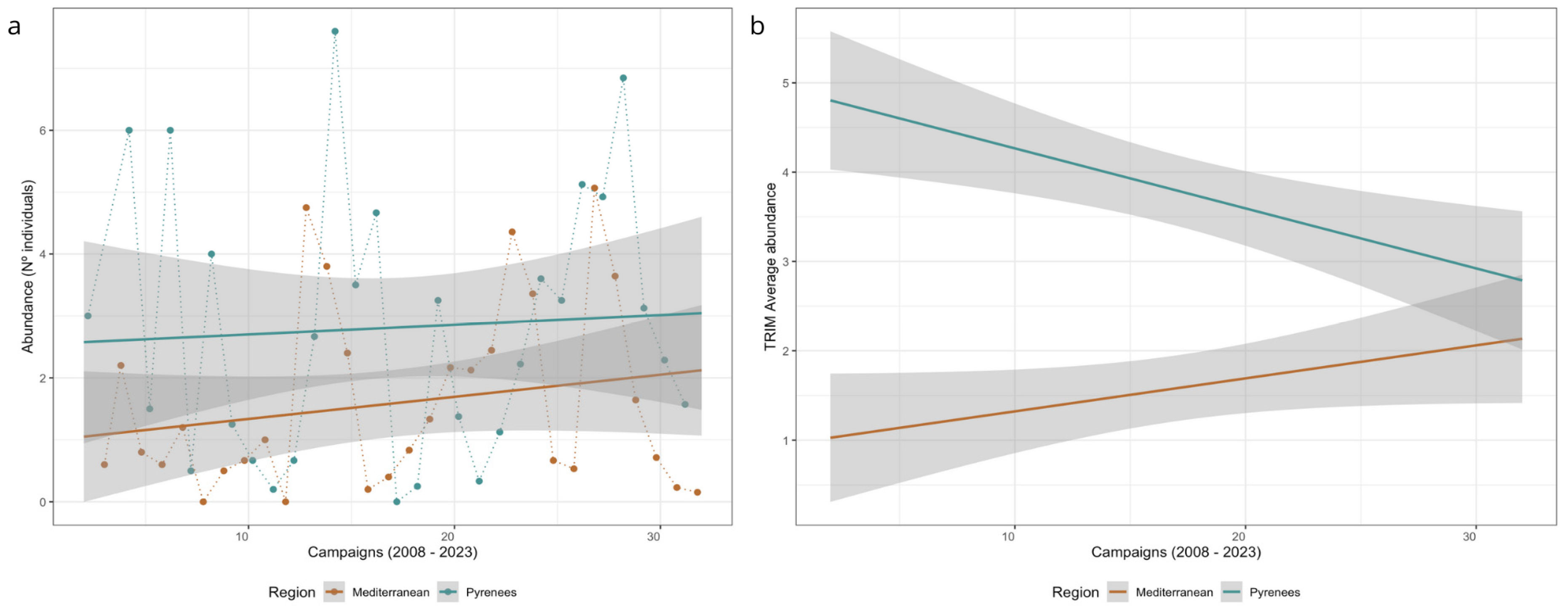
3. Results
3.1. Weather
3.2. Land Cover Change (2007–2022)
3.3. Sampling Effort and Capture Success
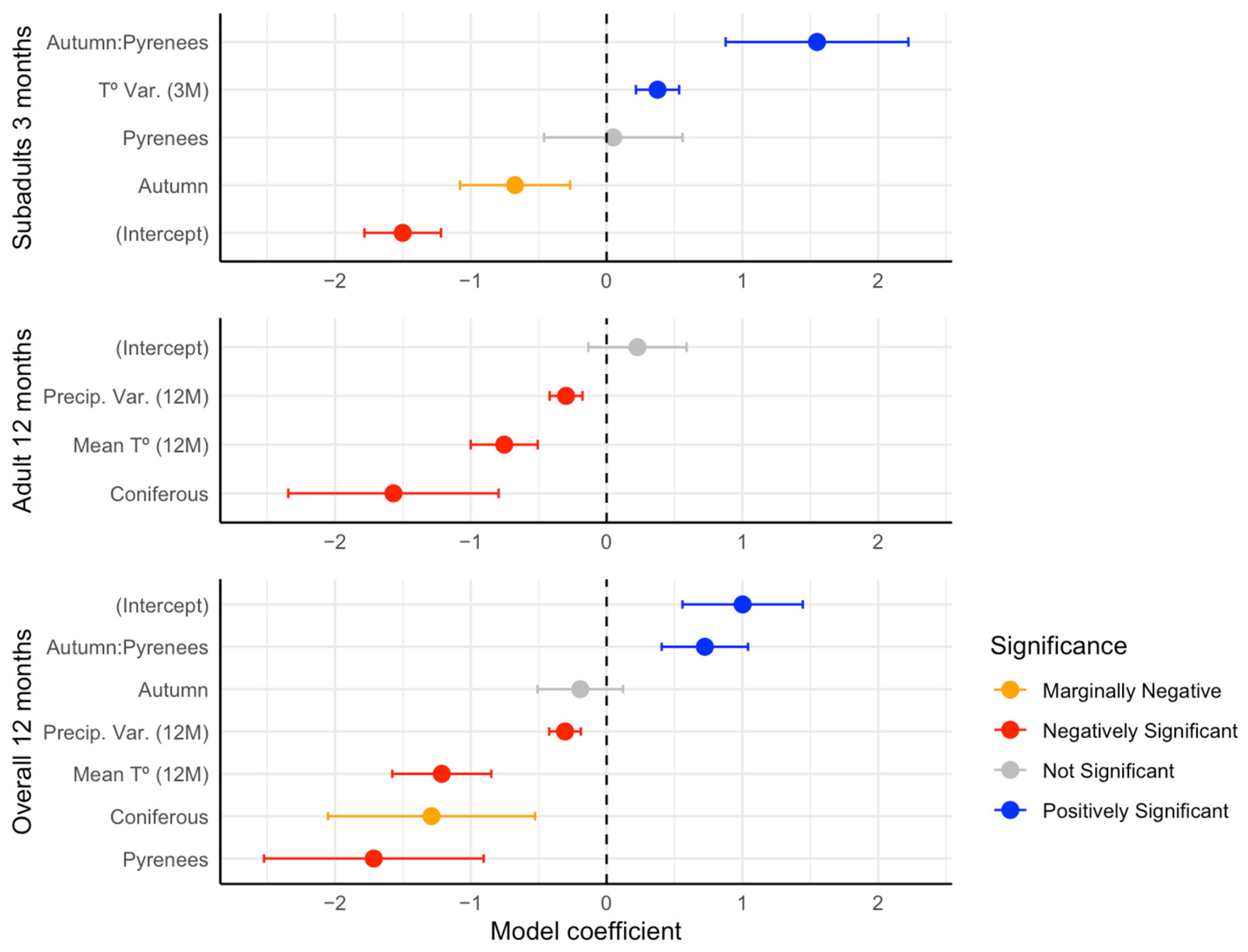
3.4. Abundance Models
3.5. Occupancy Models
4. Discussion
4.1. Population Dynamics of Bank Voles: The Effects of Climate and Habitat
4.2. Occupancy Dynamics of Bank Voles
4.3. Future Research
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jaureguiberry, P.; Titeux, N.; Wiemers, M.; Bowler, D.E.; Coscieme, L.; Golden, A.S.; Guerra, C.A.; Jacob, U.; Takahashi, Y.; Settele, J.; et al. The Direct Drivers of Recent Global Anthropogenic Biodiversity Loss. Sci. Adv. 2022, 8, eabm9982. [Google Scholar] [CrossRef] [PubMed]
- de Chazal, J.; Rounsevell, M.D.A. Land-Use and Climate Change within Assessments of Biodiversity Change: A Review. Glob. Environ. Chang. 2009, 19, 306–315. [Google Scholar] [CrossRef]
- Schröter, D.; Cramer, W.; Leemans, R.; Prentice, I.C.; Araújo, M.B.; Arnell, N.W.; Bondeau, A.; Bugmann, H.; Carter, T.R.; Gracia, C.A.; et al. Ecosystem Service Supply and Vulnerability to Global Change in Europe. Science 2005, 310, 1333–1337. [Google Scholar] [CrossRef] [PubMed]
- Cervera, T.; Pino, J.; Marull, J.; Padró, R.; Tello, E. Understanding the Long-Term Dynamics of Forest Transition: From Deforestation to Afforestation in a Mediterranean Landscape (Catalonia, 1868–2005). Land Use Policy 2019, 80, 318–331. [Google Scholar] [CrossRef]
- IPCC. Climate Change 2001: Impacts, Adaptation, and Vulnerability: Contribution of Working Group II to the Third Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2001; ISBN 978-0-521-01500-4. [Google Scholar]
- Schumacher, S.; Bugmann, H. The Relative Importance of Climatic Effects, Wildfires and Management for Future Forest Landscape Dynamics in the Swiss Alps. Glob. Chang. Biol. 2006, 12, 1435–1450. [Google Scholar] [CrossRef]
- Wang, C.-J.; Zhang, Z.-X.; Wan, J.-Z. Vulnerability of Global Forest Ecoregions to Future Climate Change. Glob. Ecol. Conserv. 2019, 20, e00760. [Google Scholar] [CrossRef]
- Resco de Dios, V.; Hedo, J.; Cunill Camprubí, À.; Thapa, P.; Martínez del Castillo, E.; Martínez de Aragón, J.; Bonet, J.A.; Balaguer-Romano, R.; Díaz-Sierra, R.; Yebra, M.; et al. Climate Change Induced Declines in Fuel Moisture May Turn Currently Fire-Free Pyrenean Mountain Forests into Fire-Prone Ecosystems. Sci. Total Environ. 2021, 797, 149104. [Google Scholar] [CrossRef]
- Seidl, R.; Rammer, W.; Lexer, M.J. Climate Change Vulnerability of Sustainable Forest Management in the Eastern Alps. Clim. Chang. 2011, 106, 225–254. [Google Scholar] [CrossRef]
- Bolòs, O. Corologia de La Flora Dels Països Catalans = Chorology of the Flora of Catalan Countries: Volum Introductori; Institut d’Estudis Catalans: Barcelona, Spain, 1985; Volume 1. [Google Scholar]
- Nuet, J.; Panareda, J.M.; Romo, A.M. Vegetació de Catalunya, 1st ed.; Eumo Editorial: Mumbai, India, 1991. [Google Scholar]
- Sanz-Elorza, M.; Dana, E.D.; González, A.; Sobrino, E. Changes in the High-mountain Vegetation of the Central Iberian Peninsula as a Probable Sign of Global Warming. Ann. Bot. 2003, 92, 273–280. [Google Scholar] [CrossRef]
- Pérez-García, N.; Font, X.; Ferré, A.; Carreras, J. Drastic Reduction in the Potential Habitats for Alpine and Subalpine Vegetation in the Pyrenees Due to Twenty-First-Century Climate Change. Reg. Environ. Chang. 2013, 13, 1157–1169. [Google Scholar] [CrossRef]
- Esteban, P.; Prohom Durán, M.J.; Aguilar Anfrons, E.; Mestre, O. Evolució recent de la temperatura i la precipitació a Andorra (1934–2008): Resultats anuals i estacionals. Rev. CENMA 2010, 5, 22–33. [Google Scholar]
- Engler, R.; Randin, C.F.; Thuiller, W.; Dullinger, S.; Zimmermann, N.E.; Araújo, M.B.; Pearman, P.B.; Le Lay, G.; Piedallu, C.; Albert, C.H.; et al. 21st Century Climate Change Threatens Mountain Flora Unequally across Europe. Glob. Chang. Biol. 2011, 17, 2330–2341. [Google Scholar] [CrossRef]
- Morán-Ordóñez, A.; Ramsauer, J.; Coll, L.; Brotons, L.; Ameztegui, A. Ecosystem Services Provision by Mediterranean Forests Will Be Compromised above 2 °C Warming. Glob. Chang. Biol. 2021, 27, 4210–4222. [Google Scholar] [CrossRef] [PubMed]
- Rowe, R.J. Legacies of Land Use and Recent Climatic Change: The Small Mammal Fauna in the Mountains of Utah. Am. Nat. 2007, 170, 2. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Nichols, J.D.; Royle, J.A.; Pollock, K.H.; Bailey, L.; Hines, J.E. Occupancy Estimation and Modeling: Inferring Patterns and Dynamics of Species Occurrence; Elsevier: Amsterdam, The Netherlands, 2017; ISBN 978-0-12-407245-9. [Google Scholar]
- Hutterer, R.; Kryštufek, B.; Yigit, N.; Mitsainas, G.; Palomo, L.; Henttonen, H.; Vohralík, V.; Zagorodnyuk, I.; Juškaitis, R.; Meinig, H.; et al. The IUCN Red List of Threatened Species 2021: E.T4973A197520967 2016. IUCN Myodes Glareolus. Available online: https://www.iucnredlist.org/species/4973/197520967 (accessed on 5 March 2024).
- Butet, A.; Delettre, Y.R. Diet Differentiation between European Arvicoline and Murine Rodents. Acta Theriol. 2011, 56, 297–304. [Google Scholar] [CrossRef]
- Canova, L.; Fasola, M. Communities of Small Mammals in Six Biotopes of Northern Italy. Acta Theriol. 1991, 36, 73–86. [Google Scholar] [CrossRef]
- Wijnhoven, S.; Van Der Velde, G.; Leuven, R.S.E.W.; Smits, A.J.M. Flooding Ecology of Voles, Mice and Shrews: The Importance of Geomorphological and Vegetational Heterogeneity in River Floodplains. Acta Theriol. 2005, 50, 453–472. [Google Scholar] [CrossRef]
- van Apeldoorn, R.C.; Oostenbrink, W.T.; van Winden, A.; van der Zee, F.F. Effects of Habitat Fragmentation on the Bank Vole, Clethrionomys Glareolus, in an Agricultural Landscape. Oikos 1992, 65, 265–274. [Google Scholar] [CrossRef]
- Mazurkiewicz, M. Factors Influencing the Distribution of the Bank Vole in Forest Habitats. Acta Theriol. 1994, 39, 113–126. [Google Scholar] [CrossRef]
- Balčiauskas, L.; Jasiulionis, M.; Stirkė, V.; Balčiauskienė, L. Temporal Changes in Bank Vole Populations Indicate Species Decline. Diversity 2024, 16, 546. [Google Scholar] [CrossRef]
- Luque-Larena, J.J.; Gosálbez, J. Atlas y Libro Rojo de los Mamíferos Terrestres de España. In Atlas y Libro Rojo de los Mamíferos Terrestres de España; ICONA: Madrid, Spain, 2007. [Google Scholar]
- Escalante, M.A.; Horníková, M.; Marková, S.; Kotlík, P. Niche Differentiation in a Postglacial Colonizer, the Bank Vole Clethrionomys Glareolus. Ecol. Evol. 2021, 11, 8054–8070. [Google Scholar] [CrossRef] [PubMed]
- Żmihorski, M.; Gryz, J.; Krauze-Gryz, D.; Olczyk, A.; Osojca, G. The Tawny Owl Strix aluco as a Material Collector in Faunistic Investigations: The Case Study of Small Mammals in NE Poland. Acta Zool. Litu. 2011, 21, 185–191. [Google Scholar] [CrossRef]
- Torre, I.; Raspall, A.; Arrizabalaga, A.; Díaz, M. SEMICE: An Unbiased and Powerful Monitoring Protocol for Small Mammals in the Mediterranean Region. Mamm. Biol. 2018, 88, 161–167. [Google Scholar] [CrossRef]
- Torre, I.; Arrizabalaga, A. Habitat Preferences of the Bank vole Myodes glareolus in a Mediterranean Mountain Range. Acta Theriol. 2008, 53, 241–250. [Google Scholar] [CrossRef]
- Imholt, C.; Reil, D.; Eccard, J.A.; Jacob, D.; Hempelmann, N.; Jacob, J. Quantifying the Past and Future Impact of Climate on Outbreak Patterns of Bank Voles (Myodes glareolus). Pest Manag. Sci. 2015, 71, 166–172. [Google Scholar] [CrossRef]
- Araújo, M.B.; Guilhaumon, F.; Neto, D.R.; Pozo, I.; Calmaestra, R. Impactos, Vulnerabilidad y Adaptación al Cambio Climático de La Biodiversidad Española. 2 Fauna de Vertebrados; Dirección general de medio Natural y Política Forestal. Ministerio de Medio Ambiente, y Medio Rural y Marino: Madrid, Spain, 2011. [Google Scholar]
- Araújo, M.B.; Peterson, A.T. Uses and Misuses of Bioclimatic Envelope Modeling. Ecology 2012, 93, 1527–1539. [Google Scholar] [CrossRef]
- Navarro, L.M.; Pereira, H.M. Rewilding Abandoned Landscapes in Europe. In Rewilding European Landscapes; Pereira, H.M., Navarro, L.M., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 3–23. ISBN 978-3-319-12039-3. [Google Scholar]
- Seoane, J.; Carrascal, L.M. Interspecific Differences in Population Trends of Spanish Birds Are Related to Habitat and Climatic Preferences. Glob. Ecol. Biogeogr. 2008, 17, 111–121. [Google Scholar] [CrossRef]
- Rowe, K.C.; Rowe, K.M.C.; Tingley, M.W.; Koo, M.S.; Patton, J.L.; Conroy, C.J.; Perrine, J.D.; Beissinger, S.R.; Moritz, C. Spatially Heterogeneous Impact of Climate Change on Small Mammals of Montane California. Proc. R. Soc. B Biol. Sci. 2015, 282, 20141857. [Google Scholar] [CrossRef]
- Torre, I.; Gracia-Quintas, L.; Arrizabalaga, A.; Baucells, J.; Díaz, M. Are Recent Changes in the Terrestrial Small Mammal Communities Related to Land Use Change? A Test Using Pellet Analyses. Ecol. Res. 2015, 30, 813–819. [Google Scholar] [CrossRef]
- Regos, A.; D’Amen, M.; Titeux, N.; Herrando, S.; Guisan, A.; Brotons, L. Predicting the Future Effectiveness of Protected Areas for Bird Conservation in Mediterranean Ecosystems under Climate Change and Novel Fire Regime Scenarios. Divers. Distrib. 2016, 22, 83–96. [Google Scholar] [CrossRef]
- Torre, I.; Díaz, M. Assessing the Effects of Landscape Change on the Occupancy Dynamics of the Greater White-Toothed Shrew Crocidura Russula. Life 2022, 12, 1230. [Google Scholar] [CrossRef] [PubMed]
- Palomo, L.J.; Gisbert, J.; Blanco, J.C. Atlas y Libro Rojo de los Mamíferos Terrestres de España; Organismo Autónomo de Parques Nacionales: Madrid, Spain, 2007. [Google Scholar]
- Torre, I.; Raspall, A.; Arrizabalaga, A.; Díaz, M. Evaluating Trap Performance and Volunteers’ Experience in Small Mammal Monitoring Programs Based on Citizen Science: The SEMICE Case Study. Mamm. Biol. 2019, 95, 26–30. [Google Scholar] [CrossRef]
- Caceres, N.C.; Nápoli, R.P.; Hannibal, W. Differential Trapping Success for Small Mammals Using Pitfall and Standard Cage Traps in a Woodland Savannah Region of Southwestern Brazil. Mammalia 2011, 75, 45–52. [Google Scholar] [CrossRef]
- Nicolas, V.; Colyn, M. Relative Efficiency of Three Types of Small Mammal Traps in an African Rainforest. Belg. J. Zool. 2006, 136, 107. [Google Scholar]
- Torre, I.; Bustamante, P.; Flaquer, C.; Oliveira, F.G. Is Bedding Material a More Effective Thermal Insulator than Trap Cover for Small Mammal Trapping? A Field Experiment. J. Therm. Biol. 2023, 118, 103738. [Google Scholar] [CrossRef]
- Palau, O.; Torre, I. Testing the Efficacy of the Heslinga Live Trap for Small Mammal Community Assessments. Mammal Res. 2024, 69, 23–31. [Google Scholar] [CrossRef]
- Torre, I.; Freixas, L.; Arrizabalaga, A.; Díaz, M. The Efficiency of Two Widely Used Commercial Live-Traps to Develop Monitoring Protocols for Small Mammal Biodiversity. Ecol. Indic. 2016, 66, 481–487. [Google Scholar] [CrossRef]
- Cuadrat, J.M.; Serrano-Notivoli, R.; Prohom, M.; Cunillera, J.; Tejedor, E.; Saz, M.Á.; de Luis, M.; Llabrés-Brustenga, A.; Soubeyroux, J.-M. Climate of the Pyrenees: Extremes Indices and Long-Term Trends. Sci. Total Environ. 2024, 933, 173052. [Google Scholar] [CrossRef]
- Sans-Fuentes, M.A.; Ventura, J. Distribution Patterns of the Small Mammals (Insectivora and Rodentia) in a Transitional Zone between the Eurosiberian and the Mediterranean Regions. J. Biogeogr. 2000, 27, 755–764. [Google Scholar] [CrossRef]
- Bronson, F.H. Climate Change and Seasonal Reproduction in Mammals. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 3331–3340. [Google Scholar] [CrossRef]
- Gurnell, J.; Flowerdew, J.R. Live Trapping Small Mammals: A Practical Guide; Mammal Society: London, UK, 2006; ISBN 978-0-906282-54-0. [Google Scholar]
- Sikes, R.S. the Animal Care and Use Committee of the American Society of Mammalogists 2016 Guidelines of the American Society of Mammalogists for the Use of Wild Mammals in Research and Education. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef] [PubMed]
- Servei Meteorològic de Catalunya Generalitat de Catalunya El temps a Catalunya—Servei Meteorològic de Catalunya. Available online: https://www.meteo.cat/ (accessed on 12 August 2024).
- Hijmans, R.J. The Raster Package; CRAN Team: Stamford, CN, USA, 2015. [Google Scholar]
- Nikam, B.R.; Kumar, P.; Garg, V.; Thakur, P.K.; Aggarwal, S.P. Comparative Evaluation of Different Potential Evapotranspiration Estimation Approaches. Int. J. Res. Eng. Technol. 2014, 3, 544–552. [Google Scholar] [CrossRef]
- Thornthwaite, C.W. An Approach toward a Rational Classification of Climate. Geogr. Rev. 1948, 38, 55–94. [Google Scholar] [CrossRef]
- Torre, I.; Bastardas-Llabot, J.; Arrizabalaga, A.; Díaz, M. Population Dynamics of Small Endotherms under Global Change: Greater White-Toothed Shrews Crocidura russula in Mediterranean Habitats. Sci. Total Environ. 2020, 705, 135799. [Google Scholar] [CrossRef]
- Torre, I.; Puig-Montserrat, X.; Díaz, M. Global Change Effects on Mediterranean Small Mammal Population Dynamics: Demography of Algerian Mice (Mus spretus) along Land Use and Climate Gradients. Sci. Total Environ. 2023, 863, 160875. [Google Scholar] [CrossRef]
- Clarke, J.R. The Reproductive Biology of the Bank Vole (Clethrionomys glareolus) and the Wood Mouse (Apodemus sylvaticus). Symp. Zool. Soc. Lond. 1985, 55, 33–59. [Google Scholar]
- Díaz, M.; Torre, I.; Arrizabalaga, A. Relative Roles of Density and Rainfall on the Short-Term Regulation of Mediterraneanwood Mouse Apodemus sylvaticus Populations. Acta Theriol. 2010, 55, 251–260. [Google Scholar] [CrossRef]
- Easterling, D.R.; Meehl, G.A.; Parmesan, C.; Changnon, S.A.; Karl, T.R.; Mearns, L.O. Climate Extremes: Observations, Modeling, and Impacts. Science 2000, 289, 2068–2074. [Google Scholar] [CrossRef]
- Thomas, R.; Vaughan, I.; Lello, J. Data Analysis with R Statistical Software. In A Guidebook for Scientists; Eco-Explore: Scotland, Australia, 2013. [Google Scholar]
- Generalitat de Catalunya Sòls. Available online: http://agricultura.gencat.cat/ca/ambits/agricultura/sols/ (accessed on 12 August 2024).
- Prévot-Julliard, A.; Henttonen, H.; Yoccoz, N.G.; Stenseth, N.C. Delayed Maturation in Female Bank Voles: Optimal Decision or Social Constraint? J. Anim. Ecol. 1999, 68, 684–697. [Google Scholar] [CrossRef]
- Kim, J.H. Multicollinearity and Misleading Statistical Results. Korean J. Anesthesiol. 2019, 72, 558–569. [Google Scholar] [CrossRef]
- Bates, D.; Maechler, M.; Bolker, B.; Walker, S. Lme4: Linear Mixed-Effects Models Using “Eigen” and S4; 1.1-35.5; CRAN Team: Stamford, CN, USA, 2003. [Google Scholar]
- Zeileis, A.; Kleiber, C.; Jackman, S. Regression Models for Count Data in R. J. Stat. Softw. 2008, 27, 1–25. [Google Scholar] [CrossRef]
- Meyer, D.; Zeileis, A.; Hornik, K.; Meyer, M.D.; KernSmooth, S. The Vcd Package; CRAN Team: Stamford, CN, USA, 2007. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Walker, N.; Saveliev, A.A.; Smith, G.M. Mixed Effects Models and Extensions in Ecology with R; Statistics for Biology and Health; Springer: New York, NY, USA, 2009; ISBN 978-0-387-87457-9. [Google Scholar]
- Zuur, A.F.; Ieno, E.N.; Elphick, C.S. A Protocol for Data Exploration to Avoid Common Statistical Problems. Methods Ecol. Evol. 2010, 1, 3–14. [Google Scholar] [CrossRef]
- Bartoń, K. MuMIn: Multi-Model Inference; 1.47.5; CRAN Team: Stamford, CN, USA, 2010. [Google Scholar]
- Cavanaugh, J.E. Unifying the Derivations for the Akaike and Corrected Akaike Information Criteria. Stat. Probab. Lett. 1997, 33, 201–208. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023. [Google Scholar]
- Fiske, I.; Chandler, R. Unmarked: An R Package for Fitting Hierarchical Models of Wildlife Occurrence and Abundance. J. Stat. Softw. 2011, 43, 1–23. [Google Scholar] [CrossRef]
- Mackenzie, D.I. Modeling the Probability of Resource Use: The Effect of, and Dealing with, Detecting a Species Imperfectly. J. Wildl. Manag. 2006, 70, 367–374. [Google Scholar] [CrossRef]
- Torre, I.; Jaime-González, C.; Díaz, M. Habitat Suitability for Small Mammals in Mediterranean Landscapes: How and Why Shrubs Matter. Sustainability 2022, 14, 1562. [Google Scholar] [CrossRef]
- Bogaart, P.; Van der Loo, M.; Pannekoek, J. Rtrim: Trends and Indices for Monitoring Data; CRAN Team: Stamford, CN, USA, 2016. [Google Scholar]
- Lionello, P.; Scarascia, L. The Relation between Climate Change in the Mediterranean Region and Global Warming. Reg. Environ. Chang. 2018, 18, 1481–1493. [Google Scholar] [CrossRef]
- Castién, E. Breeding, Abundance and Population Structure of the Bank Vole Clethrionomys glareolus (Schreber, 1780) in the Western Pyrenees. Miscel Lània Zoològica 1998, 21.2, 25–34. [Google Scholar]
- Marková, S.; Lanier, H.C.; Escalante, M.A.; Da Cruz, M.O.R.; Horníková, M.; Konczal, M.; Weider, L.J.; Searle, J.B.; Kotlík, P. Local Adaptation and Future Climate Vulnerability in a Wild Rodent. Nat. Commun. 2023, 14, 7840. [Google Scholar] [CrossRef]
- Bobretsov, A.V.; Lukyanova, L.E.; Bykhovets, N.M.; Petrov, A.N. Impact of Climate Change on Population Dynamics of Forest Voles (Myodes) in Northern Pre-Urals: The Role of Landscape Effects. Contemp. Probl. Ecol. 2017, 10, 215–223. [Google Scholar] [CrossRef]
- Kiseleva, N.V. Long-Term Population Dynamics of the Bank Vole in the Ilmen Nature Reserve. Russ. J. Ecol. 2020, 51, 188–194. [Google Scholar] [CrossRef]
- Flowerdew, J.R.; Hall, S.J.G.; Brown, J.C. Small Rodents, Their Habitats, and the Effects of Flooding at Wicken Fen, Cambridgeshire. J. Zool. 1977, 182, 323–342. [Google Scholar] [CrossRef]
- Drake, J.M. Population Effects of Increased Climate Variation. Proc. R. Soc. B Biol. Sci. 2005, 272, 1823–1827. [Google Scholar] [CrossRef]
- Huitu, O.; Norrdahl, K.; Korpimäki, E. Landscape Effects on Temporal and Spatial Properties of Vole Population Fluctuations. Oecologia 2003, 135, 209–220. [Google Scholar] [CrossRef]
- Appleby, S.M.; Balkenhol, N. Douglas Fir and Norway Spruce Have Similar Effects on Small Mammal Density, but Not Survival, in Central European Managed Forests. Mamm. Biol. 2024, 104, 25–39. [Google Scholar] [CrossRef]
- Panzacchi, M.; Linnell, J.D.C.; Melis, C.; Odden, M.; Odden, J.; Gorini, L.; Andersen, R. Effect of Land-Use on Small Mammal Abundance and Diversity in a Forest–Farmland Mosaic Landscape in South-Eastern Norway. For. Ecol. Manag. 2010, 259, 1536–1545. [Google Scholar] [CrossRef]
- Barbier, S.; Gosselin, F.; Balandier, P. Influence of Tree Species on Understory Vegetation Diversity and Mechanisms Involved—A Critical Review for Temperate and Boreal Forests. For. Ecol. Manag. 2008, 254, 1–15. [Google Scholar] [CrossRef]
- Oro, D.; Freixas, L.; Bartrina, C.; Míguez, S.; Torre, I. Direct and Indirect Effects of Climate and Seed Dynamics on the Breeding Performance of a Seed Predator at the Distribution Edge. Ecol. Evol. 2024, 14, e70104. [Google Scholar] [CrossRef]
- Selås, V. Explaining Bank Vole Cycles in Southern Norway 1980–2004 from Bilberry Reports 1932–1977 and Climate. Oecologia 2006, 147, 625–631. [Google Scholar] [CrossRef]
- MacKenzie, D.I.; Nichols, J.D.; Lachman, G.B.; Droege, S.; Andrew Royle, J.; Langtimm, C.A. Estimating Site Occupancy Rates When Detection Probabilities Are Less than One. Ecology 2002, 83, 2248–2255. [Google Scholar] [CrossRef]
- Zhigalski, O.A.; Kshnyasev, I.A. Population Cycles of the Bank Vole in the Range Optimum. Russ. J. Ecol. 2000, 31, 345–352. [Google Scholar] [CrossRef]
- Amori, G.; Locasciulli, O.; Tuccinardi, P.; Riga, F. Ecological Structure of a Population of Clethrionomys glareolus in Central Italy: An Eight-Year Study. Pol. J. Ecol. 2000, 48, 125–132. [Google Scholar]
- Yoccoz, N.G.; Mesnager, S. Are Alpine Bank Voles Larger and More Sexually Dimorphic Because Adults Survive Better? Oikos 1998, 82, 85–98. [Google Scholar] [CrossRef]
- Llanos-Guerrero, C.; Freixas-Mora, L.; Vilella, M.; Bartrina, C.; Torre, I. Seed Availability and Small Mammal Populations: Insights from Mediterranean Forests. Forests 2024, 15, 1148. [Google Scholar] [CrossRef]
- Selås, V. Evidence for Different Bottom-up Mechanisms in Wood Mouse (Apodemus sylvaticus) and Bank Vole (Myodes glareolus) Population Fluctuations in Southern Norway. Mammal Res. 2020, 65, 267–275. [Google Scholar] [CrossRef]
- Selås, V.; Framstad, E.; Rolstad, J.; Sonerud, G.A.; Spidsø, T.K.; Wegge, P. Bilberry Seed Production Explains Spatiotemporal Synchronicity in Bank Vole Population Fluctuations in Norway. Ecol. Res. 2021, 36, 409–419. [Google Scholar] [CrossRef]
- Yoccoz, N.G.; Chr, N.; Henttonen, H.; Prévot-Julliard, A.-C. Effects of Food Addition on the Seasonal Density- Dependent Structure of Bank Vole Clethrionomys glareolus Populations. J. Anim. Ecol. 2001, 70, 713–720. [Google Scholar] [CrossRef]
- Johnsen, K.; Boonstra, R.; Boutin, S.; Devineau, O.; Krebs, C.J.; Andreassen, H.P. Surviving Winter: Food, but Not Habitat Structure, Prevents Crashes in Cyclic Vole Populations. Ecol. Evol. 2017, 7, 115–124. [Google Scholar] [CrossRef]
- Johnsen, K.; Devineau, O.; Andreassen, H.P. The Effects of Winter Climate and Intrinsic Factors on Survival of Cyclic Vole Populations in Southeastern Norway. Ann. Zool. Fenn. 2018, 55, 173–185. [Google Scholar] [CrossRef]
- Gorosito, I.L.; Marziali Bermúdez, M.; Busch, M. Advantages of Combining Generalized Linear Models and Occupancy Models to Find Indicators of Habitat Selection: Small Mammals in Agroecosystems as a Case Study. Ecol. Indic. 2018, 85, 1–10. [Google Scholar] [CrossRef]


| Pyrenees | Mediterranean | |||
|---|---|---|---|---|
| 2008 | 2023 | 2008 | 2023 | |
| Temperature | 5.52° ± 0.45 °C | 7.61° ± 0.49 °C | 11.75° ± 0.40 °C | 13.22° ± 0.46 °C |
| Precipitation | 1652.57 ± 36.19 mm | 921.45 ± 32.93 mm | 1248.58 ± 59.85 mm | 510.63 ± 28.84 mm |
| Pyrenees | Mediterranean | |||
|---|---|---|---|---|
| 2007 | 2022 | 2007 | 2022 | |
| Forest | 77.9 ± 5.29 | 84.7 ± 4.24 | 85.8 ± 4.24 | 85.0 ± 4.32 |
| Open | 19.9 ± 4.54 | 15 ± 4.23 | 9.35 ± 4.09 | 10.2 ± 3.95 |
| Crops | 0.30 ± 0.19 | 0 | 3.25 ± 1.29 | 2.85 ± 1.07 |
| Urban | 0.29 ± 0.20 | 0.35 ± 0.20 | 1.48 ± 0.61 | 1.86 ± 0.66 |
| Total Abundance | Adults | Subadults | |
|---|---|---|---|
| Intercept | 1.00 ** | 227 | −1.50 *** |
| (0.44) | (0.36) | (0.28) | |
| Coniferous forest | −1.28 * | −1.57 ** | |
| (0.76) | (0.77) | ||
| Autumn | −0.19 | −0.67 * | |
| (0.31) | (0.40) | ||
| Pyrenees region | −1.71 ** | 0.04 | |
| (0.80) | (0.51) | ||
| Precipitation Variance | −0.30 *** | −0.29 ** | |
| (12 month before) | (0.11) | (0.12) | |
| Mean temperature | −1.21 *** | −0.75 *** | |
| (12 month before) | (0.36) | (0.24) | |
| Temperature Variance | 0.37 ** | ||
| (3 month before) | (0.15) | ||
| Autumn:Pyrenees region | 0.72 ** | 1.55 ** | |
| (0.31) | (0.67) | ||
| R2m | 0.16 | 0.14 | 0.05 |
| R2c | 0.53 | 0.53 | 0.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de la Huerta-Schliemann, L.; Vilella, M.; Freixas, L.; Torre, I. Effects of Climate and Land Use on the Population Dynamics of the Bank Vole (Clethrionomys glareolus) in the Southernmost Part of Its Range. Animals 2025, 15, 839. https://doi.org/10.3390/ani15060839
de la Huerta-Schliemann L, Vilella M, Freixas L, Torre I. Effects of Climate and Land Use on the Population Dynamics of the Bank Vole (Clethrionomys glareolus) in the Southernmost Part of Its Range. Animals. 2025; 15(6):839. https://doi.org/10.3390/ani15060839
Chicago/Turabian Stylede la Huerta-Schliemann, Lucía, Marc Vilella, Lídia Freixas, and Ignasi Torre. 2025. "Effects of Climate and Land Use on the Population Dynamics of the Bank Vole (Clethrionomys glareolus) in the Southernmost Part of Its Range" Animals 15, no. 6: 839. https://doi.org/10.3390/ani15060839
APA Stylede la Huerta-Schliemann, L., Vilella, M., Freixas, L., & Torre, I. (2025). Effects of Climate and Land Use on the Population Dynamics of the Bank Vole (Clethrionomys glareolus) in the Southernmost Part of Its Range. Animals, 15(6), 839. https://doi.org/10.3390/ani15060839








