Renal Biomarkers in Companion Animals—A Review
Simple Summary
Abstract
1. Introduction
2. General Aspects of Renal Disease Biomarkers
3. Markers of Glomerular Filtration Rate (GFR)
3.1. Serum Creatinine (sCr)
3.2. Symmetric Dimethylarginine (SDMA)
3.3. Cystatin C (CysC)
4. Markers of Glomerular Damage and Dysfunction
4.1. Albumin (Alb)
4.2. Immunoglobulins (IgG, IgM, and IgA)
5. Markers of Tubular Damage/Dysfunction
5.1. Cystatin B (CysB) and Clusterin (Clust)
5.2. Neutrophil Gelatinase-Associated Lipocalin (NGAL) and Kidney Injury Molecule-1 (KIM-1)
5.3. Retinol Binding Protein (RBP)
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Polzin, D.J. Chronic Kidney Disease in Small Animals. Vet. Clin. Small Anim. Pract. 2011, 41, 15–30. [Google Scholar] [CrossRef]
- Marino, C.L.; Lascelles, B.D.X.; Vaden, S.L.; Gruen, M.E.; Marks, S.L. Prevalence and Classification of Chronic Kidney Disease in Cats Randomly Selected from Four Age Groups and in Cats Recruited for Degenerative Joint Disease Studies. J. Feline Med. Surg. 2014, 16, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Heiene, R. IRIS Kidney—Education—Creatinine in Dogs. Available online: https://www.iris-kidney.com/new-page-1 (accessed on 27 December 2024).
- De Loor, J.; Daminet, S.; Smets, P.; Maddens, B.; Meyer, E. Urinary Biomarkers for Acute Kidney Injury in Dogs. J. Vet. Intern. Med. 2013, 27, 998–1010. [Google Scholar] [CrossRef]
- Cowgill, L.D.; Polzin, D.J.; Elliott, J.; Nabity, M.B.; Segev, G.; Grauer, G.F.; Brown, S.; Langston, C.; van Dongen, A.M. Is Progressive Chronic Kidney Disease a Slow Acute Kidney Injury? Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 995–1013. [Google Scholar] [CrossRef]
- McKenna, M.; Pelligand, L.; Elliott, J.; Cotter, D.; Jepson, R. Relationship between Serum Iohexol Clearance, Serum SDMA Concentration, and Serum Creatinine Concentration in Non-Azotemic Dogs. J. Vet. Intern. Med. 2020, 34, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Ghys, L.; Paepe, D.; Smets, P.; Lefebvre, H.; Delanghe, J.; Daminet, S. Cystatin C: A New Renal Marker and Its Potential Use in Small Animal Medicine. J. Vet. Intern. Med. 2014, 28, 1152–1164. [Google Scholar] [CrossRef]
- Cianciolo, R.; Hokamp, J.; Nabity, M. Advances in the Evaluation of Canine Renal Disease. Vet. J. 2016, 215, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Gregory, F. IRIS Kidney—Education—Using Urine Specific Gravity. Available online: https://www.iris-kidney.com/urine-specific-gravity (accessed on 28 December 2024).
- Cobrin, A.R.; Blois, S.L.; Kruth, S.A.; Abrams-Ogg, A.C.G.; Dewey, C. Biomarkers in the Assessment of Acute and Chronic Kidney Diseases in the Dog and Cat. J. Small Anim. Pract. 2013, 54, 647–655. [Google Scholar] [CrossRef]
- Kovarikova, S. Urinary Biomarkers of Renal Function in Dogs and Cats: A Review. Vet. Med. 2015, 60, 589–602. [Google Scholar] [CrossRef]
- Heiene, R. IRIS Kidney—Education—Biomarkers of Kidney Disease: Potential Utilities. Available online: https://www.iris-kidney.com/biomarkers-of-urinary-tract-disease-potential-utilities (accessed on 26 December 2024).
- Wasung, M.E.; Chawla, L.S.; Madero, M. Biomarkers of Renal Function, Which and When? Clin. Chim. Acta 2015, 438, 350–357. [Google Scholar] [CrossRef]
- Hokamp, J.A.; Nabity, M.B. Renal Biomarkers in Domestic Species. Vet. Clin. Pathol. 2016, 45, 28–56. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.P.; Lefebvre, H.P.; Watson, A.D.J. Creatinine in the Dog: A Review. Vet. Clin. Pathol. 2003, 32, 162–179. [Google Scholar] [CrossRef]
- Mack, R.M.; Hegarty, E.; McCrann, D.J.; Michael, H.T.; Grauer, G.F. Longitudinal Evaluation of Symmetric Dimethylarginine and Concordance of Kidney Biomarkers in Cats and Dogs. Vet. J. 2021, 276, 105732. [Google Scholar] [CrossRef] [PubMed]
- Loane, S.C.; Thomson, J.M.; Williams, T.L.; McCallum, K.E. Evaluation of Symmetric Dimethylarginine in Cats with Acute Kidney Injury and Chronic Kidney Disease. J. Vet. Intern. Med. 2022, 36, 1669–1676. [Google Scholar] [CrossRef]
- Couto, C.G.; Murphy, R.; Coyne, M.; Drake, C. Serum Symmetric Dimethylarginine Concentration in Greyhound Pups and Adults. Top. Companion Anim. Med. 2021, 45, 100558. [Google Scholar] [CrossRef] [PubMed]
- Hall, J.A.; Yerramilli, M.; Obare, E.; Yerramilli, M.; Jewell, D.E. Comparison of Serum Concentrations of Symmetric Dimethylarginine and Creatinine as Kidney Function Biomarkers in Cats with Chronic Kidney Disease. J. Vet. Intern. Med. 2014, 28, 1676–1683. [Google Scholar] [CrossRef]
- Nabity, M.B.; Lees, G.E.; Cianciolo, R.; Boggess, M.M.; Steiner, J.M.; Suchodolski, J.S. Urinary Biomarkers of Renal Disease in Dogs with X-Linked Hereditary Nephropathy. J. Vet. Intern. Med. 2012, 26, 282–293. [Google Scholar] [CrossRef]
- Dahlem, D.P.; Neiger, R.; Schweighauser, A.; Francey, T.; Yerramilli, M.; Obare, E.; Steinbach, S.M.L. Plasma Symmetric Dimethylarginine Concentration in Dogs with Acute Kidney Injury and Chronic Kidney Disease. J. Vet. Intern. Med. 2017, 31, 799–804. [Google Scholar] [CrossRef]
- dos Santos, F.M.; Veado, J.C.C.; Ribeiro, V.M.; da Costa-Val, A.P. Evaluation of Physiological Biomarkers as Possible Predictive Factors and Prognosis Markers of Kidney Injury in Dogs Naturally Infected with Leishmania infantum. Arch. Vet. Med. 2023, 16, 83–102. [Google Scholar] [CrossRef]
- Kohnken, R.; Himmel, L.; Logan, M.; Peterson, R.; Biswas, S.; Dunn, C.; LeRoy, B. Symmetric Dimethylarginine Is a Sensitive Biomarker of Glomerular Injury in Rats. Toxicol. Pathol. 2022, 50, 176–185. [Google Scholar] [CrossRef]
- DeMonaco, S.M.; Panciera, D.L.; Morre, W.A.; Conway, T.; Werre, S. Symmetric Dimethylarginine in Hyperthyroid Cats before and after Treatment with Radioactive Iodine. J. Feline Med. Surg. 2020, 22, 531–538. [Google Scholar] [CrossRef]
- Nabity, M.B.; Lees, G.E.; Boggess, M.M.; Yerramilli, M.; Obare, E.; Yerramilli, M.; Rakitin, A.; Aguiar, J.; Relford, R. Symmetric Dimethylarginine Assay Validation, Stability, and Evaluation as a Marker for the Early Detection of Chronic Kidney Disease in Dogs. J. Vet. Intern. Med. 2015, 29, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Relford, R.; Robertson, J.; Clements, C. Symmetric Dimethylarginine: Improving the Diagnosis and Staging of Chronic Kidney Disease in Small Animals. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 941–960. [Google Scholar] [CrossRef] [PubMed]
- Michael, H.T.; Mack, R.M.; Hegarty, E.; McCrann, D.J.; Grauer, G.F. A Longitudinal Study of the Persistence of Increased Creatinine and Concordance between Kidney Biomarkers in Cats and Dogs. Vet. J. 2021, 276, 105729. [Google Scholar] [CrossRef] [PubMed]
- Gordin, E.; Viitanen, S.; Gordin, D.; Szlosek, D.; Peterson, S.; Spillmann, T.; Labato, M.A. A Clinical Study on Urinary Clusterin and Cystatin B in Dogs with Spontaneous Acute Kidney Injury. Vet. Sci. 2024, 11, 200. [Google Scholar] [CrossRef]
- de Oliveira Paes-Leme, F.; de Souza, E.M.; Ceregatti, M.G.; Campos, M.T.G.; de Melo, P.D.V.; da Costa-Val, A.P. Cystatin C Assay Validation Using the Immunoturbidimetric Method to Evaluate the Renal Function of Healthy Dogs and Dogs with Acute Renal Injury. Vet. World 2022, 15, 1595–1600. [Google Scholar] [CrossRef]
- Pressler, B.M. Clinical Approach to Advanced Renal Function Testing in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2013, 43, 1193–1208. [Google Scholar] [CrossRef]
- Monti, P.; Benchekroun, G.; Berlato, D.; Archer, J. Initial Evaluation of Canine Urinary Cystatin C as a Marker of Renal Tubular Function. J. Small Anim. Pract. 2012, 53, 254–259. [Google Scholar] [CrossRef]
- Poświatowska-Kaszczyszyn, I. Usefulness of Serum Cystatin C Measurement for Assessing Renal Function in Cats. Bull. Vet. Inst. Pulawy 2012, 56, 235–239. [Google Scholar] [CrossRef]
- Davis, J.; Rossi, G.; Miller, D.W.; Cianciolo, R.E.; Raisis, A.L. Ability of Different Assay Platforms to Measure Renal Biomarker Concentrations during Ischaemia-Reperfusion Acute Kidney Injury in Dogs. Res. Vet. Sci. 2021, 135, 547–554. [Google Scholar] [CrossRef]
- Antognoni, M.T.; Siepi, D.; Porciello, F.; Fruganti, G. Use of Serum Cistatin C Determination as a Marker of Renal Function in the Dog. Vet. Res. Commun. 2005, 29 (Suppl. S2), 265–267. [Google Scholar] [CrossRef] [PubMed]
- Ghys, L.F.E.; Paepe, D.; Taffin, E.R.L.; Vandermeulen, E.; Duchateau, L.; Smets, P.M.Y.; Delanghe, J.; Daminet, S. Serum and Urinary Cystatin C in Cats with Feline Immunodeficiency Virus Infection and Cats with Hyperthyroidism. J. Feline Med. Surg. 2016, 18, 658–665. [Google Scholar] [CrossRef]
- Williams, T.L.; Dillon, H.; Elliott, J.; Syme, H.M.; Archer, J. Serum Cystatin C Concentrations in Cats with Hyperthyroidism and Chronic Kidney Disease. J. Vet. Intern. Med. 2016, 30, 1083–1089. [Google Scholar] [CrossRef]
- Braun, J.P.; Perxachs, A.; Péchereau, D.; De La Farge, F. Plasma Cystatin C in the Dog: Reference Values and Variations with Renal Failure. Comp. Clin. Path 2002, 11, 44–49. [Google Scholar] [CrossRef]
- Wehner, A.; Hartmann, K.; Hirschberger, J. Utility of Serum Cystatin C as a Clinical Measure of Renal Function in Dogs. J. Am. Anim. Hosp. Assoc. 2008, 44, 131–138. [Google Scholar] [CrossRef]
- Miyagawa, Y.; Akabane, R.; Ogawa, M.; Nagakawa, M.; Miyakawa, H.; Takemura, N. Serum Cystatin C Concentration Can Be Used to Evaluate Glomerular Filtration Rate in Small Dogs. J. Vet. Med. Sci. 2020, 82, 1828. [Google Scholar] [CrossRef]
- Ghys, L.F.E.; Paepe, D.; Lefebvre, H.P.; Taffin, E.R.L.; Hesta, M.; Delanghe, J.R.; Smets, P.; Vandendriessche, V.; Daminet, S. The Effect of Feeding, Storage and Anticoagulant on Feline Serum Cystatin, C. Vet. J. 2015, 206, 91–96. [Google Scholar] [CrossRef]
- Ghys, L.F.E.; Paepe, D.; Duchateau, L.; Taffin, E.R.L.; Marynissen, S.; Delanghe, J.; Daminet, S. Biological Validation of Feline Serum Cystatin C: The Effect of Breed, Age and Sex and Establishment of a Reference Interval. Vet. J. 2015, 204, 168–173. [Google Scholar] [CrossRef] [PubMed]
- Almy, F.S.; Christopher, M.M.; King, D.P.; Brown, S.A. Evaluation of Cystatin C as an Endogenous Marker of Glomerular Filtration Rate in Dogs. J. Vet. Intern. Med. 2002, 16, 45–51. [Google Scholar] [CrossRef]
- Pasa, S.; Bayramli, G.; Atasoy, A.; Karul, A.; Ertug, S.; Ozensoy Toz, S. Evaluation of Serum Cystatin-C in Dogs with Visceral Leishmaniasis. Vet. Res. Commun. 2009, 33, 529–534. [Google Scholar] [CrossRef]
- de Oliveira Frazilio, F.; de Almeida Borges, F.; de Souza, A.I.; Palumbo, M.I.P.; do Nascimento Ramos, C.A.; Freire, D.H.; Galvão, A.L.B.; de Freitas, M.G.; Barbosa, F.B. Biomarkers and Renal Arterial Resistive Index in Dogs Naturally Infected with Leishmania Infantum. Parasitol. Res. 2018, 117, 3399–3405. [Google Scholar] [CrossRef] [PubMed]
- García-Martínez, J.D.; Martinez-Subiela, S.; Tvarijonaviciute, A.; Caldin, M.; Ceron, J.J. Urinary Ferritin and Cystatin C Concentrations at Different Stages of Kidney Disease in Leishmaniotic Dogs. Res. Vet. Sci. 2015, 99, 204–207. [Google Scholar] [CrossRef]
- Paepe, D.; Ghys, L.F.E.; Smets, P.; Lefebvre, H.P.; Croubels, S.; Daminet, S. Routine Kidney Variables, Glomerular Filtration Rate and Urinary Cystatin C in Cats with Diabetes Mellitus, Cats with Chronic Kidney Disease and Healthy Cats. J. Feline Med. Surg. 2015, 17, 880–888. [Google Scholar] [CrossRef]
- Ghys, L.F.E.; Paepe, D.; Lefebvre, H.P.; Reynolds, B.S.; Croubels, S.; Meyer, E.; Delanghe, J.R.; Daminet, S. Evaluation of Cystatin C for the Detection of Chronic Kidney Disease in Cats. J. Vet. Intern. Med. 2016, 30, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Kongtasai, T.; Paepe, D.; Meyer, E.; Mortier, F.; Marynissen, S.; Stammeleer, L.; Defauw, P.; Daminet, S. Renal Biomarkers in Cats: A Review of the Current Status in Chronic Kidney Disease. J. Vet. Intern. Med. 2022, 36, 379–396. [Google Scholar] [CrossRef]
- Pagitz, M.; Frommlet, F.; Schwendenwein, I. Evaluation of Biological Variance of Cystatin c in Comparison with Other Endogenous Markers of Glomerular Filtration Rate in Healthy Dogs. J. Vet. Intern. Med. 2007, 21, 402–409. [Google Scholar] [CrossRef]
- Vlasakova, K.; Erdos, Z.; Troth, S.P.; McNulty, K.; Chapeau-Campredon, V.; Mokrzycki, N.; Muniappa, N.; Gu, Y.Z.; Holder, D.; Bailey, W.J.; et al. Evaluation of the Relative Performance of 12 Urinary Biomarkers for Renal Safety across 22 Rat Sensitivity and Specificity Studies. Toxicol. Sci. 2014, 138, 3–20. [Google Scholar] [CrossRef] [PubMed]
- Syme, H.M.; Markwell, P.J.; Pfeiffer, D.; Elliott, J. Survival of Cats with Naturally Occurring Chronic Renal Failure Is Related to Severity of Proteinuria. J. Vet. Intern. Med. 2006, 20, 410–414. [Google Scholar] [CrossRef]
- Raila, J.; Brunnberg, L.; Schweigert, F.J.; Kohn, B. Influence of Kidney Function on Urinary Excretion of Albumin and Retinol-Binding Protein in Dogs with Naturally Occurring Renal Disease. Am. J. Vet. Res. 2010, 71, 1387–1394. [Google Scholar] [CrossRef]
- Smets, P.M.Y.; Meyer, E.; Maddens, B.E.J.; Duchateau, L.; Daminet, S. Urinary Markers in Healthy Young and Aged Dogs and Dogs with Chronic Kidney Disease. J. Vet. Intern. Med. 2010, 24, 65–72. [Google Scholar] [CrossRef]
- Vaden, S.L.; Elliott, J. Management of Proteinuria in Dogs and Cats with Chronic Kidney Disease. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 1115–1130. [Google Scholar] [CrossRef] [PubMed]
- Falus, F.A.; Vizi, Z.; Szabó, K.É.; Müller, L.; Reiczigel, J.; Balogh, N.; Manczur, F. Establishment of a Reference Interval for Urinary Albumin-to-creatinine Ratio in Dogs. Vet. Clin. Pathol. 2022, 51, 585. [Google Scholar] [CrossRef]
- Whittemore, J.C.; Miyoshi, Z.; Jensen, W.A.; Radecki, S.V.; Lappin, M.R. Association of Microalbuminuria and the Urine Albumin-to-Creatinine Ratio with Systemic Disease in Cats. J. Am. Vet. Med. Assoc. 2007, 230, 1165–1169. [Google Scholar] [CrossRef] [PubMed]
- Lyon, S.D.; Sanderson, M.W.; Vaden, S.L.; Lappin, M.R.; Jensen, W.A.; Grauer, G.E. Comparison of Urine Dipstick, Sulfosalicylic Acid, Urine Protein-to-Creatinine Ratio, and Species-Specific ELISA Methods for Detection of Albumin in Urine Samples of Cats and Dogs. J. Am. Vet. Med. Assoc. 2010, 236, 874–879. [Google Scholar] [CrossRef] [PubMed]
- Vaden, S.L.; Turman, C.A.; Harris, T.L.; Marks, S.L. The Prevalence of Albuminuria in Dogs and Cats in an ICU or Recovering from Anesthesia. J. Vet. Emerg. Crit. Care 2010, 20, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghazlat, S.A.; Langston, C.E.; Greco, D.S.; Reine, N.J.; May, S.N.; Shofer, F.S. The Prevalence of Microalbuminuria and Proteinuria in Cats with Diabetes Mellitus. Top. Companion Anim. Med. 2011, 26, 154–157. [Google Scholar] [CrossRef]
- Schaefer, H.; Kohn, B.; Schweigert, F.J.; Raila, J. Quantitative and Qualitative Urine Protein Excretion in Dogs with Severe Inflammatory Response Syndrome. J. Vet. Intern. Med. 2011, 25, 1292–1297. [Google Scholar] [CrossRef]
- Herring, I.P.; Panciera, D.L.; Werre, S.R. Longitudinal Prevalence of Hypertension, Proteinuria, and Retinopathy in Dogs with Spontaneous Diabetes Mellitus. J. Vet. Intern. Med. 2014, 28, 488. [Google Scholar] [CrossRef]
- Defauw, P.; Schoeman, J.P.; Smets, P.; Goddard, A.; Meyer, E.; Liebenberg, C.; Daminet, S. Assessment of Renal Dysfunction Using Urinary Markers in Canine Babesiosis Caused by Babesia Rossi. Vet. Parasitol. 2012, 190, 326–332. [Google Scholar] [CrossRef][Green Version]
- Hrovat, A.; Schoeman, J.P.; de Laat, B.; Meyer, E.; Smets, P.; Goddard, A.; Nagel, S.; Daminet, S. Evaluation of Snake Envenomation-Induced Renal Dysfunction in Dogs Using Early Urinary Biomarkers of Nephrotoxicity. Vet. J. 2013, 198, 239–244. [Google Scholar] [CrossRef]
- Zaragoza, C.; Barrera, R.; Centeno, F.; Tapia, J.A.; Mañé, M.C. Characterization of Renal Damage in Canine Leptospirosis by Sodium Dodecyl Sulphate-Polyacrylamide Gel Electrophoresis (SDS-PAGE) and Western Blotting of the Urinary Proteins. J. Comp. Pathol. 2003, 129, 169–178. [Google Scholar] [CrossRef]
- Smets, P.M.Y.; Lefebvre, H.P.; Meij, B.P.; Croubels, S.; Meyer, E.; Van de Maele, I.; Daminet, S. Long-Term Follow-Up of Renal Function in Dogs after Treatment for ACTH-Dependent Hyperadrenocorticism. J. Vet. Intern. Med. 2012, 26, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Maddens, B.; Heiene, R.; Smets, P.; Svensson, M.; Aresu, L.; van der Lugt, J.; Daminet, S.; Meyer, E. Evaluation of Kidney Injury in Dogs with Pyometra Based on Proteinuria, Renal Histomorphology, and Urinary Biomarkers. J. Vet. Intern. Med. 2011, 25, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Segev, G.; Vaden, S.; Ross, S.; Dufayet, C.; Cohn, L.A.; Farace, G.; Szlosek, D.; Ouyang, Z.; Peterson, S.; Beall, M.; et al. Urinary Cystatin B Differentiates Progressive versus Stable IRIS Stage 1 Chronic Kidney Disease in Dogs. J. Vet. Intern. Med. 2023, 37, 2251. [Google Scholar] [CrossRef]
- Yerramilli, M.; Farace, G.; Quinn, J.; Yerramilli, M. Kidney Disease and the Nexus of Chronic Kidney Disease and Acute Kidney Injury: The Role of Novel Biomarkers as Early and Accurate Diagnostics. Vet. Clin. N. Am. Small Anim. Pract. 2016, 46, 961–993. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Avital, Y.; Peterson, S.; Ouyang, Z.; Yerramilli, M.; Aroch, I.; Segev, G. Urinary Cystatin B as a Marker of Acute Kidney Injury in Cats. Vet. J. 2024, 308, 106262. [Google Scholar] [CrossRef]
- Harjen, H.J.; Anfinsen, K.P.; Hultman, J.; Moldal, E.R.; Szlosek, D.; Murphy, R.; Friis, H.; Peterson, S.; Rørtveit, R. Evaluation of Urinary Clusterin and Cystatin B as Biomarkers for Renal Injury in Dogs Envenomated by the European Adder (Vipera verus). Top. Companion Anim. Med. 2022, 46, 100586. [Google Scholar] [CrossRef]
- Starybrat, D.; Jepson, R.; Bristow, P.; Peterson, S.; Yerramilli, M.; Yerramilli, M.; Chang, Y.M.; Cortellini, S. Prospective Evaluation of Novel Biomarkers of Acute Kidney Injury in Dogs Following Cardiac Surgery under Cardiopulmonary Bypass. J. Vet. Emerg. Crit. Care 2022, 32, 733–742. [Google Scholar] [CrossRef]
- Le Sueur, A.N.V.; de Souza, A.A.L.; Paes, A.C.; Takahira, R.K.; Melchert, A.; Okamoto, A.S.; Coyne, M.; Murphy, R.; Szlosek, D.; Peterson, S.; et al. Novel Renal Injury Markers in Dogs with Ehrlichiosis. PLoS ONE 2023, 18, e0293545. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, H.J.; Do, S.H. Early Detection of Chronic Kidney Disease Using Plasma Neutrophil Gelatinase-Associated Lipocalin and Kidney Injury Molecule-1 in Small-Breed Dogs: A Retrospective Pilot Study. Animals 2024, 14, 2313. [Google Scholar] [CrossRef]
- Scheemaeker, S.; Meyer, E.; Schoeman, J.P.; Defauw, P.; Duchateau, L.; Daminet, S. Urinary Neutrophil Gelatinase-Associated Lipocalin as an Early Biomarker for Acute Kidney Injury in Dogs. Vet. J. 2020, 255, 105423. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, T.; Mori, K.; Mukoyama, M.; Kasahara, M.; Yokoi, H.; Saito, Y.; Yoshioka, T.; Ogawa, Y.; Imamaki, H.; Kusakabe, T.; et al. Urinary Neutrophil Gelatinase-Associated Lipocalin Levels Reflect Damage to Glomeruli, Proximal Tubules, and Distal Nephrons. Kidney Int. 2009, 75, 285–294. [Google Scholar] [CrossRef]
- Segev, G.; Palm, C.; Leroy, B.; Cowgill, L.D.; Westropp, J.L. Evaluation of Neutrophil Gelatinase-Associated Lipocalin as a Marker of Kidney Injury in Dogs. J. Vet. Intern. Med. 2013, 27, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.L.; Lin, Y.S.; Hu, Y.Y.; Wong, M.L.; Lin, F.Y.; Lee, Y.J. Neutrophil Gelatinase-Associated Lipocalin in Dogs with Naturally Occurring Renal Diseases. J. Vet. Intern. Med. 2014, 28, 437–442. [Google Scholar] [CrossRef]
- Kim, Y.M.; Polzin, D.J.; Rendahl, A.; Granick, J.L. Urinary Neutrophil Gelatinase-Associated Lipocalin in Dogs with Stable or Progressive Kidney Disease. J. Vet. Intern. Med. 2019, 33, 654–661. [Google Scholar] [CrossRef] [PubMed]
- Ahn, H.J.; Hyun, C. Evaluation of Serum Neutrophil Gelatinase-Associated Lipocalin (NGAL) Activity in Dogs with Chronic Kidney Disease. Vet. Rec. 2013, 173, 452. [Google Scholar] [CrossRef]
- Peris, M.P.; Morales, M.; Ares-Gómez, S.; Esteban-Gil, A.; Gómez-Ochoa, P.; Gascón, M.; Moreno, B.; Castillo, J.A. Neutrophil Gelatinase-Associated Lipocalin (NGAL) Is Related with the Proteinuria Degree and the Microscopic Kidney Findings in Leishmania-Infected Dogs. Microorganisms 2020, 8, 1966. [Google Scholar] [CrossRef]
- van den Berg, M.F.; Schoeman, J.P.; Defauw, P.; Whitehead, Z.; Breemersch, A.; Goethals, K.; Daminet, S.; Meyer, E. Assessment of Acute Kidney Injury in Canine Parvovirus Infection: Comparison of Kidney Injury Biomarkers with Routine Renal Functional Parameters. Vet. J. 2018, 242, 8–14. [Google Scholar] [CrossRef]
- Sasaki, A.; Sasaki, Y.; Iwama, R.; Shimamura, S.; Yabe, K.; Takasuna, K.; Ichijo, T.; Furuhama, K.; Satoh, H. Comparison of Renal Biomarkers with Glomerular Filtration Rate in Susceptibility to the Detection of Gentamicin-Induced Acute Kidney Injury in Dogs. J. Comp. Pathol. 2014, 151, 264–270. [Google Scholar] [CrossRef]
- Zhou, X.; Ma, B.; Lin, Z.; Qu, Z.; Huo, Y.; Wang, J.; Li, B. Evaluation of the Usefulness of Novel Biomarkers for Drug-Induced Acute Kidney Injury in Beagle Dogs. Toxicol. Appl. Pharmacol. 2014, 280, 30–35. [Google Scholar] [CrossRef]
- Adedeji, A.O.; Sonee, M.; Chen, Y.; Lynch, K.; Peron, K.; King, N.; McDuffie, J.E.; Vinken, P. Evaluation of Novel Urinary Biomarkers in Beagle Dogs with Amphotericin B-Induced Kidney Injury. Int. J. Toxicol. 2023, 42, 146–155. [Google Scholar] [CrossRef]
- Gu, Y.Z.; Vlasakova, K.; Troth, S.P.; Peiffer, R.L.; Tournade, H.; Pasello dos Santos, F.R.; Glaab, W.E.; Sistare, F.D. Performance Assessment of New Urinary Translational Safety Biomarkers of Drug-Induced Renal Tubular Injury in Tenofovir-Treated Cynomolgus Monkeys and Beagle Dogs. Toxicol. Pathol. 2018, 46, 553–563. [Google Scholar] [CrossRef]
- Segev, G.; Daminet, S.; Meyer, E.; De Loor, J.; Cohen, A.; Aroch, I.; Bruchim, Y. Characterization of Kidney Damage Using Several Renal Biomarkers in Dogs with Naturally Occurring Heatstroke. Vet. J. 2015, 206, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.J.; Hu, Y.Y.; Lin, Y.S.; Chang, C.T.; Lin, F.Y.; Wong, M.L.; Kuo-Hsuan, H.; Hsu, W.L. Urine Neutrophil Gelatinase-Associated Lipocalin (NGAL) as a Biomarker for Acute Canine Kidney Injury. BMC Vet. Res. 2012, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Troia, R.; Sabetti, M.C.; Crosara, S.; Quintavalla, C.; Romito, G.; Mazzoldi, C.; Fidanzio, F.; Cescatti, M.; Bertazzolo, W.; Giunti, M.; et al. Evaluation of Urinary Neutrophil Gelatinase-Associated Lipocalin to Detect Renal Tubular Damage in Dogs with Stable Myxomatous Mitral Valve Disease. J. Vet. Intern. Med. 2022, 36, 2053–2062. [Google Scholar] [CrossRef]
- Wu, P.H.; Hsu, W.L.; Tsai, P.S.J.; Wu, V.C.; Tsai, H.J.; Lee, Y.J. Identification of Urine Neutrophil Gelatinase-Associated Lipocalin Molecular Forms and Their Association with Different Urinary Diseases in Cats. BMC Vet. Res. 2019, 15, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kongtasai, T.; Meyer, E.; Paepe, D.; Marynissen, S.; Smets, P.; Mortier, F.; Demeyere, K.; Vandermeulen, E.; Stock, E.; Buresova, E.; et al. Liver-Type Fatty Acid-Binding Protein and Neutrophil Gelatinase-Associated Lipocalin in Cats with Chronic Kidney Disease and Hyperthyroidism. J. Vet. Intern. Med. 2021, 35, 1376–1388. [Google Scholar] [CrossRef]
- Bonventre, J.V. Kidney Injury Molecule-1 (KIM-1): A Specific and Sensitive Biomarker of Kidney Injury. Scand. J. Clin. Lab. Invest. Suppl. 2008, 241, 78–83. [Google Scholar] [CrossRef]
- Lippi, I.; Perondi, F.; Meucci, V.; Bruno, B.; Gazzano, V.; Guidi, G. Clinical Utility of Urine Kidney Injury Molecule-1 (KIM-1) and Gamma-Glutamyl Transferase (GGT) in the Diagnosis of Canine Acute Kidney Injury. Vet. Res. Commun. 2018, 42, 95–100. [Google Scholar] [CrossRef]
- Harjen, H.J.; Nicolaysen, T.V.; Negard, T.; Lund, H.; Sævik, B.K.; Anfinsen, K.P.; Moldal, E.R.; Zimmer, K.E.; Rørtveit, R. Serial Serum Creatinine, SDMA and Urinary Acute Kidney Injury Biomarker Measurements in Dogs Envenomated by the European Adder (Vipera Berus). BMC Vet. Res. 2021, 17, 1–13. [Google Scholar] [CrossRef]
- Dias, C.S.; Paz, L.N.; Solcà, M.S.; Portela, R.W.D.; Bittencourt, M.V.; Pinna, M.H. Kidney Injury Molecule-1 in the Detection of Early Kidney Injury in Dogs with Leptospirosis. Comp. Immunol. Microbiol. Infect. Dis. 2021, 76, 101637. [Google Scholar] [CrossRef]
- Asma Idress, M.; Deepa, P.M.; Rathish, R.L.; Vinodkumar, K.; Pradeep, M. Diagnostic Efficacy of Urinary Neutrophil Gelatinase-Associated Lipocalin and Kidney Injury Molecule-1 for Early Detection of Acute Kidney Injury in Dogs with Leptospirosis or Babesiosis. Vet. Res. Commun. 2024, 48, 2813–2818. [Google Scholar] [CrossRef] [PubMed]
- McDuffie, J.E.; Chen, Y.; Ma, J.Y.; Lee, S.; Lynch, K.M.; Hamlin, D.M.; Nguyen, L.; Rizzolio, M.; Sonee, M.; Snook, S. Cisplatin Nephrotoxicity in Male Beagle Dogs: Next-Generation Protein Kidney Safety Biomarker Tissue Expression and Related Changes in Urine. Toxicol. Res. 2016, 5, 1202–1215. [Google Scholar] [CrossRef] [PubMed]
- Wagoner, M.P.; Yang, Y.; McDuffie, J.E.; Klapczynski, M.; Buck, W.; Cheatham, L.; Eisinger, D.; Sace, F.; Lynch, K.M.; Sonee, M.; et al. Evaluation of Temporal Changes in Urine-Based Metabolomic and Kidney Injury Markers to Detect Compound Induced Acute Kidney Tubular Toxicity in Beagle Dogs. Curr. Top. Med. Chem. 2017, 17, 2767–2780. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhou, X.; Qu, Z.; Sun, L.; Cheng, G.; Yang, Y.; Miao, Y.; Chen, X.; Li, B. Urinary Biomarker Evaluation for Early Detection of Gentamycin-Induced Acute Kidney Injury. Toxicol. Lett. 2019, 300, 73–80. [Google Scholar] [CrossRef]
- Bland, S.K. Kidney Injury Molecule 1: A Potential Biomarker of Renal Injury in the Cat. Ph.D. Thesis, University of Guelph, Guelph, ON, Canada, 2014. [Google Scholar]
- Zheng, J.S.; Zhu, T.T.; Ruan, H.R. Screening of Early Diagnostic Markers of Gentamicin-Induced Acute Kidney Injury in Canines. J. Vet. Res. 2019, 63, 405–411. [Google Scholar] [CrossRef]
- Raila, J.; Forterre, S.; Schweigert, F.J. Levels of Retinol and Retinyl Esters in Plasma and Urine of Dogs with Urolithiasis. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2003, 50, 380–382. [Google Scholar] [CrossRef]
- Raila, J.; Aupperle, H.; Raila, G.; Schoon, H.A.; Schweigert, F.J. Renal Pathology and Urinary Protein Excretion in a 14-Month-Old Bernese Mountain Dog with Chronic Renal Failure. J. Vet. Med. A Physiol. Pathol. Clin. Med. 2007, 54, 131–135. [Google Scholar] [CrossRef]
- van Hoek, I.; Lefebvre, H.P.; Peremans, K.; Meyer, E.; Croubels, S.; Vandermeulen, E.; Kooistra, H.; Saunders, J.H.; Binst, D.; Daminet, S. Short- and Long-Term Follow-up of Glomerular and Tubular Renal Markers of Kidney Function in Hyperthyroid Cats after Treatment with Radioiodine. Domest. Anim. Endocrinol. 2009, 36, 45–56. [Google Scholar] [CrossRef]
- van Hoek, I.; Daminet, S.; Notebaert, S.; Janssens, I.; Meyer, E. Immunoassay of Urinary Retinol Binding Protein as a Putative Renal Marker in Cats. J. Immunol. Methods 2008, 329, 208–213. [Google Scholar] [CrossRef]
- Chacar, F.; Kogika, M.; Sanches, T.R.; Caragelasco, D.; Martorelli, C.; Rodrigues, C.; Capcha, J.M.C.; Chew, D.; Andrade, L. Urinary Tamm-Horsfall Protein, Albumin, Vitamin D-Binding Protein, and Retinol-Binding Protein as Early Biomarkers of Chronic Kidney Disease in Dogs. Physiol. Rep. 2017, 5, e13262. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.N.; Brown, E. Todays Veterinary Practice. Clinical Application of Renal Biomarkers. September/October. 2022. Available online: https://todaysveterinarypractice.com/wp-content/uploads/sites/4/2022/08/TVP-2022-0910_Renal_Biomarkers.pdf (accessed on 9 March 2025).
- Obert, L.A.; Elmore, S.A.; Ennulat, D.; Frazier, K.S. A Review of Specific Biomarkers of Chronic Renal Injury and Their Potential Application in Nonclinical Safety Assessment Studies. Toxicol. Pathol. 2021, 49, 996–1023. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhang, A.; Chu, W. Phillyrin Is an Effective Inhibitor of Quorum Sensing with Potential as an Anti-Pseudomonas Aeruginosa Infection Therapy. J. Vet. Med. Sci. 2019, 81, 473. [Google Scholar] [CrossRef]
- Hamlin, D.M.; Schultze, A.E.; Coyne, M.J.; McCrann, D.J.; Mack, R.; Drake, C.; Murphy, R.E.; Cross, J.; Strong-Townsend, M.; Yerramilli, M.; et al. Evaluation of Renal Biomarkers, Including Symmetric Dimethylarginine, Following Gentamicin-Induced Proximal Tubular Injury in the Rat. Kidney360 2021, 3, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Kuleš, J.; Bilić, P.; Beer Ljubić, B.; Gotić, J.; Crnogaj, M.; Brkljačić, M.; Mrljak, V. Glomerular and Tubular Kidney Damage Markers in Canine Babesiosis Caused by Babesia Canis. Ticks Tick Borne Dis. 2018, 9, 1508–1517. [Google Scholar] [CrossRef]
- Winiarczyk, D.; Adaszek, Ł.; Madany, J.; Winiarczyk, M.; Winiarczyk, S. Utility of Urinary Markers in the Assessment of Renal Dysfunction in Familial Glomerulonephritis in Dobermann Dogs. J. Vet. Res. 2020, 64, 181. [Google Scholar] [CrossRef]
- Khalil, G.M.; Mahran, K.M.A.; Ali, M.E.; Salem, S.I. Feline Renal Diseases: Novel versus Traditional Biomarkers. Egypt. J. Vet. Sci. 2024, 1–19. [Google Scholar] [CrossRef]
- Liu, D.J.X.; Meyer, E.; Broeckx, B.J.G.; Daminet, S.; Delanghe, J.R.; Stock, E.; Bogaerts, E.; Hesta, M.; Vanderperren, K. Variability of Serum Concentrations of Cystatin C and Urinary Retinol-Binding Protein, Neutrophil Gelatinase-Associated Lipocalin, Immunoglobulin G, and C-Reactive Protein in Dogs. J. Vet. Intern. Med. 2018, 32, 1659–1664. [Google Scholar] [CrossRef]
- Grauer, G.F. Early Detection of Renal Damage and Disease in Dogs and Cats. Vet. Clin. N. Am. Small Anim. Pract. 2005, 35, 581–596. [Google Scholar] [CrossRef]
- Cheon, J.H.; Kim, S.Y.; Son, J.Y.; Kang, Y.R.; An, J.H.; Kwon, J.H.; Song, H.S.; Moon, A.; Lee, B.M.; Kim, H.S. Pyruvate Kinase M2: A Novel Biomarker for the Early Detection of Acute Kidney Injury. Toxicol. Res. 2016, 32, 47–56. [Google Scholar] [CrossRef]
- Vaidya, V.S.; Ferguson, M.A.; Bonventre, J.V. Biomarkers of Acute Kidney Injury. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 463–493. [Google Scholar] [CrossRef] [PubMed]
- Coyne, M.J.; Eric Schultze, A.; McCrann, D.J.; Murphy, R.E.; Cross, J.; Strong-Townsend, M.; Drake, C.; Mack, R. Evaluation of Renal Injury and Function Biomarkers, Including Symmetric Dimethylarginine (SDMA), in the Rat Passive Heymann Nephritis (PHN) Model. PLoS ONE 2022, 17, e0269085. [Google Scholar] [CrossRef] [PubMed]
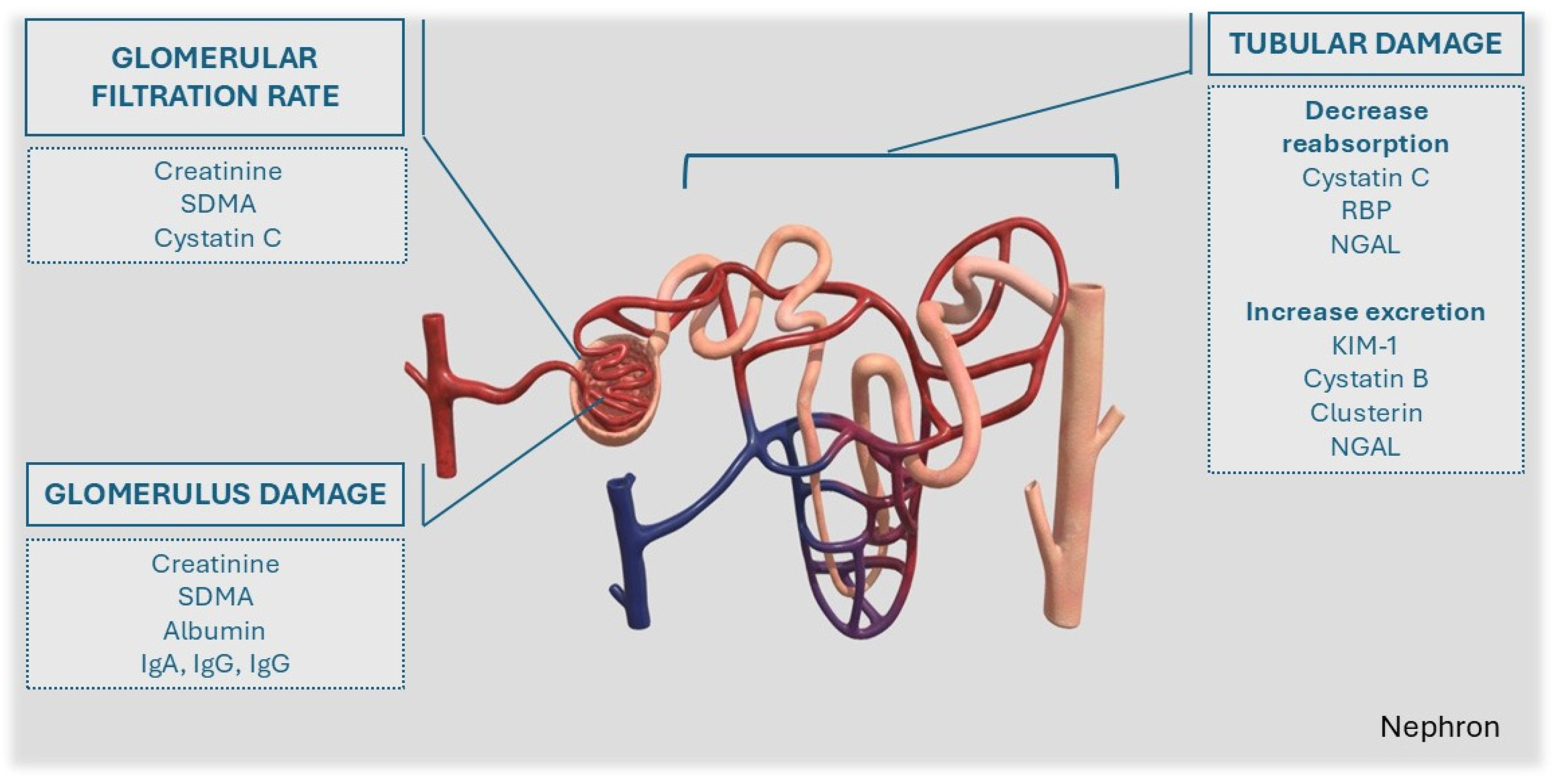
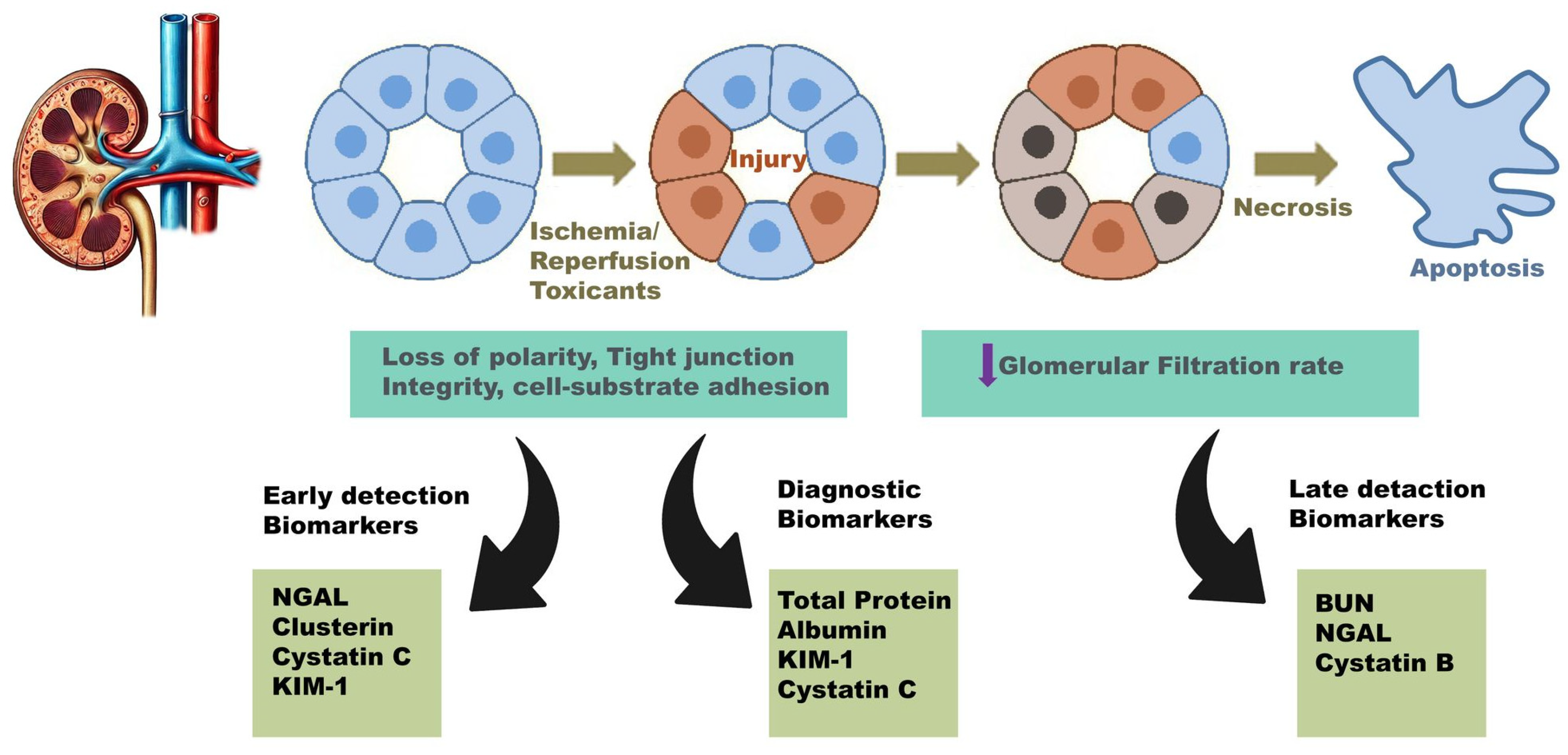
| Characteristic | Reason | References |
|---|---|---|
| Detectable in urine and/or plasma | It can be assessed routinely and serves as an indicator of kidney function. | [5] |
| Unique and specific to the kidney | Should reflect specific kidney damage very early. | [4,13] |
| Provides insights into the etiology and location of the injury | It affects the glomeruli or tubules, or it should be prerenal, renal, or postrenal. | [4,13] |
| Reflects the severity and potential for recovery | Indicate kidney injury or repair processes and predict the likelihood of recovery. | [13] |
| Increases rapidly and reliably in response to kidney disease | The absence of a biomarker may predict resolution of the active phase. | [5] |
| Chemically stable | Does not interfere with drugs and should be stable over time and across different temperatures and pH levels. | [13] |
| Biomarker | Samples | Species | Advantages | Disadvantages | CKD, AKD, or Both | References |
|---|---|---|---|---|---|---|
| Cr | Serum | Cats and dogs | Widely available. Inexpensive. Familiar assay. Most accurate in steady state GFR. | Non-linear relationship with GFR. Proportional to patient muscle mass. Influenced by pre- and post-renal azotemia and hydration status. Higher creatinine levels in breeds with increased muscle mass. | Both | [10,14] |
| SDMA | Serum | Cat and dogs | Increases progressively with increased renal impairment and progressive nephron loss in animals and humans with CKD. | Intra-individual and analytical variations are higher than those of serum creatinine. May be influenced by diseases such as diabetes mellitus, neoplasia (lymphoma), and nephrolithiasis. | CKD | [10,14,106] |
| Cyst C | Serum Urine | Cats and dogs | Good marker of GFR in early stages of renal disease. Demonstrated utility in human clinics and in animals. | Questionable effects of age and weight in dogs. Not consistently shown to be superior to creatinine as a marker of GFR. Diabetes in cats can influence the results. | AKD: Dogs CKD: Dogs and cats | [10,14,107] |
| Alb | Urine | Cats and dogs | High specificity for renal injury. | Immunoassays can underestimate low-level injury due to resorption/excretion of variably sized non-immunoreactive albumin fragments | AKD | [107] |
| Igs | Urine | Dogs | Can be helpful in diagnosing and monitoring glomerulonephritis. | Hematuria, pyuria, bacteriuria, and treatment with hydrocortisone can influence the results. Requires specialized laboratory techniques. | Both | [14,107] |
| Cyst B | Urine Serum | Dogs | Particularly valuable for detecting acute and active injury to renal tubular epithelial cells in early stages. | While valuable for tubular injury, it may not be as sensitive for other forms of kidney disease. | AKD | [28,48] |
| Clust | Urine | Dogs | May be an early indicator of renal injury. Could provide insights into the severity and progression of kidney disease. | Clusterin is involved in multiple biological processes; so, its elevation may not always be specific to kidney disease. Variability in results depending on the method of testing. | AKD: Dogs | [14,72] |
| NGAL | Urine Serum | Dogs | Good at predicting the progression of AKI to CKD. In a toxicity case, NGAL increased significantly several days before creatinine. | Hematuria and pyuria may cause assay interference. Malignancy, inflammation, and infection may decrease specificity. AKI marker with a large dynamic range in many species. | AKD: Dogs CKD: Dogs | [77,82,108] [8,20,79] |
| Kim-1 | Urine Serum | Cats and dogs | Can potentially detect kidney injury earlier than creatinine. | Current dog assays are problematic. Undetectable in healthy cats. | Both, but mostly with acute processes. | [48,107] |
| RBP | Urine | Cats and dogs | Useful for monitoring chronic disease due to progressive increases in later disease stages | Wide intra-individual variation in feline CKD and hyperthyroidism. The availability and standardization of RBP assays in veterinary medicine may be limited. | AKD and CKD: Dogs | [101,102] |
| Cause of AKD/CKD | Conclusions | Sample Size | Limitations | Reference |
|---|---|---|---|---|
| X-linked hereditary nephropathy (XLHN) | All urinary biomarkers elevated prior to an increase in sCr, but typically after the onset of proteinuria. uRBP/c may serve as a promising noninvasive tool for the diagnosis and monitoring of tubular injury and dysfunction in dogs with this pathology | 25 dogs with XLHN and 19 unaffected | The small sample size and the lack of a control group. The study did not evaluate the biomarkers in dogs with other forms of renal disease, which could limit the generalizability of the findings. | [20] |
| 22 different toxicants | Kim-1, Clust, and ALB showed the highest performance for detecting renal tubular injury. ALB used to detect glomerular injury. NGAL was the most nonspecific biomarker. | 22 rats | Inability to differentiate the cause of Clusterin increases. The damage localization was unclear. | [50] |
| Gentamicin | CysC was the most sensitive indicator of kidney injury in dogs. | 8 dogs: 4 gentamicin group 4 control group. | The small size, the short duration of gentamicin administration (7 days), and the use of only male dogs. | [82] |
| NGAL and Clust were the most sensitive biomarkers. | 12 dogs | Small sample size. The findings may not be directly applicable to other dog breeds or species [82]. | [83] | |
| SDMA was a more immediate biomarker for detecting gentamicin-induced toxicity compared to sCysC, BUN, and sCr. | 80 rats | Only male rats were used in the study, and it is possible that the results would have been different if female rats had been used. | [109] | |
| Headstrock | URBP and UNGAL were increased in all dogs with heatstroke. | 20 dogs | Small sample size and the absence of a control group of healthy dogs. The variability in heatstroke severity, which could have influenced the biomarker results. The information about pre-existing renal disease is not included. | [86] |
| Tenofovir disoproxil fumarate | Kim-1 and Clust were the most sensitive. CystC, RBP, NGAL, and ALB showed improved sensitivity over BUN and SCr. | 24 animals: 12 monkeys and 12 dogs. | Small sample size and limited time points: more frequent sampling might have provided a more detailed picture of the biomarker changes over time. Further research is needed to understand how these biomarkers relate to specific pathological processes. | [85] |
| Babesia spp. | Urinary ALB and IgG indicated glomerular damage. Elevated levels of KIM-1 and RBP suggested proximal tubular damage. | 42 dogs naturally infected with Babesia canis and 14 healthy dogs. | The researchers had to merge some of their predefined groups, which resulted in a smaller sample size for the analysis of certain markers. The creatine cut-off might be questionable because it does not account for differences in gender and muscle mass among the dogs. | [110] |
| Familiar glomerulonephritis in Doberman dogs | Urinary IgG can serve as a marker for glomerular function. uRBP has been identified as a marker for proximal tubular dysfunction. | 20 Doberman Pinschers | The study population consisted only of Doberman Pinschers, limiting the generalizability of the findings to other breeds. A direct comparison with healthy dogs would have strengthened the conclusions. The disease stage at the time of sampling could affect the biomarker levels. Limited number of biomarkers. | [111] |
| Ischemia–reperfusion (IR) | Only NGAL showed a significant increase following IR. In contrast, sCysC was not useful in identifying early AKD related to IR in dogs. | 12 dogs | The study involved a relatively small number of dogs. The study was conducted in dogs, and the findings may not be directly applicable to other species. The study compared the performance of different assay platforms for measuring renal biomarkers. The findings may not be generalizable to other assay platforms. | [33] |
| Poisoning by the European adder (snake) | KIM-1, NGAL, and ALB were indicative of renal tubular injury 12–36 h after envenomation. The observation of elevated AKI biomarkers at 36 h post-envenomation suggests poor prognosis. SDMA exhibited limited diagnostic utility in this context. | 20 dogs | Small sample size and the absence of a control group of healthy dogs. The severity of envenomation, which could have influenced the biomarker results. The study does not explicitly consider the pre-existing renal disease. | [70] |
| Amphoterinicin B | uClust was the most sensitive biomarker. | 12 dogs | Small sample size, the absence of a control group of healthy dogs, and the individual variation in response to amphotericin B. | [84] |
| Progressive feline kidney disease | SDMA and KIM-1 were sensitive biomarkers for early diagnosis and indicated an improvement in kidney function and repair. Can be potentially effective follow-up tools. | 86 cats: 68 were assigned to the diseased group and 18 to the treated group. | Further research is needed to examine the biological variability of UPC in cats with kidney diseases and overt renal proteinuria, as well as in cats with elevated UPC ratios. | [112] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pereira, A.F.; Jota Baptista, C.; Faustino-Rocha, A.; Oliveira, P.A.; Coelho, A.C. Renal Biomarkers in Companion Animals—A Review. Animals 2025, 15, 818. https://doi.org/10.3390/ani15060818
Pereira AF, Jota Baptista C, Faustino-Rocha A, Oliveira PA, Coelho AC. Renal Biomarkers in Companion Animals—A Review. Animals. 2025; 15(6):818. https://doi.org/10.3390/ani15060818
Chicago/Turabian StylePereira, Ana Filipa, Catarina Jota Baptista, Ana Faustino-Rocha, Paula A. Oliveira, and Ana Cláudia Coelho. 2025. "Renal Biomarkers in Companion Animals—A Review" Animals 15, no. 6: 818. https://doi.org/10.3390/ani15060818
APA StylePereira, A. F., Jota Baptista, C., Faustino-Rocha, A., Oliveira, P. A., & Coelho, A. C. (2025). Renal Biomarkers in Companion Animals—A Review. Animals, 15(6), 818. https://doi.org/10.3390/ani15060818









