Breed-Specific Responses and Ruminal Microbiome Shifts in Dairy Cows Under Heat Stress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Diets
2.2. Sample Collection and Measurement
2.3. DNA Extraction and MiSeq Sequencing
2.4. Sequence Bioinformatic Analysis
2.5. Data Analysis
3. Results
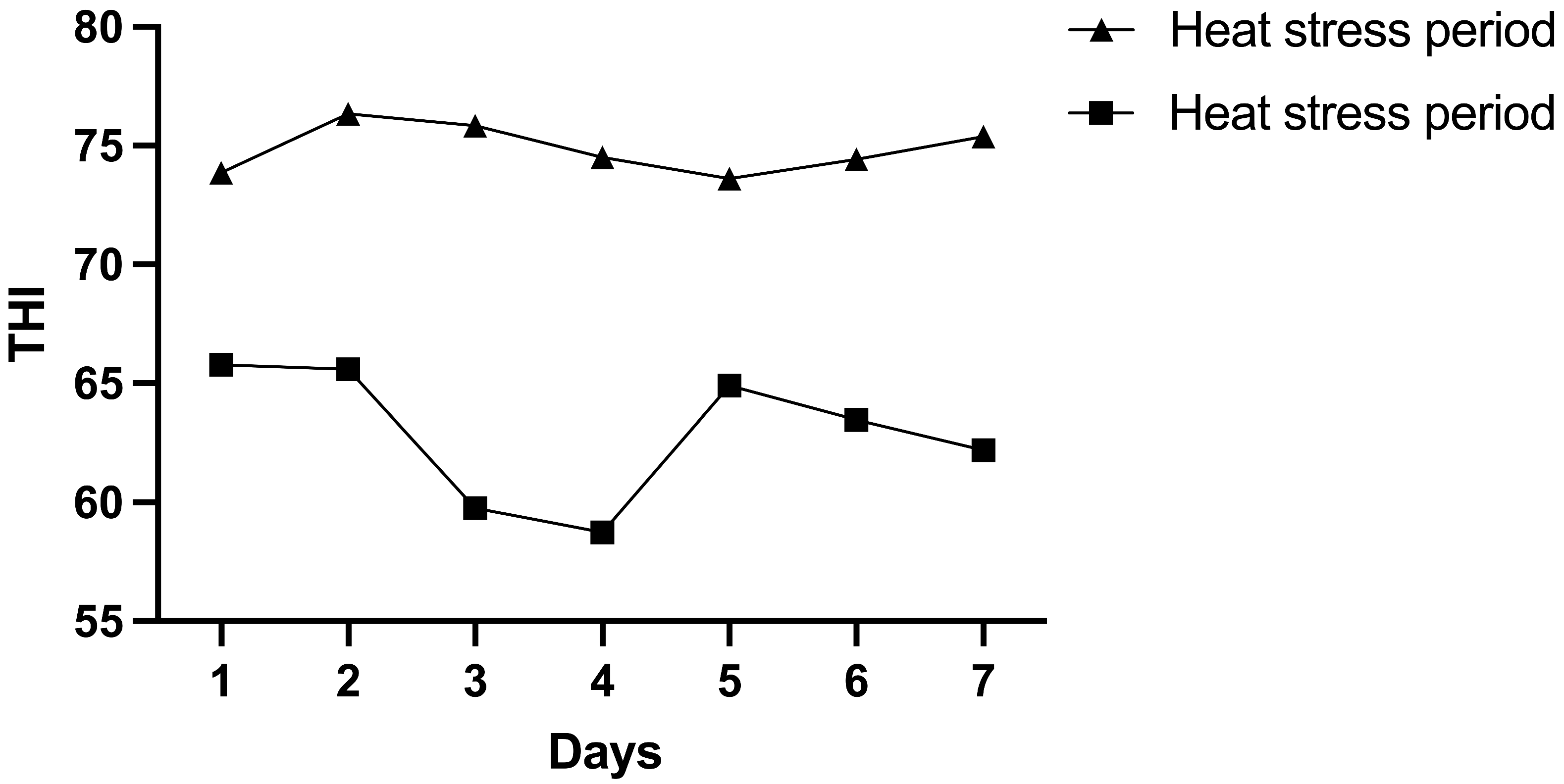
3.1. Heat Stress Response Differences Between Holstein and Jersey Cows
3.2. Differences in Rumen Fermentation Parameters Between Holstein and Jersey Cows
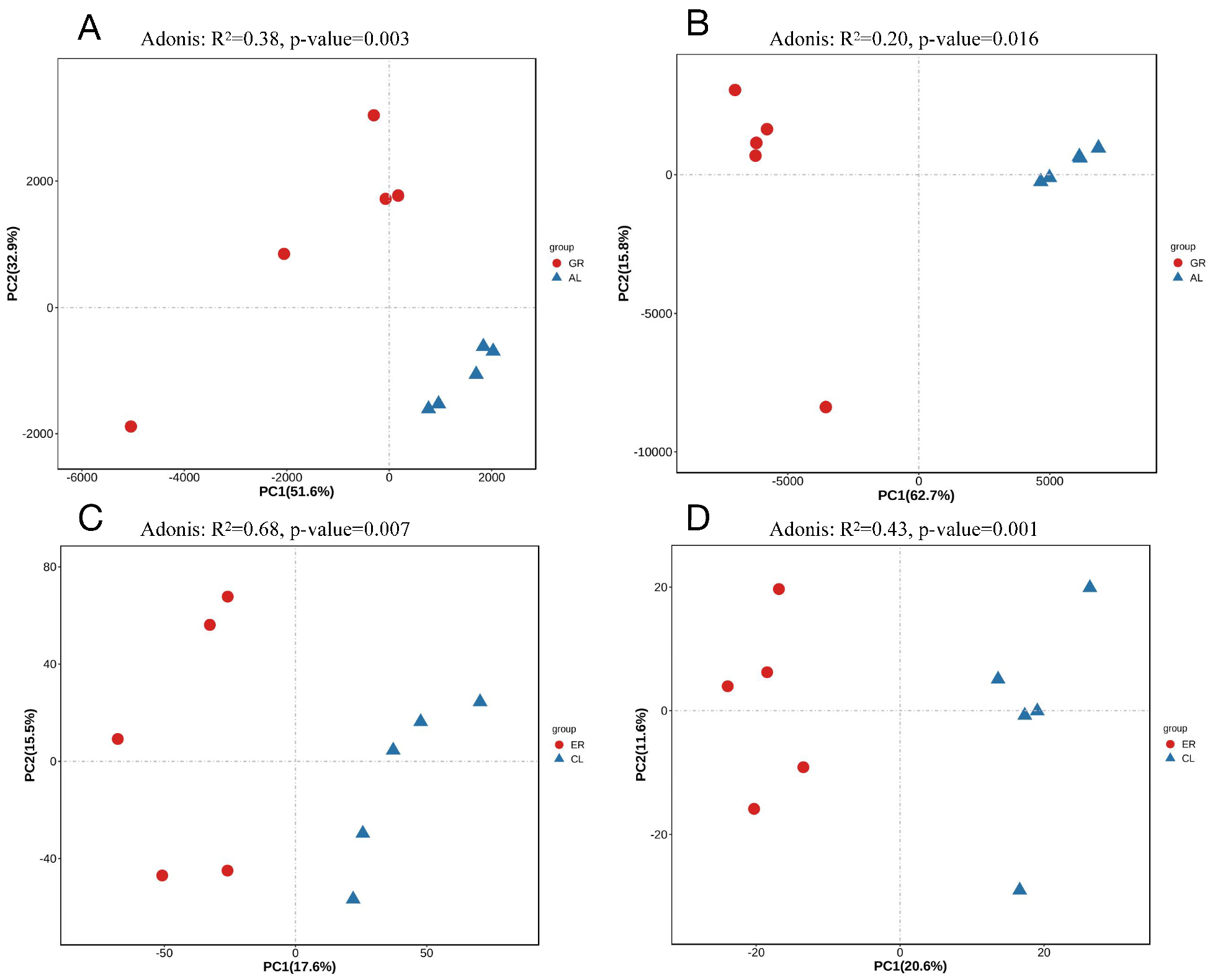
3.3. Heat Stress Impacts Microbiota Diversity
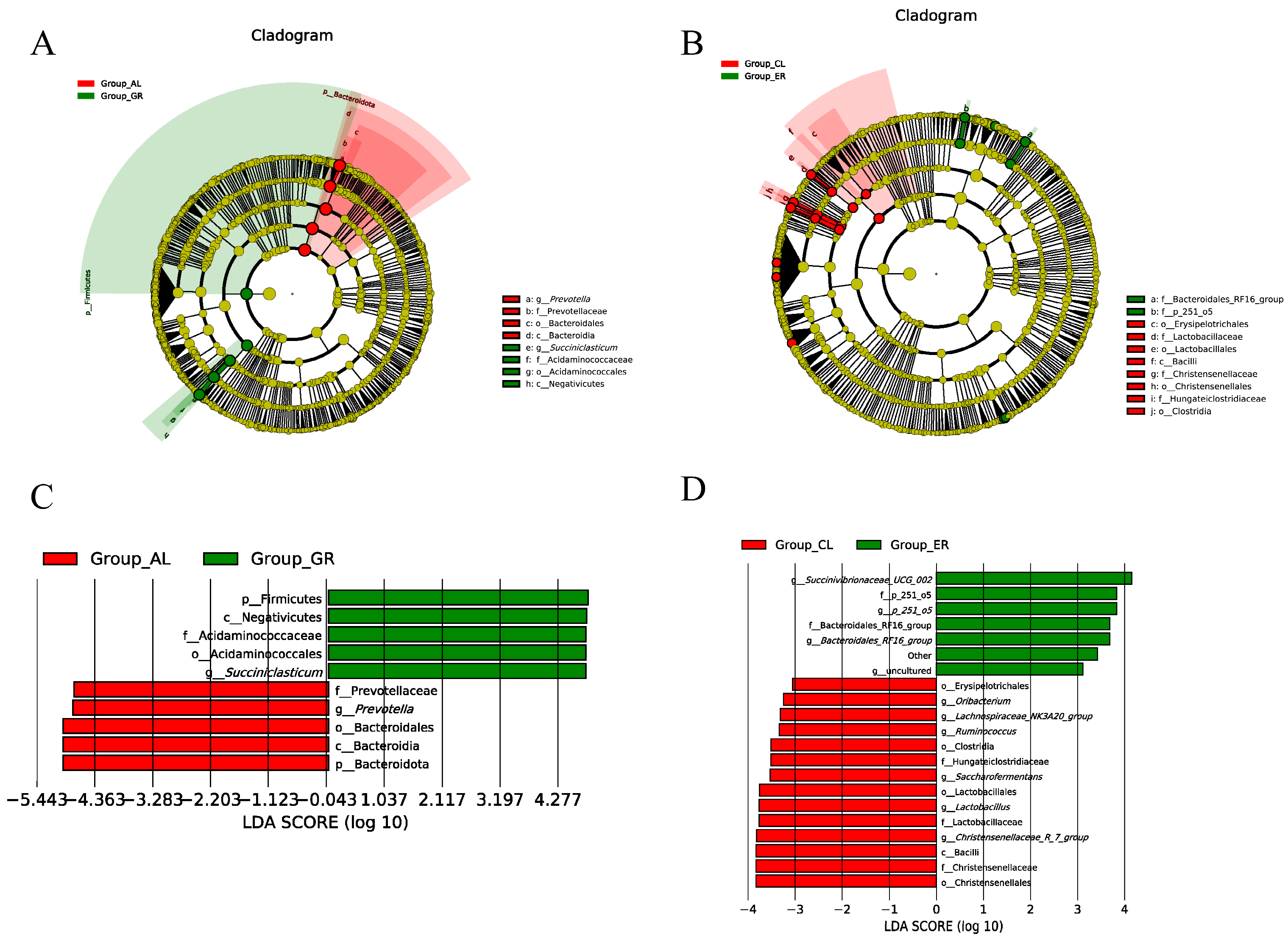
3.4. The Effect of Heat Stress on the Ruminal Microbiota Communities in Holstein and Jersey Cows
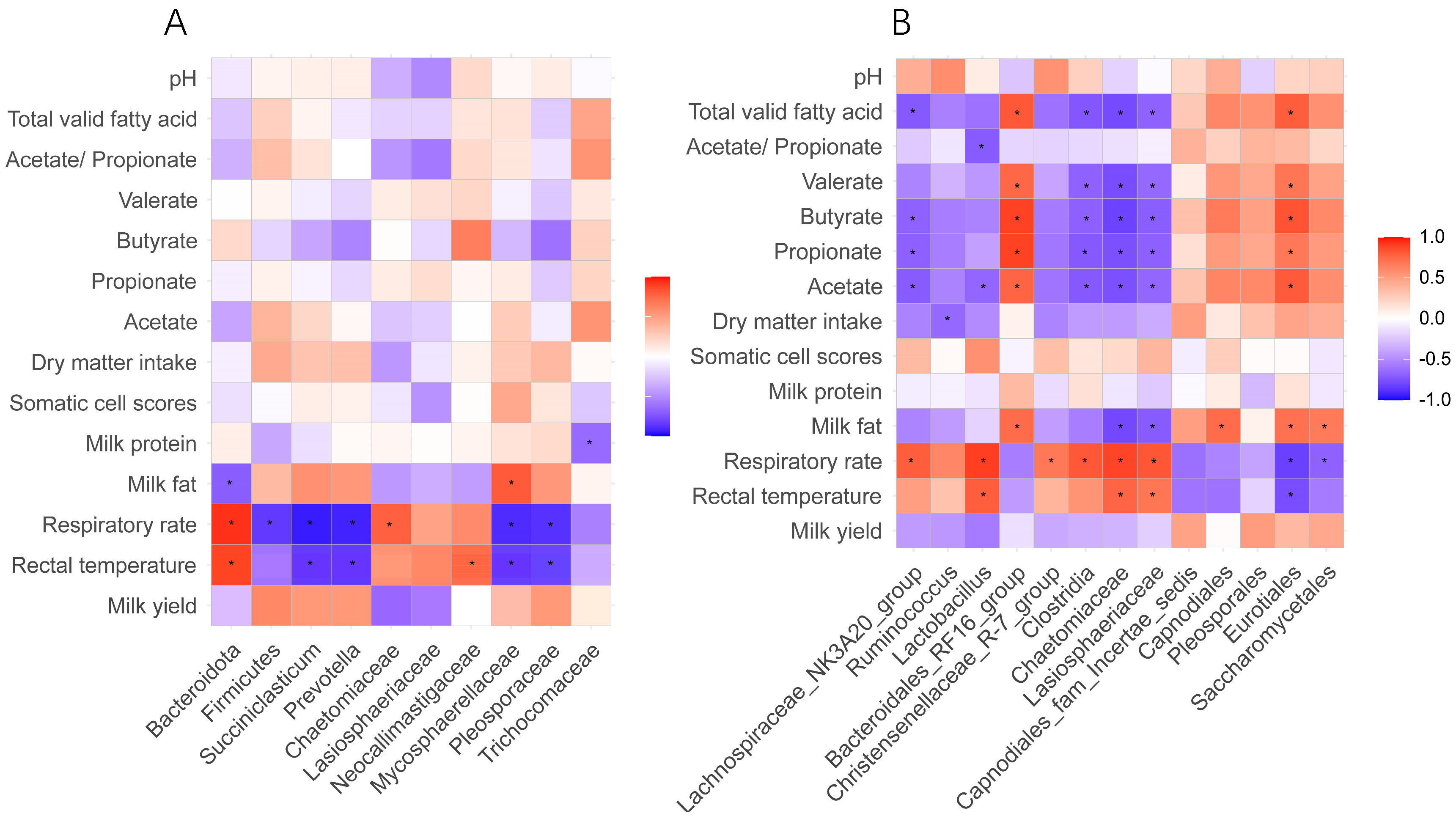
3.5. Correlation Between Differential Ruminal Microorganisms, Heat Stress Response Indices, and Rumen Fermentation Performance
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Rockett, P.L.; Campos, I.L.; Baes, C.F.; Tulpan, D.; Miglior, F.; Schenkel, F.S. Phenotypic analysis of heat stress in Holsteins using test-day production records and NASA POWER meteorological data. J. Dairy Sci. 2023, 106, 1142–1158. [Google Scholar] [CrossRef] [PubMed]
- Tian, H.; Wang, W.Y.; Zheng, N.; Cheng, J.B.; Li, S.L.; Zhang, Y.D.; Wang, J.Q. Identification of diagnostic biomarkers and metabolic pathway shifts of heat-stressed lactating dairy cows. J. Proteom. 2015, 125, 17–28. [Google Scholar] [CrossRef]
- Ren, Y.F.; Yang, Z.P.; Ling, F.H.; Xiao, L.W. Risk Zoning of Heat Stress Risk Zoning of Dairy Cows in Jiangsu Province and Its Characteristics Affected by Climate Chang. Sci. Agric. Sin. 2022, 55, 4513–4525. (In Chinese) [Google Scholar]
- Becker, C.A.; Collier, R.J.; Stone, A.E. Invited review: Physiological and behavioral effects of heat stress in dairy cows. J. Dairy Sci. 2020, 103, 6751–6770. [Google Scholar] [CrossRef] [PubMed]
- Collier, R.J.; Gebremedhin, K.G. Thermal Biology of Domestic Animals. Annu. Rev. Anim. Biosci. 2015, 3, 513–532. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.S.; Scharf, B.; Weaber, R.L.; Eichen, P.A.; Spiers, D.E. Patterns of heat response and adaptation on summer pasture: A comparison of heat-sensitive (Angus) and -tolerant (Romosinuano) cattle. J. Therm. Biol. 2012, 37, 344–350. [Google Scholar] [CrossRef]
- Berman, A.; Folman, Y.; Kaim, M.; Mamen, M.; Herz, Z.; Wolfenson, D.; Arieli, A.; Graber, Y. Upper critical temperatures and forced ventilation effects for high-yielding dairy cows in a subtropical climate. J. Dairy Sci. 1985, 68, 1488–1495. [Google Scholar] [CrossRef]
- Smith, D.L.; Smith, T.; Rude, B.J.; Ward, S.H. Short communication: Comparison of the effects of heat stress on milk and component yields and somatic cell score in Holstein and Jersey cows. J. Dairy Sci. 2013, 96, 3028–3033. [Google Scholar] [CrossRef]
- Bernabucci, U.; Basirico, L.; Morera, P.; Dipasquale, D.; Vitali, A.; Cappelli, F.P.; Calamari, L. Effect of summer season on milk protein fractions in Holstein cows. J. Dairy Sci. 2015, 98, 1815–1827. [Google Scholar] [CrossRef]
- Ozturk, H.; Gur, G. Rumen physiology: Microorganisms, fermentation and manipulation. Ankara Univ. Vet. Fak. 2021, 68, 423–434. [Google Scholar] [CrossRef]
- Ree, Z.H.; Yao, R.J.; Liu, Q.; Deng, Y.T.; Shen, L.H.; Deng, H.D.; Zuo, Z.C.; Wang, Y.; Deng, J.L.; Cui, H.M.; et al. Effects of antibacterial peptides on rumen fermentation function and rumen microorganisms in goats. PLoS ONE 2019, 14, e0221815. [Google Scholar] [CrossRef]
- Liu, H.J.; Hu, L.Y.; Han, X.P.; Zhao, N.; Xu, T.W.; Ma, L.; Wang, X.G.; Zhang, X.L.; Kang, S.P.; Zhao, X.Q.; et al. Tibetan Sheep Adapt to Plant Phenology in Alpine Meadows by Changing Rumen Microbial Community Structure and Function. Front. Microbiol. 2020, 11, 587558. [Google Scholar] [CrossRef] [PubMed]
- Carabano, M.J.; Ramon, M.; Diaz, C.; Molina, A.; Perez-Guzman, M.D.; Serradilla, J.M. BREEDING AND GENETICS SYMPOSIUM: Breeding for resilience to heat stress effects in dairy ruminants. A comprehensive review. J. Anim. Sci. 2017, 95, 1813–1826. [Google Scholar] [CrossRef]
- Sirohi, S.K.; Choudhury, P.K.; Puniya, A.K.; Singh, D.; Dagar, S.S.; Singh, N. Ribosomal ITS1 sequence-based diversity analysis of anaerobic rumen fungi in cattle fed on high fiber diet. Ann. Microbiol. 2013, 63, 1571–1577. [Google Scholar] [CrossRef]
- Denman, S.E.; Morgavi, D.P.; McSweeney, C.S. Review: The application of omics to rumen microbiota function. Animal 2018, 12, S233–S245. [Google Scholar] [CrossRef] [PubMed]
- He, J.W.; Guo, H.D.; Zheng, W.J.; Xue, Y.Q.; Zhao, R.Q.; Yao, W. Heat stress affects fecal microbial and metabolic alterations of primiparous sows during late gestation. J. Anim. Sci. Biotechnol. 2019, 10, 84. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.H.; Kim, M.H.; Kim, S.B.; Son, J.K.; Lee, J.H.; Joo, S.S.; Gu, B.H.; Park, T.; Park, B.Y.; Kim, E.T. Differential Dynamics of the Ruminal Microbiome of Jersey Cows in a Heat Stress Environment. Animals 2020, 10, 1127. [Google Scholar] [CrossRef]
- Gujar, G.; Tiwari, M.; Yadav, N.; Monika, D. Heat stress adaptation in cows—Physiological responses and underlying molecular mechanisms. J. Therm. Biol. 2023, 118, 103740. [Google Scholar] [CrossRef]
- Park, T.; Ma, L.; Gao, S.; Bu, D.; Yu, Z. Heat stress impacts the multi-domain ruminal microbiota and some of the functional features independent of its effect on feed intake in lactating dairy cows. J. Anim. Sci. Biotechnol. 2022, 13, 71. [Google Scholar] [CrossRef]
- Ceciliani, F.; Maggiolino, A.; Biscarini, F.; Dadi, Y.; De Matos, L.; Cremonesi, P.; Landi, V.; De Palo, P.; Lecchi, C. Heat stress has divergent effects on the milk microbiota of Holstein and Brown Swiss cows. J. Dairy Sci. 2024, 107, 11639–11654. [Google Scholar] [CrossRef]
- Feldsine, P.; Abeyta, C.; Andrews, W.H. AOAC INTERNATIONAL Methods Committee guidelines for validation of qualitative and quantitative food microbiological official methods of analysis. J. AOAC Int. 2002, 85, 1187–1200. [Google Scholar] [CrossRef] [PubMed]
- Benni, S.; Pastell, M.; Bonora, F.; Tassinari, P.; Torreggiani, D. A generalised additive model to characterise dairy cows’ responses to heat stress. Animal 2020, 14, 418–424. [Google Scholar] [CrossRef]
- Dado-Senn, B.; Ouellet, V.; Lantigua, V.; Van Os, J.; Laporta, J. Methods for detecting heat stress in hutch-housed dairy calves in a continental climate. J. Dairy Sci. 2023, 106, 1039–1050. [Google Scholar] [CrossRef]
- Ul Umar, S.I.; Konwar, D.; Khan, A.; Bhat, M.A.; Javid, F.; Jeelani, R.; Nabi, B.; Najar, A.A.; Kumar, D.; Brahma, B. Delineation of temperature-humidity index (THI) as indicator of heat stress in riverine buffaloes (Bubalus bubalis) of a sub-tropical Indian region. Cell Stress Chaperones 2021, 26, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mehrotra, S.; Singh, G.; Narayanan, K.; Das, G.K.; Soni, Y.K.; Singh, M.; Mahla, A.S.; Srivastava, N.; Verma, M.R. Sustained delivery of exogenous melatonin influences biomarkers of oxidative stress and total antioxidant capacity in summer-stressed anestrous water buffalo (Bubalus bubalis). Theriogenology 2015, 83, 1402–1407. [Google Scholar] [CrossRef]
- Tao, S.; Thompson, I.M.; Monteiro, A.P.A.; Hayen, M.J.; Young, L.J.; Dahl, G.E. Effect of cooling heat-stressed dairy cows during the dry period on insulin response. J. Dairy Sci. 2012, 95, 5035–5046. [Google Scholar] [CrossRef]
- Ungerfeld, E.M. Shifts in metabolic hydrogen sinks in the methanogenesis-inhibited ruminal fermentation: A meta-analysis. Front. Microbiol. 2015, 6, 37. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Chen, Y.X.; Wang, Z.C.; Li, Z.P.; Ahmad, M.J.; Li, M.; Gao, T.Y.; Liu, S.H. The Effect of Exogenous Melatonin on Milk Somatic Cell Count in Buffalo. Pak. Vet. J. 2021, 41, 152–155. [Google Scholar] [CrossRef]
- Fabris, T.F.; Laporta, J.; Skibiel, A.L.; Corra, F.N.; Senn, B.D.; Wohigemuth, S.E.; Dahl, G.E. Effect of heat stress during early, late, and entire dry period on dairy cattle. J. Dairy Sci. 2019, 102, 5647–5656. [Google Scholar] [CrossRef]
- Marai, I.F.M.; Haeeb, A.A.M. Buffalo’s biological functions as affected by heat stress—A review. Livest. Sci. 2010, 127, 89–109. [Google Scholar] [CrossRef]
- Beecher, M.; Buckley, F.; Waters, S.M.; Boland, T.N.; Enriquez-Hidalgo, D.; Deighton, M.H.; O’Donovan, M.; Lewis, E. Gastrointestinal tract size, total-tract digestibility, and rumen microflora in different dairy cow genotypes. J. Dairy Sci. 2014, 97, 3906–3917. [Google Scholar] [CrossRef] [PubMed]
- Mehaba, N.; Coloma-Garcia, W.; Such, X.; Caja, G.; Salama, A.A.K. Heat stress affects some physiological and productive variables and alters metabolism in dairy ewes. J. Dairy Sci. 2021, 104, 1099–1110. [Google Scholar] [CrossRef]
- El-Tahawy, A.S.; El-Far, A.H. Influences of somatic cell count on milk composition and dairy farm profitability. Int. J. Dairy Technol. 2010, 63, 463–469. [Google Scholar] [CrossRef]
- Li, Y.; Fang, L.Y.; Xue, F.G.; Mao, S.Y.; Xiong, B.H.; Ma, Z.; Jiang, L.S. Effects of bamboo leaf extract on the production performance, rumen fermentation parameters, and rumen bacterial communities of heat-stressed dairy cows. Anim. Biosci. 2021, 34, 1784–1793. [Google Scholar] [CrossRef] [PubMed]
- Nanas, I.; Chouzouris, T.M.; Dadouli, K.; Dovolou, E.; Stamperna, K.; Barbagianni, M.; Valasi, I.; Tsiaras, A.; Amiridis, G.S. A study on stress response and fertility parameters in phenotypically thermotolerant and thermosensitive dairy cows during summer heat stress. Reprod. Domest. Anim. 2020, 55, 1774–1783. [Google Scholar] [CrossRef]
- Hassan, F.; Tang, Z.; Ebeid, H.M.; Li, M.; Peng, K.; Liang, X.; Yang, C. Consequences of herbal mixture supplementation on milk performance, ruminal fermentation, and bacterial diversity in water buffaloes. PeerJ 2021, 9, e11241. [Google Scholar] [CrossRef] [PubMed]
- Zou, C.X.; Gu, Q.C.; Zhou, X.K.; Xia, Z.S.; Muhammad, W.I.; Tang, Q.F.; Liang, M.Z.; Lin, B.; Qin, G.S. Ruminal microbiota composition associated with ruminal fermentation parameters and milk yield in lactating buffalo in Guangxi, China-A preliminary study. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1374–1379. [Google Scholar] [CrossRef]
- McAllister, T.A.; Newbold, C.J. Redirecting rumen fermentation to reduce methanogenesis. Aust. J. Exp. Agric. 2008, 48, 7–13. [Google Scholar] [CrossRef]
- Saleem, F.; Bouatra, S.; Guo, A.C.; Psychogios, N.; Mandal, R.; Dunn, S.M.; Ametaj, B.N.; Wishart, D.S. The Bovine Ruminal Fluid Metabolome. Metabolomics 2013, 9, 360–378. [Google Scholar] [CrossRef]
- Zhou, R.; Wu, J.; Lang, X.; Liu, L.; Casper, D.P.; Wang, C.; Zhang, L.; Wei, S. Effects of oregano essential oil on in vitro ruminal fermentation, methane production, and ruminal microbial community. J. Dairy Sci. 2020, 103, 2303–2314. [Google Scholar] [CrossRef]
- Chen, S.Y.; Wang, J.; Peng, D.D.; Li, G.; Chen, J.; Gu, X.H. Exposure to heat-stress environment affects the physiology, circulation levels of cytokines, and microbiome in dairy COWS. Sci. Rep. 2018, 8, 14606. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.Z.; Zhang, Y.D.; Yu, Z.T.; Xu, Q.B.; Zheng, N.; Zhao, S.G.; Huang, G.X.; Wang, J.Q. Ruminal microbiota-host interaction and its effect on nutrient metabolism. Anim. Nutr. 2021, 7, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Park, T.; Ma, L.; Ma, Y.; Zhou, X.Q.; Bu, D.P.; Yu, Z.T. Dietary energy sources and levels shift the multi-kingdom microbiota and functions in the rumen of lactating dairy cows. J. Anim. Sci. Biotechnol. 2020, 11, 66. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.Q.Q.; Xi, Y.M.M.; Wang, Z.D.D.; Zeng, H.F.F.; Han, Z.Y. Combined signature of rumen microbiome and metabolome in dairy cows with different feed intake levels. J. Anim. Sci. 2020, 98, skaa070. [Google Scholar] [CrossRef]
- Emerson, E.L.; Weimer, P.J. Fermentation of model hemicelluloses by Prevotella strains and Butyrivibrio fibrisolvens in pure culture and in ruminal enrichment cultures. Appl. Microbiol. Biotechnol. 2017, 101, 4269–4278. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, L.; Pang, F.; Zheng, Z.; Teng, Z.; Miao, T.; Fu, T.; Rushdi, H.E.; Yang, L.; Gao, T.; et al. Novel insights into heat tolerance using metabolomic and high-throughput sequencing analysis in dairy cows rumen fluid. Animal 2022, 16, 100478. [Google Scholar] [CrossRef]
- Ma, Z.Z.; Cheng, Y.Y.; Wang, S.Q.; Ge, J.Z.; Shi, H.P.; Kou, J.C. Positive effects of dietary supplementation of three probiotics on milk yield, milk composition and intestinal flora in Sannan dairy goats varied in kind of probiotics. J. Anim. Physiol. Anim. Nutr. 2020, 104, 44–55. [Google Scholar] [CrossRef]
- Zhan, J.S.; Liu, M.M.; Wu, C.X.; Su, X.S.; Zhan, K.; Zhao, G.Q. Effects of alfalfa flavonoids extract on the microbial flora of dairy cow rumen. Asian-Australas. J. Anim. Sci. 2017, 30, 1261–1269. [Google Scholar] [CrossRef]
- Boutard, M.; Cerisy, T.; Nogue, P.Y.; Alberti, A.; Weissenbach, J.; Salanoubat, M.; Tolonen, A.C. Functional Diversity of Carbohydrate-Active Enzymes Enabling a Bacterium to Ferment Plant Biomass. PLoS Genet. 2014, 10, e1004773. [Google Scholar] [CrossRef]
- Beimforde, C.; Feldberg, K.; Nylinder, S.; Rikkinen, J.; Tuovila, H.; Dorfelt, H.; Gube, M.; Jackson, D.J.; Reitner, J.; Seyfullah, L.J.; et al. Estimating the Phanerozoic history of the Ascomycota lineages: Combining fossil and molecular data. Mol. Phylogenet. Evol. 2014, 78, 386–398. [Google Scholar] [CrossRef]
- Zhao, C.C.; Wang, L.M.; Ke, S.L.; Chen, X.H.; Kenez, A.; Xu, W.; Wang, D.D.; Zhang, F.; Li, Y.; Cui, Z.H.; et al. Yak rumen microbiome elevates fiber degradation ability and alters rumen fermentation pattern to increase feed efficiency. Anim. Nutr. 2022, 11, 201–214. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Bello-Perez, E.; Cancino-Padilla, N.; Romero, J. Technical note: Use of internal transcribed spacer for ruminal yeast identification in dairy cows. Animal 2016, 10, 1949–1954. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, P.; Liu, X.; Zhang, C.; Lu, Q.; Xi, D.; Yang, R.; Wang, S.; Bai, W.; Yang, Z.; et al. The Composition of Fungal Communities in the Rumen of Gayals (Bos frontalis), Yaks (Bos grunniens), and Yunnan and Tibetan Yellow Cattle (Bos taurs). Pol. J. Microbiol. 2019, 68, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Steenwyk, J.L.; Lind, A.L.; Ries, L.N.A.; Dos Reis, T.F.; Silva, L.P.; Almeida, F.; Bastos, R.W.; Fraga da Silva, T.F.C.; Bonato, V.L.D.; Pessoni, A.M.; et al. Pathogenic Allodiploid Hybrids of Aspergillus Fungi. Curr. Biol. 2020, 30, 2495–2507.e7. [Google Scholar] [CrossRef]
- Pratt, C.J.; Meili, C.H.; Jones, A.L.; Jackson, D.K.; England, E.E.; Wang, Y.; Hartson, S.; Rogers, J.; Elshahed, M.S.; Youssef, N.H. Anaerobic fungi in the tortoise alimentary tract illuminate early stages of host-fungal symbiosis and Neocallimastigomycota evolution. Nat. Commun. 2024, 15, 2714. [Google Scholar] [CrossRef]
- Butkovich, L.V.; Leggieri, P.A.; Lillington, S.P.; Navaratna, T.A.; Swift, C.L.; Malinov, N.G.; Zalunardo, T.R.; Vining, O.B.; Lipzen, A.; Wang, M.; et al. Separation of life stages within anaerobic fungi (Neocallimastigomycota) highlights differences in global transcription and metabolism. Fungal Genet. Biol. 2025, 176, 103958. [Google Scholar] [CrossRef]
- Talhi, I.; Dehimat, L.; Jaouani, A.; Cherfia, R.; Berkani, M.; Almomani, F.; Vasseghian, Y.; Chaouche, N.K. Optimization of thermostable proteases production under agro-wastes solid-state fermentation by a new thermophilic Mycothermus thermophilus isolated from a hydrothermal spring Hammam Debagh, Algeria. Chemosphere 2022, 286, 131479. [Google Scholar] [CrossRef]
- Fang, C.; Su, Y.; He, X.; Han, L.; Qu, H.; Zhou, L.; Huang, G. Membrane-covered composting significantly decreases methane emissions and microbial pathogens: Insight into the succession of bacterial and fungal communities. Sci. Total Environ. 2022, 845, 157343. [Google Scholar] [CrossRef]

| Ingredients | Content | Nutrient Levels | Content |
|---|---|---|---|
| Corm silage | 41.20 | NEL (MJ/kg) | 6.36 |
| Alfalfa hay | 8.24 | CP | 18.47 |
| Wheat straw | 4.14 | EE | 4.85 |
| Beer slack | 20.60 | NDF | 33.39 |
| Concentrate supplement 1 | 24.42 | ADF | 18.97 |
| NaCl | 0.55 | Ca | 0.95 |
| Na2CO3 | 0.85 | P | 0.50 |
| Total | 100.00 |
| Index | Thermo-Neutral | Heat Stress | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Holstein Jersey SEM | Holstein | Jersey | SEM | Breed | State | Breed × State | |||
| Respiratory rate, bpm | 39.70 | 38.38 | 3.06 | 66.13 A | 65.42 A | 6.29 | 0.812 | 0.001 | 0.692 |
| 37.93 | 37.54 | 0.16 | 38.62 Aa | 38.12 | 0.22 | 0.009 | <0.001 | 0.560 | |
| Dry matter intake, kg/d | 14.76 | 11.70 | 1.33 | 13.90 | 11.02 | 0.84 | 0.060 | 0.126 | 0.609 |
| Milk yield, kg/d | 26.06 A | 20.00 A | 3.11 | 21.26 | 16.94 | 2.15 | 0.054 | 0.014 | 0.651 |
| Protein, % | 3.63 | 4.31 Aa | 0.26 | 3.28 | 3.99 a | 0.32 | 0.001 | 0.882 | 0.749 |
| Fat, % | 5.12 | 7.65 a | 0.93 | 4.81 | 7.38 a | 0.91 | 0.014 | 0.007 | 0.803 |
| SCS | 3.70 | 4.36 | 1.10 | 3.51 | 4.65 | 0.83 | 0.230 | 0.940 | 0.744 |
| Index | Thermo-Neutral | Heat Stress | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Holstein Jersey SEM | Holstein | Jersey | SEM | Breed | State | Breed × State | |||
| pH | 6.72 | 6.66 | 0.32 | 6.59 | 6.59 | 0.75 | 0.925 | 0.686 | 0.917 |
| Acetate, mmol/L | 55.55 | 59.84 A | 9.37 | 51.18 a | 37.64 | 5.18 | 0.402 | 0.026 | 0.118 |
| Propionate, mmol/L | 15.26 | 15.17 A | 1.97 | 15.53 a | 10.5 | 1.60 | 0.064 | 0.104 | 0.072 |
| Butyrate, mmol/L | 6.69 | 6.90 A | 1.22 | 8.07 a | 3.89 | 0.80 | 0.017 | 0.283 | 0.009 |
| Valerate, mmol/L | 0.76 | 0.69 A | 0.09 | 0.80 a | 0.52 | 0.12 | 0.032 | 0.364 | 0.170 |
| A/P | 3.59 | 3.97 | 0.29 | 3.46 | 3.65 | 0.35 | 0.238 | 0.364 | 0.670 |
| TVFA, mmol/L | 79.65 | 83.89 A | 12.52 | 78.33 a | 53.72 | 6.84 | 0.175 | 0.044 | 0.063 |
| Index | Thermo-Neutral | Heat Stress | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Holstein | Jersey | SEM | Holstein | Jersey | SEM | Breed | State | Breed × State | |
| 16S rRNA gene | |||||||||
| Goods coverage | 0.96 | 0.98 | 0.00 | 0.98 A | 0.98 | 0.00 | 0.044 | <0.001 | 0.051 |
| Chao1 | 8147.61 A | 7834.82 A | 268.55 | 4428.32 | 4490.99 | 147.84 | 0.427 | <0.001 | 0.238 |
| Simpson indices | 0.99 | 0.99 | 0.00 | 1.00 | 1.00 | 0.00 | 0.240 | 0.137 | 0.193 |
| Shannon | 10.04 A | 10.16 A | 0.47 | 9.37 | 9.47 | 0.31 | 0.564 | 0.002 | 0.958 |
| Observed species | 6000.60 Aa | 5362.12 A | 470.72 | 3555.28 | 3551.58 | 200.73 | 0.024 | 0.421 | 0.001 |
| ITS | |||||||||
| Goods coverage | 0.99 | 0.99 Aa | 0.00 | 0.99 A | 0.99 | 0.00 | 0.004 | <0.001 | 0.180 |
| Chao1 | 417.17 | 690.77 a | 19.34 | 501.36 | 412.41 A | 80.60 | 0.041 | 0.032 | <0.001 |
| Simpson indices | 0.84 | 0.98 a | 0.01 | 0.94 A | 0.93 A | 0.02 | <0.001 | 0.065 | <0.001 |
| Shannon | 4.09 | 7.26 Aa | 0.68 | 5.97 A | 5.52 | 0.60 | <0.001 | 0.727 | <0.001 |
| Observed species | 343.14 | 599.44 Aa | 136.90 | 473.70 | 399.20 | 127.52 | 0.043 | 0.412 | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Guo, M.; Liang, Y.; Zhou, F.; Zhang, H.; Li, M.; Yang, Z.; Karrow, N.; Mao, Y. Breed-Specific Responses and Ruminal Microbiome Shifts in Dairy Cows Under Heat Stress. Animals 2025, 15, 817. https://doi.org/10.3390/ani15060817
Wang Z, Guo M, Liang Y, Zhou F, Zhang H, Li M, Yang Z, Karrow N, Mao Y. Breed-Specific Responses and Ruminal Microbiome Shifts in Dairy Cows Under Heat Stress. Animals. 2025; 15(6):817. https://doi.org/10.3390/ani15060817
Chicago/Turabian StyleWang, Zichen, Mengling Guo, Yan Liang, Fuzhen Zhou, Huiming Zhang, Mingxun Li, Zhangping Yang, Niel Karrow, and Yongjiang Mao. 2025. "Breed-Specific Responses and Ruminal Microbiome Shifts in Dairy Cows Under Heat Stress" Animals 15, no. 6: 817. https://doi.org/10.3390/ani15060817
APA StyleWang, Z., Guo, M., Liang, Y., Zhou, F., Zhang, H., Li, M., Yang, Z., Karrow, N., & Mao, Y. (2025). Breed-Specific Responses and Ruminal Microbiome Shifts in Dairy Cows Under Heat Stress. Animals, 15(6), 817. https://doi.org/10.3390/ani15060817





