Comparative Effects of Dietary Supplementations with Microencapsulated Sodium Butyrate, Glycerol Monolaurate and Tributyrin on Growth, Immunity, and Gut Health in Black Sea Bream
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Formulation and Composition of Diets
2.2. Animal Management, Experimental Location and Parameters
2.3. Sample Collection
2.4. Sample Analytical Methods
2.5. Digestive Enzyme Activity
2.6. Serum Parameters
2.7. Intestinal Histology
2.7.1. H&E Staining Sample
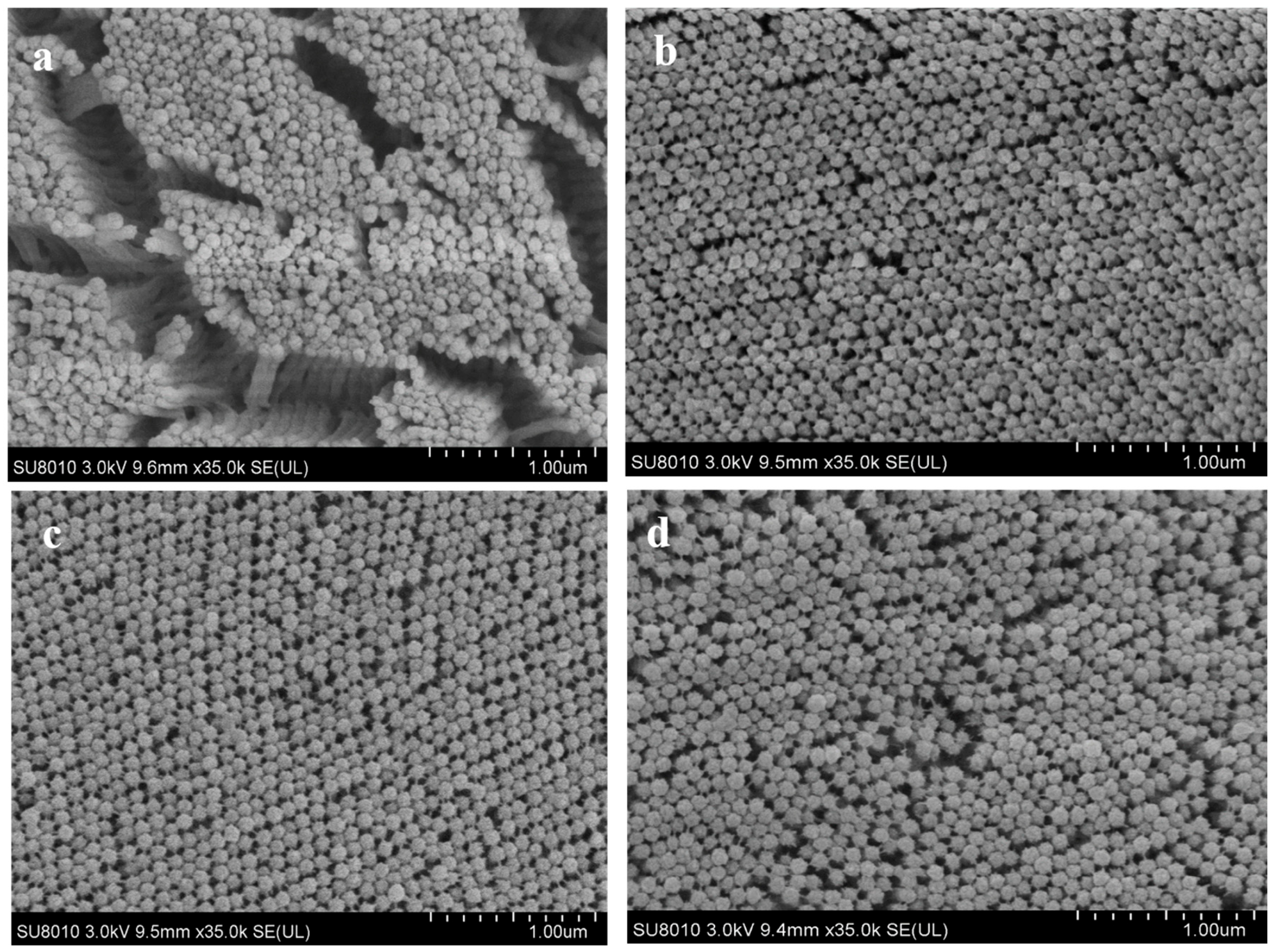
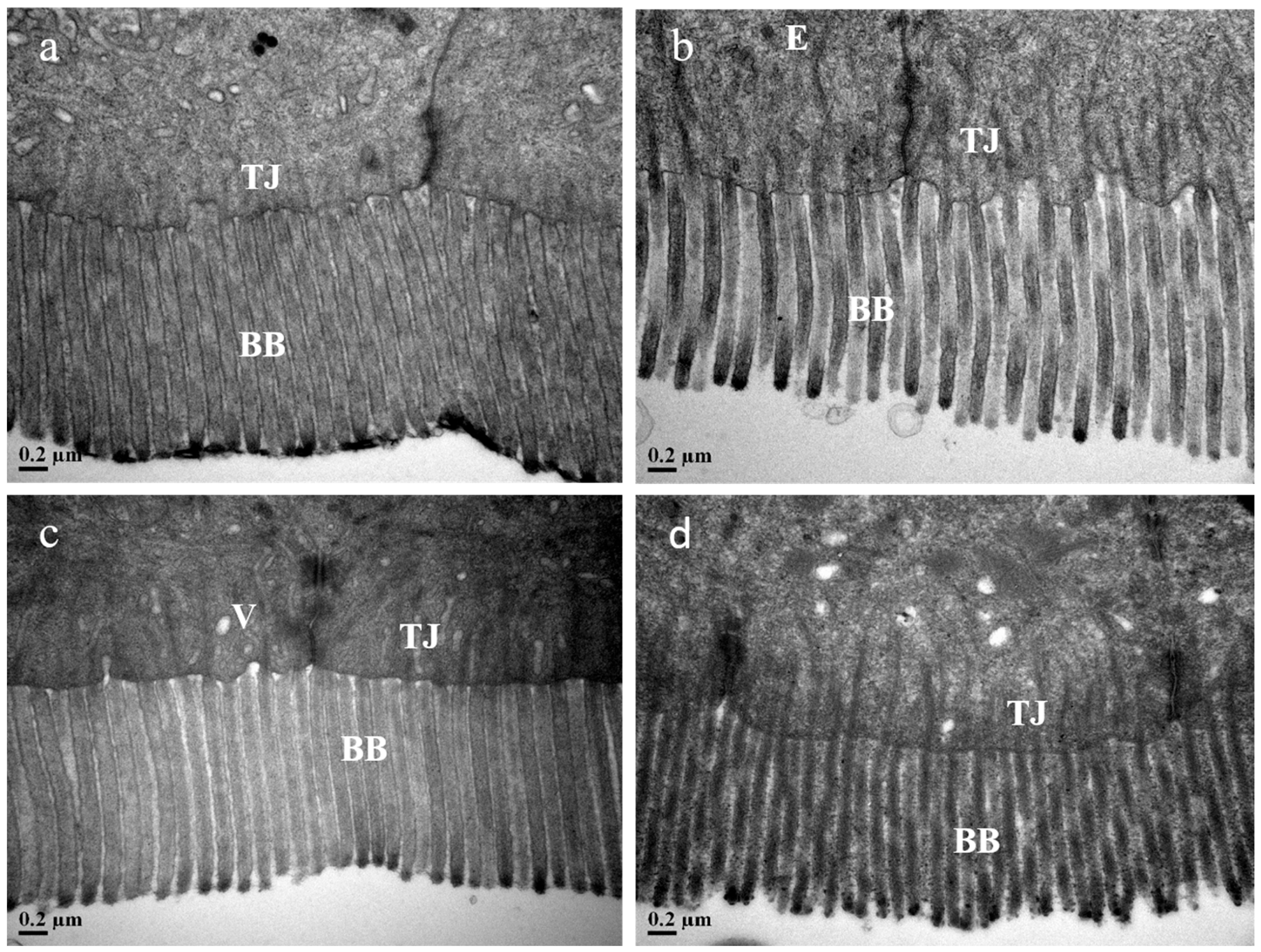
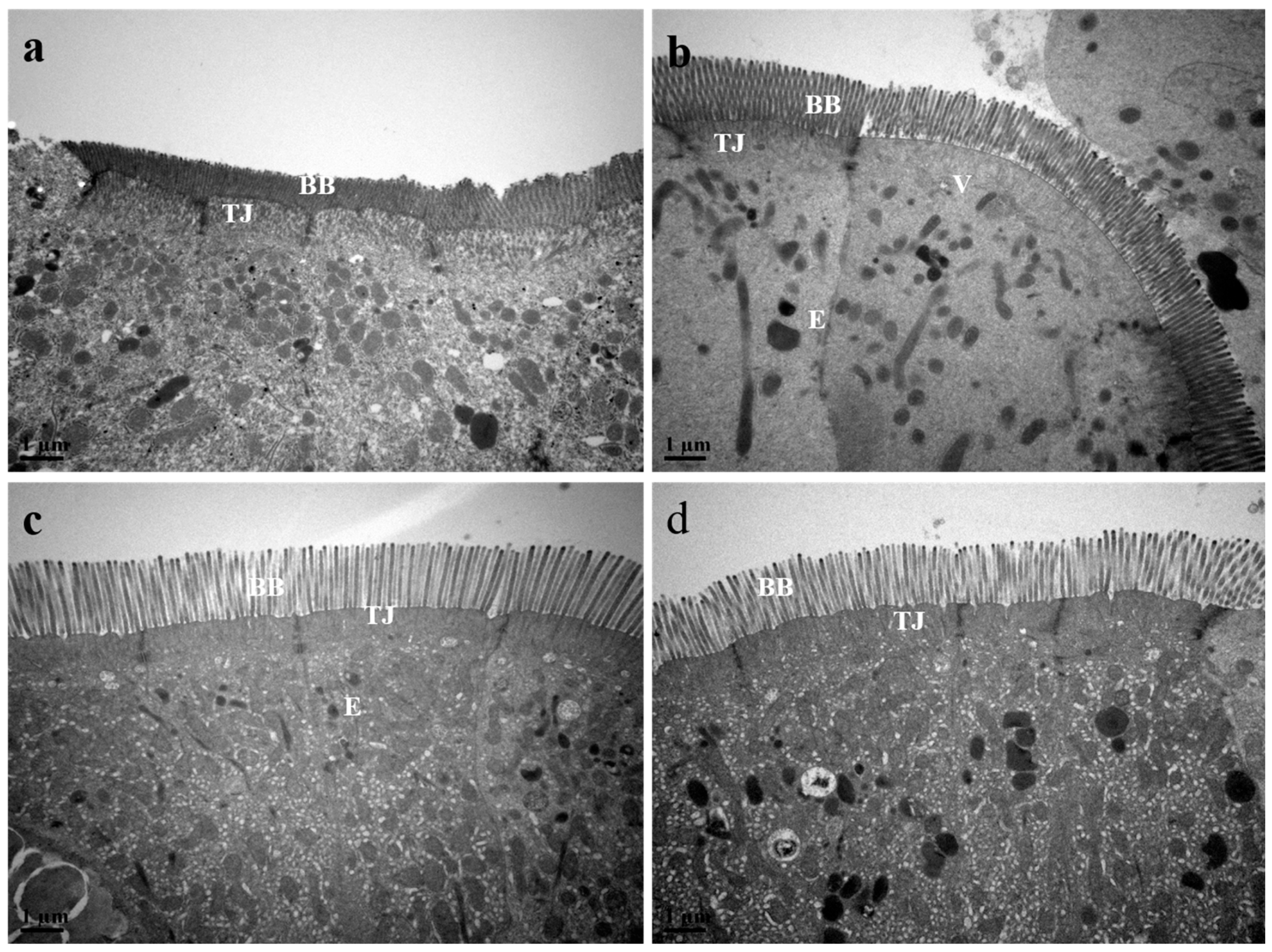
2.7.2. Scanning Electron Microscopy (SEM) Examination
2.8. Analysis of Gene Expression
RNA Extraction and Quantitative Real-Time PCR Analysis (qRT-PCR)
2.9. Microbiota Analysis
2.10. Statistical Analysis and Calculation
Final average body weight (FBW, g).
Weight gain rate (WGR, %) = 100 × (final body weight − initial bodyweight)/initial body weight
Specific growth rate (SGR, %/day) = 100 × (ln final body weight − ln initial body weight)/days.
Mean feed intake (MFI, g fish−1 d−1) = air dry fed in g/(fish in g × day)
Feed conversion ratio (FCR) = dry feed weight (g)/wet weight gain (g)
Protein efficiency ratio (PER) = wet weight gain (g)/total protein intake (g).
Condition factor (CF, g cm−3) = 100 × [(final body weight in g)/(final body length in cm)3]
Hepatosomatic index (HSI, %) = 100 × (liver weight in g/body weight in g).
Intraperitoneal fat ratio (IPR %) = 100 × (intraperitoneal fat weight in g/body weight in g).
Viscerosomatic index (VSI, %) = 100 × (viscera weight/body weight).
Protein productive value (PPV, %) = 100 × protein gain (g)/total protein intake (g).
Survival rate (SR, %) = 100 × (final fish number/initial fish number)
3. Results
3.1. Growth Performance and Feed Utilization
3.2. Composition of Whole Body and Dorsal Muscle
3.3. Digestive Enzyme Activity
3.4. Serum Biochemical, Immune and Antioxidant Parameters
3.5. Biochemical, Immune, and Antioxidant Parameters in the Hindgut and Midgut
3.6. Biochemical, Immune, and Antioxidant Parameters in the Liver
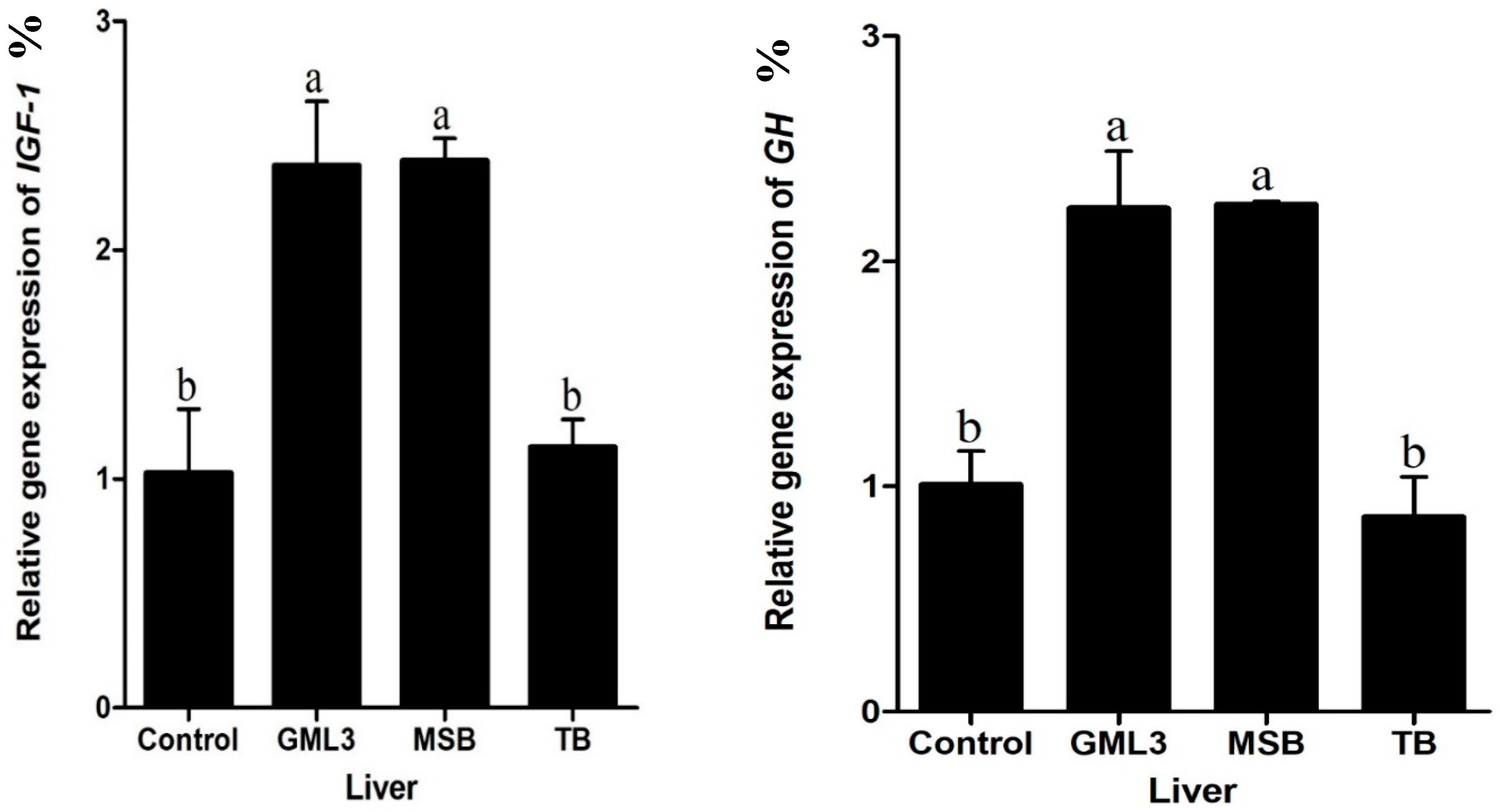
3.7. Liver Gene Expression
3.8. Intestinal Gene Expression
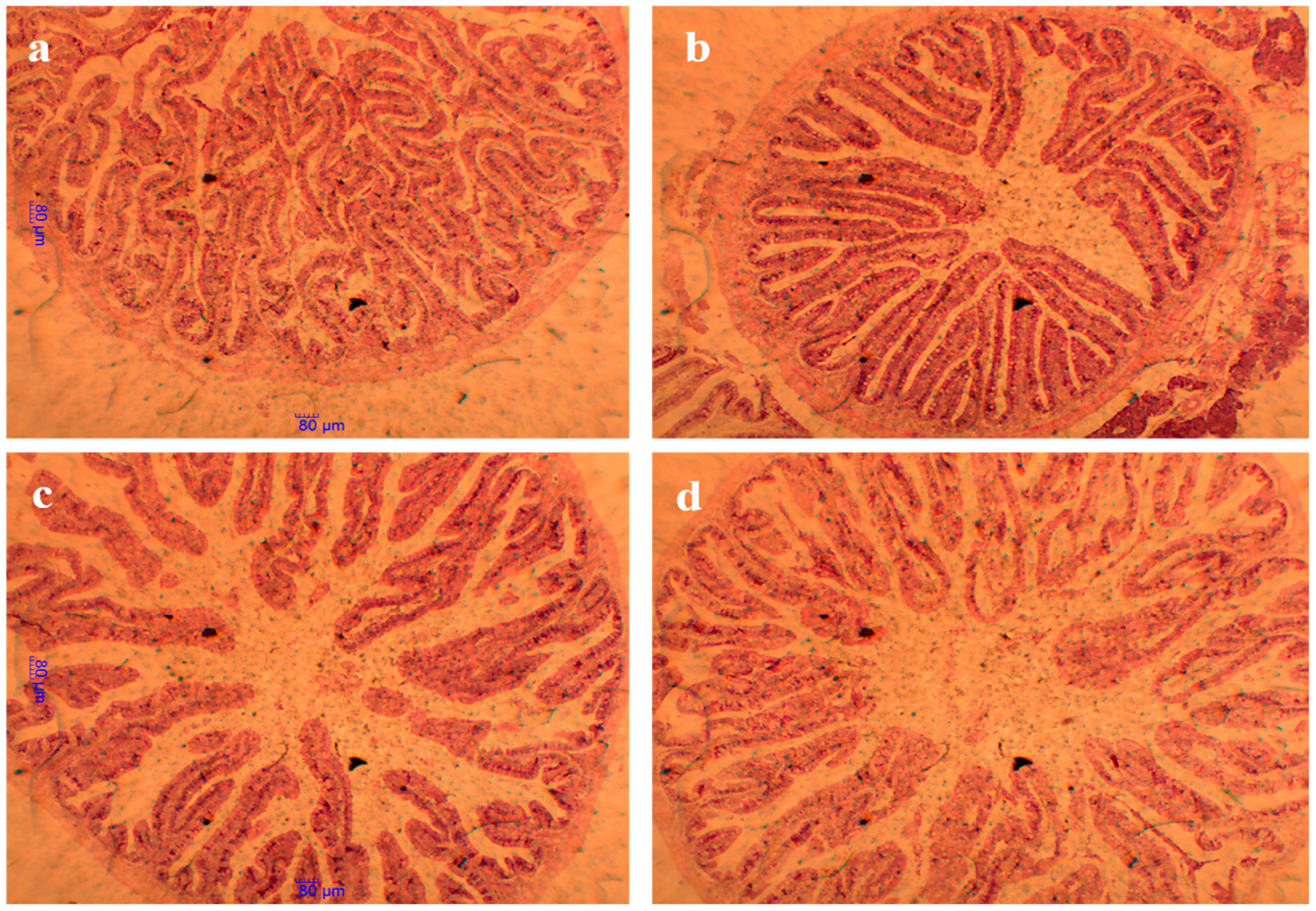
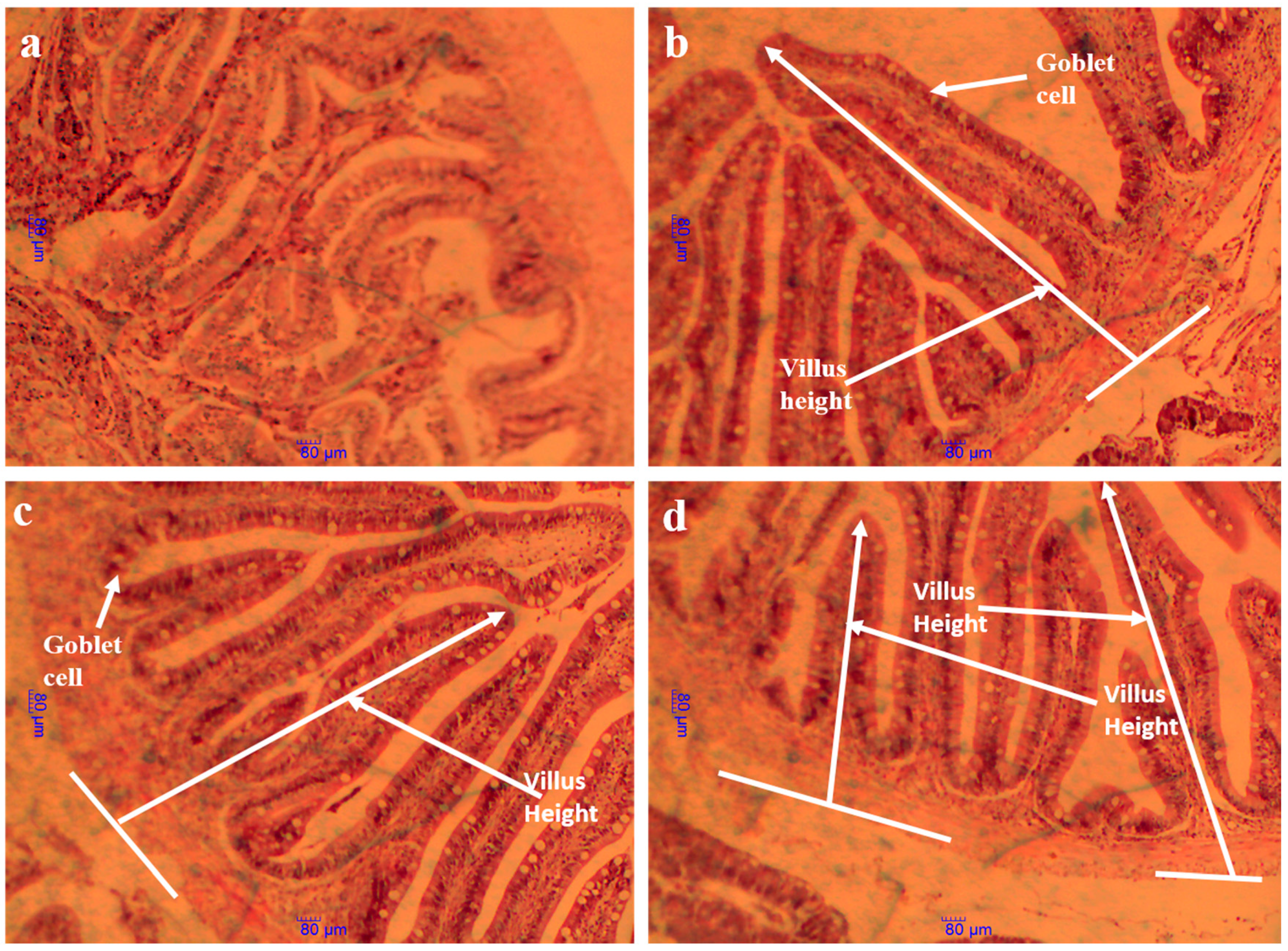
3.9. Intestinal Histology Structure and Morphometric Parameters
3.10. Intestine Histological Structure
3.11. Analysis of the Composition and Relative Abundance of the Microbial Community
3.11.1. Overview of Sequencing Data for Intestinal Microbiota
3.11.2. Alpha Diversity and Coverage Analysis of the Intestinal Microbiota
3.11.3. Phylum Level
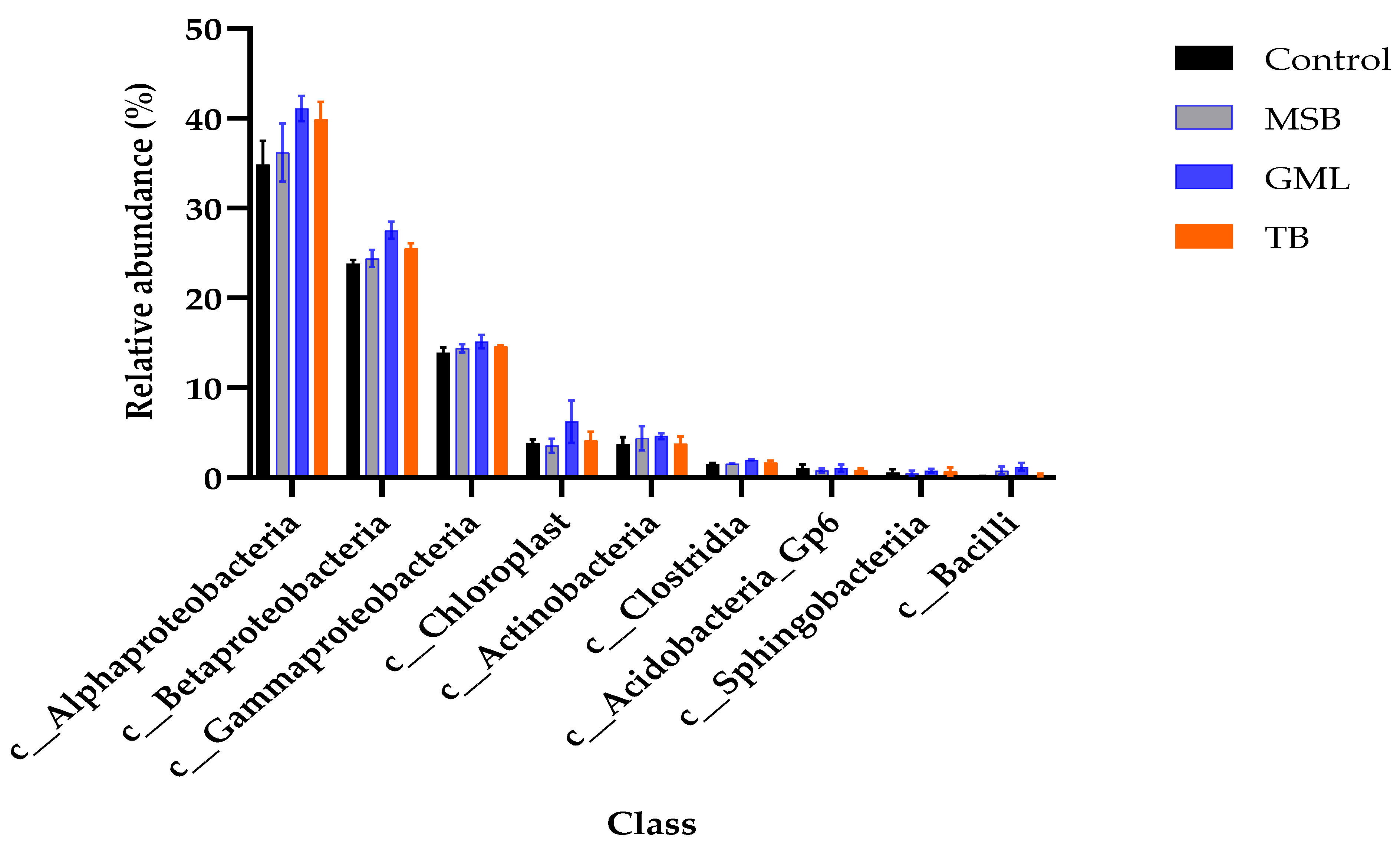
3.11.4. Class Level
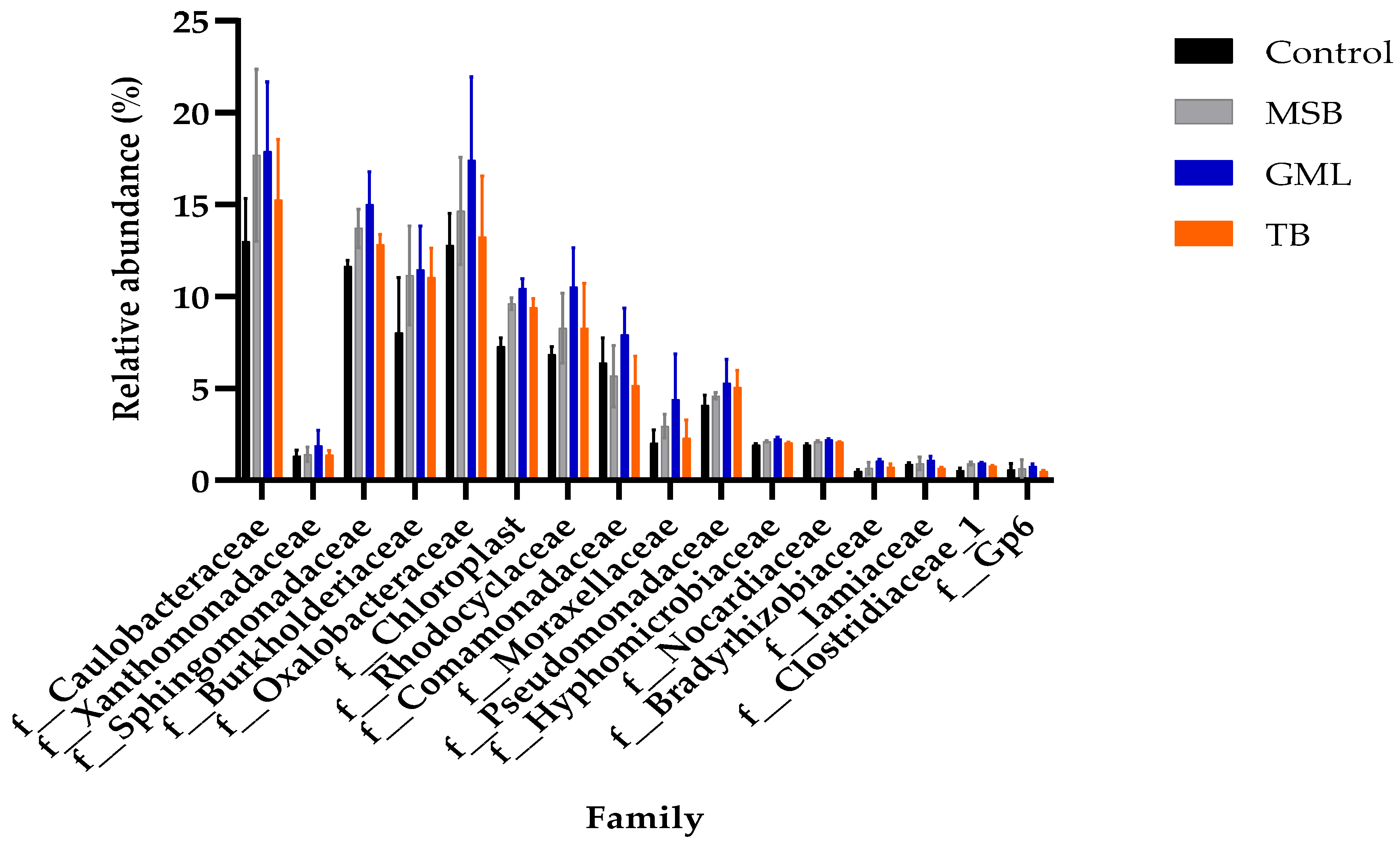
3.11.5. Family Level
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sofia, F. The State of World Fisheries and Aquaculture 2018-Meeting the Sustainable Development Goals; Fisheries and Aquaculture Department, Food and Agriculture Organization of the United Nations: Rome, Italy, 2018. [Google Scholar]
- FAO. The State of World Fisheries and Aquaculture. 2024. Available online: https://openknowledge.fao.org/handle/20.500.14283/cd0683en (accessed on 7 February 2025).
- Hague, M. The Economic and Feasibility of Aquaculture in Northern Tanzania. Bachelor’s Dissertation, University of Stirling, Stirling, UK, 1992. [Google Scholar]
- Gatlin, D.M. Dietary Supplements for the Health and Quality of Cultured Fish; Cabi: Wallingford, UK, 2007. [Google Scholar]
- Jena, J.; Aravindakshan, P.; Chandra, S.; Muduli, H.; Ayyappan, S. Comparative evaluation of growth and survival of Indian major carps and exotic carps in raising fingerlings. J. Aquac. Trop. 1998, 13, 143–149. [Google Scholar]
- Enes, P.; Panserat, S.; Kaushik, S.; Oliva-Teles, A. Effect of normal and waxy maize starch on growth, food utilization and hepatic glucose metabolism in European sea bass (Dicentrarchus labrax) juveniles. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2006, 143, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. Fatty acids, inflammation and intestinal health in pigs. J. Anim. Sci. Biotechnol. 2015, 6, 41. [Google Scholar] [CrossRef] [PubMed]
- Leeson, S.; Namkung, H.; Antongiovanni, M.; Lee, E. Effect of butyric acid on the performance and carcass yield of broiler chickens. Poult. Sci. 2005, 84, 1418–1422. [Google Scholar] [CrossRef]
- Scheppach, W. Effects of short chain fatty acids on gut morphology and function. Gut 1994, 35, S35–S38. [Google Scholar] [CrossRef]
- Bugaut, M. Occurrence, absorption and metabolism of short chain fatty acids in the digestive tract of mammals. Comp. Biochem. Physiol. Part B Comp. Biochem. 1987, 86, 439–472. [Google Scholar] [CrossRef]
- Załęski, A.; Banaszkiewicz, A.; Walkowiak, J. Butyric acid in irritable bowel syndrome. Prz. Gastroenterol. 2013, 8, 350–353. [Google Scholar] [CrossRef]
- Snel, J.; Harmsen, H.; Van der Wielen, P.; Williams, B. Dietary strategies to influence the gastrointestinal microflora of young animals, and its potential to improve intestinal health. Nutr. Health Gastrointest. Tract 2002, 37–69. [Google Scholar] [CrossRef]
- Guilloteau, P.; Zabielski, R.; David, J.-C.; Blum, J.W.; Morisset, J.A.; Biernat, M.; Woliński, J.; Laubitz, D.; Hamon, Y. Sodium-butyrate as a growth promoter in milk replacer formula for young calves. J. Dairy Sci. 2009, 92, 1038–1049. [Google Scholar] [CrossRef]
- Ma, X.; Fan, P.X.; Li, L.; Qiao, S.; Zhang, G.; Li, D. Butyrate promotes the recovering of intestinal wound healing through its positive effect on the tight junctions. J. Anim. Sci. 2012, 90, 266–268. [Google Scholar] [CrossRef]
- Liu, W.; Yang, Y.; Zhang, J.; Gatlin, D.M.; Ringo, E.; Zhou, Z. Effects of dietary microencapsulated sodium butyrate on growth, intestinal mucosal morphology, immune response and adhesive bacteria in juvenile common carp (Cyprinus carpio) pre-fed with or without oxidised oil. Br. J. Nutr. 2014, 112, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Sadek, K. Impact of dietary supplementation of sodium butyrate and/or protexin on the growth performance, some blood parameters, and immune response of Oreochromis niloticus. Int. J. Agric. Innov. Res. 2015, 3, 7. [Google Scholar]
- Wu, Y.; Zhou, Y.; Lu, C.; Ahmad, H.; Zhang, H.; He, J.; Zhang, L.; Wang, T. Influence of butyrate loaded clinoptilolite dietary supplementation on growth performance, development of intestine and antioxidant capacity in broiler chickens. PLoS ONE 2016, 11, e0154410. [Google Scholar] [CrossRef] [PubMed]
- Yoo, C.B.; Jones, P.A. Epigenetic therapy of cancer: Past, present and future. Nat. Rev. Drug Discov. 2006, 5, 37–50. [Google Scholar] [CrossRef]
- Thompson, J.L.; Hinton, M. Antibacterial activity of formic and propionic acids in the diet of hens on Salmonellas in the crop. Br. Poult. Sci. 1997, 38, 59–65. [Google Scholar] [CrossRef]
- Song, B.; Li, H.; Wu, Y.; Zhen, W.; Wang, Z.; Xia, Z.; Guo, Y. Effect of microencapsulated sodium butyrate dietary supplementation on growth performance and intestinal barrier function of broiler chickens infected with necrotic enteritis. Anim. Feed Sci. Technol. 2017, 232, 6–15. [Google Scholar] [CrossRef]
- Han, Y.; Tang, C.; Zhao, Q.; Zhan, T.; Zhang, K.; Han, Y.; Zhang, J. Effects of dietary supplementation with combinations of organic and medium chain fatty acids as replacements for chlortetracycline on growth performance, serum immunity, and fecal microbiota of weaned piglets. J. Livest. Sci. 2018, 216, 210–218. [Google Scholar] [CrossRef]
- Zentek, J.; Buchheit-Renko, S.; Ferrara, F.; Vahjen, W.; Van Kessel, A.; Pieper, R. Nutritional and physiological role of medium-chain triglycerides and medium-chain fatty acids in piglets. Anim. Health Res. Rev. 2011, 12, 83–93. [Google Scholar] [CrossRef]
- Wang, J.; Wang, X.; Li, J.; Chen, Y.; Yang, W.; Zhang, L. Effects of dietary coconut oil as a medium-chain fatty acid source on performance, carcass composition and serum lipids in male broilers. J Asian-Australas. J. Anim. Sci. 2015, 28, 223. [Google Scholar] [CrossRef]
- Bach, A.C.; Ingenbleek, Y.; Frey, A. The usefulness of dietary medium-chain triglycerides in body weight control: Fact or fancy? J. Lipid Res. 1996, 37, 708–726. [Google Scholar] [CrossRef]
- Li, Q.; Estes, J.D.; Schlievert, P.M.; Duan, L.; Brosnahan, A.J.; Southern, P.J.; Reilly, C.S.; Peterson, M.L.; Schultz-Darken, N.; Brunner, K.G. Glycerol monolaurate prevents mucosal SIV transmission. Nature 2009, 458, 1034–1038. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.S.; Sandouk, A.; Houtman, J.C. Glycerol Monolaurate (GML) inhibits human T cell signaling and function by disrupting lipid dynamics. Sci. Rep. 2016, 6, 30225. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, H.; Cui, Y.; Zhao, G.; Feng, F. Antibacterial interactions of monolaurin with commonly used antimicrobials and food components. J. Food Sci. 2009, 74, M418–M421. [Google Scholar] [CrossRef] [PubMed]
- Schlievert, P.; Deringer, J.R.; Kim, M.H.; Projan, S.J.; Novick, R. Effect of glycerol monolaurate on bacterial growth and toxin production. Antimicrob. Agents Chemother. 1992, 36, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Dierick, N.; Decuypere, J.; Degeyter, I. The combined use of whole Cuphea seeds containing medium chain fatty acids and an exogenous lipase in piglet nutrition. Arch. Anim. Nutr. 2003, 57, 49–63. [Google Scholar] [CrossRef]
- Clemente, J.C.; Ursell, L.K.; Parfrey, L.W.; Knight, R. The impact of the gut microbiota on human health: An integrative view. Cell 2012, 148, 1258–1270. [Google Scholar] [CrossRef]
- Heidor, R.; Festa Ortega, J.; de Conti, A.; Prates Ong, T.; Salvador Moreno, F. Anticarcinogenic actions of tributyrin, a butyric acid prodrug. Curr. Drug Targets 2012, 13, 1720–1729. [Google Scholar] [CrossRef]
- Hou, Y.; Wang, L.; Yi, D.; Ding, B.; Chen, X.; Wang, Q.; Zhu, H.; Liu, Y.; Yin, Y.; Gong, J. Dietary supplementation with tributyrin alleviates intestinal injury in piglets challenged with intrarectal administration of acetic acid. Br. J. Nutr. 2014, 111, 1748–1758. [Google Scholar] [CrossRef]
- Ullah, S.; Zhang, G.; Zhang, J.; Shengli, T.; Wang, L.; Kalhoro, H.; Chen, Y.; Volatiana, J.A.; Sagada, G.; Zheng, L.; et al. Effects of microencapsulated sodium butyrate supplementation on growth performance, intestinal development and antioxidative capacity of juvenile black sea bream (Acanthopagrus schlegelii). Aquac. Res. 2020, 51, 4893–4904. [Google Scholar] [CrossRef]
- Ullah, S.; Zhang, J.; Feng, F.; Shen, F.; Qiufen, M.; Wang, J.; Ur Rahman, T.; Haleem, A.; Zhao, M.; Shao, Q. Effect of Dietary Supplementation of Glycerol Monolaurate on Growth Performance, Digestive Enzymes, Serum Immune and Antioxidant Parameters, and Intestinal Morphology in Black Sea Bream. Animals 2024, 14, 2963. [Google Scholar] [CrossRef]
- Volatiana, J.A.; Wang, L.; Gray, N.; Tong, S.; Zhang, G.; Shao, Q. Tributyrin-supplemented high-soya bean meal diets of juvenile black sea bream, Acanthopagrus schlegelii: Study on growth performance and intestinal morphology and structure. Aquac. Res. 2020, 51, 135–146. [Google Scholar] [CrossRef]
- Shao, Q.; Ma, J.; Xu, Z.; Hu, W.; Xu, J.; Xie, S. Dietary phosphorus requirement of juvenile black seabream, Sparus macrocephalus. Aquaculture 2008, 277, 92–100. [Google Scholar] [CrossRef]
- Bureau, D.; Harris, A.; Cho, C. Apparent digestibility of rendered animal protein ingredients for rainbow trout (Oncorhynchus mykiss). Aquaculture 1999, 180, 345–358. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 16th ed.; Association of Official Analytical Chemists, AOAC: Arlington, VA, USA, 1995. [Google Scholar]
- Chang, H.Y.; Lin, T.H.; Anraku, K.; Shao, Y.T. The Effects of Continuous Acoustic Stress on ROS Levels and Antioxidant-related Gene Expression in the Black Porgy (Acanthopagrus schlegelii). Zool. Stud. 2018, 57, e59. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Odle, J. New Insights into the Utilization of Medium-Chain Triglycerides by the Neonate: Observations from a Piglet Model1,2. J. Nutr. 1997, 127, 1061–1067. [Google Scholar] [CrossRef]
- Wang, H.-B.; Wang, P.-Y.; Wang, X.; Wan, Y.-L.; Liu, Y.-C. Butyrate Enhances Intestinal Epithelial Barrier Function via Up-Regulation of Tight Junction Protein Claudin-1 Transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Anang, D.; Rusul, G.; Bakar, J.; Ling, F.H. Effects of lactic acid and lauricidin on the survival of Listeria monocytogenes, Salmonella enteritidis and Escherichia coli O157: H7 in chicken breast stored at 4 C. Food Control 2007, 18, 961–969. [Google Scholar] [CrossRef]
- Fortuoso, B.F.; dos Reis, J.H.; Gebert, R.R.; Barreta, M.; Griss, L.G.; Casagrande, R.A.; de Cristo, T.G.; Santiani, F.; Campigotto, G.; Rampazzo, L.; et al. Glycerol monolaurate in the diet of broiler chickens replacing conventional antimicrobials: Impact on health, performance and meat quality. Microb. Pathog. 2019, 129, 161–167. [Google Scholar] [CrossRef]
- Ng, W.; Koh, C. Application of organic acids in aquafeeds: Impacts on fish growth, nutrient utilization and disease resistance. In Standards for Acidifiers, Principles for the Use of Organic Acids in Animal Nutrition; Nottingham University Press: Nottingham, UK, 2011; pp. 49–58. [Google Scholar]
- Hong, S.M.; Hwang, J.H.; Kim, I.H. Effect of Medium-chain Triglyceride (MCT) on Growth Performance, Nutrient Digestibility, Blood Characteristics in Weanling Pigs. Asian-Australas. J. Anim. Sci. 2012, 25, 1003–1008. [Google Scholar] [CrossRef]
- De Snoeck, S.; van der Wolf, P.; Swart, W.; Heiiman, E.; Ebbinge, B. The effect of the application of mono-lauric acid with glycerol mono-laurate in weaned piglets, on the use of antimicrobials in sow herds. Agric. Food Sci. 2011, 346–348. [Google Scholar] [CrossRef]
- Sun, L.; Liu, Z.; Hao, G.; Wang, S.; Zhou, L.; Feng, J.; Yu, Z.; Lu, S. Effects of sodium butyrate on protein metabolism and its related gene expression of triploid crucian carp (Carassius auratus tripl). Chin. J. Anim. Nutr. 2013, 25, 2775–2782. [Google Scholar]
- Ng, W.-K.; Lim, P.-K.; Sidek, H. The influence of a dietary lipid source on growth, muscle fatty acid composition and erythrocyte osmotic fragility of hybrid tilapia. Fish Physiol. Biochem. 2001, 25, 301–310. [Google Scholar] [CrossRef]
- Ringo, E.; Gatesoupe, F.-J. Lactic acid bacteria in fish: A review. Aquaculture 1998, 160, 177–203. [Google Scholar] [CrossRef]
- Brenes, A.; Smith, M.; Guenter, W.; Marquardt, R.R. Effect of Enzyme Supplementation on the Performance and Digestive Tract Size of Broiler Chickens Fed Wheat- and Barley-Based Diets. Poult. Sci. 1993, 72, 1731–1739. [Google Scholar] [CrossRef]
- Liu, M.; Guo, W.; Wu, F.; Qu, Q.; Tan, Q.; Gong, W. Dietary supplementation of sodium butyrate may benefit growth performance and intestinal function in juvenile grass carp (Ctenopharyngodon idellus). Aquac. Res. 2017, 48, 4102–4111. [Google Scholar] [CrossRef]
- Chwen, L.T.; Foo, H.L.; Thanh, N.T.; Choe, D. Growth performance, plasma fatty acids, villous height and crypt depth of preweaning piglets fed with medium chain triacylglycerol. Asian-Australas. J. Anim. Sci. 2013, 26, 700–704. [Google Scholar] [CrossRef]
- Wang, J.; Yang, M.; Xu, S.; Lin, Y.; Che, L.; Fang, Z.; Wu, D. Comparative effects of sodium butyrate and flavors on feed intake of lactating sows and growth performance of piglets. Anim. Sci. J. 2014, 85, 683–689. [Google Scholar] [CrossRef]
- Ullah, S.; Zhang, J.; Xu, B.; Tegomo, A.F.; Sagada, G.; Zheng, L.; Wang, L.; Shao, Q. Effect of dietary supplementation of lauric acid on growth performance, antioxidative capacity, intestinal development and gut microbiota on black sea bream (Acanthopagrus schlegelii). PLoS ONE 2022, 17, e0262427. [Google Scholar] [CrossRef]
- Berges, J.; Mulholland, M. Nitrogen in the Marine Environment; Elsevier: Amsterdam, The Netherlands, 2008. [Google Scholar]
- Hwang, P.; Tsai, Y. Effects of arsenic on osmoregulation in the tilapia Oreochromis mossambicus reared in seawater. Mar. Biol. 1993, 117, 551–558. [Google Scholar] [CrossRef]
- Ahmad, H.; Tian, J.; Wang, J.; Khan, M.A.; Wang, Y.; Zhang, L.; Wang, T. Effects of dietary sodium selenite and selenium yeast on antioxidant enzyme activities and oxidative stability of chicken breast meat. J. Agric. Food Chem. 2012, 60, 7111–7120. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Zhao, K.; Whiteman, M. The gastrointestinal tract: A major site of antioxidant action? Free Radic. Res. 2000, 33, 819–830. [Google Scholar] [CrossRef] [PubMed]
- Buscaino, G.; Filiciotto, F.; Buffa, G.; Bellante, A.; Di Stefano, V.; Assenza, A.; Fazio, F.; Caola, G.; Mazzola, S. Impact of an acoustic stimulus on the motility and blood parameters of European sea bass (Dicentrarchus labrax L.) and gilthead sea bream (Sparus aurata L.). Mar. Environ. Res. 2010, 69, 136–142. [Google Scholar] [CrossRef]
- Fazio, F.; Marafioti, S.; Arfuso, F.; Piccione, G.; Faggio, C. Comparative study of the biochemical and haematological parameters of four wild Tyrrhenian fish species. Vet. Med. 2013, 58, 576–581. [Google Scholar] [CrossRef]
- Zhang, J.; Zhou, F.; Wang, L.l.; Shao, Q.; Xu, Z.; Xu, J. Dietary protein requirement of juvenile black sea bream, Sparus macrocephalus. J. World Aquac. Soc. 2010, 41, 151–164. [Google Scholar] [CrossRef]
- Tezcan, F.; Gultekin-Ozguven, M.; Diken, T.; Ozcelik, B.; Erim, F.B. Antioxidant activity and total phenolic, organic acid and sugar content in commercial pomegranate juices. Food Chem. 2009, 115, 873–877. [Google Scholar] [CrossRef]
- Wang, J.; Yoo, J.; Kim, H.; Lee, J.; Kim, I. Nutrient digestibility, blood profiles and fecal microbiota are influenced by chitooligosaccharide supplementation of growing pigs. Livest. Sci. 2009, 125, 298–303. [Google Scholar] [CrossRef]
- Jahns, F.; Wilhelm, A.; Jablonowski, N.; Mothes, H.; Greulich, K.O.; Glei, M. Butyrate modulates antioxidant enzyme expression in malignant and non-malignant human colon tissues. Mol. Carcinog. 2015, 54, 249–260. [Google Scholar] [CrossRef]
- Orchel, A.; Gruchlik, A.; Weglarz, L.; Dzierzewicz, Z. Influence of sodium butyrate on antioxidative enzymes activity in Caco-2 cell lines. Acta Pol. Pharm. 2006, 63, 441–442. [Google Scholar]
- Gessner, D.K.; Gröne, B.; Couturier, A.; Rosenbaum, S.; Hillen, S.; Becker, S.; Erhardt, G.; Reiner, G.; Ringseis, R.; Eder, K. Dietary fish oil inhibits pro-inflammatory and ER stress signalling pathways in the liver of sows during lactation. PLoS ONE 2015, 10, e0137684. [Google Scholar] [CrossRef]
- Fang, C.; Sun, H.; Wu, J.; Niu, H.; Feng, J. Effects of sodium butyrate on growth performance, haematological and immunological characteristics of weanling piglets. J. Anim. Physiol. Anim. Nutr. 2014, 98, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Wang, Y.; Zhang, Y.; Song, Y.; Zhang, X.; Lin, Y.; Che, L.; Xu, S.; Wu, D.; Xue, B. Effects of dietary combinations of organic acids and medium chain fatty acids as a replacement of zinc oxide on growth, digestibility and immunity of weaned pigs. Anim. Feed Sci. Technol. 2015, 208, 145–157. [Google Scholar] [CrossRef]
- Ullah, S.; Feng, F.; Shen, F.; Xiao, N.; Jian, J.; Zhao, M.; Ullah Dawar, F.; Zhang, J.; Shao, Q. Effect of dietary glycerol monolaurate supplementation on gene expression, intestinal mucosa and microbiota of juvenile black sea bream (Acanthopagrus schlegelii). Isr. J. Aquac. Bamidgeh 2024, 776, 1–17. [Google Scholar] [CrossRef]
- Le Roith, D.; Bondy, C.; Yakar, S.; Liu, J.-L.; Butler, A. The somatomedin hypothesis: 2001. Endocr. Rev. 2001, 22, 53–74. [Google Scholar] [CrossRef]
- Clemmons, D.R. Use of mutagenesis to probe IGF-binding protein structure/function relationships. Endocr. Rev. 2001, 22, 800–817. [Google Scholar] [CrossRef]
- Abid, A.; Davies, S.; Waines, P.; Emery, M.; Castex, M.; Gioacchini, G.; Carnevali, O.; Bickerdike, R.; Romero, J.; Merrifield, D. Dietary synbiotic application modulates Atlantic salmon (Salmo salar) intestinal microbial communities and intestinal immunity. Fish Shellfish Immunol. 2013, 35, 1948–1956. [Google Scholar] [CrossRef]
- Lazado, C.C.; Caipang, C.M.A. Mucosal immunity and probiotics in fish. Fish Shellfish Immunol. 2014, 39, 78–89. [Google Scholar] [CrossRef]
- Liu, W.; Ren, P.; He, S.; Xu, L.; Yang, Y.; Gu, Z.; Zhou, Z. Comparison of adhesive gut bacteria composition, immunity, and disease resistance in juvenile hybrid tilapia fed two different Lactobacillus strains. Fish Shellfish Immunol. 2013, 35, 54–62. [Google Scholar] [CrossRef]
- Selim, K.M.; Reda, R.M. Improvement of immunity and disease resistance in the Nile tilapia, Oreochromis niloticus, by dietary supplementation with Bacillus amyloliquefacien. Fish Shellfish Immunol. 2015, 44, 496–503. [Google Scholar] [CrossRef]
- Standen, B.; Peggs, D.; Rawling, M.; Foey, A.; Davies, S.; Santos, G.; Merrifield, D. Dietary administration of a commercial mixed-species probiotic improves growth performance and modulates the intestinal immunity of tilapia, Oreochromis niloticus. Fish Shellfish Immunol. 2016, 49, 427–435. [Google Scholar] [CrossRef]
- Lie, K.K.; Torresen, O.K.; Solbakken, M.H.; Ronnestad, I.; Tooming-Klunderud, A.; Nederbragt, A.J.; Jentoft, S.; Sæle, O. Loss of stomach, loss of appetite? Sequencing of the ballan wrasse (Labrus bergylta) genome and intestinal transcriptomic profiling illuminate the evolution of loss of stomach function in fish. BMC Genom. 2018, 19, 186. [Google Scholar] [CrossRef] [PubMed]
- De Benedetto, A.; Rafaels, N.M.; McGirt, L.Y.; Ivanov, A.I.; Georas, S.N.; Cheadle, C.; Berger, A.E.; Zhang, K.; Vidyasagar, S.; Yoshida, T. Tight junction defects in patients with atopic dermatitis. J. Allergy Clin. Immunol. 2011, 127, 773–786.e7. [Google Scholar] [CrossRef] [PubMed]
- Park, H.H.; Lo, Y.-C.; Lin, S.-C.; Wang, L.; Yang, J.K.; Wu, H. The death domain superfamily in intracellular signaling of apoptosis and inflammation. Annu. Rev. Immunol. 2007, 25, 561–586. [Google Scholar] [CrossRef]
- Xu, J.; Jiang, S.; Li, Y.; Li, M.; Cheng, Q.; Zhao, D.; Yang, B.; Jia, Z.; Wang, L.; Song, L. Caspase-3 serves as an intracellular immune receptor specific for lipopolysaccharide in oyster Crassostrea gigas. Dev. Comp. Immunol. 2016, 61, 1–12. [Google Scholar] [CrossRef]
- Wu, M.-H.; Jin, X.-K.; Yu, A.-Q.; Zhu, Y.-T.; Li, D.; Li, W.-W.; Wang, Q. Caspase-mediated apoptosis in crustaceans: Cloning and functional characterization of EsCaspase-3-like protein from Eriocheir sinensis. Fish Shellfish Immunol. 2014, 41, 625–632. [Google Scholar] [CrossRef]
- Xiong, Y.; Miyamoto, N.; Shibata, K.; Valasek, M.A.; Motoike, T.; Kedzierski, R.M.; Yanagisawa, M. Short-chain fatty acids stimulate leptin production in adipocytes through the G protein-coupled receptor GPR41. Proc. Natl. Acad. Sci. USA 2004, 101, 1045–1050. [Google Scholar] [CrossRef]
- Cera, K.; Mahan, D.; Cross, R.; Reinhart, G.; Whitmoyer, R. Effect of age, weaning and postweaning diet on small intestinal growth and jejunal morphology in young swine. J. Anim. Sci. 1988, 66, 574–584. [Google Scholar] [CrossRef]
- Volatiana, J.A.; Sagada, G.; Xu, B.; Zhang, J.; Ng, W.K.; Shao, Q. Effects of butyrate glycerides supplementation in high soybean meal diet on growth performance, intestinal morphology and antioxidative status of juvenile black sea bream, Acanthopagrus schlegelii. Aquac. Nutr. 2020, 26, 15–25. [Google Scholar] [CrossRef]
- Owen, M.; Waines, P.; Bradley, G.; Davies, S. The effect of dietary supplementation of sodium butyrate on the growth and microflora of Clarias gariepinus (Burchell 1822). In Proceedings of the XII International Symposium Fish Nutrition and Feeding, Biarritz, Spain, 28 May–1 June 2006. [Google Scholar]
- Sun, L.; Liu, Z.; Hao, G.; Zhou, L.; Lu, S.; Zhang, J.; Xiao, T. Effects of sodium butyrate on growth and intestinal cell proliferation of Carassius auratus. J. Fish. Sci. China 2013, 20, 893–901. [Google Scholar] [CrossRef]
- Nabuurs, M.; Hoogendoorn, A.; Van Der Molen, E.; Van Osta, A. Villus height and crypt depth in weaned and unweaned pigs, reared under various circumstances in the Netherlands. Res. Vet. Sci. 1993, 55, 78–84. [Google Scholar] [CrossRef]
- Cerezuela, R.; Fumanal, M.; Tapia-Paniagua, S.T.; Meseguer, J.; Moriñigo, M.Á.; Esteban, M.Á. Changes in intestinal morphology and microbiota caused by dietary administration of inulin and Bacillus subtilis in gilthead sea bream (Sparus aurata L.) specimens. Fish Shellfish Immunol. 2013, 34, 1063–1070. [Google Scholar] [CrossRef] [PubMed]
- Tadiparthi, R.; Bansal, A.; Wani, S.; Mathur, S.; Hall, S.; Rastogi, A.; Higbee, A.; Gaddam, S.; Sharma, P. Dilated intercellular spaces and lymphocytes on biopsy relate to symptoms in erosive GERD but not NERD. Aliment. Pharmacol. Ther. 2011, 33, 1202–1208. [Google Scholar] [CrossRef] [PubMed]
- Jang, W.J.; Hasan, M.T.; Lee, B.-J.; Hur, S.W.; Lee, S.; Kim, K.W.; Lee, E.-W.; Kong, I.-S. Effect of dietary differences on changes of intestinal microbiota and immune-related gene expression in juvenile olive flounder (Paralichthys olivaceus). Aquaculture 2020, 527, 735442. [Google Scholar] [CrossRef]
- Bergsson, G.; Steingrímsson, O.; Thormar, H. Bactericidal effects of fatty acids and monoglycerides on Helicobacter pylori. Int. J. Antimicrob. Agents 2002, 20, 258–262. [Google Scholar] [CrossRef] [PubMed]
- Yu, C.; Zhang, J.; Qin, Q.; Liu, J.; Xu, J.; Xu, W. Berberine improved intestinal barrier function by modulating the intestinal microbiota in blunt snout bream (Megalobrama amblycephala) under dietary high-fat and high-carbohydrate stress. Fish Shellfish Immunol. 2020, 102, 336–349. [Google Scholar] [CrossRef]
- Kormas, K.A.; Meziti, A.; Mente, E.; Frentzos, A. Dietary differences are reflected on the gut prokaryotic community structure of wild and commercially reared sea bream (Sparus aurata). Microbiologyopen 2014, 3, 718–728. [Google Scholar] [CrossRef]
- Ray, A.K.; Ghosh, K.; Ringo, E. Enzyme-producing bacteria isolated from fish gut: A review. Aquac. Nutr. 2012, 18, 465–492. [Google Scholar] [CrossRef]
- Ghanbari, M.; Kneifel, W.; Domig, K. A new view of the fish gut microbiome: Advances from next-generation sequencing. Aquaculture 2015, 448, 464–475. [Google Scholar] [CrossRef]
- Estruch, G.; Collado, M.; Penaranda, D.; Tomas Vidal, A.; Jover Cerda, M.; Perez Martinez, G.; Martinez-Llorens, S. Impact of fishmeal replacement in diets for gilthead sea bream (Sparus aurata) on the gastrointestinal microbiota determined by pyrosequencing the 16S rRNA gene. PLoS ONE 2015, 10, e0136389. [Google Scholar] [CrossRef]
- Rimoldi, S.; Gliozheni, E.; Ascione, C.; Gini, E.; Terova, G. Effect of a specific composition of short-and medium-chain fatty acid 1-Monoglycerides on growth performances and gut microbiota of gilthead sea bream (Sparus aurata). PeerJ 2018, 6, e5355. [Google Scholar] [CrossRef]
- Kollanoor, A.; Vasudevan, P.; Nair, M.K.M.; Hoagland, T.; Venkitanarayanan, K. Inactivation of bacterial fish pathogens by medium-chain lipid molecules (caprylic acid, monocaprylin and sodium caprylate). Aquac. Res. 2007, 38, 1293–1300. [Google Scholar] [CrossRef]
- Mo, Q.; Fu, A.; Deng, L.; Zhao, M.; Li, Y.; Zhang, H.; Feng, F. High-dose glycerol monolaurate up-regulated beneficial indigenous microbiota without inducing metabolic dysfunction and systemic inflammation: New insights into its antimicrobial potential. Nutrients 2019, 11, 1981. [Google Scholar] [CrossRef]


| Ingredient | Diets | |||
|---|---|---|---|---|
| Control | MSB | GML | TB | |
| FM | 19.9 | 19.9 | 19.9 | 19.9 |
| SBM | 43.5 | 36.0 | 43.5 | 45.0 |
| Soy protein concentrate | 4.0 | 4.0 | 4.0 | 4.0 |
| Soy lecithin | 2.0 | 2.0 | 2.0 | 2.0 |
| Corn oil | 6.4 | 6.4 | 6.4 | 6.4 |
| Fish oil | 3.0 | 3.0 | 3.0 | 3.0 |
| α-starch | 7.0 | 7.0 | 7.0 | 7.0 |
| Squid liver meal | 3.0 | 3.0 | 3.0 | 3.0 |
| MSB | 0.0 | 0.24 | 0.0 | 0.0 |
| GML | 0.0 | 0.0 | 0.04 | 0.0 |
| TB | 0.0 | 0.0 | 0.0 | 0.22 |
| Ca(H2PO4)2·H2O | 2.5 | 2.5 | 2.5 | 2.5 |
| CaCO3 | 0.7 | 0.7 | 0.7 | 0.7 |
| Alpha cellulose | 3.94 | 11.20 | 3.90 | 2.22 |
| Vitamins | 0.75 | 0.75 | 0.75 | 0.75 |
| Minerals | 0.75 | 0.75 | 0.75 | 0.75 |
| Y2O3 | 0.1 | 0.1 | 0.1 | 0.1 |
| Phytase | 0.05 | 0.05 | 0.05 | 0.05 |
| L-carnitine | 0.2 | 0.2 | 0.2 | 0.2 |
| CMC | 0.5 | 0.5 | 0.5 | 0.5 |
| Carrageenan | 0.2 | 0.2 | 0.2 | 0.2 |
| Methionine | 0.8 | 0.8 | 0.8 | 0.8 |
| Lysine | 0.21 | 0.21 | 0.21 | 0.21 |
| Taurine | 0.5 | 0.5 | 0.5 | 0.5 |
| Total | 100 | 100 | 100 | 100 |
| Nutrient contents | ||||
| Protein | 38.36 | 38.35 | 38.37 | 38.35 |
| Lipid | 14.11 | 14.10 | 14.13 | 14.12 |
| Carbohydrates | 27.43 | 27.41 | 27.42 | 27.42 |
| P/E ratio | 1.98 | 1.99 | 1.97 | 1.96 |
| P available | 0.78 | 0.77 | 0.79 | 0.77 |
| Energy kJ g−1 | 19.35 | 19.36 | 19.34 | 19.36 |
| Total phosphorus | 1.38 | 1.39 | 1.37 | 1.38 |
| Calcium | 0.98 | 0.96 | 0.98 | 0.97 |
| Ca/P | 0.71 | 0.70 | 0.69 | 0.72 |
| Methionine | 1.39 | 1.37 | 1.38 | 1.37 |
| Lysine | 2.82 | 2.81 | 2.83 | 2.81 |
| Arginine | 2.48 | 2.47 | 2.46 | 2.49 |
| Fish oil | 4.55 | 4.54 | 4.56 | 4.54 |
| Target Genes | Nucleotide Sequence (5′–3′) | Product Size (bp) | Accession No: Or Publication | Amplification Efficiency |
|---|---|---|---|---|
| IGF-1 | Forward: GTGGACGAGTGCTGCTTCCAA Reverse: GTGCCCTGCGGTACTAACCT | 2299 | AF030573.1 | 97.43 |
| GH | Forward: GCCGTCAGCTTTCCTGATGATG Reverse: GGAGGAGACCTGCGACTACC | 2469 | AF502071.1 | 98.54 |
| IL-1 | Forward: GAATCAAGGAGGGAGACAGGA Reverse: GTAGAGGAAGACAGAGACCAA | 986 | JQ973887.1 | 98.65 |
| TGF | Forward: TGTCTCCCCTACCCGCCGTCATC Reverse: ACCTCGCCTCCCGCTTCATCACT | 3751 | OQ248005.1 | 97.76 |
| CCK | Forward: AGCCCAAGGCACTCTAGACA Reverse: GTTCTGGGCAGCTGTAGAGG | 153 | Cluster-15370.85974 | 97.47 |
| CLDN1 | Forward: ACTGTTAGGGTTTTTCCTGTCTC Reverse: GTGATGATGTTGTCCCCGATGTA | 229 | Cluster-15370.120273 | 98.98 |
| Caspase-3 | Forward: AGTCAGTCGAGCAGATGAAACA Reverse: GGAGAAAGCGTAGAGGAAGTC | 290 | Cluster-15370.106619 | 97.35 |
| GPR41 | Forward: CGCTGCTCGTGTTCGCTCTATG Reverse: GGTCCAGACAGGCGTTGAAGGT | 398 | MH381812.1 | 97.74 |
| β-actin | Forward: TATCGTCATGGACTCCGGTG Reverse: TGATGTCACGCACGATTTCC | 187 | (Jiao et al., 2006) | 98.53 |
| Parameters | Diets | |||
|---|---|---|---|---|
| Control | MSB | GML | TB | |
| IBW | 1.55 ± 0.01 | 1.54 ± 0.01 | 1.55 ± 0.01 | 1.54 ± 0.01 |
| FBW | 18.00 ± 0.57 b | 21.81 ± 1.85 a | 21.86 ± 1.12 a | 19.64 ± 1.65 ab |
| WG (%) | 1007.73 ± 35.39 b | 1224.12 ± 159.60 ab | 1324.80 ± 104.38 a | 1167.40 ± 107.74 ab |
| SGR (% d−1) | 4.24 ± 0.06 b | 4.52 ± 0.21 ab | 4.66 ± 0.11 a | 4.45 ± 0.15 ab |
| MFI (g fish−1 day−1) | 0.46 ± 0.07 | 0.48 ± 0.05 | 0.32 ± 0.26 | 0.43 ± 0.03 |
| FCR | 1.24 ± 1.21 | 1.23 ± 0.11 | 1.24 ± 0.12 | 1.22 ± 1.01 |
| PER | 1.85 ± 0.12 bc | 1.99 ± 0.12 ab | 2.04 ± 0.03 a | 1.98 ± 0.04 ab |
| CF (g cm−3) | 2.95 ± 0.08 | 2.91 ± 0.07 | 2.95 ± 0.05 | 2.95 ± 0.04 |
| HSI (%) | 1.79 ± 0.09 | 1.75 ± 0.05 | 1.83 ± 0.09 | 1.88 ± 0.09 |
| IPF (%) | 2.18 ± 0.22 | 2.06 ± 0.01 | 2.22 ± 0.19 | 2.60 ± 0.45 |
| VSI (%) | 7.38 ± 0.60 | 7.71 ± 0.12 | 7.85 ± 0.18 | 8.40 ± 0.75 |
| PPV (%) | 27.13 ± 1.89 ab | 33.92 ± 2.22 a | 30.34 ± 2.65 b | 30.01 ± 1.64 ab |
| SR (%) | 99.16 ± 1.44 | 99.16 ± 1.44 | 99.16 ± 1.44 | 100.00 ± 0.00 |
| Parameters | Diets | |||
|---|---|---|---|---|
| Control | MSB | GML | TB | |
| Whole body | ||||
| Moisture | 68.24 ± 0.63 | 68.42 ± 0.71 | 66.79 ± 2.79 | 68.39 ± 0.91 |
| Protein | 17.50 ± 0.15 b | 17.76 ± 0.18 ab | 17.85 ± 0.13 a | 17.67 ± 0.14 b |
| Lipid | 11.92 ± 0.12 | 13.39 ± 0.19 | 13.33 ± 0.31 | 12.92 ± 0.14 |
| Ash | 4.81 ± 0.31 | 4.74 ± 0.24 | 4.73 ± 0.22 | 4.82 ± 0.23 |
| Dorsal muscle | ||||
| Moisture | 82.68 ± 11.63 | 78.06 ± 7.93 | 82.57 ± 6.59 | 78.35 ± 6.57 |
| Protein | 18.52 ± 0.42 | 18.75 ± 0.18 | 18.77 ± 0.15 | 18.70 ± 0.04 |
| Lipid | 4.63 ± 0.16 | 4.66 ± 0.57 | 4.73 ± 0.12 | 4.65 ± 0.40 |
| Ash | 1.57 ± 0.13 a | 0.12 ± 0.06 b | 0.10 ± 0.02 b | 0.11 ± 0.14 b |
| Parameters | Diets | |||
|---|---|---|---|---|
| Control | MSB | GML | TB | |
| TP (g L−1) | 3.99 ± 0.86 | 3.83 ± 0.34 | 4.29 ± 0.92 | 4.14 ± 0.70 |
| Trypsin (U mgprot−1) | 3032.39 ± 1374.15 | 2873.47 ± 295.54 | 3011.35 ± 613.74 | 2801.45 ± 474.30 |
| Lipase (U gprot−1) | 0.65 ± 0.14 | 0.93 ± 0.36 | 0.54 ± 0.21 | 0.69 ± 0.27 |
| Amylase (U mgprot−1) | 3.74 ± 1.48 | 3.15 ± 0.25 | 2.94 ± 1.33 | 3.31 ± 0.71 |
| Parameters | Diets | |||
|---|---|---|---|---|
| Control | MSB | GML | TB | |
| TP (g L−1) | 32.11 ± 4.61 | 36.34 ± 8.72 | 43.77 ± 9.83 | 37.67 ± 2.34 |
| ALB (g L−1) | 13.65 ± 3.27 | 11.97 ± 1.17 | 12.74 ± 2.15 | 11.46 ± 0.13 |
| SOD (U mL−1) | 143.34 ± 28.17 | 152.07 ± 38.33 | 173.88 ± 6.28 | 163.15 ± 13.47 |
| MDA (nmol mL−1) | 15.85 ± 2.26 | 14.43 ± 2.33 | 14.29 ± 1.80 | 14.81±1.54 |
| GSH-Px (U mL−1) | 178.24 ± 17.16 ab | 190.96±4.56 ab | 215.21±17.25 a | 170.38 ± 11.23 b |
| CAT (U mL−1) | 2.58 ± 0.26 b | 4.99 ± 0.70 a | 4.09 ± 1.34 a | 3.73±0.79 ab |
| T-AOC (U mL−1) | 1.92 ± 0.73 | 1.75 ± 0.16 | 2.05 ± 0.23 | 1.54 ± 0.13 |
| LZM (U mL−1) | 66.66 ± 17.97 | 71.43 ± 0.00 | 69.05 ± 14.86 | 60.71 ± 15.15 |
| ALT (U L−1) | 3.34 ± 0.51 | 2.99 ± 0.71 | 2.92 ± 0.92 | 2.89 ± 0.95 |
| AST (U L−1) | 10.25 ± 1.56 | 7.05 ± 0.97 | 7.52 ± 4.24 | 9.89 ± 1.92 |
| T-CHO (mmol L−1) | 10.36 ± 3.22 | 9.08 ± 0.63 | 9.62 ± 2.11 | 9.19 ± 0.63 |
| IgM (mg mL−1) | 2.04 ± 0.85 | 2.68 ± 0.44 | 1.91 ± 0.54 | 1.87 ± 0.78 |
| C3 (μg mL−1) | 276.56 ± 142.42 | 340.93 ± 108.76 | 272.96 ± 71.95 | 240.09 ± 38.45 |
| C4 (μg mL−1) | 142.63 ± 80.69 | 202.59 ± 61.38 | 127.75 ± 59.29 | 133.19 ± 37.71 |
| Parameters | Diets | |||
|---|---|---|---|---|
| Control | MSB | GML | TB | |
| Hindgut | ||||
| TP (g L−1) | 3.74 ± 1.48 | 3.15 ± 0.25 | 2.94 ± 1.33 | 3.31 ± 0.71 |
| LZM (U mL−1) | 15.94 ± 6.13 | 15.95 ± 4.78 | 11.78 ± 5.518 | 15.63 ± 1.44 |
| ALB (g L−1) | 22.62 ± 0.14 | 25.31 ± 2.54 | 23.21 ± 0.86 | 22.93 ± 2.70 |
| T-AOC (U mL−1) | 8.21 ± 0.54 bc | 10.07 ± 0.45 a | 10.11 ± 0.47 a | 9.64 ± 1.04 b |
| IgM (mg mL−1) | 1.03 ± 0.02 ab | 1.45 ± 0.13 ab | 1.78 ± 0.03 a | 1.28 ± 0.04 ab |
| Midgut | ||||
| TP (g L−1) | 2.50 ± 0.27 | 2.45 ± 0.16 | 2.42 ± 0.04 | 2.47 ± 0.54 |
| LZM (U mL−1) | 18.62 ± 0.79 b | 19.29 ± 2.59 b | 26.03 ± 3.38 a | 18.79 ± 5.11 b |
| ALB (g L−1) | 20.32 ± 0.14 | 20.39 ± 0.84 | 20.42 ± 0.24 | 20.17 ± 0.64 |
| T-AOC (U mL−1) | 4.87 ± 1.53 | 5.31 ± 1.34 | 6.57 ± 0.43 | 5.00 ± 1.10 |
| IgM (mg mL−1) | 2.08 ± 0.06 | 2.30 ± 0.09 | 2.18 ± 0.19 | 2.07 ± 0.34 |
| Parameters | Diets | |||
|---|---|---|---|---|
| Control | MSB | GML | TB | |
| TP (g L−1) | 4.49 ± 0.71 | 4.46 ± 0.65 | 4.75 ± 0.78 | 4.29 ± 0.59 |
| SOD (U mgprot−1) | 300.06 ± 34.35 | 309.32 ± 43.40 | 315.36 ± 65.04 | 323.72 ± 73.45 |
| MDA (nmol mgprot−1) | 6.10 ± 0.27 | 7.33 ± 3.56 | 4.72 ± 0.48 | 5.01 ± 1.61 |
| GSH-Px (U mgprot−1) | 9.53 ± 1.55 | 8.44 ± 1.34 | 12.06 ± 4.61 | 11.79 ± 2.76 |
| CAT (U mgprot−1) | 22.23 ± 4.72 | 25.40 ± 2.79 | 23.54 ± 4.08 | 21.37 ± 1.94 |
| T-AOC (mmol gprot−1) | 0.15 ± 0.05 | 0.14 ± 0.05 | 0.13 ± 0.04 | 0.14 ± 0.04 |
| ALT (U gprot−1) | 14.74 ± 7.06 b | 20.37 ± 8.04 ab | 28.63 ± 5.01 a | 16.97 ± 2.99 b |
| AST (U gprot−1) | 53.17 ± 12.72 | 59.08 ± 0.51 | 65.99 ± 7.02 | 66.26 ± 23.45 |
| Parameters | Diets | |||
|---|---|---|---|---|
| Control | MSB | GML | TB | |
| VH (μm) | 432.82 ± 3.54 c | 502.49 ± 4.48 a | 504.83 ± 10.49 a | 483.04 ± 17.29 b |
| CD (μm) | 84.09 ± 16.31 b | 97.43 ± 2.32 ab | 98.36 ± 2.51 a | 95.59 ± 2.15 ab |
| VH/CD (μm) | 7.08 ± 0.25 b | 7.98 ± 0.88 ab | 8.73 ± 0.04 a | 7.87±0.34 ab |
| GC/VH | 15.18 ± 1.15 b | 24.23 ± 2.10 a | 26.35 ± 2.25 a | 23.70 ± 2.10 a |
| Parameters | Diets | |||
|---|---|---|---|---|
| Control | MSB | GML | TB | |
| Raw tags | 79,583 | 83,191 | 85,738 | 81,348 |
| Valid tags | 70,274 | 78,153 | 79,137 | 72,089 |
| Coverage | 1.00 | 1.00 | 1.00 | 1.00 |
| Index | Diets | |||
|---|---|---|---|---|
| Control | MSB | GML | TB | |
| Observed species | 191.58 ± 2.26 d | 232.31 ± 3.40 b | 285.310 ± 1.67 a | 214.69 ± 3.90 c |
| Shannon | 3.51 ± 0.01 c | 4.23 ± 0.12 b | 4.64 ± 0.12 a | 4.18 ± 0.03 b |
| Simpson | 0.93 ± 0.02 b | 1.00 ± 0.03 a | 1.03 ± 0.01 a | 0.98 ± 0.02 ab |
| Chao1 | 230.67 ± 2.76 b | 258.16 ± 9.17 a | 265.81 ± 8.91 a | 251.72 ± 2.07 a |
| Phylum | Diets | |||
|---|---|---|---|---|
| Control | MSB | GML | TB | |
| P__Proteobacteria | 80.15 ± 3.56 c | 87.92 ± 2.94 ab | 92.095 ± 4.81 a | 83.28 ± 1.29 bc |
| p__Cyanobacteria | 17.07 ± 0.31 a | 16.05 ± 1.57 ab | 14.49 ± 0.49 b | 15.09 ± 0.50 b |
| p__Actinobacteria | 7.05 ± 0.69 | 6.01 ± 2.73 | 7.15 ±1.79 | 5.63 ± 0.28 |
| p__Firmicutes | 5.47 ± 0.49 b | 5.90 ± 0.11 ab | 7.39 ± 0.98 a | 6.74 ± 0.96 ab |
| p__Acidobacteria | 2.24 ± 0.95 | 2.57 ± 0.96 | 2.90 ± 0.31 | 2.85 ± 0.20 |
| p__Bacteroidetes | 1.11 ± 0.54 | 1.19 ± 1.00 | 1.39 ± 1.08 | 1.22 ± 1.06 |
| Class | Diets | |||
|---|---|---|---|---|
| Control | MSB | GML | TB | |
| c__Alphaproteobacteria | 34.82 ± 2.68 b | 36.19 ± 3.26 ab | 41.10 ± 1.39 a | 39.85 ± 1.96 ab |
| c__Betaproteobacteria | 23.82 ± 0.40 c | 24.39 ± 0.97 bc | 27.53 ± 0.94 a | 25.47 ± 0.63 b |
| c__Gammaproteobacteria | 13.89 ± 0.60 b | 14.38 ± 0.46 ab | 15.14 ± 0.76 a | 14.61 ± 0.11 ab |
| c__Chloroplast | 3.84 ± 0.44 | 3.56 ± 0.79 | 6.26 ± 2.36 | 4.16 ± 0.97 |
| c__Actinobacteria | 3.69 ± 0.85 | 4.41 ± 1.33 | 4.64 ± 0.31 | 3.77 ± 0.85 |
| c__Clostridia | 1.48 ± 0.16 b | 1.56 ± 0.04 b | 1.96 ± 0.04 a | 1.67 ± 0.24 ab |
| c__Acidobacteria_Gp6 | 1.02 ± 0.46 | 0.80 ± 0.22 | 1.06 ± 0.41 | 0.79 ± 0.22 |
| c__Sphingobacteriia | 0.55 ± 0.37 | 0.49± 0.28 | 0.76 ± 0.23 | 0.68 ± 0.48 |
| c__Bacilli | 0.16 ± 0.05 b | 0.78 ± 0.44 ab | 1.21 ± 0.42 a | 0.26 ± 0.16 b |
| Family | Diets | |||
|---|---|---|---|---|
| Control | MSB | GML | TB | |
| f__Caulobacteraceae | 12.99 ± 2.36 | 17.69 ± 4.67 | 17.91 ± 3.77 | 15.26 ± 3.28 |
| f__Xanthomonadaceae | 1.34 ± 0.30 | 1.42 ± 0.39 | 1.88 ± 0.84 | 1.39 ± 0.22 |
| f__Sphingomonadaceae | 11.66 ± 0.30 b | 13.71 ± 1.05 ab | 15.02 ± 1.76 b | 12.85 ± 0.53 ab |
| f__Burkholderiaceae | 8.03 ± 3.00 | 11.16 ± 2.69 | 11.47 ± 2.36 | 11.05 ± 1.59 |
| f__Oxalobacteraceae | 12.79 ± 1.73 | 14.66 ± 2.92 | 17.42 ± 4.52 | 13.24 ± 3.33 |
| f__Chloroplast | 7.29 ± 0.48 c | 9.61 ± 0.33 ab | 10.44 ± 0.54 a | 9.41 ± 0.47 b |
| f__Rhodocyclaceae | 6.84 ± 0.44 | 8.29 ± 1.89 | 10.54 ± 2.11 | 8.28 ± 2.45 |
| f__Comamonadaceae | 6.39 ± 1.37 | 5.67 ± 1.68 | 7.93 ± 1.43 | 5.18 ± 1.58 |
| f__Moraxellaceae | 2.04 ± 0.69 | 2.95 ± 0.64 | 4.41 ± 2.47 | 2.29 ± 0.99 |
| f__Pseudomonadaceae | 4.10 ± 0.53 | 4.60 ± 0.17 | 5.27 ± 1.32 | 5.08 ± 0.91 |
| f__Hyphomicrobiaceae | 1.93 ± 0.07 c | 2.12 ± 0.04 b | 2.22 ± 0.04 a | 2.09 ± 0.01 b |
| f__Nocardiaceae | 0.50 ± 0.09 b | 0.67 ± 0.32 b | 1.77 ± 0.72 a | 0.62 ± 0.26 b |
| f__Bradyrhizobiaceae | 0.50 ± 0.09 b | 0.67 ± 0.32 ab | 1.07 ± 0.09 a | 0.740 ± 0.18 ab |
| f__Iamiaceae | 0.89 ± 0.07 ab | 0.94 ± 0.34 ab | 1.11 ± 0.21 a | 0.68 ± 0.05 b |
| f__Clostridiaceae_1 | 0.54 ± 0.13c | 0.93 ± 0.09 ab | 0.97 ± 0.02 a | 0.80 ± 0.02 b |
| f__Gp6 | 0.60 ± 0.31 | 0.65 ± 0.46 | 0.78 ± 0.13 | 0.52 ± 0.03 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ullah, S.; Feng, F.; Zhao, M.; Zhang, J.; Shao, Q. Comparative Effects of Dietary Supplementations with Microencapsulated Sodium Butyrate, Glycerol Monolaurate and Tributyrin on Growth, Immunity, and Gut Health in Black Sea Bream. Animals 2025, 15, 810. https://doi.org/10.3390/ani15060810
Ullah S, Feng F, Zhao M, Zhang J, Shao Q. Comparative Effects of Dietary Supplementations with Microencapsulated Sodium Butyrate, Glycerol Monolaurate and Tributyrin on Growth, Immunity, and Gut Health in Black Sea Bream. Animals. 2025; 15(6):810. https://doi.org/10.3390/ani15060810
Chicago/Turabian StyleUllah, Sami, Fengqin Feng, Minjie Zhao, Jinzhi Zhang, and Qingjun Shao. 2025. "Comparative Effects of Dietary Supplementations with Microencapsulated Sodium Butyrate, Glycerol Monolaurate and Tributyrin on Growth, Immunity, and Gut Health in Black Sea Bream" Animals 15, no. 6: 810. https://doi.org/10.3390/ani15060810
APA StyleUllah, S., Feng, F., Zhao, M., Zhang, J., & Shao, Q. (2025). Comparative Effects of Dietary Supplementations with Microencapsulated Sodium Butyrate, Glycerol Monolaurate and Tributyrin on Growth, Immunity, and Gut Health in Black Sea Bream. Animals, 15(6), 810. https://doi.org/10.3390/ani15060810









