Non-Coding RNAs in Regulating Fat Deposition in Farm Animals
Simple Summary
Abstract
1. Introduction
2. Molecular Regulation of Fat Deposition
3. Regulatory ncRNAs and Fat Deposition
3.1. miRNAs Regulating Fat Deposition in Farm Animals
3.1.1. miRNAs Positively Regulating Fat Deposition
3.1.2. miRNAs Negatively Regulating Fat Deposition
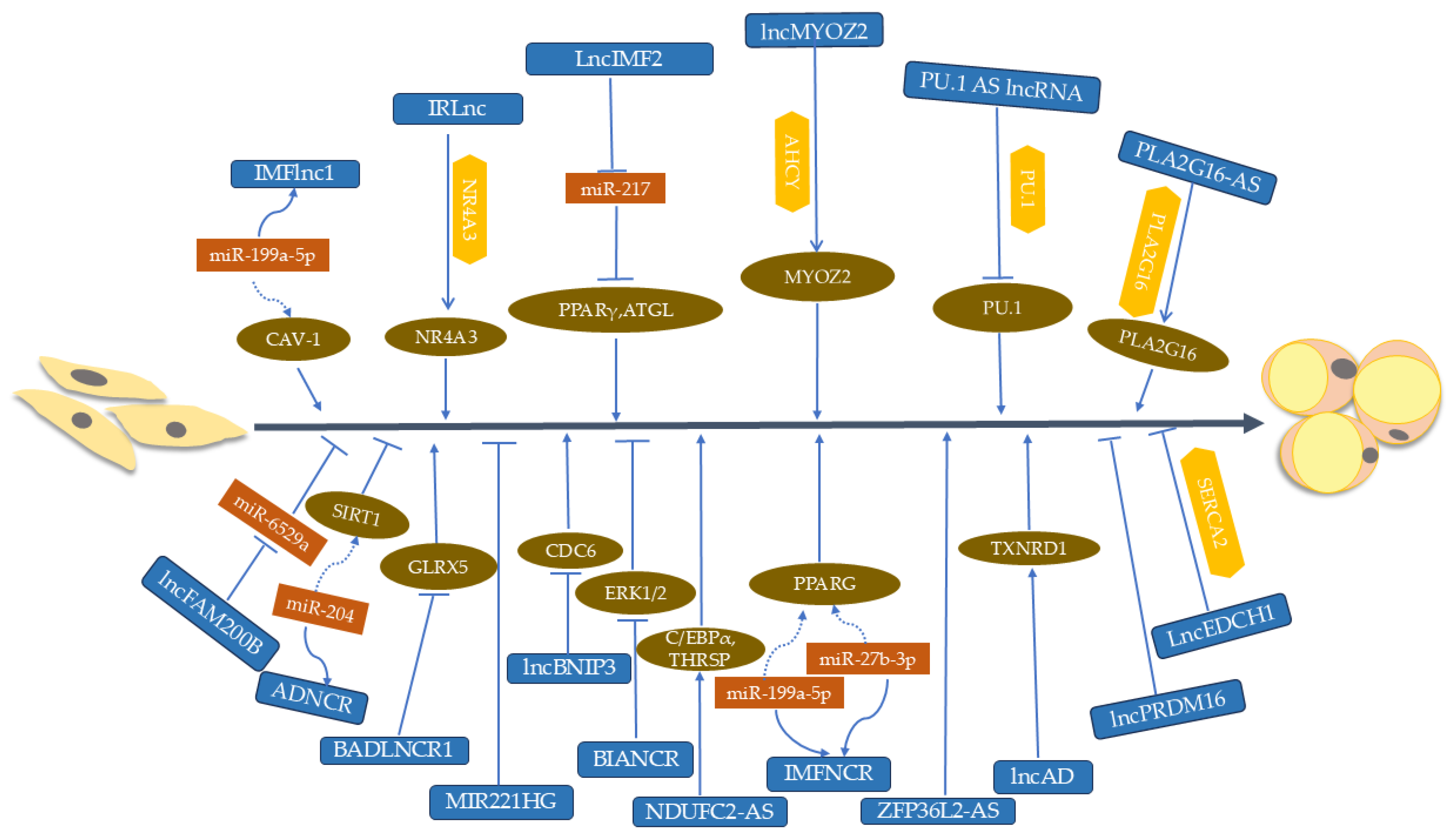
3.2. lncRNAs Regulating Fat Deposition in Farm Animals
3.2.1. Screening of lncRNAs in Adipose Tissue
3.2.2. lncRNAs Positively Regulating Fat Deposition
3.2.3. lncRNAs Negatively Regulating Fat Deposition
3.3. circRNAs Regulating Fat Deposition in Farm Animals
3.3.1. Screening of DE circRNAs in Adipose Tissue
3.3.2. circRNAs Positively Regulating Fat Deposition
3.3.3. circRNAs Negatively Regulating Fat Deposition
4. Conclusions
5. Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Frigolet, M.E.; Gutiérrez-Aguilar, R. The colors of adipose tissue. Gac. Medica Mex. 2020, 156, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Mancuso, P. The role of adipokines in chronic inflammation. ImmunoTargets Ther. 2016, 5, 47–56. [Google Scholar] [CrossRef]
- Guerreiro, O.; Alves, S.P.; Soldado, D.; Cachucho, L.; Almeida, J.M.; Francisco, A.; Santos-Silva, J.; Bessa, R.J.B.; Jeronimo, E. Inclusion of the aerial part and condensed tannin extract from Cistus ladanifer L. in lamb diets—Effects on growth performance, carcass and meat quality and fatty acid composition of intramuscular and subcutaneous fat. Meat Sci. 2020, 160, 107945. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Lane, M.D. Adipogenesis: From stem cell to adipocyte. Annu. Rev. Biochem. 2012, 81, 715–736. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Mottillo, E.P.; Granneman, J.G. Adipose tissue plasticity from WAT to BAT and in between. Biochim. Biophys. Acta 2014, 1842, 358–369. [Google Scholar] [CrossRef] [PubMed]
- Hausman, D.B.; DiGirolamo, M.; Bartness, T.J.; Hausman, G.J.; Martin, R.J. The biology of white adipocyte proliferation. Obes. Rev. Off. J. Int. Assoc. Study Obes. 2001, 2, 239–254. [Google Scholar] [CrossRef]
- Sarjeant, K.; Stephens, J.M. Adipogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008417. [Google Scholar] [CrossRef]
- Lei, Z.; Wu, H.; Xiong, Y.; Wei, D.; Wang, X.; Luoreng, Z.; Cai, X.; Ma, Y. ncRNAs regulate bovine adipose tissue deposition. Mol. Cell. Biochem. 2021, 476, 2837–2845. [Google Scholar] [CrossRef]
- Xu, B.; Gerin, I.; Miao, H.; Vu-Phan, D.; Johnson, C.N.; Xu, R.; Chen, X.W.; Cawthorn, W.P.; MacDougald, O.A.; Koenig, R.J. Multiple roles for the non-coding RNA SRA in regulation of adipogenesis and insulin sensitivity. PLoS ONE 2010, 5, e14199. [Google Scholar] [CrossRef]
- Otto, T.C.; Lane, M.D. Adipose development: From stem cell to adipocyte. Crit. Rev. Biochem. Mol. Biol. 2005, 40, 229–242. [Google Scholar] [CrossRef]
- Li, Y.; Jin, D.; Xie, W.; Wen, L.; Chen, W.; Xu, J.; Ding, J.; Ren, D. PPAR-γ and Wnt Regulate the Differentiation of MSCs into Adipocytes and Osteoblasts Respectively. Curr. Stem Cell Res. Ther. 2018, 13, 185–192. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Yang, C.; Luo, J.; Wei, Y.; Wang, W.; Zhong, Y. Adiponectin promotes preadipocyte differentiation via the PPARγ pathway. Mol. Med. Rep. 2018, 17, 428–435. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhou, Y.; Lei, W.; Zhang, K.; Shi, J.; Hu, Y.; Shu, G.; Song, J. Signal transducer and activator of transcription 3 (STAT3) regulates adipocyte differentiation via peroxisome-proliferator-activated receptor gamma (PPARgamma). Biol. Cell 2009, 102, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Oishi, Y.; Manabe, I.; Tobe, K.; Tsushima, K.; Shindo, T.; Fujiu, K.; Nishimura, G.; Maemura, K.; Yamauchi, T.; Kubota, N.; et al. Krüppel-like transcription factor KLF5 is a key regulator of adipocyte differentiation. Cell Metab. 2005, 1, 27–39. [Google Scholar] [CrossRef]
- Banerjee, S.S.; Feinberg, M.W.; Watanabe, M.; Gray, S.; Haspel, R.L.; Denkinger, D.J.; Kawahara, R.; Hauner, H.; Jain, M.K. The Krüppel-like factor KLF2 inhibits peroxisome proliferator-activated receptor-gamma expression and adipogenesis. J. Biol. Chem. 2003, 278, 2581–2584. [Google Scholar] [CrossRef]
- Tanaka, T.; Yoshida, N.; Kishimoto, T.; Akira, S. Defective adipocyte differentiation in mice lacking the C/EBPbeta and/or C/EBPdelta gene. EMBO J. 1997, 16, 7432–7443. [Google Scholar] [CrossRef]
- Tang, Q.Q.; Otto, T.C.; Lane, M.D. CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc. Natl. Acad. Sci. USA 2003, 100, 850–855. [Google Scholar] [CrossRef]
- Guo, L.; Li, X.; Tang, Q.Q. Transcriptional regulation of adipocyte differentiation: A central role for CCAAT/enhancer-binding protein (C/EBP) β. J. Biol. Chem. 2015, 290, 755–761. [Google Scholar] [CrossRef]
- Wu, Z.; Rosen, E.D.; Brun, R.; Hauser, S.; Adelmant, G.; Troy, A.E.; McKeon, C.; Darlington, G.J.; Spiegelman, B.M. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell 1999, 3, 151–158. [Google Scholar] [CrossRef]
- Zhang, M.; Shao, Y.; Gao, B.; Chen, J.; Zhang, P.; Hu, Y.; Ding, S. Erchen Decoction Mitigates Lipid Metabolism Disorder by the Regulation of PPARγ and LPL Gene in a High-Fat Diet C57BL/6 Mice Model. Evid.-Based Complement. Altern. Med. 2020, 2020, 9102475. [Google Scholar] [CrossRef]
- Kim, J.B.; Spiegelman, B.M. ADD1/SREBP1 promotes adipocyte differentiation and gene expression linked to fatty acid metabolism. Genes Dev. 1996, 10, 1096–1107. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.D. Regulation of fatty acid synthase gene expression: An approach for reducing fat accumulation. J. Anim. Sci. 1993, 71, 1957–1965. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.H.; Edkins, A.L.; Hoppe, H.C.; Prinsloo, E. Dynamic Mitochondrial Localisation of STAT3 in the Cellular Adipogenesis Model 3T3-L1. J. Cell Biochem. 2015, 116, 1232–1240. [Google Scholar] [CrossRef] [PubMed]
- Fox, K.E.; Colton, L.A.; Erickson, P.F.; Friedman, J.E.; Cha, H.C.; Keller, P.; MacDougald, O.A.; Klemm, D.J. Regulation of cyclin D1 and Wnt10b gene expression by cAMP-responsive element-binding protein during early adipogenesis involves differential promoter methylation. J. Biol. Chem. 2008, 283, 35096–35105. [Google Scholar] [CrossRef]
- Qin, L.; Chen, Y.; Niu, Y.; Chen, W.; Wang, Q.; Xiao, S.; Li, A.; Xie, Y.; Li, J.; Zhao, X.; et al. A deep investigation into the adipogenesis mechanism: Profile of microRNAs regulating adipogenesis by modulating the canonical Wnt/beta-catenin signaling pathway. BMC Genom. 2010, 11, 320. [Google Scholar] [CrossRef]
- Zhang, H.; Nøohr, J.; Jensen, C.H.; Petersen, R.K.; Bachmann, E.; Teisner, B.; Larsen, L.K.; Mandrup, S.; Kristiansen, K. Insulin-like growth factor-1/insulin bypasses Pref-1/FA1-mediated inhibition of adipocyte differentiation. J. Biol. Chem. 2003, 278, 20906–20914. [Google Scholar] [CrossRef]
- Zhu, D.; Shi, S.; Wang, H.; Liao, K. Growth arrest induces primary-cilium formation and sensitizes IGF-1-receptor signaling during differentiation induction of 3T3-L1 preadipocytes. J. Cell Sci. 2009, 122 Pt 15, 2760–2768. [Google Scholar] [CrossRef]
- Lee, R.A.; Harris, C.A.; Wang, J.C. Glucocorticoid Receptor and Adipocyte Biology. Nucl. Receptor. Res. 2018, 5, 101373. [Google Scholar] [CrossRef]
- Pantoja, C.; Huff, J.T.; Yamamoto, K.R. Glucocorticoid signaling defines a novel commitment state during adipogenesis in vitro. Mol. Biol. Cell 2008, 19, 4032–4041. [Google Scholar] [CrossRef]
- Suh, J.M.; Gao, X.; McKay, J.; McKay, R.; Salo, Z.; Graff, J.M. Hedgehog signaling plays a conserved role in inhibiting fat formation. Cell Metab. 2006, 3, 25–34. [Google Scholar] [CrossRef]
- Aouadi, M.; Laurent, K.; Prot, M.; Le Marchand-Brustel, Y.; Binétruy, B.; Bost, F. Inhibition of p38MAPK increases adipogenesis from embryonic to adult stages. Diabetes 2006, 55, 281–289. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Lisanti, M.P.; Scherer, P.E. Specific inhibitors of p38 mitogen-activated protein kinase block 3T3-L1 adipogenesis. J. Biol. Chem. 1998, 273, 32111–32120. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Berg, A.H.; Lewis, R.Y.; Lin, A.; Lisanti, M.P.; Scherer, P.E. Constitutively active mitogen-activated protein kinase kinase 6 (MKK6) or salicylate induces spontaneous 3T3-L1 adipogenesis. J. Biol. Chem. 1999, 274, 35630–35638. [Google Scholar] [CrossRef] [PubMed]
- MacDougald, O.A.; Lane, M.D. Transcriptional regulation of gene expression during adipocyte differentiation. Annu. Rev. Biochem. 1995, 64, 345–373. [Google Scholar] [CrossRef]
- Piergentili, R.; Zaami, S.; Cavaliere, A.F.; Signore, F.; Scambia, G.; Mattei, A.; Marinelli, E.; Gulia, C.; Perelli, F. Non-Coding RNAs as Prognostic Markers for Endometrial Cancer. Int. J. Mol. Sci. 2021, 22, 3151. [Google Scholar] [CrossRef]
- Wu, J.; Yu, H.; Huang, H.; Shu, P.; Peng, X. Functions of noncoding RNAs in glial development. Dev. Neurobiol. 2021, 81, 877–891. [Google Scholar] [CrossRef]
- Cho, I.S.; Kim, J.; Seo, H.Y.; Lim, D.H.; Hong, J.S.; Park, Y.H.; Park, D.C.; Hong, K.C.; Whang, K.Y.; Lee, Y.S. Cloning and characterization of microRNAs from porcine skeletal muscle and adipose tissue. Mol. Biol. Rep. 2010, 37, 3567–3574. [Google Scholar] [CrossRef]
- Li, G.; Wu, Z.; Li, X.; Ning, X.; Li, Y.; Yang, G. Biological role of microRNA-103 based on expression profile and target genes analysis in pigs. Mol. Biol. Rep. 2011, 38, 4777–4786. [Google Scholar] [CrossRef]
- Beutler, B.; Greenwald, D.; Hulmes, J.D.; Chang, M.; Pan, Y.C.; Mathison, J.; Ulevitch, R.; Cerami, A. Identity of tumour necrosis factor and the macrophage-secreted factor cachectin. Nature 1985, 316, 552–554. [Google Scholar] [CrossRef]
- Torti, F.M.; Dieckmann, B.; Beutler, B.; Cerami, A.; Ringold, G.M. A macrophage factor inhibits adipocyte gene expression: An in vitro model of cachexia. Science 1985, 229, 867–869. [Google Scholar] [CrossRef]
- Li, H.; Chen, X.; Guan, L.; Qi, Q.; Shu, G.; Jiang, Q.; Yuan, L.; Xi, Q.; Zhang, Y. MiRNA-181a regulates adipogenesis by targeting tumor necrosis factor-alpha (TNF-alpha) in the porcine model. PLoS ONE 2013, 8, e71568. [Google Scholar]
- Dong, P.; Mai, Y.; Zhang, Z.; Mi, L.; Wu, G.; Chu, G.; Yang, G.; Sun, S. MiR-15a/b promote adipogenesis in porcine pre-adipocyte via repressing FoxO1. Acta Biochim. Biophys. Sin. 2014, 46, 565–571. [Google Scholar] [CrossRef]
- Ning, X.; Liu, S.; Qiu, Y.; Li, G.; Li, Y.; Li, M.; Yang, G. Expression Profiles and Biological Roles of miR-196a in Swine. Genes 2016, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Zhang, Z.; Zhou, X.; Wang, Z.; Wang, G.; Han, Z. Effects of microRNA-143 in the differentiation and proliferation of bovine intramuscular preadipocytes. Mol. Biol. Rep. 2011, 38, 4273–4280. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.Y.; Shao, J.; Yin, B.Z.; Zhang, L.M.; Fang, J.C.; Zhang, J.S.; Xia, G.J. Bovine bta-microRNA-1271 Promotes Preadipocyte Differentiation by Targeting Activation Transcription Factor 3. Biochemistry 2020, 85, 749–757. [Google Scholar] [CrossRef]
- Xu, H.; Shao, J.; Fang, J.; Yin, B.; Zhang, L.; Zhang, J.; Xia, G. miR-381 Targets KCTD15 to Regulate Bovine Preadipocyte Differentiation In Vitro. Horm. Metab. Res. 2021, 53, 63–70. [Google Scholar] [CrossRef]
- Yang, Z.; Ma, X.; Zhang, D.; Li, B.; Gao, N.; Li, X.; Mei, C.; Zan, L. Bta-miR-330 promotes bovine intramuscular pre-adipocytes adipogenesis via targeting SESN3 to activate the Akt-mTOR signaling pathway. Int. J. Biol. Macromol. 2024, 275 Pt 1, 133650. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, W.; Li, H.; Xiang, H.; Zhang, C.; Du, Z.; Huang, L.; Zhu, J. MiR-196a Promotes Lipid Deposition in Goat Intramuscular Preadipocytes by Targeting MAP3K1 and Activating PI3K-Akt Pathway. Cells 2024, 13, 1459. [Google Scholar] [CrossRef]
- Zhai, B.; Li, H.; Li, S.; Gu, J.; Zhang, H.; Zhang, Y.; Li, H.; Tian, Y.; Li, G.; Wang, Y. Transcriptome analysis reveals FABP5 as a key player in the development of chicken abdominal fat, regulated by miR-122-5p targeting. BMC Genom. 2023, 24, 386. [Google Scholar] [CrossRef]
- Li, G.; Chen, Y.; Jin, W.; Zhai, B.; Li, Y.; Sun, G.; Li, H.; Kang, X.; Tian, Y. Effects of miR-125b-5p on Preadipocyte Proliferation and Differentiation in Chicken. Mol. Biol. Rep. 2021, 48, 491–502. [Google Scholar] [CrossRef]
- Li, G.; Fu, S.; Chen, Y.; Jin, W.; Zhai, B.; Li, Y.; Sun, G.; Han, R.; Wang, Y.; Tian, Y.; et al. MicroRNA-15a Regulates the Differentiation of Intramuscular Preadipocytes by Targeting ACAA1, ACOX1 and SCP2 in Chickens. Int. J. Mol. Sci. 2019, 20, 4063. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, D.H.; Li, F.; Sun, J.W.; Jiang, R.R.; Li, Z.J.; Han, R.L.; Li, G.X.; Liu, X.J.; Kang, X.T.; et al. Integrated Analysis of MiRNA and Genes Associated with Meat Quality Reveals that Gga-MiR-140-5p Affects Intramuscular Fat Deposition in Chickens. Cell Physiol. Biochem. 2018, 46, 2421–2433. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Chen, Y.; Zhang, Y.; Zhang, Y.; Chen, L.; Mo, D. Up-regulated miR-145 expression inhibits porcine preadipocytes differentiation by targeting IRS1. Int. J. Biol. Sci. 2012, 8, 1408–1417. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Xi, Q.Y.; Cheng, X.; Dong, T.; Zhu, X.T.; Shu, G.; Wang, L.N.; Jiang, Q.Y.; Zhang, Y.L. miR-146a-5p inhibits TNF-alpha-induced adipogenesis via targeting insulin receptor in primary porcine adipocytes. J. Lipid Res. 2016, 57, 1360–1372. [Google Scholar] [CrossRef]
- Wu, W.; Xu, K.; Li, M.; Zhang, J.; Wang, Y. MicroRNA-29b/29c targeting CTRP6 influences porcine adipogenesis via the AKT/PKA/MAPK Signalling pathway. Adipocyte 2021, 10, 264–274. [Google Scholar] [CrossRef]
- Du, J.; Xu, Y.; Zhang, P.; Zhao, X.; Gan, M.; Li, Q.; Ma, J.; Tang, G.; Jiang, Y.; Wang, J.; et al. MicroRNA-125a-5p Affects Adipocytes Proliferation, Differentiation and Fatty Acid Composition of Porcine Intramuscular Fat. Int. J. Mol. Sci. 2018, 19, 501. [Google Scholar] [CrossRef]
- Shi, X.E.; Li, Y.F.; Jia, L.; Ji, H.L.; Song, Z.Y.; Cheng, J.; Wu, G.F.; Song, C.C.; Zhang, Q.L.; Zhu, J.Y.; et al. MicroRNA-199a-5p affects porcine preadipocyte proliferation and differentiation. Int. J. Mol. Sci. 2014, 15, 8526–8538. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Y.; Chen, X.; Peng, Y.; Chen, F.; He, Y.; Pang, W.; Yang, G.; Yu, T. MiR-127 attenuates adipogenesis by targeting MAPK4 and HOXC6 in porcine adipocytes. J. Cell Physiol. 2019, 234, 21838–21850. [Google Scholar] [CrossRef]
- Shan, B.; Yan, M.; Yang, K.; Lin, W.; Yan, J.; Wei, S.; Wei, W.; Chen, J.; Zhang, L. MiR-218-5p Affects Subcutaneous Adipogenesis by Targeting ACSL1, a Novel Candidate for Pig Fat Deposition. Genes 2022, 13, 260. [Google Scholar] [CrossRef]
- Zhang, D.; Hao, W.; Zhu, R.; Wang, L.; Wu, X.; Tian, M.; Liu, D.; Yang, X. MiR-26a Inhibits Porcine Adipogenesis by Regulating ACADM and ACSL1 Genes and Cell Cycle Progression. Animals 2024, 14, 3491. [Google Scholar] [CrossRef]
- Zhang, A.; Lu, L.; Yang, F.; Luo, T.; Yang, S.; Yang, P.; Li, X.; Deng, X.; Qiu, Y.; Chen, L.; et al. Effects of miR-29c on proliferation and adipogenic differentiation of porcine bone marrow mesenchymal stromal cells. Adipocyte 2024, 13, 2365211. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wei, D.; Cheng, G.; Li, S.; Wang, L.; Wang, Y.; Wang, X.; Zhang, S.; Wang, H.; Zan, L. Bta-miR-130a/b regulates preadipocyte differentiation by targeting PPARG and CYP2U1 in beef cattle. Mol. Cell Probes 2018, 42, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Zhao, X.; Shan, X.; Zhu, Y.; Wang, S.; Chen, H.; Li, H.; Ma, Y. MiR-107 Regulates Adipocyte Differentiation and Adipogenesis by Targeting Apolipoprotein C-2 (APOC2) in Bovine. Genes 2022, 13, 1467. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, D.; Yang, Z.; Sun, M.; Gao, N.; Mei, C.; Zan, L. bta-miR-484 Inhibits Bovine Intramuscular Adipogenesis by Regulating Mitotic Clonal Expansion via the MAP3K9/JNK/CCND1 Axis. J. Agric. Food Chem. 2024, 73, 1062–1074. [Google Scholar] [CrossRef]
- Hu, C.; Yang, M.; Feng, X.; Wang, S.; Ma, Y.; Ma, Y. miR-10167-3p targets TCF7L1 to inhibit bovine adipocyte differentiation and promote bovine adipocyte proliferation. Genomics 2024, 116, 110903. [Google Scholar] [CrossRef]
- Chen, M.; Zhang, C.; Wu, Z.; Guo, S.; Lv, W.; Song, J.; Hao, B.; Bai, J.; Zhang, X.; Xu, H.; et al. Bta-miR-365-3p-targeted FK506-binding protein 5 participates in the AMPK/mTOR signaling pathway in the regulation of preadipocyte differentiation in cattle. Anim. Biosci. 2024, 37, 1156–1167. [Google Scholar] [CrossRef]
- Liu, H.; Li, B.; Qiao, L.; Liu, J.; Ren, D.; Liu, W. miR-340-5p inhibits sheep adipocyte differentiation by targeting ATF7. Anim. Sci. J. 2020, 91, e13462. [Google Scholar] [CrossRef]
- Deng, K.; Ren, C.; Fan, Y.; Liu, Z.; Zhang, G.; Zhang, Y.; You, P.; Wang, F. miR-27a is an important adipogenesis regulator associated with differential lipid accumulation between intramuscular and subcutaneous adipose tissues of sheep. Domest. Anim. Endocrinol. 2020, 71, 106393. [Google Scholar] [CrossRef]
- Pan, Y.; Jing, J.; Qiao, L.; Liu, J.; An, L.; Li, B.; Ren, D.; Liu, W. MiRNA-seq reveals that miR-124-3p inhibits adipogenic differentiation of the stromal vascular fraction in sheep via targeting C/EBPalpha. Domest. Anim. Endocrinol. 2018, 65, 17–23. [Google Scholar] [CrossRef]
- Luo, M.; Wang, L.; Xiao, C.; Zhou, M.; Li, M.; Li, H. miR136 regulates proliferation and differentiation of small tail han sheep preadipocytes. Adipocyte 2023, 12, 2173966. [Google Scholar] [CrossRef]
- Xue, S.; Liu, K.; Zhao, L.; Zhou, L.; Gao, X.; Liu, L.; Liu, N.; He, J. The role of miR-369-3p in proliferation and differentiation of preadipocytes in Aohan fine-wool sheep. Arch. Anim. Breed. 2023, 66, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Pan, Y.; Zhao, B.; Qiao, L.; Liu, J.; Liang, Y.; Liu, W. MiR-33a inhibits the adipogenic differentiation of ovine adipose-derived stromal vascular fraction cells by targeting SIRT6. Domest. Anim. Endocrinol. 2021, 74, 106513. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.; Yuan, Z.; Li, T.; Wang, H.; Wei, C. The Effects of DDI1 on Inducing Differentiation in Ovine Preadipocytes via Oar-miR-432. Int. J. Mol. Sci. 2023, 24, 11567. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Li, F.; Ma, X.; Sun, J.; Jiang, R.; Tian, Y.; Han, R.; Li, G.; Wang, Y.; Li, Z.; et al. gga-miRNA-18b-3p Inhibits Intramuscular Adipocytes Differentiation in Chicken by Targeting the ACOT13 Gene. Cells 2019, 8, 556. [Google Scholar] [CrossRef]
- Tian, W.; Hao, X.; Nie, R.; Ling, Y.; Zhang, B.; Zhang, H.; Wu, C. Integrative analysis of miRNA and mRNA profiles reveals that gga-miR-106-5p inhibits adipogenesis by targeting the KLF15 gene in chickens. J. Anim. Sci. Biotechnol. 2022, 13, 81. [Google Scholar] [CrossRef]
- Zhu, S.; Zhang, B.; Zhu, T.; Wang, D.; Liu, C.; Liu, Y.; He, Y.; Liang, W.; Li, W.; Han, R.; et al. miR-128-3p inhibits intramuscular adipocytes differentiation in chickens by downregulating FDPS. BMC Genom. 2023, 24, 540. [Google Scholar] [CrossRef]
- Li, F.; Li, D.; Zhang, M.; Sun, J.; Li, W.; Jiang, R.; Han, R.; Wang, Y.; Tian, Y.; Kang, X.; et al. miRNA-223 targets the GPAM gene and regulates the differentiation of intramuscular adipocytes. Gene 2019, 685, 106–113. [Google Scholar] [CrossRef]
- Spizzo, R.; Almeida, M.I.; Colombatti, A.; Calin, G.A. Long non-coding RNAs and cancer: A new frontier of translational research? Oncogene 2012, 31, 4577–4587. [Google Scholar] [CrossRef]
- Ulitsky, I.; Bartel, D.P. lincRNAs: Genomics, evolution, and mechanisms. Cell 2013, 154, 26–46. [Google Scholar] [CrossRef]
- Matsumoto, A.; Pasut, A.; Matsumoto, M.; Yamashita, R.; Fung, J.; Monteleone, E.; Saghatelian, A.; Nakayama, K.I.; Clohessy, J.G.; Pandolfi, P.P. mTORC1 and muscle regeneration are regulated by the LINC00961-encoded SPAR polypeptide. Nature 2017, 541, 228–232. [Google Scholar] [CrossRef]
- Wu, P.; Mo, Y.; Peng, M.; Tang, T.; Zhong, Y.; Deng, X.; Xiong, F.; Guo, C.; Wu, X.; Li, Y.; et al. Emerging role of tumor-related functional peptides encoded by lncRNA and circRNA. Mol. Cancer 2020, 19, 22. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, X.; Li, A.; Xie, L.; Miao, X. Differential regulation of mRNAs and lncRNAs related to lipid metabolism in two pig breeds. Oncotarget 2017, 8, 87539–87553. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.; Zhang, X.; Li, A.; Xie, L.; Miao, X. Genome-Wide Analysis of mRNAs and lncRNAs of Intramuscular Fat Related to Lipid Metabolism in Two Pig Breeds. Cell. Physiol. Biochem. 2018, 50, 2406–2422. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Ma, C.; Wang, W.; Wang, H.; Jiang, Y. Comparative Transcriptomic Analysis of mRNAs, miRNAs and lncRNAs in the Longissimus dorsi Muscles between Fat-Type and Lean-Type Pigs. Biomolecules 2022, 12, 1294. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Wang, Y.; Wang, J. Comprehensive Analysis of Differentially Expressed mRNAs, lncRNAs and circRNAs Related to Intramuscular Fat Deposition in Laiwu Pigs. Genes 2022, 13, 1349. [Google Scholar] [CrossRef]
- Bao, G.; Li, S.; Zhao, F.; Wang, J.; Liu, X.; Hu, J.; Shi, B.; Wen, Y.; Zhao, L.; Luo, Y. Comprehensive Transcriptome Analysis Reveals the Role of lncRNA in Fatty Acid Metabolism in the Longissimus Thoracis Muscle of Tibetan Sheep at Different Ages. Front. Nutr. 2022, 9, 847077. [Google Scholar] [CrossRef]
- Liu, T.; Feng, H.; Yousuf, S.; Xie, L.; Miao, X. Differential regulation of mRNAs and lncRNAs related to lipid metabolism in Duolang and Small Tail Han sheep. Sci. Rep. 2022, 12, 11157. [Google Scholar] [CrossRef]
- Han, F.; Li, J.; Zhao, R.; Liu, L.; Li, L.; Li, Q.; He, J.; Liu, N. Identification and co-expression analysis of long noncoding RNAs and mRNAs involved in the deposition of intramuscular fat in Aohan fine-wool sheep. BMC Genom. 2021, 22, 98. [Google Scholar] [CrossRef]
- He, X.; Wu, R.; Yun, Y.; Qin, X.; Chen, L.; Han, Y.; Wu, J.; Sha, L.; Borjigin, G. Transcriptome analysis of messenger RNA and long noncoding RNA related to different developmental stages of tail adipose tissues of sunite sheep. Food Sci. Nutr. 2021, 9, 5722–5734. [Google Scholar] [CrossRef]
- Ma, L.; Zhang, M.; Jin, Y.; Erdenee, S.; Hu, L.; Chen, H.; Cai, Y.; Lan, X. Comparative Transcriptome Profiling of mRNA and lncRNA Related to Tail Adipose Tissues of Sheep. Front. Genet. 2018, 9, 365. [Google Scholar] [CrossRef]
- Bakhtiarizadeh, M.R.; Salami, S.A. Identification and Expression Analysis of Long Noncoding RNAs in Fat-Tail of Sheep Breeds. G3 Genes Genomes Genet. 2019, 9, 1263–1276. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Sun, X.; Cai, H.; Sun, Y.; Plath, M.; Li, C.; Lan, X.; Lei, C.; Lin, F.; Bai, Y.; et al. Long non-coding RNA ADNCR suppresses adipogenic differentiation by targeting miR-204. Biochim. Biophys. Acta 2016, 1859, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Z.; Song, Q.; Dong, B.; Bi, Y.; Bai, H.; Jiang, Y.; Chang, G.; Chen, G. Transcriptome Sequencing to Identify Important Genes and lncRNAs Regulating Abdominal Fat Deposition in Ducks. Animals 2022, 12, 1256. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zhang, X.; Han, K.; Zhang, G.; Wang, J.; Xie, K.; Xue, Q. Genome-Wide Analysis of lncRNA and mRNA Expression During Differentiation of Abdominal Preadipocytes in the Chicken. G3 Genes Genomes Genet. 2017, 7, 953–966. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, T.; Zhang, S.; Huang, J.; Zhang, G.; Xie, K.; Wang, J.; Wu, H.; Dai, G. Identification of Long Non-Coding RNA-Associated Competing Endogenous RNA Network in the Differentiation of Chicken Preadipocytes. Genes 2019, 10, 795. [Google Scholar] [CrossRef]
- Wang, J.; Chen, M.Y.; Chen, J.F.; Ren, Q.L.; Zhang, J.Q.; Cao, H.; Xing, B.S.; Pan, C.Y. LncRNA IMFlnc1 promotes porcine intramuscular adipocyte adipogenesis by sponging miR-199a-5p to up-regulate CAV-1. BMC Mol. Cell Biol. 2020, 21, 77. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Y.; Ji, M.; Rong, X.; Zhang, Y.; Yang, S.; Lu, C.; Cai, C.; Gao, P.; Guo, X.; et al. The long non-coding RNA lncMYOZ2 mediates an AHCY/MYOZ2 axis to promote adipogenic differentiation in porcine preadipocytes. BMC Genom. 2022, 23, 700. [Google Scholar] [CrossRef]
- Yi, X.; He, Z.; Tian, T.; Kou, Z.; Pang, W. LncIMF2 promotes adipogenesis in porcine intramuscular preadipocyte through sponging MiR-217. Anim. Biotechnol. 2023, 34, 268–279. [Google Scholar] [CrossRef]
- Wei, N.; Wang, Y.; Xu, R.X.; Wang, G.Q.; Xiong, Y.; Yu, T.Y.; Yang, G.S.; Pang, W.J. PU. 1 antisense lncRNA against its mRNA translation promotes adipogenesis in porcine preadipocytes. Anim. Genet. 2015, 46, 133–140. [Google Scholar] [CrossRef]
- Zhang, S.; Kang, Z.; Cai, H.; Jiang, E.; Pan, C.; Dang, R.; Lei, C.; Chen, H.; Lan, X. Identification of novel alternative splicing of bovine lncRNA lncFAM200B and its effects on preadipocyte proliferation. J. Cell Physiol. 2021, 236, 601–611. [Google Scholar] [CrossRef]
- Ran, H.; Yang, Y.; Luo, M.; Liu, X.; Yue, B.; Chai, Z.; Zhong, J.; Wang, H. Molecular Regulation of Yak Preadipocyte Differentiation and Proliferation by LncFAM200B and ceRNA Regulatory Network Analysis. Cells 2022, 11, 2366. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Yang, X.; Zhang, D.; Zhang, W.; Wang, X.; Xie, K.; He, J.; Mei, C.; Zan, L. RNA-seq analysis reveals the critical role of the novel lncRNA BIANCR in intramuscular adipogenesis through the ERK1/2 signaling pathway. J. Anim. Sci. Biotechnol. 2023, 14, 21. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, Q.; Wang, S.; Wei, X.; Li, F.; Ma, Y. High-Throughput RNA Sequencing Reveals NDUFC2-AS lncRNA Promotes Adipogenic Differentiation in Chinese Buffalo (Bubalus bubalis L). Genes 2019, 10, 689. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Ma, X.; Li, H.; Li, X.; Wang, J.; Zan, L. SERPINE1AS2 regulates intramuscular adipogenesis by inhibiting PAI1 protein expression. Int. J. Biol. Macromol. 2024, 275 Pt 2, 133592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Li, F.; Sun, J.W.; Li, D.H.; Li, W.T.; Jiang, R.R.; Li, Z.J.; Liu, X.J.; Han, R.L.; Li, G.X.; et al. LncRNA IMFNCR Promotes Intramuscular Adipocyte Differentiation by Sponging miR-128-3p and miR-27b-3p. Front. Genet. 2019, 10, 42. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, X.; Zhai, Y.; Zhang, D.; Sui, L.; Li, W.; Jiang, R.; Han, R.; Li, G.; Li, Z.; et al. Comprehensive Transcriptome Analysis of lncRNAs Reveals the Role of lncAD in Chicken Intramuscular and Abdominal Adipogenesis. J. Agric. Food Chem. 2020, 68, 3678–3688. [Google Scholar] [CrossRef]
- Cai, B.; Ma, M.; Zhang, J.; Kong, S.; Zhou, Z.; Li, Z.; Abdalla, B.A.; Xu, H.; Zhang, X.; Lawal, R.A.; et al. Long noncoding RNA ZFP36L2-AS functions as a metabolic modulator to regulate muscle development. Cell Death Dis. 2022, 13, 389. [Google Scholar] [CrossRef]
- Ma, X.; He, Y.; Liu, C.; Zhu, T.; Li, D.; Li, W.; Sun, G.; Kang, X. Long Noncoding RNA 6302 Regulates Chicken Preadipocyte Differentiation by Targeting SLC22A16. Genes 2024, 15, 758. [Google Scholar] [CrossRef]
- Sun, Y.; Cai, R.; Wang, Y.; Zhao, R.; Qin, J.; Pang, W. A Newly Identified LncRNA LncIMF4 Controls Adipogenesis of Porcine Intramuscular Preadipocyte through Attenuating Autophagy to Inhibit Lipolysis. Animals 2020, 10, 926. [Google Scholar] [CrossRef]
- Shi, M.; Yang, S.; Zhao, X.; Sun, D.; Li, Y.; Yang, J.; Li, M.; Cai, C.; Guo, X.; Li, B.; et al. Effect of LncRNA LOC106505926 on myogenesis and Lipogenesis of porcine primary cells. BMC Genom. 2024, 25, 530. [Google Scholar] [CrossRef]
- Cai, H.; Li, M.; Jian, W.; Song, C.; Huang, Y.; Lan, X.; Lei, C.; Chen, H. A novel lncRNA BADLNCR1 inhibits bovine adipogenesis by repressing GLRX5 expression. J. Cell. Mol. Med. 2020, 24, 7175–7186. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Gao, Q.; Tian, Z.; Lu, X.; Sun, Y.; Chen, Z.; Zhang, H.; Mao, Y.; Yang, Z. MIR221HG Is a Novel Long Noncoding RNA that Inhibits Bovine Adipocyte Differentiation. Genes 2019, 11, 29. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wang, J.; Li, B.; Sun, B.; Yu, S.; Wang, X.; Zan, L. Long Non-Coding RNA BNIP3 Inhibited the Proliferation of Bovine Intramuscular Preadipocytes via Cell Cycle. Int. J. Mol. Sci. 2023, 24, 4234. [Google Scholar] [CrossRef]
- Cai, B.; Ma, M.; Zhang, J.; Wang, Z.; Kong, S.; Zhou, Z.; Lian, L.; Zhang, J.; Li, J.; Wang, Y.; et al. LncEDCH1 improves mitochondrial function to reduce muscle atrophy by interacting with SERCA2. Mol. Ther. Nucleic Acids 2022, 27, 319–334. [Google Scholar] [CrossRef]
- Xu, T.; Wu, J.; Han, P.; Zhao, Z.; Song, X. Circular RNA expression profiles and features in human tissues: A study using RNA-seq data. BMC Genom. 2017, 18 (Suppl. S6), 680. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Zhou, L.; Hao, X.; Wang, L.; Han, F.; Liu, L.; Duan, X.; Guo, F.; He, J.; Liu, N. Identification and Characterization of Circular RNAs in Association With the Deposition of Intramuscular Fat in Aohan Fine-Wool Sheep. Front. Genet. 2021, 12, 759747. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, J.; Zheng, Q.; Wang, S.; Wei, X.; Li, F.; Shang, J.; Lei, C.; Ma, Y. Characterization of Circular RNAs in Chinese Buffalo (Bubalus bubalis) Adipose Tissue: A Focus on Circular RNAs Involved in Fat Deposition. Animals 2019, 9, 403. [Google Scholar] [CrossRef]
- Yousuf, S.; Li, A.; Feng, H.; Lui, T.; Huang, W.; Zhang, X.; Xie, L.; Miao, X. Genome-Wide Expression Profiling and Networking Reveals an Imperative Role of IMF-Associated Novel CircRNAs as ceRNA in Pigs. Cells 2022, 11, 2638. [Google Scholar] [CrossRef]
- Li, Q.; Wang, L.; Xing, K.; Yang, Y.; Abiola Adetula, A.; Liu, Y.; Yi, G.; Zhang, H.; Sweeney, T.; Tang, Z. Identification of circRNAs Associated with Adipogenesis Based on RNA-seq Data in Pigs. Genes 2022, 13, 2062. [Google Scholar] [CrossRef]
- Li, X.; Zhang, H.; Wang, Y.; Li, Y.; Wang, Y.; Zhu, J.; Lin, Y. Chi-Circ_0006511 Positively Regulates the Differentiation of Goat Intramuscular Adipocytes via Novel-miR-87/CD36 Axis. Int. J. Mol. Sci. 2022, 23, 12295. [Google Scholar] [CrossRef]
- Zhang, S.; Jiang, E.; Kang, Z.; Bi, Y.; Liu, H.; Xu, H.; Wang, Z.; Lei, C.; Chen, H.; Lan, X. CircRNA Profiling Reveals an Abundant circBDP1 that Regulates Bovine Fat Development by Sponging miR-181b/miR-204 Targeting Sirt1/TRARG1. J. Agric. Food Chem. 2022, 70, 14312–14328. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Zhang, S.; Yue, B.; Zhang, S.; Jiang, E.; Chen, H.; Lan, X. CircRNA Profiling Reveals CircPPARgamma Modulates Adipogenic Differentiation via Sponging miR-92a-3p. J. Agric. Food Chem. 2022, 70, 6698–6708. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Zhang, S.; Jiang, E.; Wang, X.; Wang, Z.; Chen, H.; Lan, X. circFLT1 and lncCCPG1 Sponges miR-93 to Regulate the Proliferation and Differentiation of Adipocytes by Promoting lncSLC30A9 Expression. Mol. Ther. Nucleic Acids 2020, 22, 484–499. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Zhao, J.; Li, F.; Aloufi, B.H.; Alshammari, A.M.; Ma, Y. Weighted Gene Co-expression Network Analysis Revealed That CircMARK3 Is a Potential CircRNA Affects Fat Deposition in Buffalo. Front. Vet. Sci. 2022, 9, 946447. [Google Scholar] [CrossRef]
- Shen, X.; Tang, J.; Huang, Y.; Lan, X.; Lei, C.; Chen, H. CircRNF111 Contributes to Adipocyte Differentiation by Elevating PPARgamma Expression via miR-27a-3p. Epigenetics 2023, 18, 2145058. [Google Scholar] [CrossRef]
- Liu, Y.; Dou, Y.; Qi, K.; Li, C.; Song, C.; Li, X.; Li, X.; Qiao, R.; Wang, K.; Han, X. CircSETBP1 Acts as a MiR-149-5p Sponge to Promote Intramuscular Fat Deposition by Regulating CRTCs. J. Agric. Food Chem. 2022, 70, 12841–12851. [Google Scholar] [CrossRef]
- Li, B.; He, Y.; Wu, W.; Tan, X.; Wang, Z.; Irwin, D.M.; Wang, Z.; Zhang, S. Circular RNA Profiling Identifies Novel circPPARA that Promotes Intramuscular Fat Deposition in Pigs. J. Agric. Food Chem. 2022, 70, 4123–4137. [Google Scholar] [CrossRef]
- Wang, L.; Liang, W.; Wang, S.; Wang, Z.; Bai, H.; Jiang, Y.; Bi, Y.; Chen, G.; Chang, G. Circular RNA expression profiling reveals that circ-PLXNA1 functions in duck adipocyte differentiation. PLoS ONE 2020, 15, e0236069. [Google Scholar] [CrossRef]
- Shen, X.; Tang, J.; Ru, W.; Zhang, X.; Huang, Y.; Lei, C.; Cao, H.; Lan, X.; Chen, H. CircINSR Regulates Fetal Bovine Muscle and Fat Development. Front. Cell Dev. Biol. 2020, 8, 615638. [Google Scholar] [CrossRef]
- Zhao, B.; Zhang, H.; Zhao, D.; Liang, Y.; Qiao, L.; Liu, J.; Pan, Y.; Yang, K.; Liu, W. circINSR Inhibits Adipogenic Differentiation of Adipose-Derived Stromal Vascular Fractions through the miR-152/MEOX2 Axis in Sheep. Int. J. Mol. Sci. 2023, 24, 3501. [Google Scholar] [CrossRef]
- Jiang, R.; Li, H.; Yang, J.; Shen, X.; Song, C.; Yang, Z.; Wang, X.; Huang, Y.; Lan, X.; Lei, C.; et al. circRNA Profiling Reveals an Abundant circFUT10 that Promotes Adipocyte Proliferation and Inhibits Adipocyte Differentiation via Sponging let-7. Mol. Ther. Nucleic Acids 2020, 20, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Feng, X.; Ma, Y.; Wei, D.; Zhang, L.; Wang, S.; Ma, Y. CircADAMTS16 Inhibits Differentiation and Promotes Proliferation of Bovine Adipocytes by Targeting miR-10167-3p. Cells 2023, 12, 1175. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Zhang, W.; Raza, S.H.A.; Qu, X.; Yang, Z.; Deng, J.; Ma, J.; Aloufi, B.H.; Wang, J.; Zan, L. CircSSBP2 acts as a MiR-2400 sponge to promote intramuscular preadipocyte proliferation by regulating NDRG1. Mol. Genet. Genom. 2024, 299, 48. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Li, J.; Ji, M.; An, J.; Zhao, T.; Yang, Y.; Cai, C.; Gao, P.; Cao, G.; Guo, X.; et al. CircHOMER1 inhibits porcine adipogenesis via the miR-23b/SIRT1 axis. FASEB J. 2023, 37, e22828. [Google Scholar] [CrossRef]
- Rong, X.; Li, R.; Gong, T.; Li, H.; Zhao, X.; Cao, G.; Li, M.; Li, B.; Yang, Y.; Guo, X. CircMEF2C(2, 3) modulates proliferation and adipogenesis of porcine intramuscular preadipocytes by miR-383/671-3p/MEF2C axis. iScience 2024, 27, 109710. [Google Scholar] [CrossRef]
- Liu, X.; Bai, Y.; Cui, R.; He, S.; Zhao, X.; Wu, K.; Fang, M. Sus_circPAPPA2 Regulates Fat Deposition in Castrated Pigs through the miR-2366/GK Pathway. Biomolecules 2022, 12, 753. [Google Scholar] [CrossRef]
- Li, H.; Zhang, H.; Dai, Y.; Li, S.; Gu, J.; Wu, R.; Jia, J.; Shen, J.; Zhang, Y.; Li, H.; et al. CircITGB5 regulates the proliferation and adipogenic differentiation of chicken intramuscular preadipocytes through the miR-181b-5p/CPT1A axis. Int. J. Biol. Macromol. 2024, 283 Pt 4, 137608. [Google Scholar] [CrossRef]
- Shen, Y.; Liang, Y.; Yuan, Z.; Qiao, L.; Liu, J.; Pan, Y.; Yang, K.; Liu, W. circARID1A Inhibits Tail Fat Cell Differentiation in Guangling Large-Tailed Sheep by Regulating the miR-493-3p/YTHDF2 Axis. Int. J. Mol. Sci. 2024, 25, 12351. [Google Scholar] [CrossRef]
- Guo, W.; Ciwang, R.; Wang, L.; Zhang, S.; Liu, N.; Zhao, J.; Zhou, L.; Li, H.; Gao, X.; He, J. CircRNA-5335 Regulates the Differentiation and Proliferation of Sheep Preadipocyte via the miR-125a-3p/STAT3 Pathway. Vet. Sci. 2024, 11, 70. [Google Scholar] [CrossRef]


| Model | miRNA | Function | Direct Target(s) | Cell Culture Model |
|---|---|---|---|---|
| pig | miR-103 | Pro-adipogenic | RAI14 | preadipocytes |
| pig | miR-145 | Anti-adipogenic | IRS1 | dedifferentiated fat cells |
| pig | miR-181a | Pro-adipogenic | TNF-α | preadipocytes |
| pig | miR-199a-5p | Anti-adipogenic | CAV-1 | preadipocytes |
| pig | miR-15a/b | Pro-adipogenic | FOXO1 | preadipocytes |
| pig | miR-196a | Pro-adipogenic | -- | preadipocytes |
| pig | miR-29b/29c | Anti-adipogenic | CTRP6 | preadipocytes |
| pig | miR-125a-5p | Anti-adipogenic | KLF13 | preadipocytes |
| pig | miR-127 | Anti-adipogenic | HOXC6 | preadipocytes |
| pig | miR-146a-5p | Anti-adipogenic | IR | preadipocytes |
| pig | miR-218-5p | Anti-adipogenic | ACSL1 | preadipocytes |
| pig | miR-26a | Anti-adipogenic | ACADM, ACSL1 | preadipocytes |
| pig | miR-29c | Anti-adipogenic | IGF1 | bone marrow stromal cells |
| cattle | miR-143 | Pro-adipogenic | -- | preadipocytes |
| cattle | miR-1271 | Pro-adipogenic | ATF3 | preadipocytes |
| cattle | miR-381 | Pro-adipogenic | KCTD15 | preadipocytes |
| cattle | miR-130a/b | Anti-adipogenic | PPAR-γ, CYP2U1 | preadipocytes |
| cattle | miR-107 | Anti-adipogenic | APOC2 | adipocytes |
| cattle | miR-330 | Pro-adipogenic | SESN3 | intramuscular preadipocytes |
| cattle | miR-484 | Anti-adipogenic | MAP3K9 | intramuscular adipocytes |
| cattle | miR-10167-3p | Anti-adipogenic | TCF7L1 | preadipocytes |
| cattle | miR--365-3p | Anti-adipogenic | FKBP5 | adipocytes |
| goat | miR-196a | Pro-adipogenic | MAP3K1 | preadipocytes |
| sheep | miR-340-5p | Anti-adipogenic | ATF7 | adipocytes |
| sheep | miR-27a | Anti-adipogenic | RXRα | preadipocytes |
| sheep | miR-124-3p | Anti-adipogenic | C/EBP-α | preadipocytes |
| sheep | miR-136 | Anti-adipogenic | PPARGC1B | preadipocytes |
| sheep | miR-369-3p | Anti-adipogenic | -- | preadipocytes |
| sheep | miR-33a | Anti-adipogenic | SIRT6 | stromal vascular fraction cells |
| sheep | miR-432 | Anti-adipogenic | DDI1 | preadipocytes |
| chicken | miR-18b-3p | Anti-adipogenic | ACOT13 | adipocytes |
| chicken | miR-122-5p | Pro-adipogenic | FABP5 | preadipocytes |
| chicken | miR-106-5p | Anti-adipogenic | KLF15 | preadipocytes |
| chicken | miR-128-3p | Anti-adipogenic | FDPS | intramuscular precursor adipocytes |
| chicken | miR-223 | Anti-adipogenic | GPAM | preadipocytes |
| chicken | miR-125b-5p | Pro-adipogenic | ACSBG2 | preadipocytes |
| chicken | miR-15a | Pro-adipogenic | ACAA1, ACOX1, SCP2 | intramuscular preadipocytes |
| chicken | miR-140-5p | Pro-adipogenic | RXRG | intramuscular preadipocyte |
| Model | lncRNA | Function on Adipogenesis | Direct Target(s) | Cell Culture Model |
|---|---|---|---|---|
| pig | IMFlnc1 | promote | miR-199a-5p | intramuscular preadipocyte |
| pig | lncIMF4 | inhibit | -- | intramuscular adipocytes |
| pig | lncMYOZ2 | promote | MYOZ2 | preadipocytes |
| pig | lncIMF2 | promote | miR-217 | intramuscular preadipocytes |
| pig | PU.1 AS lncRNA | promote | PU.1 | preadipocytes |
| pig | PLA2G16-AS | -- | PLA2G16 | PK15 |
| pig | LOC106505926 | inhibit | FASN | preadipocytes |
| bovine | ADNCR | inhibit | miR-204 | preadipocytes |
| bovine | BADLNCR1 | inhibit | SREBP1/2 | adipocytes |
| bovine | MIR221HG | inhibit | -- | adipocytes |
| bovine | SERPINE1AS2 | promote | PAI1 | intramuscular adipocytes |
| yak | lncFAM200B | promote | -- | preadipocytes |
| bovine | BNIP3 | inhibit | -- | intramuscular preadipocytes |
| bovine | BIANCR | promote | -- | intramuscular adipocytes |
| buffalo | NDUFC2-AS lncRNA | promote | -- | adipocytes |
| chicken | IMFNCR | promote | miR-128-3p/miR-27b-3p | intramuscular adipocytes |
| chicken | lncAD | promote | -- | intramuscular preadipocytes |
| chicken | ZFP36L2-AS | promote | ACACA/PC | preadipocytes |
| chicken | lncEDCH1 | inhibit | SERCA2 | myoblasts |
| chicken | LNC6302 | promote | -- | abdominal preadipocyte |
| Model | circRNA | Function on Adipogenesis | Direct Target(s) | Cell Culture Model |
|---|---|---|---|---|
| goat | circ_0006511 | promote | miR-87 | intramuscular preadipocytes |
| bovine | circBDP1 | promote | miR-204/miR-181b | adipocytes |
| bovine | circPPARγ | promote | miR-92a-3p | primary adipocytes |
| bovine | circFLT1 | promote | miR-93 | adipocytes |
| bovine | circINSR | inhibit | miR-15/16 | preadipocytes |
| bovine | circRNF111 | promote | miR-27a-3p | preadipocytes |
| bovine | circFUT10 | inhibit | let-7c | adipocytes |
| bovine | circADAMTS16 | inhibit | miR-10167-3p | preadipocytes |
| bovine | circSSBP2 | inhibit | miR-2400 | intramuscular preadipocytes |
| buffalo | circMARK3 | promote | -- | buffalo adipocytes/3T3-L1 |
| pig | circSETBP1 | promote | miR-149-5p | intramuscular preadipocytes |
| pig | circPPARA | promote | miR-429/miR-200b | intramuscular preadipocytes |
| pig | circHOMER1 | inhibit | miR-23b | preadipocytes |
| pig | circPAPPA2 | inhibit | miR-2366 | preadipocyte |
| pig | circMEF2C(2, 3) | inhibit | miR-383 | intramuscular preadipocytes |
| sheep | circINSR | inhibit | miR-152 | stromal vascular fractions |
| sheep | circARID1A | inhibit | miR-493-3p | adipocytes |
| sheep | circRNA-5335 | promote | miR-125a-3p | adipocytes |
| duck | circ-PLXNA1 | promote | miR-214 | adipocytes |
| chicken | circITGB5 | inhibit | miR-181b-5p | intramuscular preadipocytes |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, J.; Zhao, X.; Wang, Y.; Wang, J. Non-Coding RNAs in Regulating Fat Deposition in Farm Animals. Animals 2025, 15, 797. https://doi.org/10.3390/ani15060797
Li J, Zhao X, Wang Y, Wang J. Non-Coding RNAs in Regulating Fat Deposition in Farm Animals. Animals. 2025; 15(6):797. https://doi.org/10.3390/ani15060797
Chicago/Turabian StyleLi, Jingxuan, Xueyan Zhao, Yanping Wang, and Jiying Wang. 2025. "Non-Coding RNAs in Regulating Fat Deposition in Farm Animals" Animals 15, no. 6: 797. https://doi.org/10.3390/ani15060797
APA StyleLi, J., Zhao, X., Wang, Y., & Wang, J. (2025). Non-Coding RNAs in Regulating Fat Deposition in Farm Animals. Animals, 15(6), 797. https://doi.org/10.3390/ani15060797





