Effect of Dietary Cannabis sativa L. Residue Supplementation on Meat Quality and Flavor-Enhancing Free Amino Acids in Broiler Chickens
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Ethics Statement
2.2. Animals, Treatments, and Experimental Design
2.3. Growth Performance
2.4. Meat Quality Analysis
2.4.1. Sample Collection
2.4.2. Physical Properties
- L1, a1, b1 were the color of the control sample;
- L2, a2, b2 were the color of the treatment sample.
2.4.3. Fatty Acid Composition
2.4.4. Free Amino Acids
2.4.5. Chemical Composition and Ribonucleotide Content
2.5. Statistical Analysis
3. Results
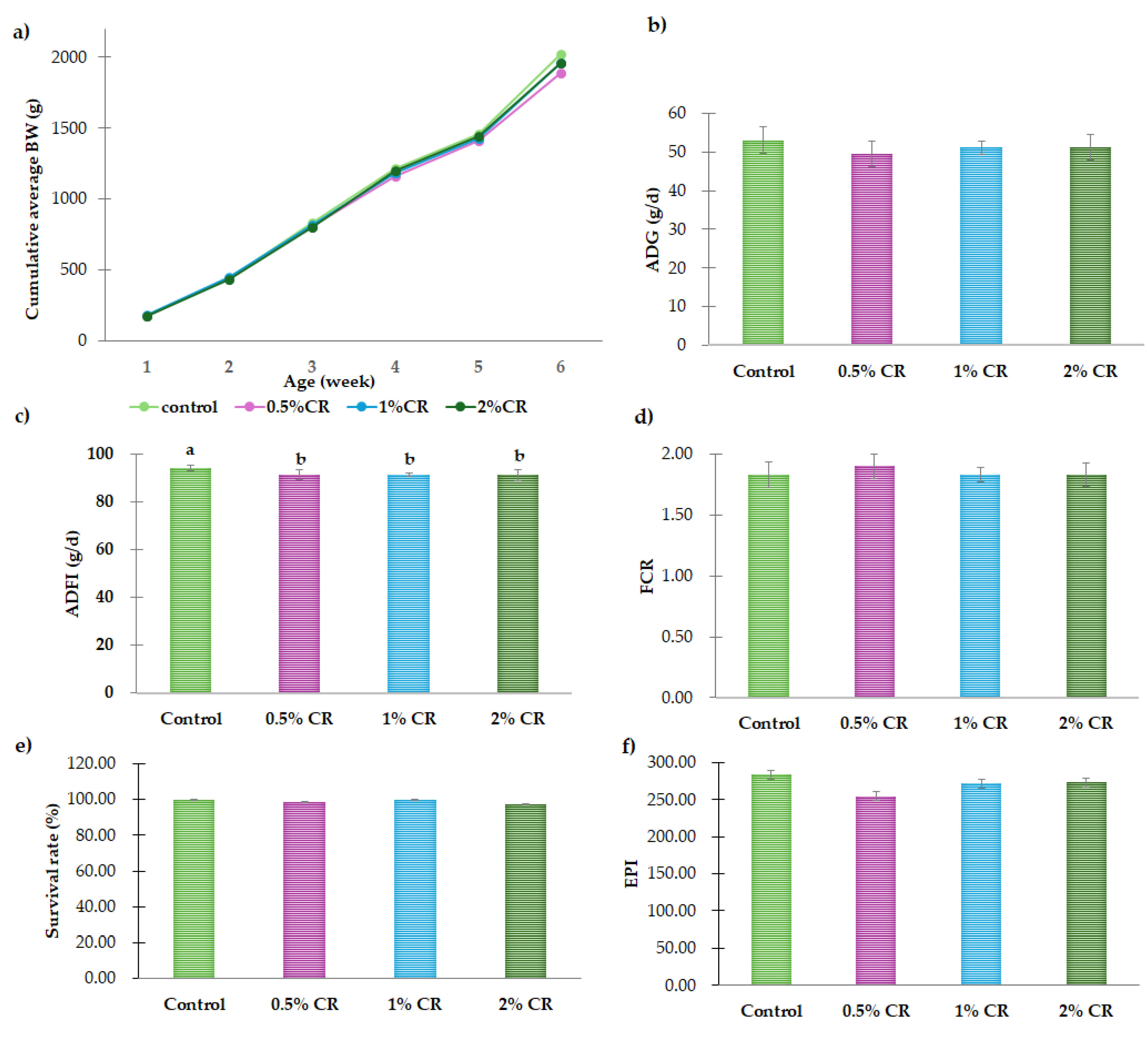
3.1. Growth Performance
3.2. Physical Properties
3.3. Fatty Acid Composition
3.4. Free Amino Acids
3.5. Chemical Composition and Ribonucleotide Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- OECD/FAO. OECD-FAO Agricultural Outlook 2023–2032; OECD-FAO Agricultural Outlook; OECD: Paris, France, 2023; ISBN 9789264619333. [Google Scholar]
- Mir, N.A.; Rafiq, A.; Kumar, F.; Singh, V.; Shukla, V. Determinants of Broiler Chicken Meat Quality and Factors Affecting Them: A Review. J. Food Sci. Technol. 2017, 54, 2997–3009. [Google Scholar] [CrossRef] [PubMed]
- Estévez, M. Oxidative Damage to Poultry: From Farm to Fork. Poult. Sci. 2015, 94, 1368–1378. [Google Scholar] [CrossRef] [PubMed]
- Balant, M.; Gras, A.; Ruz, M.; Vallès, J.; Vitales, D.; Garnatje, T. Traditional Uses of Cannabis: An Analysis of the CANNUSE Database. J. Ethnopharmacol. 2021, 279, 114362. [Google Scholar] [CrossRef]
- Jin, D.; Dai, K.; Xie, Z.; Chen, J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020, 10, 3309. [Google Scholar] [CrossRef]
- Konieczka, P.; Wojtasik-Kalinowska, I.; Poltorak, A.; Kinsner, M.; Szkopek, D.; Fotschki, B.; Juśkiewicz, J.; Banach, J.; Michalczuk, M. Cannabidiol Affects Breast Meat Volatile Compounds in Chickens Subjected to Different Infection Models. Sci. Rep. 2022, 12, 18940. [Google Scholar] [CrossRef] [PubMed]
- Bień, D.; Michalczuk, M.; Jóźwik, A.; Matuszewski, A.; Konieczka, P. Effects of Cannabis sativa Extract on Growth Performance, Meat Physicochemical Properties, and Oxidative Status in Chickens Challenged with Clostridium Perfringens and Lipopolysaccharide. Anim. Sci. Pap. Rep. 2024, 42, 81–108. [Google Scholar] [CrossRef]
- Government of Thailand. Narcotics Act (7) B.E. 2562; Royal Thai Government: Bangkok, Thailand, 2019.
- Balenović, M.; Janječić, Z.; Savić, V.; Kasap, A.; Popović, M.; Šimpraga, B.; Sokolović, M.; Bedeković, D.; Kiš, G.; Zglavnik, T.; et al. Immunostimulatory and Antibacterial Effects of Cannabis sativa L. Leaves on Broilers. Animals 2024, 14, 1159. [Google Scholar] [CrossRef]
- Sopian, Y.; Sartsook, A.; Arjin, C.; Lumsangkul, C.; Sringarm, K.; Sivapirunthep, P.; Chaosap, C. Dietary Supplementation of Cannabis sativa Residues in Broiler Chickens Affects Performance, Carcass Characteristics, Intestinal Morphology, Blood Biochemistry Profile and Oxidative Stability. Poult. Sci. 2024, 103, 104117. [Google Scholar] [CrossRef]
- National Research Council of Thailand (NRCT). Ethical Principles and Guidelines for the Procedures on Animals for Scientific Purposes; Institute of Animals for Scientific Purposes Development (IAD): Bangkok, Thailand, 2019; pp. 1–25. [Google Scholar]
- National Research Council (U.S.). Subcommittee on Poultry Nutrition. In Nutrient Requirements of Poultry; National Academy Press: Washington, DC, USA, 1994; ISBN 0309048923. [Google Scholar]
- Kamporn, K.; Deeden, B.; Klompanya, A.; Setakul, J.; Chaosap, C.; Sittigaipong, R. Effect of strain and gender on production performance, carcass characteristics and meat quality of broiler chickens. Int. J. Agric. Technol. 2022, 18, 567–578. [Google Scholar]
- Kryeziu, A.; Mestani, N.; Berisha, S.; Kamberi, M. The European performance indicators of broiler chickens as influenced by stocking density and sex. Agron. Res. 2018, 16, 483491. [Google Scholar]
- Chaosap, C.; Sivapirunthep, P.; Takeungwongtrakul, S.; Zulkifli, R.M.; Sazili, A.Q. Effects of Zn-L-Selenomethionine on Carcass Composition, Meat Characteristics, Fatty Acid Composition, Glutathione Peroxidase Activity, and Ribonucleotide Content in Broiler Chickens. Food Sci. Anim. Resour. 2020, 40, 338–349. [Google Scholar] [CrossRef]
- Mokrzycki, W.; Tatol, M. Colour difference ΔE—A survey. Mach. Graph. Vis. 2011, 20, 383–411. [Google Scholar]
- Morrison, W.R.; Smith, L.M. Preparation of Fatty Acid Methyl Esters and Dimethylacetals from Lipids with Boron Fluoride–Methanol. J. Lipid Res. 1964, 5, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Sringarm, K.; Chaiwang, N.; Wattanakul, W.; Mahinchai, P.; Satsook, A.; Norkeaw, R.; Seel-Audom, M.; Moonmanee, T.; Mekchay, S.; Sommano, S.R.; et al. Improvement of Intramuscular Fat in Longissimus Muscle of Finishing Thai Crossbred Black Pigs by Perilla Cake Supplementation in a Low-Lysine Diet. Foods 2022, 11, 907. [Google Scholar] [CrossRef] [PubMed]
- AOAC. Official Methods of Analysis, 18th ed.; AOAC International: Gaithersburg, MD, USA, 2006; ISBN 0-935584-74. [Google Scholar]
- ISO 13903:2005; First Edition Animal Feeding Stuffs-Determination of Amino Acids Content. ISO: Geneva, Switzerland, 2005. Available online: https://standards.iteh.ai/catalog/standards/sist/bf232efb-deab-4086-baa9-a7a8a8d4d0ed/iso-13903-2005 (accessed on 9 September 2024).
- Mirzapour-Kouhdasht, A.; McClements, D.J.; Taghizadeh, M.S.; Niazi, A.; Garcia-Vaquero, M. Strategies for Oral Delivery of Bioactive Peptides with Focus on Debittering and Masking. NPJ Sci. Food 2023, 7, 22. [Google Scholar] [CrossRef]
- Ding, X.; Yang, C.; Wang, P.; Yang, Z.; Ren, X. Effects of Star Anise (Illicium Verum Hook. f) and Its Extractions on Carcass Traits, Relative Organ Weight, Intestinal Development, and Meat Quality of Broiler Chickens. Poult. Sci. 2020, 99, 5673–5680. [Google Scholar] [CrossRef]
- Shen, M.M.; Zhang, L.L.; Chen, Y.N.; Zhang, Y.Y.; Han, H.L.; Niu, Y.; He, J.T.; Zhang, Y.L.; Cheng, Y.F.; Wang, T. Effects of Bamboo Leaf Extract on Growth Performance, Meat Quality, and Meat Oxidative Stability in Broiler Chickens. Poult. Sci. 2019, 98, 6787–6796. [Google Scholar] [CrossRef]
- Abdel-Wareth, A.A.A.; Kehraus, S.; Südekum, K.H. Peppermint and Its Respective Active Component in Diets of Broiler Chickens: Growth Performance, Viability, Economics, Meat Physicochemical Properties, and Carcass Characteristics. Poult. Sci. 2019, 98, 3850–3859. [Google Scholar] [CrossRef]
- Park, J.H.; Kang, S.N.; Chu, G.M.; Jin, S.K. Growth Performance, Blood Cell Profiles, and Meat Quality Properties of Broilers Fed with Saposhnikovia Divaricata, Lonicera Japonica, and Chelidonium Majus Extracts. Livest. Sci. 2014, 165, 87–94. [Google Scholar] [CrossRef]
- Lee, J.Y.; Sohn, K.H.; Rhee, S.H.; Hwang, D. Saturated Fatty Acids, but Not Unsaturated Fatty Acids, Induce the Expression of Cyclooxygenase-2 Mediated through Toll-like Receptor 4. J. Biol. Chem. 2001, 276, 16683–16689. [Google Scholar] [CrossRef]
- Jia, M.; Zhang, Y.; Gao, Y.; Ma, X. Effects of medium chain fatty acids on intestinal health of monogastric animals. Curr. Protein Pept. Sci. 2020, 21, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Delgado, G.E.; Krämer, B.K.; Lorkowski, S.; März, W.; von Schacky, C.; Kleber, M.E. Individual Omega-9 Monounsaturated Fatty Acids and Mortality—The Ludwigshafen Risk and Cardiovascular Health Study. J. Clin. Lipidol. 2017, 11, 126–135.e5. [Google Scholar] [CrossRef] [PubMed]
- Hristakieva, P.; Oblakova, M.; Ivanova, I.; Mincheva, N.; Penchev, I.; Ivanov, N.; Lalev, M. Growth Performance, Carcass Characteristics and Meat Quality of Broilers Fed Diets Supplemented with Some Dry Herbs. Bulg. J. Agric. Sci. 2023, 29, 102–109. [Google Scholar]
- Dokou, S.; Vasilopoulou, K.; Bonos, E.; Grigoriadou, K.; Savvidou, S.; Stefanakis, M.K.; Christaki, S.; Kyriakoudi, A.; Mourtzinos, I.; Tzora, A.; et al. Effects of Dietary Supplementation with Phytobiotic Encapsulated Plant Extracts on Broilers’ Performance Parameters, Welfare Traits and Meat Characteristics. Ann. Anim. Sci. 2023, 23, 1105–1118. [Google Scholar] [CrossRef]
- Isahq, M.S.; Afridi, M.S.; Ali, J.; Hussain, M.M.; Ahmad, S.; Kanwal, F. Proximate Composition, Phytochemical Screening, GC-MS Studies of Biologically Active Cannabinoids and Antimicrobial Activities of Cannabis indica. Asian Pac. J. Trop. Dis. 2015, 5, 897–902. [Google Scholar] [CrossRef]
- Nakkliang, K.; Areesantichai, C.; Rungsihirunrat, K. Assessment of Pharmacognostic Specification of Cannabis sativa Leaves in Thailand. J. Adv. Pharm. Technol. Res. 2022, 13, 226–231. [Google Scholar] [CrossRef]
- Mirzamohammad, E.; Alirezalu, A.; Alirezalu, K.; Norozi, A.; Ansari, A. Improvement of the Antioxidant Activity, Phytochemicals, and Cannabinoid Compounds of Cannabis sativa by Salicylic Acid Elicitor. Food. Sci. Nutr. 2021, 9, 6873–6881. [Google Scholar] [CrossRef]
- Atalay, S.; Gęgotek, A.; Skrzydlewska, E. Protective Effects of Cannabidiol on the Membrane Proteome of Uvb-Irradiated Keratinocytes. Antioxidants 2021, 10, 402. [Google Scholar] [CrossRef]
- Fordjour, E.; Manful, C.F.; Sey, A.A.; Javed, R.; Pham, T.H.; Thomas, R.; Cheema, M. Cannabis: A Multifaceted Plant with Endless Potentials. Front. Pharmacol. 2023, 14, 1200269. [Google Scholar] [CrossRef]
- Zandani, G.; Anavi-Cohen, S.; Assa-Glazer, T.; Gorelick, J.; Nyska, A.; Sela, N.; Bernstein, N.; Madar, Z. Cannabis Extract Effects on Metabolic Parameters and Gut Microbiota Composition in a Mice Model of NAFLD and Obesity. Evid.-Based Complement. Altern. Med. 2022, 2022, 7964018. [Google Scholar] [CrossRef]
- Manninen, H.; Rotola-Pukkila, M.; Aisala, H.; Hopia, A.; Laaksonen, T. Free Amino Acids and 5′-Nucleotides in Finnish Forest Mushrooms. Food Chem. 2018, 247, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Silvestri, C.; Paris, D.; Martella, A.; Melck, D.; Guadagnino, I.; Cawthorne, M.; Motta, A.; Di Marzo, V. Two Non-Psychoactive Cannabinoids Reduce Intracellular Lipid Levels and Inhibit Hepatosteatosis. J. Hepatol. 2015, 62, 1382–1390. [Google Scholar] [CrossRef] [PubMed]
- Mele, L.; Bidault, G.; Mena, P.; Crozier, A.; Brighenti, F.; Vidal-Puig, A.; Del Rio, D. Dietary (Poly)Phenols, Brown Adipose Tissue Activation, and Energy Expenditure: A Narrative Review. Adv. Nutr. 2017, 8, 694–704. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Wang, P.; Fu, Q.; Xiao, H.; Zhao, Y.; Li, Y.; Song, X.; Xie, H.; Song, Z. Effects of Dietary Supplementation of Anoectochilus Roxburghii Extract (ARE) on Growth Performance, Abdominal Fat Deposition, Meat Quality, and Gut Microbiota in Broilers. Poult. Sci. 2023, 102, 102842. [Google Scholar] [CrossRef]
- Ahmed, S.T.; Islam, M.M.; Bostami, A.B.M.R.; Mun, H.S.; Kim, Y.J.; Yang, C.J. Meat Composition, Fatty Acid Profile and Oxidative Stability of Meat from Broilers Supplemented with Pomegranate (Punica granatum L.) by-Products. Food. Chem. 2015, 188, 481–488. [Google Scholar] [CrossRef]
- Al-Hijazeen, M.A.; Al-Rawashdeh, M.S.; Al-Rabadi, G.J. Cooked Broiler Meat Quality Affected by Different Mediterranean Medicinal Plants in the Diet. Anim. Biosci. 2022, 35, 290–298. [Google Scholar] [CrossRef]
- Aliani, M.; Farmer, L.J.; Kennedy, J.T.; Moss, B.W.; Gordon, A. Post-Slaughter Changes in ATP Metabolites, Reducing and Phosphorylated Sugars in Chicken Meat. Meat Sci. 2013, 94, 55–62. [Google Scholar] [CrossRef]
- Li, X.; Chen, S.; Ouyang, K.H.; Wang, W.J. Effects of Polysaccharides from Yingshan Yunwu Tea on Free Amino Acids, Flavor Nucleotides and Antioxidant Abilities in Chickens. Res. Vet. Sci. 2022, 149, 11–20. [Google Scholar] [CrossRef]
| Variables | Starter Diets (1–23 Days) | Finisher Diets (24–40 Days) | ||||||
|---|---|---|---|---|---|---|---|---|
| CR | CR | |||||||
| 0% | 0.5% | 1% | 2% | 0% | 0.5% | 1% | 2% | |
| Proximate composition (%) | ||||||||
| Dry matter | 91.57 | 91.45 | 91.18 | 91.33 | 90.90 | 90.82 | 90.88 | 90.91 |
| Crude protein | 22.08 | 21.06 | 21.63 | 22.18 | 21.76 | 20.70 | 20.25 | 21.53 |
| Ether extract | 5.31 | 6.16 | 5.69 | 5.31 | 5.59 | 4.50 | 4.74 | 4.74 |
| Ash | 6.22 | 6.59 | 6.76 | 6.86 | 5.75 | 5.47 | 5.46 | 5.92 |
| Crude fiber | 3.53 | 3.50 | 3.61 | 3.46 | 3.22 | 3.32 | 2.99 | 3.21 |
| Gross energy (Cal/g) | 4002 | 3892 | 3986 | 3927 | 3950 | 3899 | 3869 | 3829 |
| Cannabinoid (mg/Kg) | ||||||||
| THC 1 | nd 3 | 8.6 | 18.1 | 30.7 | nd | 8 | 18 | 37.6 |
| CBD 2 | nd | 1.6 | 2.4 | 2.9 | nd | 1.2 | 1.3 | 4.8 |
| Variables | Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| Control | 0.5% CR | 1% CR | 2% CR | |||
| pH3 | 6.55 | 6.58 | 6.50 | 6.49 | 0.02 | 0.439 |
| pH24 | 6.14 | 6.24 | 6.10 | 6.22 | 0.02 | 0.088 |
| Lightness (L*) | 52.92 | 52.96 | 53.33 | 53.81 | 0.34 | 0.791 |
| Redness (a*) | −1.61 | −1.40 | −1.23 | −1.53 | 0.07 | 0.170 |
| Yellowness (b*) | 6.66 | 7.46 | 7.67 | 7.70 | 0.21 | 0.243 |
| Drip loss (%) | 3.03 | 3.16 | 3.31 | 3.15 | 0.09 | 0.782 |
| Thawing loss (%) | 7.43 | 7.61 | 8.42 | 8.71 | 0.32 | 0.418 |
| Cooking loss (%) | 10.71 | 11.04 | 11.78 | 10.76 | 0.37 | 0.723 |
| Shear force (kG) | 2.07 | 2.02 | 2.24 | 1.87 | 0.08 | 0.414 |
| Variables | Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| Control | 0.5% CR | 1% CR | 2% CR | |||
| C10:0 | 0.011 | 0.011 | 0.013 | 0.015 | 0.001 | 0.721 |
| C12:0 | 0.612 a | 0.512 b | 0.523 b | 0.563 a,b | 0.012 | 0.005 |
| C14:0 | 0.947 | 0.899 | 0.899 | 0.926 | 0.010 | 0.281 |
| C14:1 | 0.216 | 0.164 | 0.178 | 0.197 | 0.010 | 0.274 |
| C15:0 | 0.051 | 0.050 | 0.047 | 0.053 | 0.003 | 0.929 |
| C16:0 | 26.614 | 26.573 | 26.593 | 26.885 | 0.160 | 0.899 |
| C16:1 | 7.960 | 6.904 | 7.088 | 6.422 | 0.308 | 0.367 |
| C17:0 | 0.063 | 0.067 | 0.059 | 0.074 | 0.004 | 0.514 |
| C17:1 | 0.039 | 0.037 | 0.053 | 0.050 | 0.004 | 0.357 |
| C18:0 | 5.293 | 5.753 | 5.534 | 5.581 | 0.088 | 0.333 |
| C18:1n9c | 41.685 | 41.704 | 42.576 | 42.256 | 0.225 | 0.434 |
| C18:2n6c | 14.893 | 15.823 | 14.994 | 15.498 | 0.272 | 0.609 |
| C20:0 | 0.073 | 0.068 | 0.059 | 0.064 | 0.004 | 0.545 |
| C18:3n6 | 0.151 | 0.152 | 0.156 | 0.179 | 0.008 | 0.609 |
| C20:1n9 | 0.177 a | 0.071 b | 0.105 a,b | 0.063 b | 0.014 | 0.006 |
| C18:3n3 | 0.659 | 0.511 | 0.686 | 0.587 | 0.041 | 0.455 |
| C21:0 | 0.020 | 0.019 | 0.017 | 0.019 | 0.001 | 0.955 |
| C20:2 | 0.118 | 0.103 | 0.050 | 0.140 | 0.019 | 0.376 |
| C22:0 | 0.014 | 0.011 | 0.019 | 0.022 | 0.002 | 0.057 |
| C20:3n6 | 0.071 | 0.089 | 0.084 | 0.071 | 0.005 | 0.550 |
| C20:3n3 | 0.001 | 0.002 | 0.045 | 0.032 | 0.010 | 0.326 |
| C20:4n6 | 0.055 | 0.065 | 0.062 | 0.063 | 0.005 | 0.927 |
| C22:1n9 | 0.008 a,b | 0.010 a | 0.003 b,c | 0.001 c | 0.001 | 0.001 |
| Others | 0.269 | 0.402 | 0.157 | 0.239 | 0.044 | 0.118 |
| SFA | 33.697 | 33.962 | 33.762 | 34.200 | 0.192 | 0.807 |
| MUFA | 50.340 | 49.277 | 50.143 | 49.214 | 0.299 | 0.436 |
| PUFA | 15.846 | 16.659 | 16.045 | 16.447 | 0.294 | 0.773 |
| Omega-6 | 15.185 | 16.145 | 15.310 | 15.825 | 0.279 | 0.606 |
| Omega-3 | 0.660 | 0.514 | 0.736 | 0.622 | 0.042 | 0.323 |
| Variables | Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| Control | 0.5% CR | 1% CR | 2% CR | |||
| Aspartic acid | 0.037 a,b | 0.043 a,b | 0.031 b | 0.046 a | 0.002 | 0.011 |
| Glutamic acid | 0.279 | 0.291 | 0.340 | 0.370 | 0.014 | 0.083 |
| Histidine | 0.443 | 0.373 | 0.495 | 0.596 | 0.036 | 0.159 |
| Serine | 0.101 b | 0.135 a,b | 0.122 a,b | 0.159 a | 0.006 | 0.005 |
| Arginine | 1.730 | 2.005 | 1.578 | 1.779 | 0.060 | 0.082 |
| Glycine | 0.608 a,b | 0.696 a | 0.585 b | 0.578 b | 0.017 | 0.045 |
| Threonine | 0.272 | 0.351 | 0.358 | 0.407 | 0.023 | 0.244 |
| Alanine | 0.168 | 0.191 | 0.248 | 0.257 | 0.015 | 0.111 |
| Proline | 0.072 b | 0.081 a,b | 0.087 a,b | 0.110 a | 0.005 | 0.017 |
| Lysine | 0.426 | 0.363 | 0.350 | 0.372 | 0.016 | 0.361 |
| Valine | 0.059 | 0.058 | 0.061 | 0.072 | 0.003 | 0.193 |
| Methionine | 0.050 b | 0.062 a,b | 0.056 b | 0.075 a | 0.003 | 0.002 |
| Isoleucine | 0.099 | 0.098 | 0.105 | 0.118 | 0.004 | 0.274 |
| Leucine | 0.176 | 0.197 | 0.193 | 0.235 | 0.008 | 0.063 |
| Phenylalanine | 0.109 b | 0.127 a,b | 0.123 a,b | 0.153 a | 0.005 | 0.027 |
| Tryptophane | 0.061 | 0.049 | 0.012 | 0.035 | 0.010 | 0.347 |
| EAA 2 | 3.424 | 3.681 | 3.328 | 3.841 | 0.098 | 0.232 |
| NEAA 2 | 1.265 b | 1.438 a,b | 1.415 a,b | 1.519 a | 0.033 | 0.043 |
| Human taste classification 3 | ||||||
| Sweetness | 1.220 | 1.455 | 1.398 | 1.513 | 0.040 | 0.141 |
| Bitterness | 3.040 | 3.220 | 2.903 | 3.326 | 0.076 | 0.211 |
| Umami | 0.316 | 0.334 | 0.371 | 0.416 | 0.015 | 0.083 |
| Variables | Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| Control | 0.5%CR | 1%CR | 2%CR | |||
| Chemical composition (%) | ||||||
| Moisture | 73.78 b | 74.58 a | 74.32 a,b | 74.79 a | 0.10 | 0.003 |
| Protein | 24.66 | 23.67 | 24.24 | 23.87 | 0.14 | 0.057 |
| Ash | 1.33 | 1.32 | 1.30 | 1.29 | 0.02 | 0.380 |
| Ether extract | 0.73 a | 0.56 a,b | 0.70 a,b | 0.52 b | 0.03 | 0.007 |
| Ribonucleotide content (mg/100 g) | ||||||
| Guanosine monophosphate | 5.01 | 5.56 | 5.79 | 4.64 | 0.26 | 0.410 |
| Inosine monophosphate | 299.46 | 269.43 | 251.87 | 240.44 | 19.91 | 0.792 |
| Hypoxanthine | 27.33 | 35.11 | 28.80 | 24.86 | 1.76 | 0.203 |
| Inosine | 119.51 | 103.46 | 107.42 | 124.30 | 7.10 | 0.734 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sopian, Y.; Sahatsanon, K.; Satsook, A.; Arjin, C.; Sringarm, K.; Lumsangkul, C.; Sivapirunthep, P.; Chaosap, C. Effect of Dietary Cannabis sativa L. Residue Supplementation on Meat Quality and Flavor-Enhancing Free Amino Acids in Broiler Chickens. Animals 2025, 15, 759. https://doi.org/10.3390/ani15050759
Sopian Y, Sahatsanon K, Satsook A, Arjin C, Sringarm K, Lumsangkul C, Sivapirunthep P, Chaosap C. Effect of Dietary Cannabis sativa L. Residue Supplementation on Meat Quality and Flavor-Enhancing Free Amino Acids in Broiler Chickens. Animals. 2025; 15(5):759. https://doi.org/10.3390/ani15050759
Chicago/Turabian StyleSopian, Yusup, Katatikarn Sahatsanon, Apinya Satsook, Chaiwat Arjin, Korawan Sringarm, Chompunut Lumsangkul, Panneepa Sivapirunthep, and Chanporn Chaosap. 2025. "Effect of Dietary Cannabis sativa L. Residue Supplementation on Meat Quality and Flavor-Enhancing Free Amino Acids in Broiler Chickens" Animals 15, no. 5: 759. https://doi.org/10.3390/ani15050759
APA StyleSopian, Y., Sahatsanon, K., Satsook, A., Arjin, C., Sringarm, K., Lumsangkul, C., Sivapirunthep, P., & Chaosap, C. (2025). Effect of Dietary Cannabis sativa L. Residue Supplementation on Meat Quality and Flavor-Enhancing Free Amino Acids in Broiler Chickens. Animals, 15(5), 759. https://doi.org/10.3390/ani15050759








