Application of Branched-Chain Amino Acids Mitigates Mitochondrial Damage to Spotted Seabass (Lateolabrax maculatus) Hepatocytes Cultured in High-Glucose and High-Fat Media
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Model Establishment
2.2. Measurement of Indicators
2.2.1. Cellular Biochemical Analyses
2.2.2. Oxidative Stress-Related Indicators
Measurement of Antioxidant and Oxidative Stress Markers
Measurement of Reactive Oxygen Species (ROS) Concentration
2.2.3. Key Enzymatic Activities in Hepatocyte Metabolism
2.2.4. Mitochondrial Status
Mitochondrial Membrane Potential Detection
Mitochondrial Activity Staining
DNA Damage Detection
2.2.5. Fluorescence Quantification
2.3. Data Analysis
3. Results
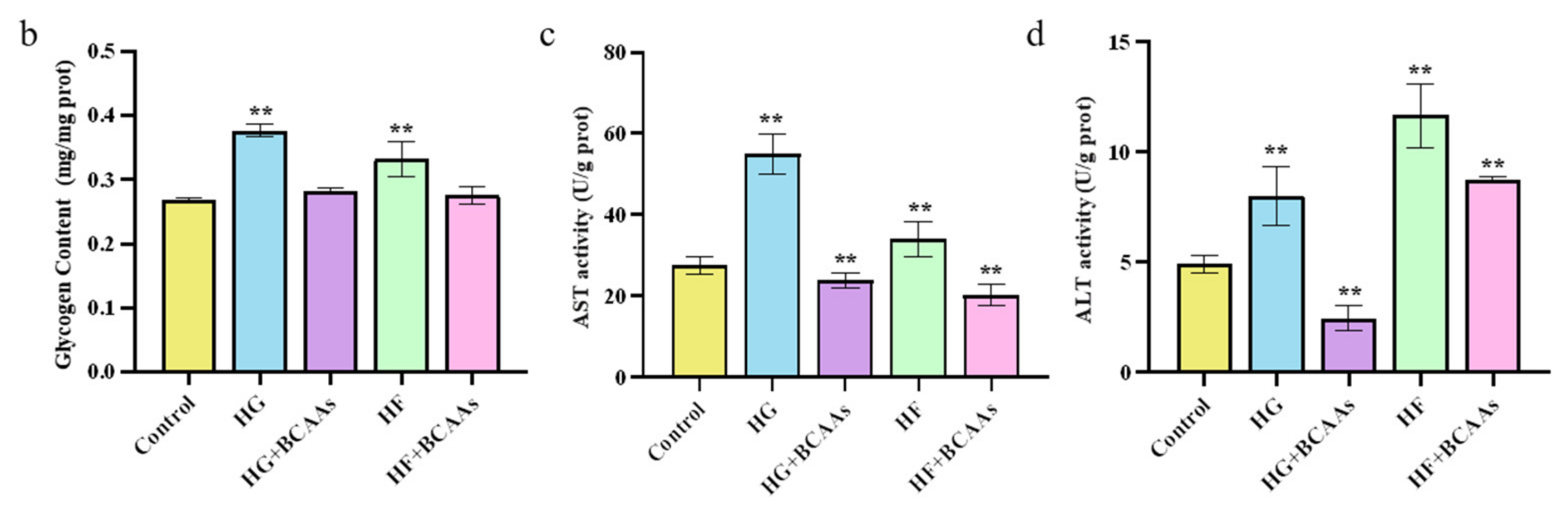
3.1. Cellular Biochemistry
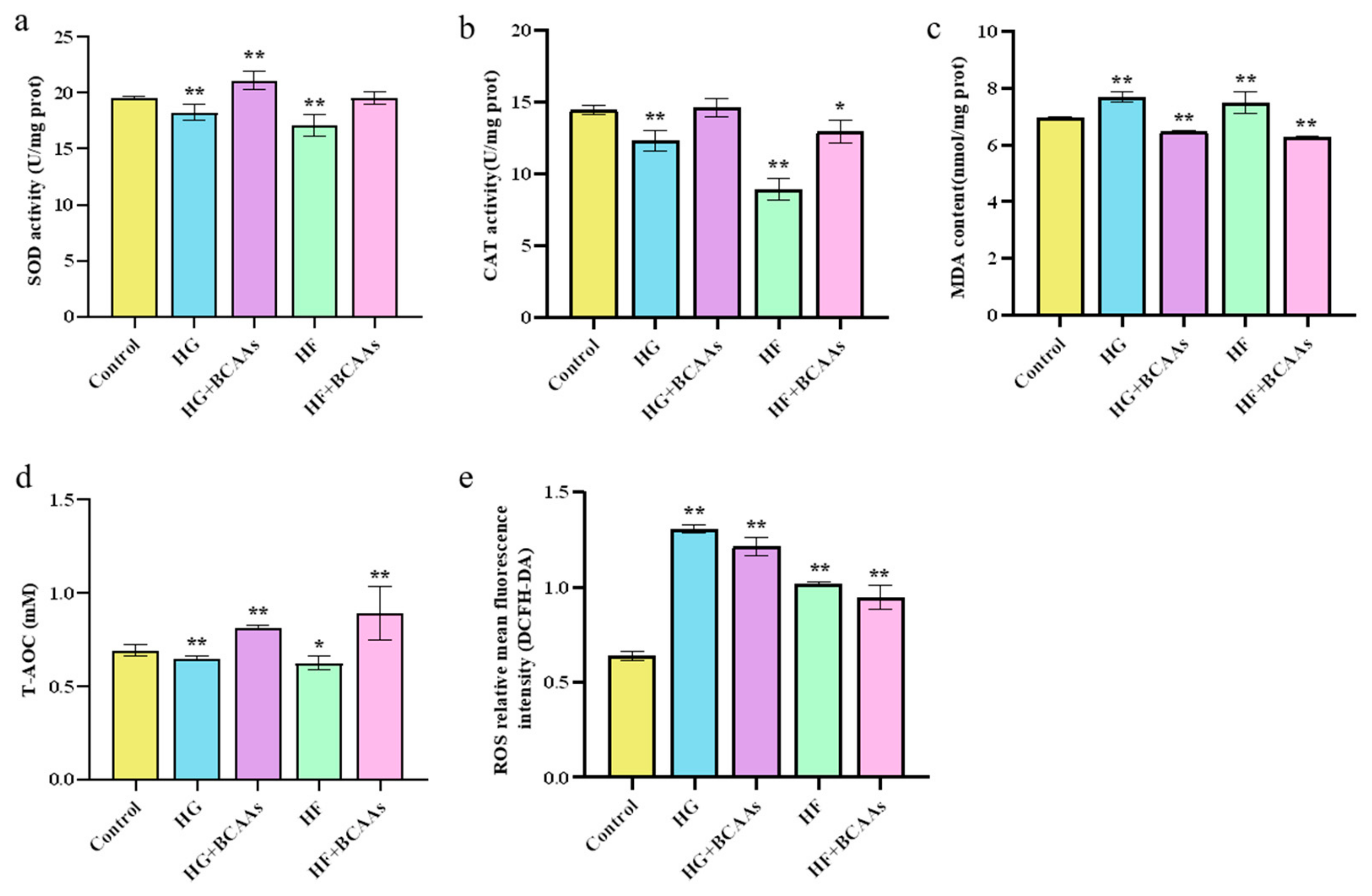
3.2. Cellular Antioxidant Defense
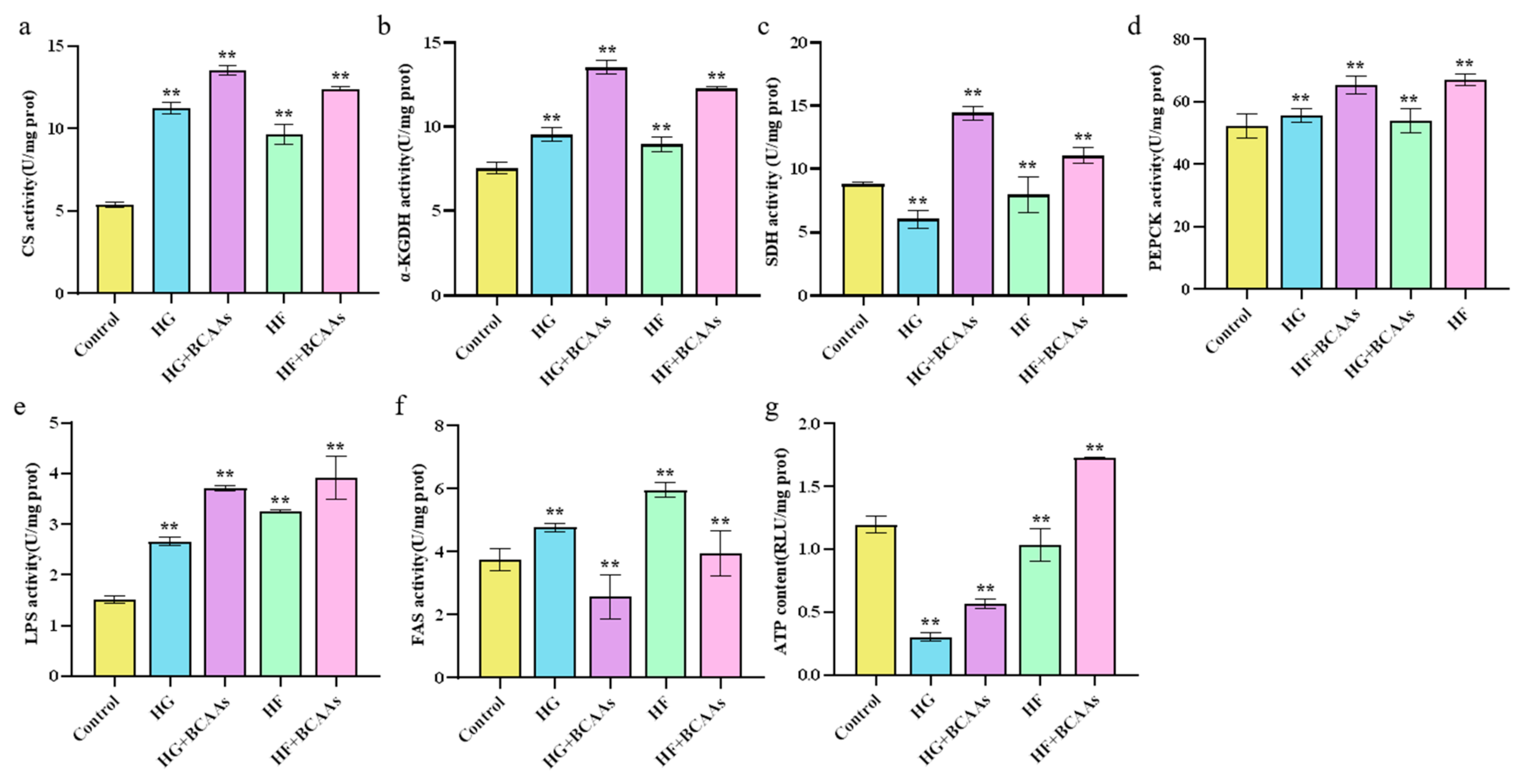
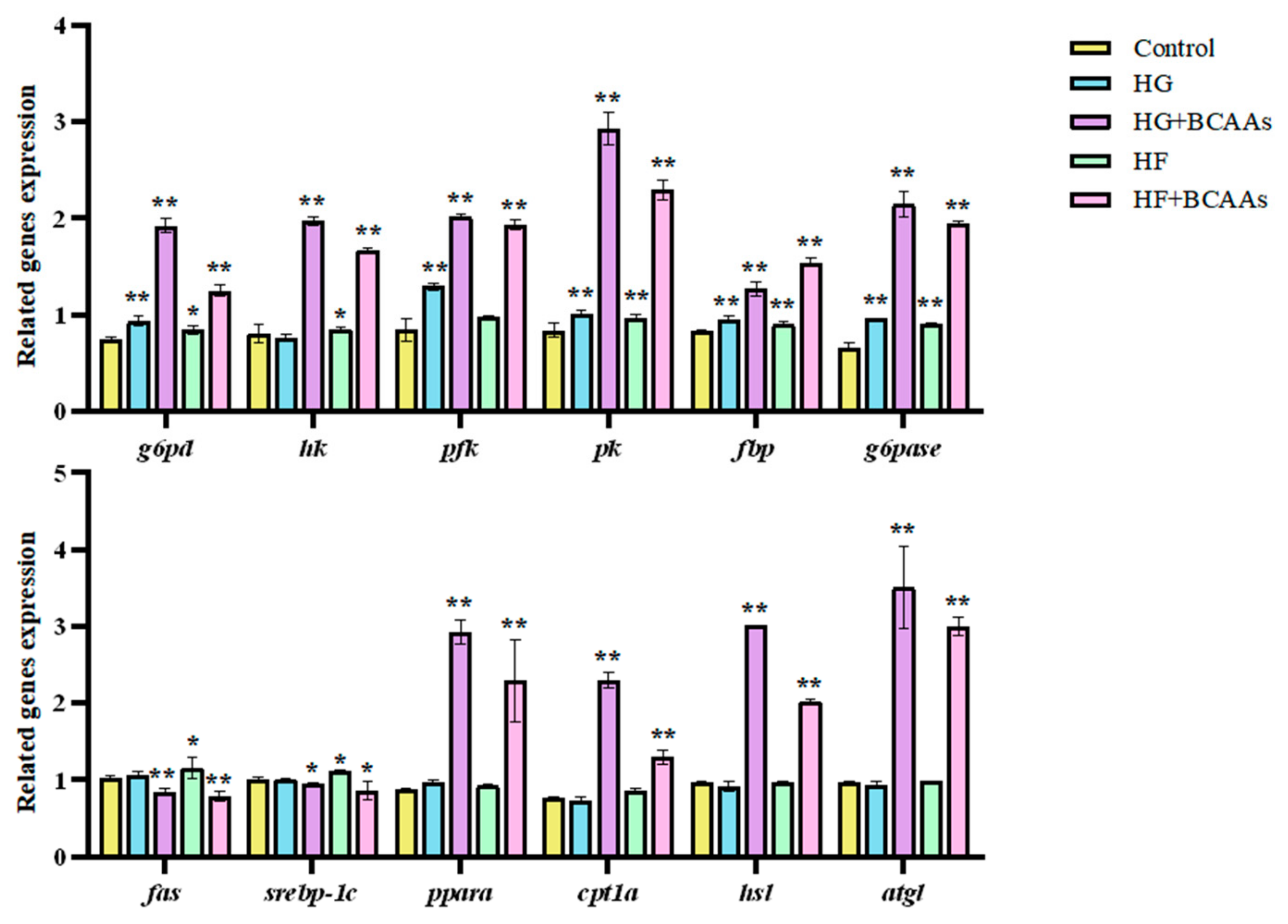
3.3. Cellular Metabolism
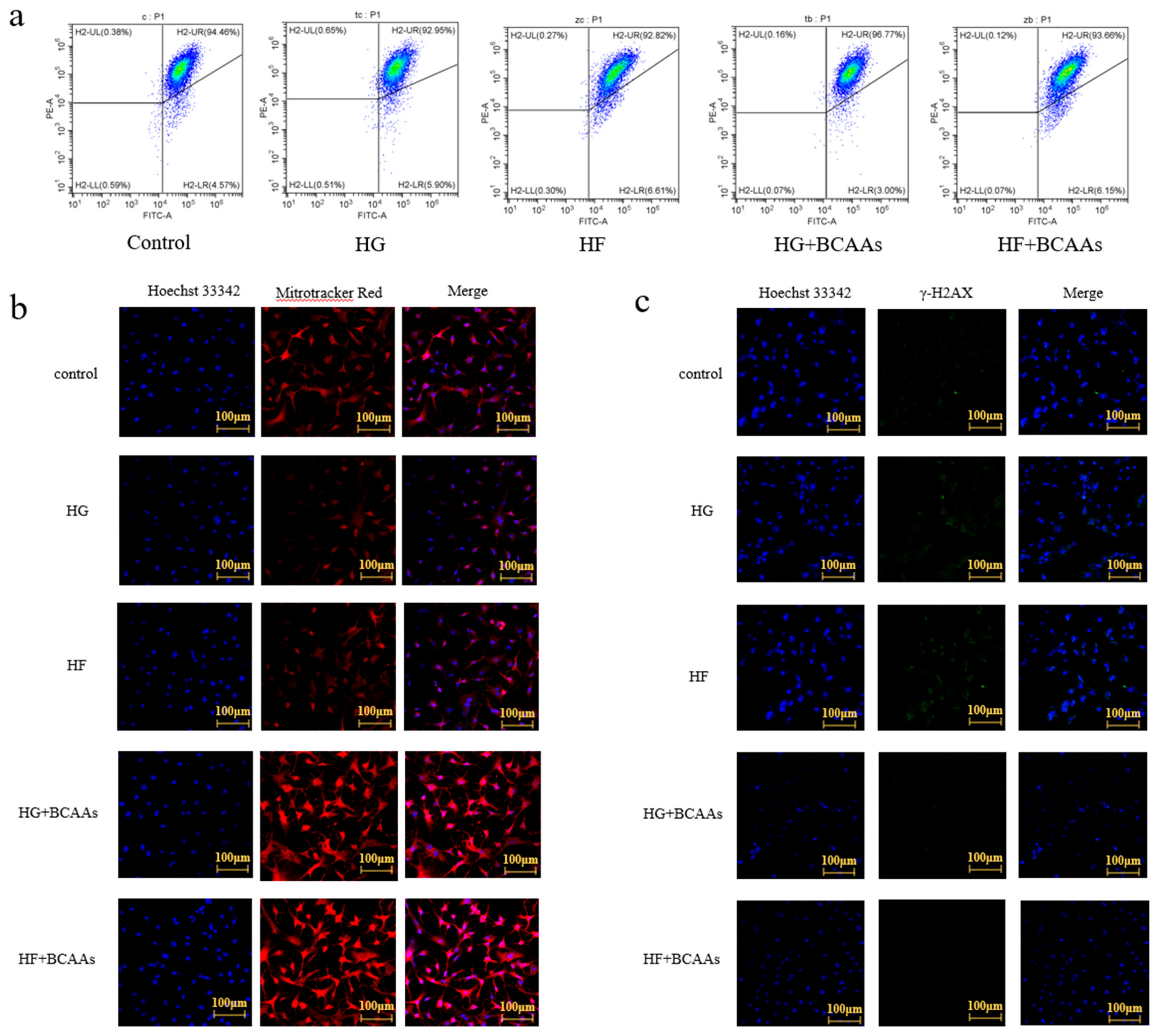
3.4. Mitochondrial Function
4. Discussion
4.1. BCAAs Supplementation Mitigated Metabolic Disorders in Hepatocytes Induced by HG or HF Load
4.2. BCAAs Supplementation Alleviated Mitochondrial Damage Caused by HG or HF Loads
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tamanna, N.; Mahmood, N. Emerging Roles of Branched-Chain Amino Acid Supplementation in Human Diseases. Int. Math. Res. Not. 2014, 2014, 235619. [Google Scholar] [CrossRef] [PubMed][Green Version]
- De Bandt, J.P.; Coumoul, X.; Barouki, R. Branched-Chain Amino Acids and Insulin Resistance, from Protein Supply to Diet-Induced Obesity. Nutrients 2022, 15, 68. [Google Scholar] [CrossRef] [PubMed]
- Bonvini, A.; Rogero, M.M.; Coqueiro, A.Y.; Raizel, R.; Bella, L.M.; Fock, R.A.; Borelli, P.; Tirapegui, J. Effects of different branched-chain amino acids supplementation protocols on the inflammatory response of LPS-stimulated RAW 264.7 macrophages. Amino Acids 2021, 53, 597–607. [Google Scholar] [CrossRef] [PubMed]
- Valerio, A.D.; Antona, G.; Nisoli, E. Branched-chain amino acids, mitochon-drial biogenesis, and healthspan: An evolutionary perspective. Aging 2011, 3, 464–478. [Google Scholar] [CrossRef]
- Blair, M.C.; Neinast, M.D.; Arany, Z. Whole-body metabolic fate of branched-chain amino acids. Biochemical. J. 2021, 478, 765–776. [Google Scholar] [CrossRef]
- Ra, S.G.; Miyazaki, T.; Ishikura, K.; Nagayama, H.; Komine, S.; Nakata, Y.; Maeda, S.; Matsuzaki, Y.; Ohmori, H. Combined effect of branched-chain amino acids and taurine supple-mentation on delayed onset muscle soreness and muscle damage inhigh-intensity eccentric exercise. J. Int. Soc. Sports Nutr. 2013, 10, 51. [Google Scholar] [CrossRef]
- Fouré, A.; Bendahan, D. Is branched-chain amino acids supplementation an efficient nutritional strategy to alleviate skeletal muscle damage? A systematic review. Nutrients 2017, 9, 1047. [Google Scholar] [CrossRef]
- Holeček, M. Branched-chain amino acid supplementation in treatment of liver cirrhosis: Updated views on how to attenuate their harmful effects on cataplerosis and ammonia formation. Nutrition 2017, 41, 80–85. [Google Scholar] [CrossRef]
- Weber, M.G.; Dias, S.S.; de Angelis, T.R.; Fernandes, E.V.; Bernardes, A.G.; Milanez, V.F.; Jussiani, E.I.; Ramos, S.d.P. The use of BCAA to decrease delayed-onset muscle soreness after a single bout of exercise:a systematic review and meta-analysis. Amino Acids. 2021, 53, 1663–1678. [Google Scholar] [CrossRef]
- Doma, K.; Singh, U.; Boullosa, D.; Connor, J.D. The effect of branched-chain amino acid on muscle damage markers and performance following strenuous exercise: A systematic review and meta-analysis. Nutr. Metab. 2021, 46, 1303–1313. [Google Scholar] [CrossRef]
- Hormoznejad, R.; Zare Javid, A.; Mansoori, A. Effect of BCAA supplemen-tation on central fatigue, energy metabolism substrate and muscle damage to the exercise: A systematic review with meta-analysis. Sport. Sci. Health 2019, 15, 265–279. [Google Scholar] [CrossRef]
- Khemtong, C.; Kuo, C.H.; Chen, C.Y.; Jaime, S.J.; Condello, G. Does branched-chain amino acids (BCAAs) supplementation attenuate muscle damage markers and soreness after resistance exercise in trained males? A meta-analysis of randomized controlled trials. Nutrients 2021, 13, 1880. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.H.; Shab-Bidar, S.; Mollahosseini, M.; Djafarian, K. Branched-chain amino acid supplementation and exercise-induced muscle damage in exercise recovery: A meta-analysis of randomized clinical trials. Nutrition 2017, 42, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Rahimlou, M.; Ramezani, A.; Mahdipour, M.; Palimi, E.; Moradipoodeh, B. Reduction of muscle injuries and improved post-exercise recovery by branched-chain amino acid supplementation: A systematic review and meta-analysis. J. Nutr. Health Aging. 2020, 8, 1–16. [Google Scholar]
- McGarrah, R.W.; White, P.J. Branched-chain amino acids in cardiovascular disease. Nat. Rev. Cardiol. 2023, 20, 77–89. [Google Scholar] [CrossRef]
- Lauer, S.M.; Omar, M.H.; Golkowski, M.G.; Kenerson, H.L.; Lee, K.-S.; Pascual, B.C.; Lim, H.C.; Forbush, K.; Smith, F.D.; Gordan, J.D.; et al. Recruitment of BAG2 to DNAJ-PKAc scaffolds promotes cell survival and resistance to drug-induced apoptosis in fibrolamellar carcinoma. Cell Rep. 2024, 43, 113678. [Google Scholar] [CrossRef]
- Lo, E.K.K.; Felicianna; Xu, J.-H.; Zhan, Q.; Zeng, Z.; El-Nezami, H. The Emerging Role of Branched-Chain Amino Acids in Liver Diseases. Biomedicines 2022, 10, 1444. [Google Scholar] [CrossRef]
- Ananieva, E.A.; Van Horn, C.G.; Jones, M.R.; Hutson, S.M. Liver BCATm transgenic mouse model reveals the important role of the liver in maintaining BCAA homeostasis. J. Nutr. Biochem. 2017, 40, 132–140. [Google Scholar] [CrossRef]
- Kamalam, B.S.; Medale, F.; Panserat, S. Utilisation of dietary carbohydrates in farmed fishes: New insights on influencing factors, biological limitations and future strategies. Aquaculture 2017, 467, 3–27. [Google Scholar] [CrossRef]
- Sari, D.N.; Ekasari, J.; Nasrullah, H.; Suprayudi, M.A.; Alimuddin, A. High carbohydrate increases amylase, plasma glucose, and gene expression related to glycolysis in giant gourami Osphronemus goramy. Fish. Physiol. Biochem. 2022, 48, 1495–1505. [Google Scholar] [CrossRef]
- Lin, S.-M.; Shi, C.-M.; Mu, M.-M.; Chen, Y.-J.; Luo, L. Effect of high dietary starch levels on growth, hepatic glucose metabolism, oxidative status and immune response of juvenile largemouth bass, Micropterus salmoides. Fish. Shellfish. Immunol. 2018, 78, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Lu, K.-L.; Wang, L.-N.; Zhang, D.-D.; Liu, W.-B.; Xu, W.-N. Berberine Attenuates Oxidative Stress and Hepatocytes Apoptosis Via Protecting Mitochondria in Blunt Snout Bream Megalobrama amblycephala Fed High- Fat Diets. Fish. Physiol. Biochem. 2017, 43, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Mao, X.; Zhang, J.; Rahimnejad, S.; Lu, K. Effects of quercetin and hydroxytyrosol supplementation in a high-fat diet on growth, lipid metabolism, and mitochondrial function in spotted seabass (Lateolabrax maculatus). Aquaculture 2024, 582, 740538. [Google Scholar] [CrossRef]
- Dong, Y.; Yu, M.; Wu, Y.; Xia, T.; Wang, L.; Song, K.; Zhang, C.; Lu, K.; Rahimnejad, S. Hydroxytyrosol Promotes the Mitochondrial Function through Activating Mitophagy. Antioxidants 2022, 11, 893. [Google Scholar] [CrossRef]
- Ren, H.J.; Ke, Y.X.; Lu, K.L.; Li, X.S.; Wang, L.; Song, K.; Zhang, C.X. Effects of high-glucose load on energy metabolism and mitochondrial function of hepatocytes from spotted seabass (Lateolabrax maculatus). J. Fish. Sci. China 2024, 31, 513–523. [Google Scholar]
- Mao, X.J. Alleviation Effect and Mechanism of Coenzyme Q10 on Excessive Fat Deposition in Liver of Lateolabrax maculatus Induced by High-Fat Diet. Master’s Thesis, Jimei University, China, Xiamen, 2024. [Google Scholar]
- Jung, S.M.; Doxsey, W.G.; Le, J.; Haley, J.A.; Mazuecos, L.; Luciano, A.K.; Li, H.; Jang, C.; Guertin, D.A. In vivo isotope tracing reveals the versatility of glucose as a brown adipose tissue substrate. Cell Rep. 2021, 36, 109459. [Google Scholar] [CrossRef]
- Zhu, J.; Pan, J.; Wang, X.; Huang, Y.; Qin, C.; Qiao, F.; Qin, J.; Chen, L. Alleviation of the adverse effect of dietary carbohydrate by supplementation of myo-inositol to the diet of Nile tilapia (Oreochromis niloticus). Animals 2020, 10, 2190. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Zhang, J.; Sang, C.; Chen, N. The impacts of dietary carbohydrate levels on growth performance, feed utilization, glycogen accumulation and hepatic glucose metabolism in hybrid grouper (Epinephelus fuscoguttatus ♀ × E. lanceolatus♂). Aquaculture 2019, 512, 734351. [Google Scholar] [CrossRef]
- Bornstein, M.R.; Neinast, M.D.; Zeng, X.; Chu, Q.; Axsom, J.; Thorsheim, C.; Li, K.; Blair, M.C.; Rabinowitz, J.D.; Arany, Z. Comprehensive quantification of metabolic flux during acute cold stress in mice. Cell Metab. 2023, 35, 2077–2092. [Google Scholar] [CrossRef]
- Qian, L.; Li, N.; Lu, X.C.; Xu, M.; Liu, Y.; Li, K.; Zhang, Y.; Hu, K.; Qi, Y.T.; Yao, J.; et al. Enhanced BCAT1 activity and BCAA metabolism promotes RhoC activity in cancer progression. Nat. Metab. 2023, 5, 1159–1173. [Google Scholar] [CrossRef]
- Wang, J.; Liu, Y.; Lian, K.; Shentu, X.; Fang, J.; Shao, J.; Chen, M.; Wang, Y.; Zhou, M.; Sun, H. BCAA catabolic defect alters glucose metabolism in lean mice. Front. Physiol. 2019, 10, 1140. [Google Scholar] [CrossRef] [PubMed]
- Hui, S.; Cowan, A.J.; Zeng, X.; Yang, L.; TeSlaa, T.; Li, X.; Bartman, C.; Zhang, Z.; Jang, C.; Wang, L.; et al. Quantitative Fluxomics of Circulating Metabolites. Cell Metab. 2020, 32, 676–688. [Google Scholar] [CrossRef] [PubMed]
- Yoneshiro, T.; Kataoka, N.; Walejko, J.M.; Ikeda, K.; Brown, Z.; Yoneshiro, M.; Crown, S.B.; Osawa, T.; Sakai, J.; McGarrah, R.W.; et al. Meta-bolic flexibility via mitochondrial BCAA carrier SLC25A44 is required for optimal fever. eLife 2021, 10, 66865. [Google Scholar] [CrossRef] [PubMed]
- Espenshade, P.J. SREBPs:Sterol-Regulated Transcription Factors. J. Cell Sci. 2006, 119, 973–976. [Google Scholar] [CrossRef]
- Brown, M.S.; Goldstein, J.L. Cholesterol Feedback: From Schoenheimer’s Bottle to Scap’s MELADL. Lipid Res. 2009, 50, S15–S27. [Google Scholar] [CrossRef]
- Dong, Y.Z.; Wei, Y.; Wang, L.; Song, K.; Zhang, C.X.; Lu, K.L.; Rahimnejad, S. Dietary n-3/n-6 PUFA ratio modulates growth performance in spotted seabass (Lateolabrax maculatus) through regulating lipid metabolism, hepatic antioxidant capacity and intestinal health. Anim. Nutr. 2023, 14, 20–31. [Google Scholar] [CrossRef]
- Athanikar, J.N.; Sanchez, H.B.; Osborne, T.F. Promoter Selective Transcriptional Synergy Mediated by Sterol Regulatory Element Binding Protein and Sp1: A Critical Role for the Btd Domain of Sp1. Mol. Cell Biol. 1997, 17, 5193–5200. [Google Scholar] [CrossRef]
- Wilentz, R.E.; Witters, L.A.; Pizer, E.S. Lipogenic Enzymes Fatty Acid Synthase and Acetyl-Coenzyme a Carboxylase are Coexpressed with Sterol Regulatory Element Binding Protein and Ki-67 in Fetal Tissues. Pediatr. Dev. Pathol. 2000, 3, 525–531. [Google Scholar] [CrossRef]
- Zimmermann, R.; Strauss, J.G.; Haemmerle, G.; Schoiswohl, G.; Birner-Gruenberger, R.; Riederer, M.; Lass, A.; Neuberger, G.; Eisenhaber, F.; Hermetter, A.; et al. Fat Mobilization in Adipose Tissue is Promoted by Adipose Triglyceride Lipase. Science 2004, 306, 1383–1386. [Google Scholar] [CrossRef]
- Schreiber, R.; Xie, H.; Schweiger, M. Of Mice and Men: The Physiological Role of Adipose Triglyceride Lipase (ATGL). Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 880–899. [Google Scholar] [CrossRef]
- Calabrese, G.; Morgan, B.; Riemer, J. Mitochondrial Glutathione: Regulation and Functions. Antioxid. Redox Signal. 2017, 27, 1162–1177. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q.; Han, T.; Li, X.; Wang, J.; Yang, Y.; Yu, X.; Zheng, P.; Liu, T.; Xu, H.; Wang, C. Effects of dietary carbohydrate levels on growth, body composition, and gene expression of key enzymes involved in hepatopancreas metabolism in mud crab Scylla paramamosain. Aquaculture 2020, 529, 735638. [Google Scholar] [CrossRef]
- Forman, H.J.; Zhang, H.; Rinna, A. Glutathione: Overview of its protective roles, measurement, and biosynthesis. Mol. Asp. Med. 2009, 30, 1–12. [Google Scholar] [CrossRef]
- Pfanner, N.; Warscheid, B.; Wiedemann, N. Mitochondrial proteins: From biogenesis to functional networks. Nat. Rev. Mol. Cell Biol. 2019, 20, 26. [Google Scholar] [CrossRef]
- Palikaras, K.; Lionaki, E. Taverarakis. Balancing Mitochondrial Biogenesis and Mitophagy to Maintain Energy Metabolism Homeostasis. Cell Death Differ. 2015, 22, 1399–1401. [Google Scholar] [CrossRef]
- Yu, E.P.K.; Bennett, M.R. The Role of Mitochondrial DNA Damage in the Development of Atherosclerosis. Free Radic. Biol. Med. 2016, 100, 223–230. [Google Scholar]
- Mao, S.; Medeiros, D.M.; Leone, T.C.; Kelly, D.P. Mitochondrial Transcription Factor A is Increased but Expression of ATP Synthase Beta Subunit and Medium-Chain Acyl-CoA Dehydrogenase Genes are Decreased in Hearts of Copper-Deficient Rats. J. Nutr. 2000, 130, 2143–2150. [Google Scholar] [CrossRef][Green Version]
- Bremer, K.; Kocha, K.; Snider, T.; Moyes, C. Sensing and Responding to Energetic Stress:The Role of the AMPK-PGC1alpha-NRF1 Axis in Control of Mitochondrial Biogenesis in Fish. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2016, 199, 4–12. [Google Scholar] [CrossRef]
- Lazarou, M.; Sliter, D.A.; Kane, L.A.; Sarraf, S.A.; Wang, C.; Burman, J.L.; Sideris, D.P.; Fogel, A.I.; Youle, R.J. The Ubiquitin Kinase PINK1 Recruits Autophagy Receptors to Induce Mitophagy. Nature 2015, 524, 309–314. [Google Scholar] [CrossRef]
- Palikaras, K.; Taverarakis, N. Mitochondrial Homeostasis: The Interplay between Mitophagy and Mitochondrial Biogenesis. Exp. Gerontol. 2014, 56, 182–188. [Google Scholar] [CrossRef]
- Beaulant, A.; Dia, M.; Pillot, B.; Chauvin, M.-A.; Ji-Cao, J.; Durand, C.; Bendridi, N.; Chanon, S.; Vieille-Marchiset, A.; Da Silva, C.C.; et al. Endoplasmic Reticulum-Mitochondria Miscommunication is an Early and Causal Trigger of Hepatic Insulin Resistance and Steatosis. J. Hepatol. 2022, 77, 710–722. [Google Scholar] [CrossRef]


| Target Gene | Forward Sequence (5′−3′) | Reversed Sequence (5′−3′) | Annealing Temperature |
|---|---|---|---|
| g6pd | ATGCTCTGTTTGGTCGCCAT | ACATCCGACAGAGCAACAGG | 60 |
| hk | CTGGCTTGTGGGGACAGATT | GAGGCTGGCCCTCTTTATCC | 60 |
| pfk-1 | CGAGGGGCTAAATGTCAGGG | AAGGGGCATTCCGGTGATTT | 60 |
| pk | GTGGCCCAATCCAAATGTCC | GCAAGAGTGAGAGTTGGGGT | 60 |
| fbp | AACTGAGAAAGTCCCCCGAC | CCGGCCAAAACCTCGTATCT | 60 |
| g6pase | CAGGTCATGGGGTACTGCTC | TTCCCGCTTTGGTTTCACCT | 60 |
| fas | AAACTGAAGCCCTGTGTGCC | CACCCTGCCTATTACATTGCTC | 60 |
| srebp-1c | CCTCACTCTGCAGCCAATCA | CGTAGTCCCACCCTCAAACC | 60 |
| ppaα | CCGTGCGTGTTTTCACCATT | AGACCAAATACATCGCCCCC | 60 |
| cpt-1α | CCTCAATGATACATCGGAACCC | CTGCGGCTCATCATCTAACG | 60 |
| hsl | CGAAACACAGAGACGGTCCA | TCATGACATCTACCAGCCGC | 60 |
| atgl | CTTCCTCTCCGCAACAAGTC | TGGTGCTGTCTGGAGTGTTC | 60 |
| drp1 | CTCGCCAACAGAAACGGAAC | TGGCACTTTGGTCTTCGACA | 60 |
| mfn1b | GTCAACGCTATGCTGAGGGA | TCATCAGAGCCCTCCGTCTT | 60 |
| mfn2 | TTCCAACGACCCAACACCAA | GTAGGCCCCCAACTGTTCAA | 60 |
| mul1 | GCTGCCGTGATACGAGTCAT | ACGTTGGACAAGGACTGGAC | 60 |
| atg5 | TCAGTCGCTGCCATTAGAGC | TCTCGTCACCTGCGAAAACT | 60 |
| pgc-1α | AACCCGACTCTTATCCCTCC | CGTATCAACGCCACAGCAC | 60 |
| pgc-1β | GTTCCTCCGAACTCCCAGTG | GCAACACCCCTCCAACTACA | 60 |
| fis1 | GTCCCGGGAGTCATCCTTTG | ACAATGAGCTGGTGAAGGGAG | 60 |
| β-actin | CAACTGGGATGACATGGAGAAG | TTGGCTTTGGGGTTCAGG | 60 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ren, H.; Ke, Y.; Li, X.; Wang, L.; Song, K.; Guardiola, F.A.; Zhang, C.; Lu, K.; Rahimnejad, S. Application of Branched-Chain Amino Acids Mitigates Mitochondrial Damage to Spotted Seabass (Lateolabrax maculatus) Hepatocytes Cultured in High-Glucose and High-Fat Media. Animals 2025, 15, 560. https://doi.org/10.3390/ani15040560
Ren H, Ke Y, Li X, Wang L, Song K, Guardiola FA, Zhang C, Lu K, Rahimnejad S. Application of Branched-Chain Amino Acids Mitigates Mitochondrial Damage to Spotted Seabass (Lateolabrax maculatus) Hepatocytes Cultured in High-Glucose and High-Fat Media. Animals. 2025; 15(4):560. https://doi.org/10.3390/ani15040560
Chicago/Turabian StyleRen, Huijuan, Yixiong Ke, Xueshan Li, Lin Wang, Kai Song, Francisco A. Guardiola, Chunxiao Zhang, Kangle Lu, and Samad Rahimnejad. 2025. "Application of Branched-Chain Amino Acids Mitigates Mitochondrial Damage to Spotted Seabass (Lateolabrax maculatus) Hepatocytes Cultured in High-Glucose and High-Fat Media" Animals 15, no. 4: 560. https://doi.org/10.3390/ani15040560
APA StyleRen, H., Ke, Y., Li, X., Wang, L., Song, K., Guardiola, F. A., Zhang, C., Lu, K., & Rahimnejad, S. (2025). Application of Branched-Chain Amino Acids Mitigates Mitochondrial Damage to Spotted Seabass (Lateolabrax maculatus) Hepatocytes Cultured in High-Glucose and High-Fat Media. Animals, 15(4), 560. https://doi.org/10.3390/ani15040560











