The Immune-Antioxidant Trade-Off Mediated by Actinobacteria Drives Niche Differentiation: Physiological and Gut Microbiota Responses of Two Cold-Adapted Brown Frog Species to Contrasting Peak Daily Habitat Temperatures
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study System, Frogs Collection and Experimental Design
2.2. Immune Capacity and Antioxidant Capacity Assays
2.2.1. Determination of Phytohemagglutinin (PHA) Response Intensity
2.2.2. Collection of Experimental Samples
2.2.3. Preparation of Blood Smears and Enumeration of Lymphocytes
2.2.4. Determination of Enzyme Activity
2.3. Guts Collection and Gut Microbiota Analysis
2.3.1. Library Sequencing and Quality Filtering
2.3.2. DADA2 Denoising and Taxonomy Classification
2.3.3. Diversity Index, Taxonomy Differential Abundance Analysis and Microbiome Function Prediction
2.4. Statistical Analysis
3. Results
3.1. Immune Capacity and Antioxidant Capacity
3.2. Gut Microbiota Analysis
3.2.1. Alpha and Beta Polymorphic Index Analysis
3.2.2. Composition Analysis of Gut Microbiota
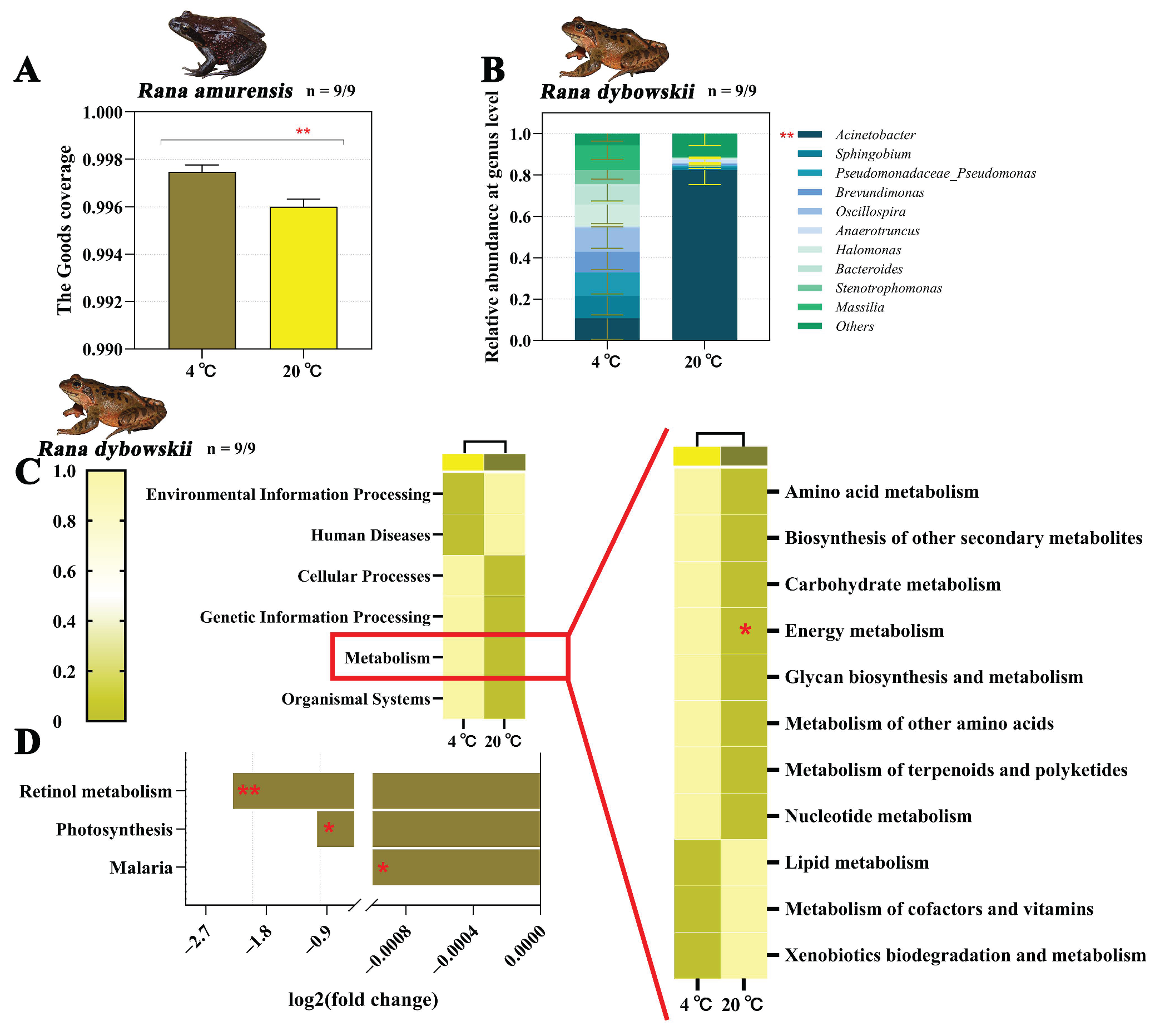
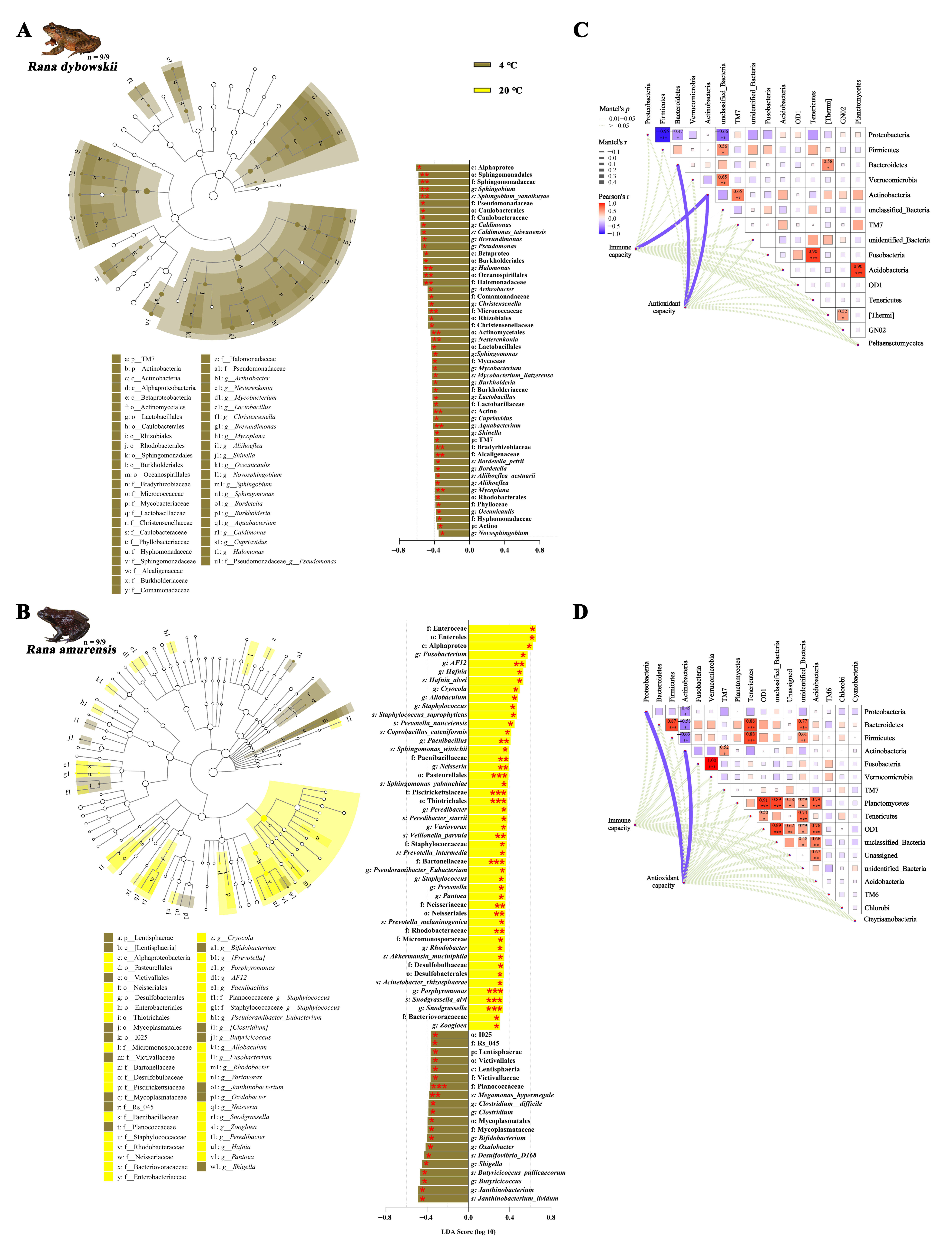
3.2.3. Differential Analysis and Functional Annotation of Microbial Community
3.3. Correlation Analysis and Redundancy Analysis
4. Discussion
4.1. Dual Effects of Contrasting Peak Daily Habitat Temperatures on Physiological Homeostasis
4.2. Dynamic Response of Gut Microbiota to Contrasting Peak Daily Habitat Temperatures
4.3. Regulatory Mechanisms of the Gut Microbiota–Host Interaction Network
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | Analysis of variance |
| ASVs | Amplicon sequence variants |
| BMI | Body mass index |
| CAT | Catalase |
| FDR | False discovery rate |
| GPCRs | G protein-coupled receptors |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| LDA | Linear discriminant analysis |
| LEfSe | Linear discriminant analysis effect size |
| LSZ | Lysozyme |
| Lym | Lymphocytes |
| MDA | Malondialdehyde |
| NMDS | Nonmetric multidimensional scaling |
| PBS | Phosphate buffered saline |
| PHA | Phytohemagglutinin |
| PICRUSt2 | Phylogenetic investigation of communities by reconstruction of unobserved states |
| POS | Preparation for oxidative stress |
| ROS | Reactive oxygen species |
| SCFAs | Short-chain fatty acids |
| SOD | Superoxide dismutase |
| Treg | T regulatory |
References
- Barnosky, A.D.; Matzke, N.; Tomiya, S.; Wogan, G.O.U.; Swartz, B.; Quental, T.B.; Marshall, C.; McGuire, J.L.; Lindsey, E.L.; Maguire, K.C.; et al. Has the Earth’s Sixth Mass Extinction Already Arrived? Nature 2011, 471, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Ceballos, G.; Ehrlich, P.R.; Raven, P.H. Vertebrates on the Brink as Indicators of Biological Annihilation and the Sixth Mass Extinction. Proc. Natl. Acad. Sci. USA 2020, 117, 13596–13602. [Google Scholar] [CrossRef] [PubMed]
- Li, W.J.; Zhang, Q.; Wang, Z.N.; Liu, X. Three-Quarters of Species’ Ranges Have Not Been Covered by Protected Areas in Global Borders. Nat. Commun. 2025, 16, 2608. [Google Scholar] [CrossRef] [PubMed]
- Pottier, P.; Kearney, M.R.; Wu, N.C.; Gunderson, A.R.; Rej, J.E.; Rivera-Villanueva, A.N.; Pollo, P.; Burke, S.; Drobniak, S.M.; Nakagawa, S. Vulnerability of Amphibians to Global Warming. Nature 2025, 639, 954–961. [Google Scholar] [CrossRef]
- Cortazar-Chinarro, M.; Corral-Lopez, A.; Lüdtke, D.U.; Tegner, F.; Luquet, E.; Laurila, A. Metamorphosis Reverses the Behavioural Phenotype in Rana arvalis Along a Latitudinal Gradient. Ecol. Evol. 2023, 15, e71945. [Google Scholar] [CrossRef]
- Kim, Y.I.; Chuang, M.F.; Borzée, A.; Kwon, S.; Jang, Y. Latitude-Induced and Behaviorally Thermoregulated Variations in Upper Thermal Tolerance of Two Anuran Species. Biology 2022, 11, 1506. [Google Scholar] [CrossRef]
- Bensouilah, S.; Zebsa, R.; Bensakhri, Z.; Youcefi, A.; Amari, H.; Lazli, A.; Houhamdi, M.; Khelifa, R. Trends to Adaptation of the Sahara Frog (Pelophylax saharicus) Larvae Across An Environmental Gradient. Biologia 2021, 77, 2857–2866. [Google Scholar]
- Leung, K.W.; Yang, S.N.; Wang, X.Y.; Tang, K.; Hu, J.H. Ecogeographical Adaptation Revisited: Morphological Variations in the Plateau Brown Frog along an Elevation Gradient on the Qinghai-Tibetan Plateau. Biology 2021, 10, 1081. [Google Scholar] [CrossRef]
- Buckley, E.M.B.; Gottesman, B.L.; Caven, A.J.; Harner, M.J.; Pijanowski, B.C. Assessing Ecological and Environmental Influences on Boreal Chorus Frog (Pseudacris maculata) Spring Calling Phenology using Multimodal Passive Monitoring Technologies. Ecol. Indic. 2021, 121, 107171. [Google Scholar] [CrossRef]
- Mueller, C.A.; Leão, C.C.B.d.P.; Atherley, K.R.; Campos, N.; Eme, J. Embryos and Tadpoles of the Eurythermal Baja California Chorus Frog (Pseudacris hypochondriaca) Show Subtle Phenotypic Changes in Response to Daily Cycling Temperatures. Ecol. Evol. Physiol. 2024, 97, 354–370. [Google Scholar] [CrossRef]
- Spranger, R.R.; Raffel, T.R.; Sinervo, B.R. Canopy Coverage, Light, and Moisture Affect Thermoregulatory Trade-Offs in an Amphibian Breeding Habitat. J. Therm. Biol. 2024, 122, 103864. [Google Scholar] [CrossRef]
- Sun, X.Q.; Zhao, L.H.; Chen, Q.H.; Wang, J.C.; Cui, J.G. Auditory Sensitivity Changes with Diurnal Temperature Variation in Little Torrent Frogs (Amolops torrentis). Bioacoustics 2019, 29, 684–696. [Google Scholar] [CrossRef]
- Zigler, A.; Straw, S.; Tokuda, I.; Bronson, E.; Riede, T. Critical Calls: Circadian and Seasonal Periodicity in Vocal Activity in a Breeding Colony of Panamanian Golden Frogs (Atelopus zeteki). PLoS ONE 2023, 18, e0286582. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Wang, J.H.; Jin, B.Y.; Chen, L.; Yang, Y.Q.; Diao, Y.Z.; Wu, L. Effect of Temperature on Metamorphosis Duration and Response Patterns to Phytohemagglutinin-P in the Tadpoles of Pelophylax nigromaculatus. Chin. J. Ecol. 2021, 40, 4019–4033. [Google Scholar]
- Weerathunga, W.A.M.T.; Rajapaksa, G. The Impact of Elevated Temperature and CO2 on Growth, Physiological and Immune Responses of Polypedates cruciger (Common Hourglass Tree Frog). Front. Zool. 2020, 17, 3. [Google Scholar] [CrossRef]
- Maniero, G.D.; Carey, C. Changes in Selected Aspects of Immune Function in the Leopard Frog, Rana pipiens, Associated with Exposure to Cold. J. Comp. Physiol. B 1997, 167, 256–263. [Google Scholar] [CrossRef]
- Niu, Y.G.; Zhang, X.J.; Zhang, H.Y.; Xu, T.S.; Men, S.K.; Storey, K.B.; Chen, Q. Antioxidant and Non-Specific Immune Defenses in Partially Freeze-Tolerant Xizang Plateau Frogs, Nanorana parkeri. J. Therm. Biol. 2021, 102, 103132. [Google Scholar] [CrossRef]
- Vaziri, G.J.; Reid, N.M.; Rittenhouse, T.A.G.; Bolnick, D.I. Winter Break? The Effect of Overwintering on Immune Gene Expression in Wood Frogs. Comp. Biochem. Phys. D 2024, 52, 101296. [Google Scholar] [CrossRef]
- Delgado-Baquerizo, M.; Guerra, C.A.; Cano-Díaz, C.; Egidi, E.; Wang, J.T.; Eisenhauer, N.; Singh, B.K.; Maestre, F.T. The Proportion of Soil-Borne Pathogens Increases with Warming at the Global Scale. Nat. Clim. Change 2020, 10, 550–554. [Google Scholar] [CrossRef]
- Hoberg, E.P.; Brooks, D.R. Evolution in Action: Climate Change, Biodiversity Dynamics and Emerging Infectious Disease. Philos. Trans. R. Soc. B 2015, 370, 20130553. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cohen, J.M.; Rohr, J.R. Review and Synthesis of the Effects of Climate Change on Amphibians. Integr. Zool. 2013, 8, 145–161. [Google Scholar] [CrossRef] [PubMed]
- Pounds, J.A.; Bustamante, M.R.; Coloma, L.A.; Consuegra, J.A.; Fogden, M.P.L.; Foster, P.N.; La Marca, E.; Masters, K.L.; Merino-Viteri, A.; Puschendorf, R.; et al. Widespread Amphibian Extinctions from Epidemic Disease Driven by Global Warming. Nature 2006, 439, 161–167. [Google Scholar] [CrossRef]
- Rohr, J.R.; Raffel, T.R. Linking Global Climate and Temperature Variability to Widespread Amphibian Declines Putatively Caused by Disease. Proc. Natl. Acad. Sci. USA 2010, 107, 8269–8274. [Google Scholar] [CrossRef]
- Scheele, B.C.; Pasmans, F.; Skerratt, L.F.; Berger, L.; Martel, A.; Beukema, W.; Acevedo, A.A.; Burrowes, P.A.; Carvalho, T.; Catenazzi, A.; et al. Amphibian Fungal Panzootic Causes Catastrophic and Ongoing Loss of Biodiversity. Science 2019, 363, 1459–1463. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.Z. The Effect of Environmental Temperature on Natural Immunity, Antioxidant Function and Energy Consumption in Bufo bufo gargarizans. Master’s Thesis, Zhejiang Normal University, Jinhua, China, 2013. [Google Scholar]
- Brooks, D.R.; Hoberg, E.P. How will Global Climate Change Affect Parasite-Host Assemblages? Trends Parasitol. 2007, 23, 571–574. [Google Scholar] [CrossRef]
- Niu, Y.G. Physiological and Biochemical Characteristics and Underlying Molecular Mechanisms of Hibernation in Nanorana parkeri. Ph.D. Thesis, Lanzhou University, Lanzhou, China, 2019. [Google Scholar]
- Wang, N. The Effect of Acute Cold Stress and Hibernation Temperature Fluctuation on Non-Specific Immunity, Antioxidative Defenses and Hsp70 Expression in the Giant Spiny Frog (Paa spinosa). Master’s Thesis, Zhejiang Normal University, Jinhua, China, 2011. [Google Scholar]
- Wang, N.; Shao, C.; Xie, Z.G.; Ling, Y.; Cheng, D.H. Viability and Changes of Physiological Functions in the Tiger Frog (Hoplobatrachus rugulosus) Exposed to Cold Stress. Acta Ecol. Sinica 2012, 32, 3538–3545. [Google Scholar] [CrossRef]
- Huang, Z.S. High Temperature Stress on the Frog Tadpole Antioxidant Enzyme Activity. J. Fish. Res. 2016, 38, 445–452. [Google Scholar]
- Ritchie, D.J.; Friesen, C.R. Invited Review: Thermal Effects on Oxidative Stress in Vertebrate Ectotherms. Comp. Biochem. Phys. A 2022, 263, 111082. [Google Scholar] [CrossRef]
- Chevalier, C.; Stojanović, O.; Colin, D.J.; Suarez-Zamorano, N.; Tarallo, V.; Veyrat-Durebex, C.; Rigo, D.; Fabbiano, S.; Stevanović, A.; Hagemann, S.; et al. Gut Microbiota Orchestrates Energy Homeostasis during Cold. Cell 2015, 163, 1360–1374. [Google Scholar] [CrossRef] [PubMed]
- Dishaw, L.J.; Flores-Torres, J.; Lax, S.; Gemayel, K.; Leigh, B.; Melillo, D.; Mueller, M.G.; Natale, L.; Zucchetti, I.; De Santis, R.; et al. The Gut of Geographically Disparate Ciona intestinalis Harbors a Core Microbiota. PLoS ONE 2014, 9, e93386. [Google Scholar] [CrossRef]
- Xue, S.Y.; Fang, J.G.; Zhang, J.H.; Jiang, Z.J.; Mao, Y.Z.; Zhao, F.Z. Effects of Temperature and Salinity on the Development of the Amphipod Crustacean Eogammarus sinensis. Chin. J. Oceanol. Limn. 2013, 31, 1010–1017. [Google Scholar] [CrossRef]
- Cao, H.W.; Shi, Y.P.; Wang, J.; Niu, Z.Y.; Wei, L.; Tian, H.B.; Yu, F.F.; Gao, L. The Intestinal Microbiota and Metabolic Profiles of Strauchbufo raddei Underwent Adaptive Changes during Hibernation. Integr. Zool. 2023, 19, 612–630. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Park, J.K.; Do, Y. Gut Microbiome Diversity and Function During Hibernation and Spring Emergence in an Aquatic Frog. PLoS ONE 2024, 19, e0298245. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Do, Y. Combined Effect of Seasons and Life History in an Anuran Strengthens the Response and Relationship Between Their Physiology and Gut Microbiota. Sci. Rep. 2024, 14, 10137. [Google Scholar] [CrossRef] [PubMed]
- Tong, Q.; Cui, L.Y.; Hu, Z.F.; Du, X.P.; Abid, H.M.; Wang, H.B. Environmental and Host Factors Shaping the Gut Microbiota Diversity of Brown Frog Rana dybowskii. Sci. Total Environ. 2020, 741, 140142. [Google Scholar] [CrossRef]
- Tong, Q.; Dong, W.J.; Xu, M.D.; Hu, Z.F.; Guo, P.; Han, X.Y.; Cui, L.Y. Characteristics and a Comparison of the Gut Microbiota in Two Frog Species at the Beginning and End of Hibernation. Front. Microbiol. 2023, 14, 1057398. [Google Scholar] [CrossRef]
- Williams, C.E.; Williams, C.L.; Logan, M.L. Climate Change is not just Global Warming: Multidimensional Impacts on Animal Gut Microbiota. Microb. Biotechnol. 2023, 16, 1736–1744. [Google Scholar] [CrossRef]
- Kohl, K.D.; Yahn, J. Effects of Environmental Temperature on the Gut Microbial Communities of Tadpoles. Environ. Microbiol. 2016, 18, 1561–1565. [Google Scholar] [CrossRef]
- Li, J.Y.; Rui, J.P.; Li, Y.L.; Tang, N.; Zhan, S.P.; Jiang, J.P.; Li, X.Z. Ambient Temperature Alters Body Size and Gut Microbiota of Xenopus tropicalis. Sci. China Life Sci. 2019, 62, 915–925. [Google Scholar] [CrossRef]
- Wiebler, J.M.; Kohl, K.D.; Lee, R.E.; Costanzo, J.P. Urea Hydrolysis by Gut Bacteria in a Hibernating Frog: Evidence for Ureanitrogen Recycling in Amphibia. Proc. R. Soc. B 2018, 285, 20180241. [Google Scholar] [CrossRef]
- Zhou, E.; Zhang, L.; He, L.; Xiao, Y.; Zhang, K.; Luo, B. Cold Exposure, Gut Microbiota and Health Implications: A Narrative Review. Sci. Total Environ. 2024, 916, 170060. [Google Scholar] [CrossRef] [PubMed]
- Eterovick, P.C.; Schmidt, R.; Sabino-Pinto, J.; Yang, C.; Künzel, S.; Ruthsatz, K. The Microbiome at the Interface Between Environmental Stress and Animal Health: An Example from the Most Threatened Vertebrate Group. Proc. R. Soc. B 2024, 291, 20240917. [Google Scholar] [CrossRef] [PubMed]
- Gaskins, H.R.; Croix, J.A.; Nakamura, N.; Nava, G.M. Impact of the Intestinal Microbiota on the Development of Mucosal Defense. Clin. Infect. Dis. 2008, 46, S80–S86. [Google Scholar] [CrossRef] [PubMed]
- Lindsay, E.C.; Metcalfe, N.B.; Llewellyn, M.S. The Potential Role of the Gut Microbiota in Shaping Host Energetics and Metabolic Rate. J. Anim. Ecol. 2020, 89, 2415–2426. [Google Scholar] [CrossRef]
- McFall-Ngai, M.; Hadfield, M.G.; Bosch, T.C.G.; Carey, H.V.; Domazet-Lošo, T.; Douglas, A.E.; Dubilier, N.; Eberl, G.; Fukami, T.; Gilbert, S.F.; et al. Animals in a Bacterial World, a New Imperative for the Life Sciences. Proc. Natl. Acad. Sci. USA 2013, 110, 3229–3236. [Google Scholar] [CrossRef]
- Gibiino, G.; Lopetuso, L.R.; Scaldaferri, F.; Rizzatti, G.; Binda, C.; Gasbarrini, A. Exploring Bacteroidetes: Metabolic Key Points and Immunological Tricks of Our Gut Commensals. Digest. Liver Dis. 2018, 50, 635–639. [Google Scholar] [CrossRef]
- Levy, M.; Thaiss, C.A.; Elinav, E. Metabolites: Messengers between the Microbiota and the Immune System. Genes Dev. 2016, 30, 1589–1597. [Google Scholar] [CrossRef]
- Li, S.P.; Heng, X.; Guo, L.Y.; Lessing, D.J.; Chu, W.H. SCFAs Improve Disease Resistance via Modulate Gut Microbiota, Enhance Immune Response and Increase Antioxidative Capacity in the Host. Fish Shellfish Immun. 2022, 120, 560–568. [Google Scholar] [CrossRef]
- Tan, D.S.Y.; Akelew, Y.; Snelson, M.; Nguyen, J.; O’Sullivan, K.M. Unravelling the Link between the Gut Microbiome and Autoimmune Kidney Diseases: A Potential New Therapeutic Approach. Int. J. Mol. Sci. 2024, 25, 4817. [Google Scholar] [CrossRef]
- Yoo, J.Y.; Groer, M.; Dutra, S.V.O.; Sarkar, A.; McSkimming, D.I. Gut Microbiota and Immune System Interactions. Microorganisms 2020, 8, 1587. [Google Scholar] [CrossRef]
- Jiménez, R.R.; Sommer, S. The Amphibian Microbiome: Natural Range of Variation, Pathogenic Dysbiosis, and Role in Conservation. Biodivers. Conserv. 2016, 26, 763–786. [Google Scholar] [CrossRef]
- Yun, B.; King, M.; Draz, M.S.; Kline, T.; Rodriguez-Palacios, A. Oxidative Reactivity Across Kingdoms in the Gut: Host Immunity, Stressed Microbiota and Oxidized Foods. Free Radical Bio. Med. 2022, 178, 97–110. [Google Scholar] [CrossRef]
- Liao, G.W.; Wang, W.Q.; Yu, J.P.; Li, J.P.; Yan, Y.M.; Liu, H.L.; Chen, B.; Fan, L.F. Integrated Analysis of Intestinal Microbiota and Transcriptome Reveals That a Coordinated Interaction of the Endocrine, Immune System and Gut Microbiota Response to Heat Stress in Litopenaeus vannamei. Dev. Comp. Immunol. 2024, 156, 105176. [Google Scholar] [CrossRef]
- Weng, F.C.H.; Yang, Y.J.; Wang, D. Functional Analysis for Gut Microbes of the Brown Tree Frog (Polypedates megacephalus) in Artificial Hibernation. BMC Genom. 2016, 17, 1024. [Google Scholar] [CrossRef]
- She, K.; Liu, P. Distribution and Protection Status of Two Kinds of Wood Frog in Heilongjiang Province. Nat. Sci. J. Harbin Norm. Univ. 2009, 25, 101–103. [Google Scholar]
- Huang, H. Comparison of Intestinal Flora Between Rana chensinensis and Rana amurensis and Preliminary Analysis of Immune Function. Master’s Thesis, Northeast Forestry University, Harbin, China, 2019. [Google Scholar]
- Xu, L.; Dong, W.Q.; Yang, X.Y.; Jia, Y.T.; Yang, B.; Xiao, X.H.; Huang, P.Y.; Chai, L.H. Comparative Study on Cold Tolerance Capacities of Two Species of Wood Frogs in Heilongjiang Province: New Evidence for the Cold Tolerance of Rana amurensis Over Rana dybowskii. Heilongjiang Anim. Sci. Vet. Med. 2020, 9, 148–150,154. [Google Scholar]
- Zhao, E.M.; Zhao, K.T.; Zhou, K.Y. Fauna Sinica Reptilia. Vol. 2 Squamata; Chinese Science Press: Beijing, China, 1999. [Google Scholar]
- Zhao, W.G. Annals of Amphibians and Reptiles in Heilongjiang Province; Science Press: Beijing, China, 2008. [Google Scholar]
- Brown, G.P.; Shilton, C.M.; Shine, R. Measuring Amphibian Immunocompetence: Validation of the Phytohemagglutinin Skin-swelling Assay in the Cane Toad, Rhinella marina. Methods Ecol. Evol. 2011, 2, 341–348. [Google Scholar] [CrossRef]
- Hawley, L.; Smalling, K.L.; Glaberman, S. Critical Review of the Phytohemagglutinin Assay for Assessing Amphibian Immunity. Conserv. Physiol. 2023, 11, coad090. [Google Scholar] [CrossRef]
- Bardocz, S.; Grant, G.; Ewen, S.W.B.; Duguid, T.J.; Brown, D.S.; Englyst, K.; Pusztai, A. Reversible Effect of Phytohaemagglutinin on the Growth and Metabolism of Rat Gastrointestinal Tract. Gut 1995, 37, 353–360. [Google Scholar] [CrossRef]
- Humphries, J.E.; Hicks, A.; Lanctôt, C.; McCallum, H.; Newell, D.; Grogan, L.F. Amphibian Cellular Immune Response to Chytridiomycosis at Metamorphic Climax. Immunol. Res. 2025, 73, 44. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef] [PubMed]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Maki, K.A.; Wolff, B.; Varuzza, L.; Green, S.J.; Barb, J.J. Multi-amplicon Microbiome Data Analysis Pipelines for Mixed Orientation Sequences Using QIIME2: Assessing Reference Database, Variable Region and Pre-Processing Bias in Classification of Mock Bacterial Community Samples. PLoS ONE 2023, 18, e0280293. [Google Scholar] [CrossRef] [PubMed]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.; Holmes, S.P. DADA2: High-Resolution Sample Inference From Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef] [PubMed]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Afgan, E.; Baker, D.; Batut, B.; van den Beek, M.; Bouvier, D.; Čech, M.; Chilton, J.; Clements, D.; Coraor, N.; Grüning, B.A.; et al. The Galaxy Platform for Accessible, Reproducible and Collaborative Biomedical Analyses: 2018 Update. Nucl. Acids. Res. 2018, 46, W537–W544. [Google Scholar] [CrossRef]
- Hung, Y.M.; Lu, T.P.; Tsai, M.H.; Lai, L.C.; Chuang, E.Y. EasyMAP: A User-Friendly Online Platform for Analyzing 16S Ribosomal DNA Sequencing Data. New Biotechnol. 2021, 63, 37–44. [Google Scholar] [CrossRef]
- Rognes, T.; Flouri, T.; Nichols, B.; Quince, C.; Mahé, F. VSEARCH: A Versatile Open Source Tool for Metagenomics. Peer J. 2016, 4, e2584. [Google Scholar] [CrossRef]
- Kanehisa, M.; Goto, S. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucl. Acids. Res. 2000, 28, 27–30. [Google Scholar] [CrossRef]
- Assis, V.R.; Robert, J.; Titon, S.C.M. Introduction to the Special Issue Amphibian Immunity: Stress, Disease and Ecoimmunology. Philos. Trans. R. Soc. B 2023, 378, 20220117. [Google Scholar] [CrossRef]
- Rollins-Smith, L.A. The Future of Amphibian Immunology: Opportunities and Challenges. Dev. Comp. Immunol. 2024, 160, 105237. [Google Scholar] [CrossRef]
- de Freitas Dutra, V.; Leal, V.N.C.; Pontillo, A. The Inflammasomes: Crosstalk Between Innate Immunity and Hematology. Inflamm. Res. 2022, 71, 1403–1416. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Do, Y. The Difference and Variation of Gut Bacterial Community and Host Physiology Can Support Adaptation During and After Overwintering in Frog Population. Integr. Zool. 2024, 19, 631–645. [Google Scholar] [CrossRef]
- Comas, M.; Zamora-Camacho, F.J.; Garrido-Bautista, J.; Moreno-Rueda, G.; MartÍN, J.; LÓPez, P. Mounting an Immune Response Reduces Male Attractiveness in a Lizard. Integr. Zool. 2024, 2024, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.K.; Li, B.; Wang, Y.; Shao, C. Effects of Shock Chilling on Malonaldehyhy Content and Superoxide Dismutase Activity in Different Tissues of Hoplobatrachus chinensis and Bufo gargarizans and Their Comparison. J. Anhui Agr. Sci. 2018, 46, 97–99. [Google Scholar]
- Dawson, N.J.; Katzenback, B.A.; Storey, K.B. Free-radical First Responders: The Characterization of CuZnSOD and MnSOD Regulation During Freezing of the Freeze-Tolerant North American Wood Frog, Rana sylvatica. BBA-Gen Subj. 2015, 1850, 97–106. [Google Scholar] [CrossRef]
- Dawson, N.J.; Storey, K.B. A Hydrogen Peroxide Safety Valve: The Reversible Phosphorylation of Catalase from the Freeze-tolerant North American Wood Frog, Rana sylvatica. BBA Gen Subj. 2016, 1860, 476–485. [Google Scholar] [CrossRef]
- Giraud-Billoud, M.; Rivera-Ingraham, G.A.; Moreira, D.C.; Burmester, T.; Castro-Vazquez, A.; Carvajalino-Fernández, J.M.; Dafre, A.; Niu, C.; Tremblay, N.; Paital, B.; et al. Twenty Years of the ‘Preparation for Oxidative Stress’ (POS) Theory: Ecophysiological Advantages and Molecular Strategies. Comp. Biochem. Phys. A 2019, 234, 36–49. [Google Scholar] [CrossRef]
- Zhao, H.L.; Zhang, C.H.; Meng, R.Y.; Ma, J.X.; Wen, R.S.; Yu, C.P. Analysis on the Mechanism of Artificial Induction of Awakening Adaptation in Rana dybowskii. Anhui. Agr. Sci. Bull. 2024, 30, 49–55. [Google Scholar]
- Fitzpatrick, M.J.; Porter, W.P.; Pauli, J.N.; Kearney, M.R.; Notaro, M.; Zuckerberg, B. Future Winters Present a Complex Energetic Landscape of Decreased Costs and Reduced Risk for a Freeze-tolerant Amphibian, the Wood Frog (Lithobates sylvaticus). Global Change Biol. 2020, 26, 6350–6362. [Google Scholar] [CrossRef]
- Zhang, C.Y.; Hu, H.L.; Wang, Y.; Zhang, L.; Ding, G.H. Combined effects of thermal environment and gene regulation on metabolic enzyme activities of major metabolic tissues in a winter-breeding amphibian. J. Therm. Biol. 2024, 125, 104000. [Google Scholar] [CrossRef]
- Candela, M.; Biagi, E.; Maccaferri, S.; Turroni, S.; Brigidi, P. Intestinal Microbiota is a Plastic Factor Responding to Environmental Changes. Trends Microbiol. 2012, 20, 385–391. [Google Scholar] [CrossRef]
- Vaziri, G.J.; Caicedo, B.; Dahrouge, N.; Ryerson, W.G.; Davenport, J.M.; Stager, M.; Jones, K.R.; Frost, C.; Seewagen, C.L.; Rittenhouse, T.A.G.; et al. Gut Microbiomes are Largely Unchanged When Exposed to Their Amphibian Host’s Latitudinally Variable Upper Thermal Limit. Comp. Biochem. Phys. A 2025, 302, 111816. [Google Scholar] [CrossRef]
- Kruger, A.; Roth, S. Temporal Variation in Skin Microbiota of Cohabitating Amphibians. Can. J. Microbiol. 2022, 68, 583–593. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.Q.; He, C.; Hua, Y.L.; Miao, Z.Y.; Li, Z.X.; Dong, B.J. Comparative Study on Gut Bacteria Diversity of Captive and Wild Rana dybowskii in Liaoning Province. J. Econ. Anim. 2025, 1, 1–12. [Google Scholar]
- Colston, T.J.; Jackson, C.R. Microbiome Evolution along Divergent Branches of the Vertebrate Tree of Life: What Is Known and Unknown. Mol. Ecol. 2016, 25, 3776–3800. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Tian, Y.N.; Wu, Y.; Ma, X. Contributions of the Interaction Between Dietary Protein and Gut Microbiota to Intestinal Health. Curr. Protein Pept. Sc. 2017, 18, 795–808. [Google Scholar] [CrossRef]
- Zhang, M.J.; Chen, H.; Liu, L.S.; Xu, L.L.; Wang, X.G.; Chang, L.M.; Chang, Q.; Lu, G.Q.; Jiang, J.P.; Zhu, L.F. The Changes in the Frog Gut Microbiome and Its Putative Oxygen-Related Phenotypes Accompanying the Development of Gastrointestinal Complexity and Dietary Shift. Front. Microbiol. 2020, 11, 162. [Google Scholar] [CrossRef]
- Dudek-Wicher, R.K.; Junka, A.; Bartoszewicz, M. The Influence of Antibiotics and Dietary Components on Gut Microbiota. Gastroenterol. Rev. 2018, 13, 85–92. [Google Scholar] [CrossRef]
- Gong, J.H.; Shen, Y.; Zhang, H.C.; Cao, M.; Guo, M.Y.; He, J.Q.; Zhang, B.Z.; Xiao, C.X. Gut Microbiota Characteristics of People with Obesity by Meta-Analysis of Existing Datasets. Nutrients 2022, 14, 2993. [Google Scholar] [CrossRef]
- Carey, H.V.; Assadi-Porter, F.M. The Hibernator Microbiome: Host-Bacterial Interactions in an Extreme Nutritional Symbiosis. Annu. Rev. Nutr. 2017, 37, 477–500. [Google Scholar] [CrossRef]
- Huang, C.H.; Liao, W.B. Seasonal Variation in Gut Microbiota Related to Diet in Fejervarya limnocharis. Animals 2021, 11, 1393. [Google Scholar] [CrossRef] [PubMed]
- Rollins, H.B.; Benard, M.F. Challenges in Predicting the Outcome of Competition Based on Climate Change-Induced Phenological and Body Size Shifts. Oecologia 2020, 193, 749–759. [Google Scholar] [CrossRef] [PubMed]
- Yang, B.; Cui, Z.Z.; Ning, M.H.; Chen, Y.; Wu, Z.J.; Huang, H.Y. Variation in the Intestinal Microbiota at Different Developmental Stages of Hynobius maoershanensis. Ecol. Evol. 2022, 12, e8712. [Google Scholar] [CrossRef]
- Jin, Y.X.; Zeng, Z.Y.; Wu, Y.; Zhang, S.B.; Fu, Z.W. Oral Exposure of Mice to Carbendazim Induces Hepatic Lipid Metabolism Disorder and Gut Microbiota Dysbiosis. Toxicol. Sci. 2015, 147, 116–126. [Google Scholar] [CrossRef]
- Xiang, J.G.; He, T.Y.; Wang, P.P.; Xie, M.; Xiang, J.; Ni, J.J. Opportunistic Pathogens are Abundant in the Gut of Cultured Giant Spiny Frog (Paa spinosa). Aquac. Res. 2018, 49, 2033–2041. [Google Scholar] [CrossRef]
- Binda, C.; Lopetuso, L.R.; Rizzatti, G.; Gibiino, G.; Cennamo, V.; Gasbarrini, A. Actinobacteria: A Relevant Minority for the Maintenance of Gut Homeostasis. Digest. Liver Dis. 2018, 50, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Shin, N.-R.; Whon, T.W.; Bae, J.-W. Proteobacteria: Microbial Signature of Dysbiosis in Gut Microbiota. Trends Biotechnol. 2015, 33, 496–503. [Google Scholar] [CrossRef]
- Ludwig, W.; Euzéby, J.; Whitman, W.B. Road Map of the Phyla Bacteroidetes, Spirochaetes, Tenericutes (Mollicutes), Acidobacteria, Fibrobacteres, Fusobacteria, Dictyoglomi, Gemmatimonadetes, Lentisphaerae, Verrucomicrobia, Chlamydiae, and Planctomycetes. In Bergey’s Manual of Systematics of Archaea and Bacteria; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 1–24. [Google Scholar]
- Sun, Q.L.; Xu, X.X.; Liu, P.; Zhao, W.G. Influence of Ambient Temperature on Body Temperature of Rana dybowskii. Chin. J. Wildl. 2016, 37, 266–270. [Google Scholar]
- Li, D.W.; Liu, J.H.; Peng, T.; Shan, H.J.; Zhang, B.Y.; Meng, F.X.; Yao, X. Comparison of Pre-Winter Thermoregulation in Between Rana dybowskii and R. amurensis. Chin. J. Wildl. 2022, 43, 145–150. [Google Scholar]
- van Dijk, L.J.A.; Fisher, B.L.; Miraldo, A.; Goodsell, R.M.; Iwaszkiewicz-Eggebrecht, E.; Raharinjanahary, D.; Rajoelison, E.T.; Łukasik, P.; Andersson, A.F.; Ronquist, F.; et al. Temperature and Water Availability Drive Insect Seasonality Across a Temperate and a Tropical Region. Proc. R. Soc. B 2024, 291, 20240090. [Google Scholar] [CrossRef] [PubMed]
- Bos, A.V.; Erkelens, M.N.; Koenders, S.T.A.; van der Stelt, M.; van Egmond, M.; Mebius, R.E. Clickable Vitamins as a New Tool to Track Vitamin A and Retinoic Acid in Immune Cells. Front. Immunol. 2021, 12, 671283. [Google Scholar] [CrossRef]
- Kloehn, J.; Blume, M.; Cobbold, S.A.; Saunders, E.C.; Dagley, M.J.; McConville, M.J. Using Metabolomics to Dissect Host-parasite Interactions. Curr. Opin. Microbiol. 2016, 32, 59–65. [Google Scholar] [CrossRef]
- Vijendravarma, R.K.; Narasimha, S.; Chakrabarti, S.; Babin, A.; Kolly, S.; Lemaitre, B.; Kawecki, T.J.; Turlings, T. Gut Physiology Mediates a Trade-Off Between Adaptation to Malnutrition and Susceptibility to Food-Borne Pathogens. Ecol. Lett. 2015, 18, 1078–1086. [Google Scholar] [CrossRef]
- Shi, Q.K.; Li, Y.; Deng, S.T.; Zhang, H.J.; Jiang, H.L.; Shen, L.; Pan, T.; Hong, P.; Wu, H.L.; Shu, Y.L. The Succession of Gut Microbiota in the Concave-Eared Torrent Frog (Odorrana tormota) Throughout Developmental History. Ecol. Evol. 2023, 13, e10094. [Google Scholar] [CrossRef]
- Voituron, Y.; Paaschburg, L.; Holmstrup, M.; Barré, H.; Ramløv, H. Survival and Metabolism of Rana arvalis during Freezing. J. Comp. Physiol. B 2008, 179, 223–230. [Google Scholar] [CrossRef]
- Kokou, F.; Sasson, G.; Nitzan, T.; Doron-Faigenboim, A.; Harpaz, S.; Cnaani, A.; Mizrahi, I. Host Genetic Selection for Cold Tolerance Shapes Microbiome Composition and Modulates its Response to Temperature. eLife 2018, 7, e36398. [Google Scholar] [CrossRef]
- Lee, Y.K.; Mazmanian, S.K. Has the Microbiota Played a Critical Role in the Evolution of the Adaptive Immune System? Science 2010, 330, 1768–1773. [Google Scholar] [CrossRef]
- Woodhams, D.C.; McCartney, J.; Walke, J.B.; Whetstone, R. The Adaptive Microbiome Hypothesis and Immune Interactions in Amphibian Mucus. Dev. Comp. Immunol. 2023, 145, 104690. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.H.; Liu, H.; Wang, H.; Lu, Z.N.; Han, X.Y.; Luo, Z.W.; Wu, L.G.; Tong, Q. From Skin to Gut: Understanding Microbial Diversity in Rana amurensis and R. dybowskii. Curr. Microbiol. 2024, 81, 354. [Google Scholar] [CrossRef]
- den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.-J.; Bakker, B.M. The Role of Short-Chain Fatty Acids in the Interplay Between Diet, Gut Microbiota, and Host Energy Metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Biller, J.D.; Takahashi, L.S. Oxidative Stress and Fish Immune System: Phagocytosis and Leukocyte Respiratory Burst Activity. An. Acad. Bras. Cienc. 2018, 90, 3403–3414. [Google Scholar] [CrossRef]
- Hissen, K.L.; He, W.; Wu, G.; Criscitiello, M.F. Immunonutrition: Facilitating Mucosal Immune Response in Teleost Intestine with Amino Acids Through Oxidant-Antioxidant Balance. Front. Immunol. 2023, 14, 1241615. [Google Scholar] [CrossRef]
- Mills, S.; Yang, B.; Smith, G.J.; Stanton, C.; Ross, R.P. Efficacy of Bifidobacterium longum Alone or in Multi-Strain Probiotic Formulations During Early Life and Beyond. Gut Microbes 2023, 15, 2186098. [Google Scholar] [CrossRef]
- Rizzatti, G.; Lopetuso, L.R.; Gibiino, G.; Binda, C.; Gasbarrini, A. Proteobacteria: A Common Factor in Human Diseases. BioMed Res. Int. 2017, 2017, 9351507. [Google Scholar] [CrossRef]
- Ahlawat, S.; Asha, N.; Sharma, K.K. Gut-Organ Axis: A Microbial Outreach and Networking. Lett. Appl. Microbiol. 2020, 72, 636–668. [Google Scholar] [CrossRef]
- Macke, E.; Tasiemski, A.; Massol, F.; Callens, M.; Decaestecker, E. Life History and Eco-Evolutionary Dynamics in Light of the Gut Microbiota. Oikos 2017, 126, 508–531. [Google Scholar] [CrossRef]
- Kefford, B.J.; Ghalambor, C.K.; Dewenter, B.; Poff, N.L.; Hughes, J.; Reich, J.; Thompson, R. Acute, Diel, and Annual Temperature Variability and the Thermal Biology of Ectotherms. Glob. Change Biol. 2022, 28, 6872–6888. [Google Scholar] [CrossRef]
- Shahvandi, M.K.; Adhikari, S.; Dumberry, M.; Mishra, S.; Soja, B. The Increasingly Dominant Role of Climate Change on Length of Day Variations. Proc. Natl. Acad. Sci. USA 2024, 121, e2406930121. [Google Scholar] [CrossRef] [PubMed]
- Turner, R.K.; Maclean, I.M.D. Microclimate–driven Trends in Spring-Emergence Phenology in a Temperate Reptile (Vipera berus): Evidence for a Potential “Climate Trap”? Ecol. Evol. 2022, 12, e8623. [Google Scholar] [CrossRef]
- Chatterjee, D.; Sivashanmugam, K. Immunomodulatory Peptides: New Therapeutic Horizons for Emerging and Re-Emerging Infectious Diseases. Front. Microbiol. 2024, 15, 1505571. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.N.; Gao, F.L.; Huang, Y.X.; Zheng, R.H.; Fang, C.; Huang, W.S.; Wang, K.J.; Bo, J. Antimicrobial Activity and Immunomodulation of Four Novel Cathelicidin Genes Isolated from the Tiger Frog Hoplobatrachus rugulosus. Comp. Biochem. Phys. C 2025, 289, 110091. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lan, Z.; Zhou, S.; Wang, C.; Liu, W.; Liu, P. The Immune-Antioxidant Trade-Off Mediated by Actinobacteria Drives Niche Differentiation: Physiological and Gut Microbiota Responses of Two Cold-Adapted Brown Frog Species to Contrasting Peak Daily Habitat Temperatures. Animals 2025, 15, 3604. https://doi.org/10.3390/ani15243604
Lan Z, Zhou S, Wang C, Liu W, Liu P. The Immune-Antioxidant Trade-Off Mediated by Actinobacteria Drives Niche Differentiation: Physiological and Gut Microbiota Responses of Two Cold-Adapted Brown Frog Species to Contrasting Peak Daily Habitat Temperatures. Animals. 2025; 15(24):3604. https://doi.org/10.3390/ani15243604
Chicago/Turabian StyleLan, Zhenying, Shuang Zhou, Chao Wang, Wanli Liu, and Peng Liu. 2025. "The Immune-Antioxidant Trade-Off Mediated by Actinobacteria Drives Niche Differentiation: Physiological and Gut Microbiota Responses of Two Cold-Adapted Brown Frog Species to Contrasting Peak Daily Habitat Temperatures" Animals 15, no. 24: 3604. https://doi.org/10.3390/ani15243604
APA StyleLan, Z., Zhou, S., Wang, C., Liu, W., & Liu, P. (2025). The Immune-Antioxidant Trade-Off Mediated by Actinobacteria Drives Niche Differentiation: Physiological and Gut Microbiota Responses of Two Cold-Adapted Brown Frog Species to Contrasting Peak Daily Habitat Temperatures. Animals, 15(24), 3604. https://doi.org/10.3390/ani15243604




