Sodium Butyrate Promotes In Vitro Development of Mouse Preantral Follicles and Improves Oocyte Quality by Regulating Steroidogenesis, Oxidative Stress, and Cytoskeleton Remodeling
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection of Mouse Preantral Follicles
2.2. Preantral Follicles for 3D In Vitro Culture
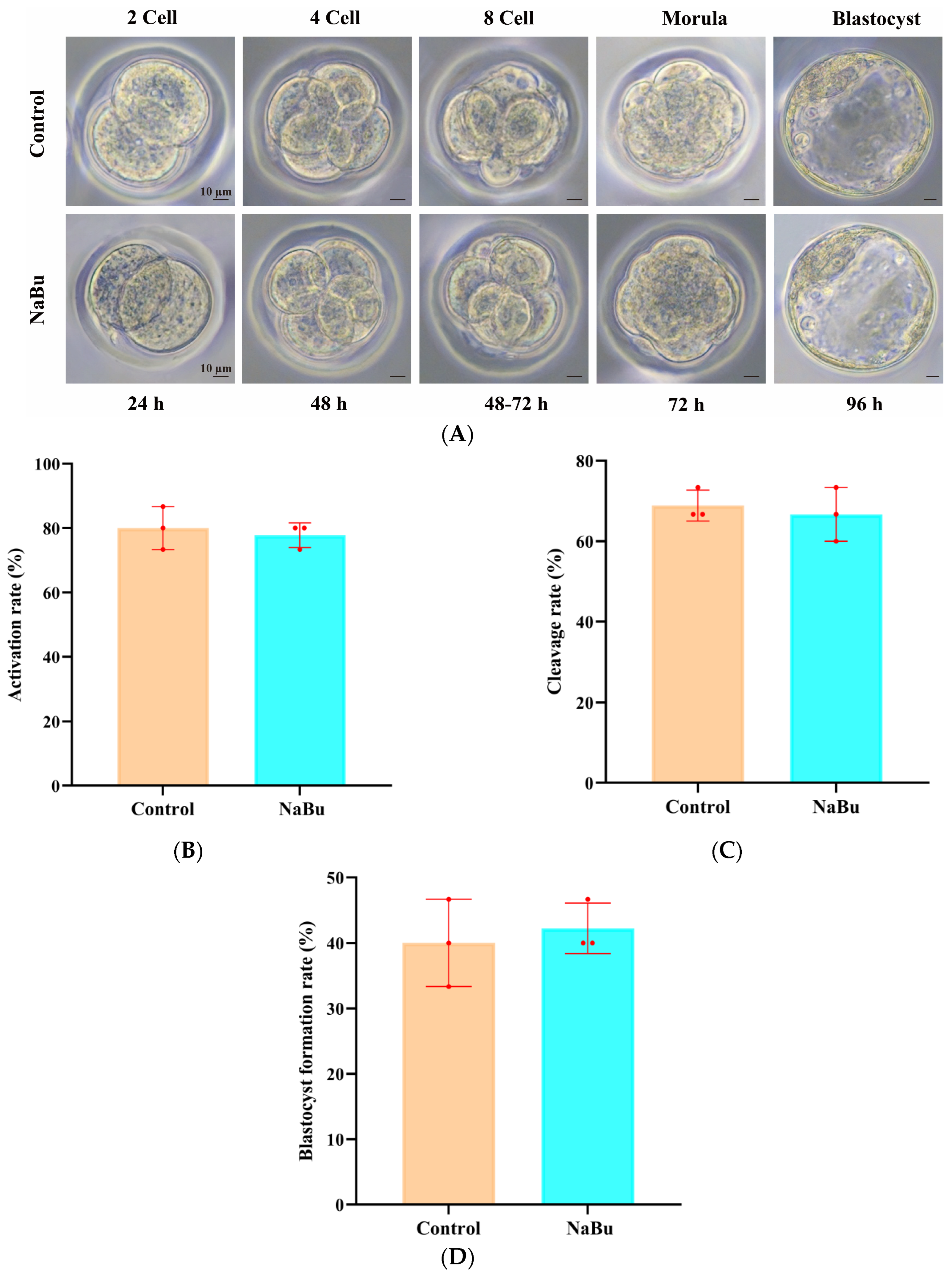
2.3. Oocyte Collection and Parthenogenetic Activation
2.4. Measurement of E2 Secretion In Vitro
2.5. Observation of Follicular Morphology and Measurement of Follicular Diameter
2.6. Evaluation of Follicular Survival, Antral Formation, and Ovulation Rates
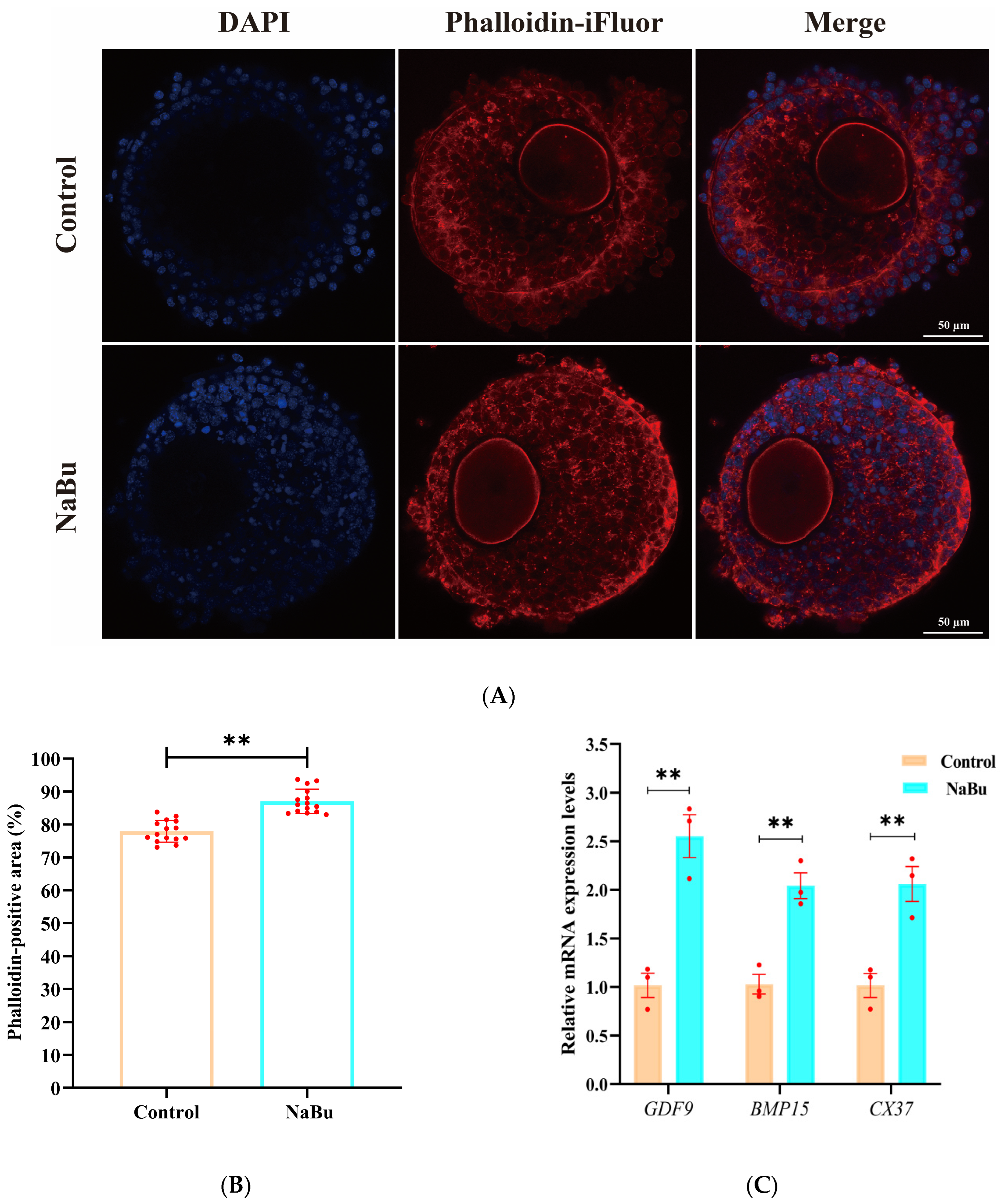
2.7. Detection of Follicular Viability and F-Actin Cytoskeleton
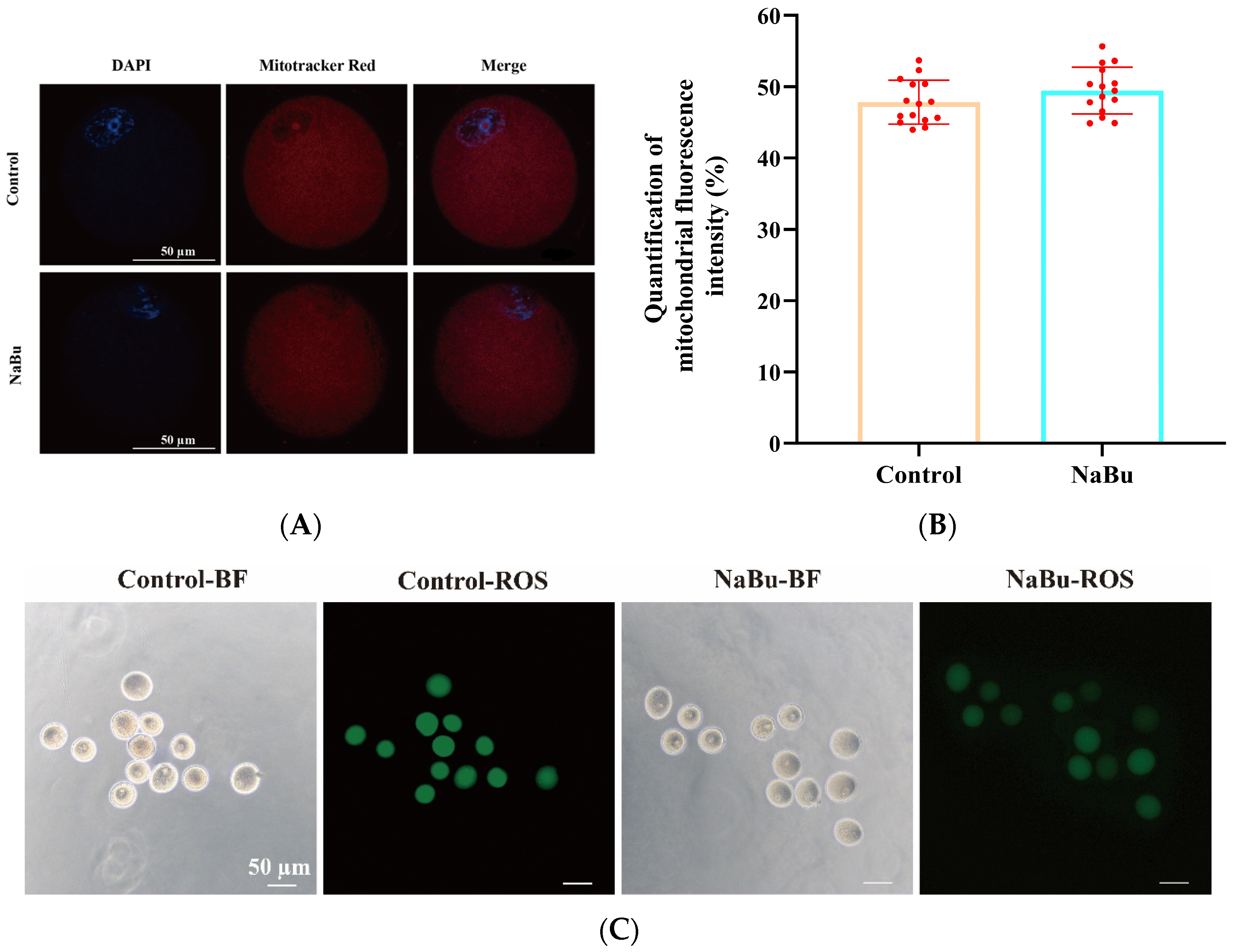
2.8. Detection of Spindle Morphology and Mitochondrial and ROS Distribution in Oocytes
2.9. RNA Extraction and Quantitative PCR (qPCR)
2.10. Statistical Analysis
3. Results
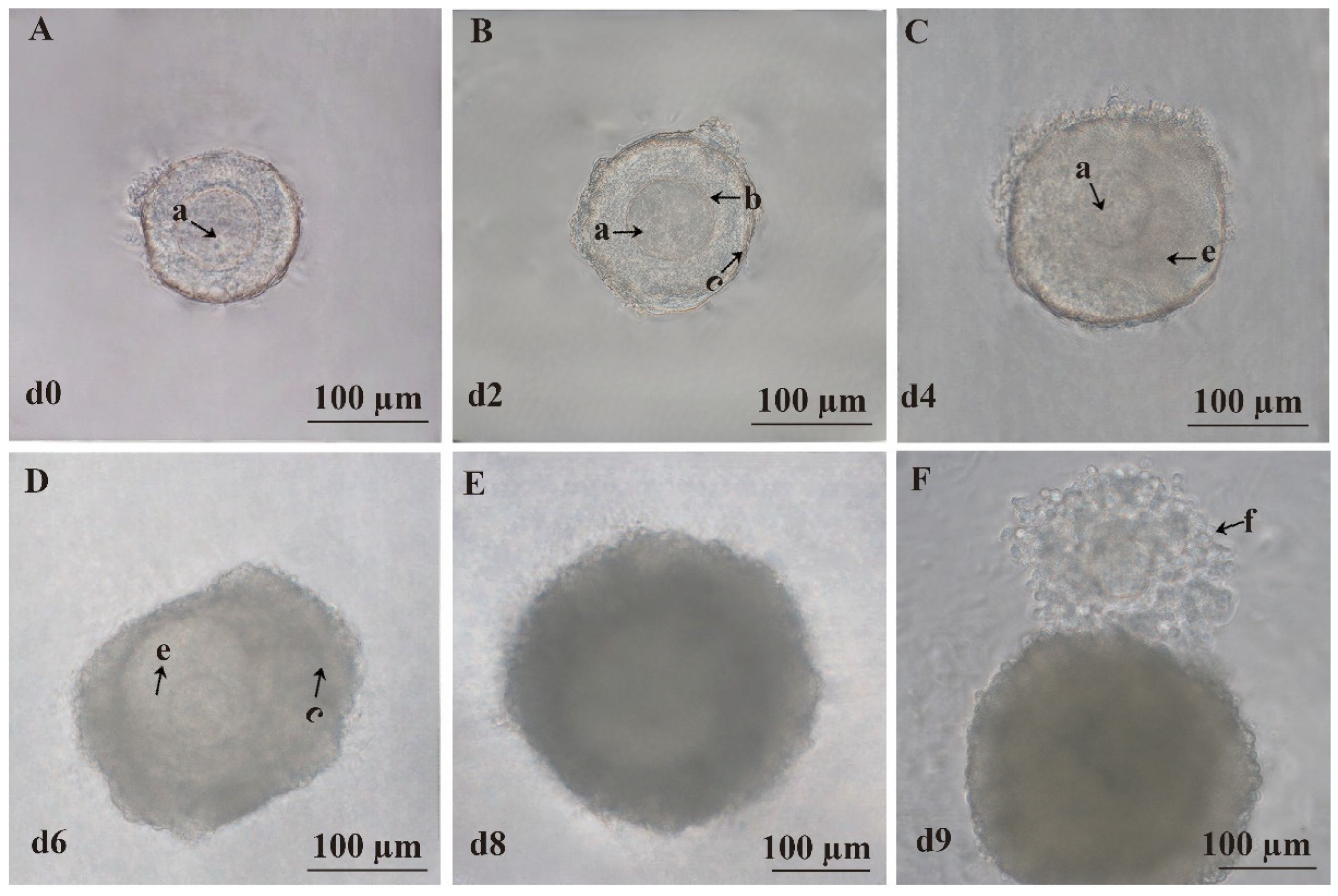
3.1. In Vitro Isolation of Mouse Preantral Follicles
3.2. In Vitro 3D Culture of Mouse Preantral Follicles
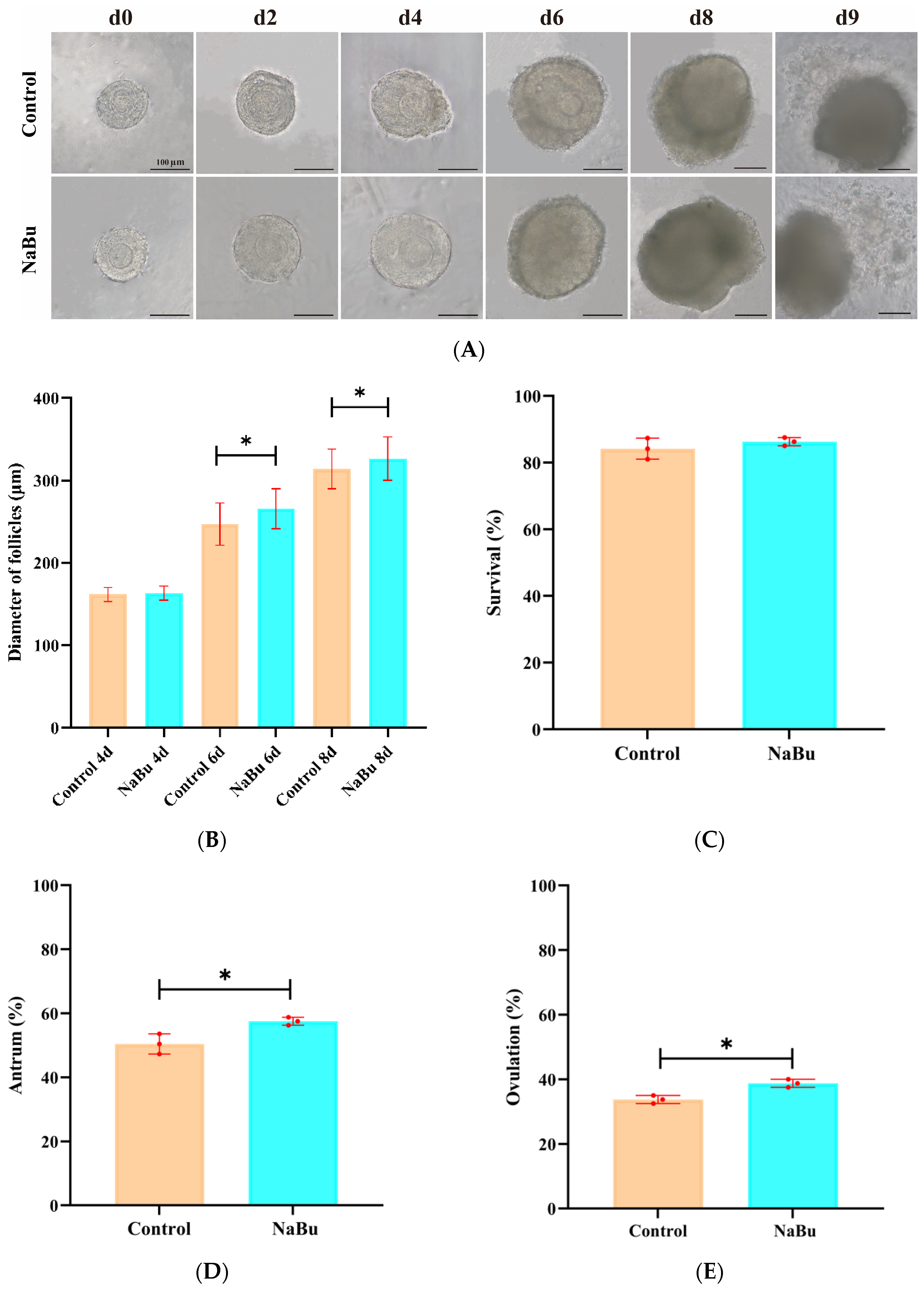
3.3. Effects of NaBu on Follicle Morphology, Diameter, Survival, Antral Formation, and Ovulation In Vitro
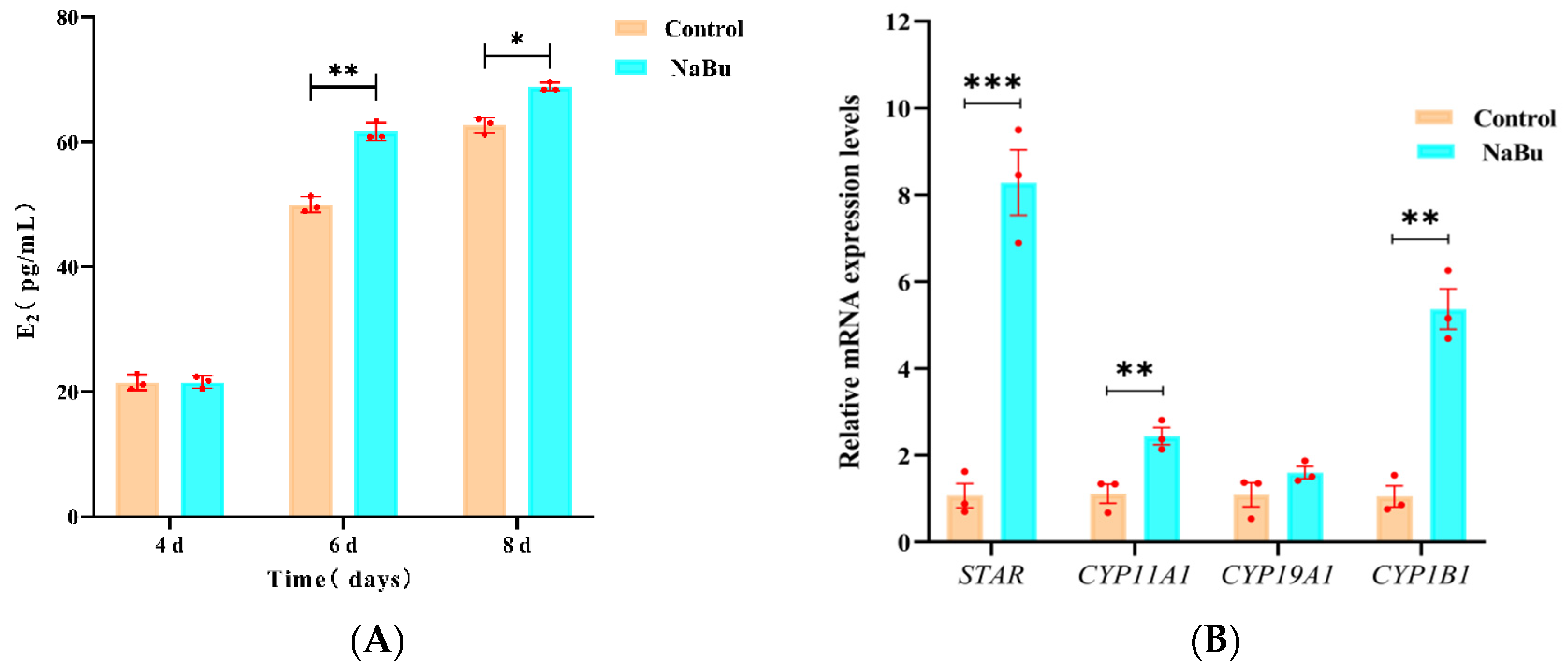
3.4. Effects of NaBu on E2 Secretion
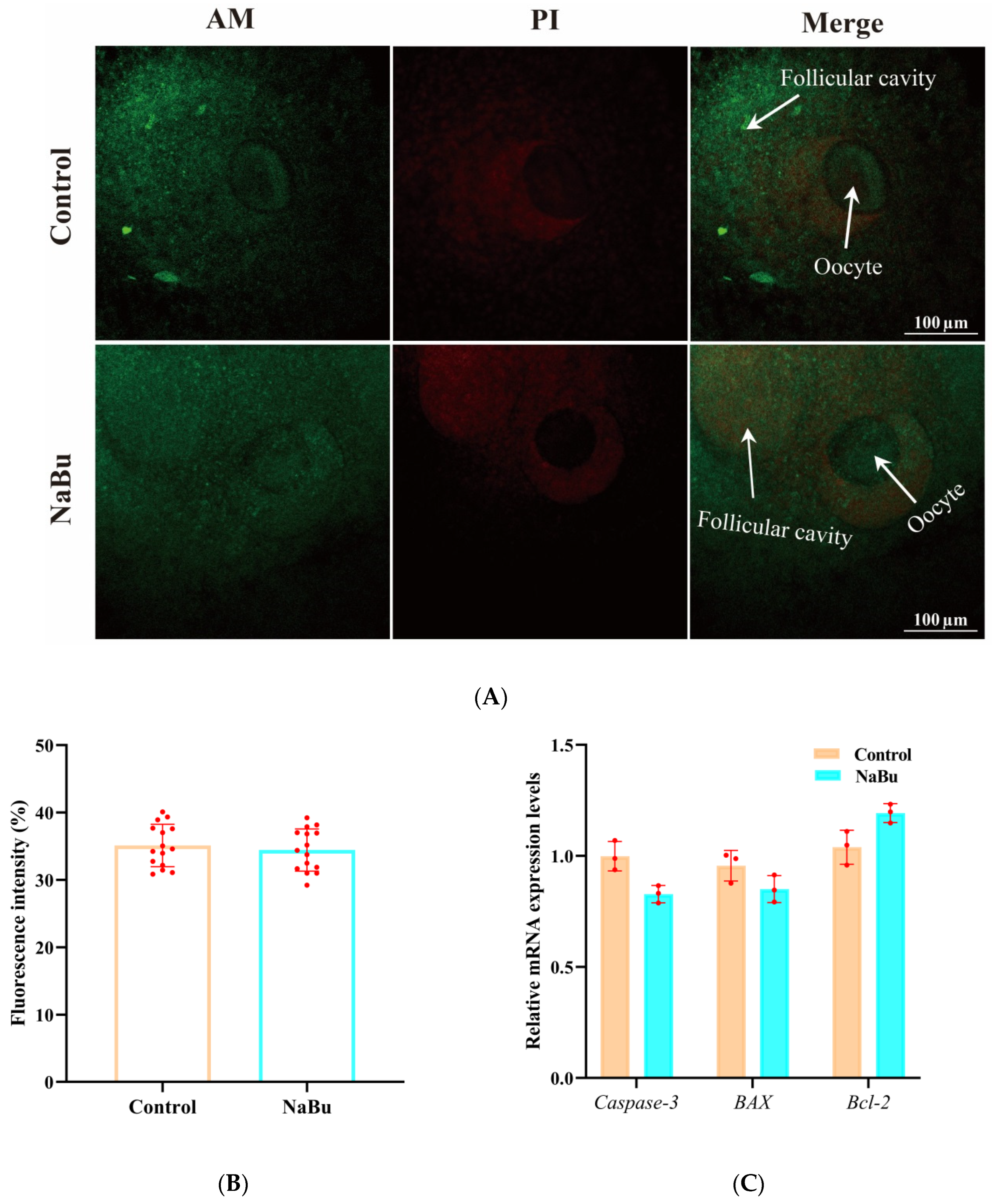
3.5. Effects of NaBu on Follicle Viability
3.6. Effect of NaBu on F-Actin Cytoskeleton Remodeling and Distribution in the Follicles
3.7. Effects of NaBu on Oocyte Spindle Structure and Chromosomal Distribution
3.8. Effects of NaBu on Mitochondrial Distribution and Oxidative Stress in Oocytes
3.9. Effects of NaBu Supplementation During Follicle Growth in 3D In Vitro Culture on the Development of Parthenogenetic Pre-Implantation Embryos
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fricke, P.M.; Wiltbank, M.C. Symposium review: The implications of spontaneous versus synchronized ovulations on the reproductive performance of lactating dairy cows. J. Dairy Sci. 2022, 105, 4679–4689. [Google Scholar] [CrossRef] [PubMed]
- Swelum, A.A.; Hashem, N.M.; Abdelnour, S.A.; Taha, A.E.; Ohran, H.; Khafaga, A.F.; El-Tarabily, K.A.; Abd El-Hack, M.E. Effects of phytogenic feed additives on the reproductive performance of animals. Saudi J. Biol. Sci. 2021, 28, 5816–5822. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar]
- Zhang, M.; Wang, Y.; Zhao, X.; Liu, C.; Wang, B.; Zhou, J. Mechanistic basis and preliminary practice of butyric acid and butyrate sodium to mitigate gut inflammatory diseases: A comprehensive review. Nutr. Res. 2021, 95, 1–18. [Google Scholar] [CrossRef]
- van der Hee, B.; Wells, J.M. Microbial Regulation of Host Physiology by Short-chain Fatty Acids. Trends Microbiol. 2021, 29, 700–712. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, X.; Cui, Y.; Wang, W.; Liu, H.; Li, Z.; Guo, Z.; Ma, S.; Li, D.; Wang, C.; et al. Consumption of Dietary Fiber from Different Sources during Pregnancy Alters Sow Gut Microbiota and Improves Performance and Reduces Inflammation in Sows and Piglets. mSystems 2021, 6, e00591-20. [Google Scholar] [CrossRef]
- Zeng, X.; Li, S.; Ye, Q.; Cai, S.; Quan, S.; Liu, L.; Zhang, S.; Chen, F.; Cai, C.; Wang, F.; et al. The Combined Use of Medium- and Short-Chain Fatty Acids Improves the Pregnancy Outcomes of Sows by Enhancing Ovarian Steroidogenesis and Endometrial Receptivity. Nutrients 2022, 14, 4405. [Google Scholar] [CrossRef]
- Xiao, C.; Zhang, L.; Zhang, B.; Kong, L.; Pan, X.; Goossens, T.; Song, Z. Dietary sodium butyrate improves female broiler breeder performance and offspring immune function by enhancing maternal intestinal barrier and microbiota. Poult. Sci. 2023, 102, 102658. [Google Scholar] [CrossRef]
- Yu, M.F.; Wang, J.L.; Yi, J.M.; Ma, L. Sodium butyrate interrupts the maturation of oocytes and enhances the development of preimplantation embryos. PLoS ONE 2019, 14, e0220479. [Google Scholar]
- Ye, Q.; Zeng, X.; Wang, S.; Zeng, X.; Yang, G.; Ye, C.; Cai, S.; Chen, M.; Li, S.; Qiao, S. Butyrate drives the acetylation of histone H3K9 to activate steroidogenesis through PPARγ and PGC1α pathways in ovarian granulosa cells. Faseb J. 2021, 35, e21316. [Google Scholar] [CrossRef] [PubMed]
- Sakaguchi, K. In Vitro Growth of Mammalian Follicles and Oocytes. Animals 2024, 14, 1355. [Google Scholar] [CrossRef]
- Feng, X.; Xiao, J.; Wang, D.; Fu, X.; Gao, J.; Jiang, M.; Li, J.; Jiang, L.; Liang, X.; Huang, Y.; et al. Butyric acid ameliorates PCOS-related reproductive dysfunction through gut-brain-ovary axis signaling and ovarian steroidogenic factor activation. Front. Endocrinol. 2025, 16, 1604302. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, Y.; Zhang, Y.; Qiu, Y.; Chang, Q.; Yu, X.; Pei, X. Optimized study of an in vitro 3D culture of preantral follicles in mice. J. Vet. Sci. 2023, 24, e4. [Google Scholar] [CrossRef]
- Li, R.; Li, E.; Kamili, G.; Ou, S.; Yang, D. Effect of resveratrol on superovulation in mice. Biomed. Pharmacother. 2022, 146, 112565. [Google Scholar] [CrossRef]
- Olaniyi, K.S.; Akintayo, C.O.; Oladimeji, T.E.; Areloegbe, S.E.; Badejogbin, O.C.; Bashir, A.-A.M.; Agan, S.U.; Fafure, A.A.; Adekeye, A.O.; Ajadi, M.B.; et al. Butyrate ameliorates ovarian failure in experimental PCOS rat model by suppression of HDAC2. Comp. Clin. Pathol. 2025, 34, 155–167. [Google Scholar] [CrossRef]
- Nascimento, D.R.; Barbalho, E.C.; Gondim Barrozo, L.; de Assis, E.I.T.; Costa, F.C.; Silva, J.R.V. The mechanisms that control the preantral to early antral follicle transition and the strategies to have efficient culture systems to promote their growth in vitro. Zygote 2023, 31, 305–315. [Google Scholar] [CrossRef]
- Heiligentag, M.; Eichenlaub-Ritter, U. Preantral follicle culture and oocyte quality. Reprod. Fertil. Dev. 2017, 30, 18–43. [Google Scholar] [CrossRef] [PubMed]
- Orisaka, M.; Orisaka, S.; Jiang, J.Y.; Craig, J.; Wang, Y.; Kotsuji, F.; Tsang, B.K. Growth differentiation factor 9 is antiapoptotic during follicular development from preantral to early antral stage. Mol. Endocrinol. 2006, 20, 2456–2468. [Google Scholar] [CrossRef]
- Samie, K.A.; Kowalewski, M.P.; Schuler, G.; Gastal, G.D.A.; Bollwein, H.; Scarlet, D. Roles of GDF9 and BMP15 in equine follicular development: In vivo content and in vitro effects of IGF1 and cortisol on granulosa cells. BMC Vet. Res. 2025, 21, 292. [Google Scholar] [CrossRef] [PubMed]
- Parrott, J.A.; Skinner, M.K. Kit-ligand/stem cell factor induces primordial follicle development and initiates folliculogenesis. Endocrinology 1999, 140, 4262–4271. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.H.; Vanderhyden, B.C. Oocyte-granulosa cell interactions during mouse follicular development: Regulation of kit ligand expression and its role in oocyte growth. Reprod. Biol. Endocrinol. 2006, 4, 19. [Google Scholar] [CrossRef]
- Kristensen, S.G.; Mamsen, L.S.; Jeppesen, J.V.; Bøtkjær, J.A.; Pors, S.E.; Borgbo, T.; Ernst, E.; Macklon, K.T.; Andersen, C.Y. Hallmarks of Human Small Antral Follicle Development: Implications for Regulation of Ovarian Steroidogenesis and Selection of the Dominant Follicle. Front. Endocrinol. 2017, 8, 376. [Google Scholar] [CrossRef]
- McNatty, K.P.; Moore, L.G.; Hudson, N.L.; Quirke, L.D.; Lawrence, S.B.; Reader, K.; Hanrahan, J.P.; Smith, P.; Groome, N.P.; Laitinen, M.; et al. The oocyte and its role in regulating ovulation rate: A new paradigm in reproductive biology. Reproduction 2004, 128, 379–386. [Google Scholar] [CrossRef]
- Sarker, M.T.; Wang, S.; Wang, S.; Xia, W.; Zhang, Y.; Jin, C.; Huang, X.; Li, K.; Elokil, A.; Lv, Y.; et al. Sodium butyrate alleviates high ambient temperature-induced oxidative stress, intestinal structural disruption, and barrier integrity for growth and production in growing layer chickens. BMC Vet. Res. 2025, 21, 131. [Google Scholar] [CrossRef]
- Berni Canani, R.; Di Costanzo, M.; Leone, L. The epigenetic effects of butyrate: Potential therapeutic implications for clinical practice. Clin. Epigenetics 2012, 4, 4. [Google Scholar] [CrossRef]
- Den Besten, G.; van Eunen, K.; Groen, A.K.; Venema, K.; Reijngoud, D.J.; Bakker, B.M. The role of short-chain fatty acids in the interplay between diet, gut microbiota, and host energy metabolism. J. Lipid Res. 2013, 54, 2325–2340. [Google Scholar] [CrossRef] [PubMed]
- Lu, N.; Li, M.; Lei, H.; Jiang, X.; Tu, W.; Lu, Y.; Xia, D. Butyric acid regulates progesterone and estradiol secretion via cAMP signaling pathway in porcine granulosa cells. J. Steroid Biochem. Mol. Biol. 2017, 172, 89–97. [Google Scholar] [CrossRef] [PubMed]
- Lange, O.; Proczko-Stepaniak, M.; Mika, A. Short-Chain Fatty Acids—A Product of the Microbiome and Its Participation in Two-Way Communication on the Microbiome-Host Mammal Line. Curr. Obes. Rep. 2023, 12, 108–126. [Google Scholar] [CrossRef]
- Pan, Y.; Pan, C.; Zhang, C. Unraveling the complexity of follicular fluid: Insights into its composition, function, and clinical implications. J. Ovarian Res. 2024, 17, 237. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Xu, X.; Liu, S. Intercellular communication in the cumulus-oocyte complex during folliculogenesis: A review. Front. Cell Dev. Biol. 2023, 11, 1087612. [Google Scholar] [CrossRef]
- Gironi, B.; Kahveci, Z.; McGill, B.; Lechner, B.D.; Pagliara, S.; Metz, J.; Morresi, A.; Palombo, F.; Sassi, P.; Petrov, P.G. Effect of DMSO on the Mechanical and Structural Properties of Model and Biological Membranes. Biophys. J. 2020, 119, 274–286. [Google Scholar] [CrossRef] [PubMed]
- Gallardo-Villagrán, M.; Paulus, L.; Leger, D.Y.; Therrien, B.; Liagre, B. Dimethyl Sulfoxide: A Bio-Friendly or Bio-Hazard Chemical? The Effect of DMSO in Human Fibroblast-like Synoviocytes. Molecules 2022, 27, 4472. [Google Scholar] [CrossRef]
- Sewer, M.B.; Li, D. Regulation of Steroid Hormone Biosynthesis by the Cytoskeleton. Lipids 2008, 43, 1109–1115. [Google Scholar] [CrossRef]
- Zhang, H.; Wei, Q.; Gao, Z.; Ma, C.; Yang, Z.; Zhao, H.; Liu, C.; Liu, J.; Zhao, X.; Ma, B. G protein-coupled receptor 30 mediates meiosis resumption and gap junction communications downregulation in goat cumulus-oocyte complexes by 17β-estradiol. J. Steroid Biochem. Mol. Biol. 2019, 187, 58–67. [Google Scholar] [CrossRef]
- Duan, J.; Chen, H.; Li, Y.; Xu, D.; Li, X.; Zhang, Z.; Cheng, J.; Yang, L.; Li, Q. 17β-Estradiol Enhances Porcine Meiosis Resumption from Autophagy-Induced Gap Junction Intercellular Communications and Connexin 43 Phosphorylation via the MEK/ERK Signaling Pathway. J. Agric. Food Chem. 2021, 69, 11847–11855. [Google Scholar] [CrossRef] [PubMed]
- Brito, I.R.; Lima, I.M.; Xu, M.; Shea, L.D.; Woodruff, T.K.; Figueiredo, J.R. Three-dimensional systems for in vitro follicular culture: Overview of alginate-based matrices. Reprod. Fertil. Dev. 2014, 26, 915–930. [Google Scholar] [CrossRef]
- Knight, P.G.; Glister, C. TGF-β superfamily members and ovarian follicle development. Reproduction 2006, 132, 191–206. [Google Scholar] [CrossRef]
- Wang, Y.; Li, H.; You, L.; Wang, S.; Bie, J.; Su, Z.; Shi, L.; Su, Y.Q. RSPO2 Coordinates with GDF9:BMP15 Heterodimers to Promote Granulosa Cell and Oocyte Development in Mice. Adv. Sci. 2025, 12, e01973. [Google Scholar] [CrossRef]
- Wu, L.; Shen, J.; Hou, Z.; Zhang, Y.; Bi, Y.; Zhang, R.; Bai, H.; Ye, W.; Chen, K.; Zhu, J.; et al. Obox1 deficiency impairs fertility in female mice. Fundam. Res. 2025, 5, 1570–1580. [Google Scholar] [CrossRef]
- Alam, M.H.; Miyano, T. Interaction between growing oocytes and granulosa cells in vitro. Reprod. Med. Biol. 2020, 19, 13–23. [Google Scholar] [CrossRef] [PubMed]
- Kordowitzki, P.; Sokołowska, G.; Wasielak-Politowska, M.; Skowronska, A.; Skowronski, M.T. Pannexins and Connexins: Their Relevance for Oocyte Developmental Competence. Int. J. Mol. Sci. 2021, 22, 5918. [Google Scholar] [CrossRef]
- Hall, P.F.; Almahbobi, G. Roles of microfilaments and intermediate filaments in adrenal steroidogenesis. Microsc. Res. Tech. 1997, 36, 463–479. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Kuai, D.; Zhang, H.; Wang, Y.; Wang, K.; Tian, W. Mdivi-1 promotes steroidogenesis in granulosa cells by inhibiting mitochondrial fission. Mol. Cell. Endocrinol. 2025, 607, 112606. [Google Scholar] [CrossRef]
- Das, D.; Arur, S. Regulation of oocyte maturation: Role of conserved ERK signaling. Mol. Reprod. Dev. 2022, 89, 353–374. [Google Scholar] [CrossRef]
- Zhao, H.; Dinh, T.H.; Wang, Y.; Yang, Y. The roles of MAPK signaling pathway in ovarian folliculogenesis. J. Ovarian Res. 2025, 18, 152. [Google Scholar] [CrossRef]
- Torkashvand, H.; Shabani, R.; Artimani, T.; Amiri, I.; Pilehvari, S.; Torkashvand, L.; Mehdizadeh, R.; Mehdizadeh, M. Oocyte competence develops: Nuclear maturation synchronously with cytoplasm maturation. Zygote 2024, 32, 421–428. [Google Scholar] [CrossRef]
- Adhikari, D.; Lee, I.W.; Yuen, W.S.; Carroll, J. Oocyte mitochondria-key regulators of oocyte function and potential therapeutic targets for improving fertility. Biol. Reprod. 2022, 106, 366–377. [Google Scholar] [CrossRef] [PubMed]
- Bao, B.; Wang, J.; Li, Y.; Feng, F.E.N.; Ji, Z.; Luoreng, Z.; Wang, X. Molecular regulation mechanism of oocyte maturation in beef cattle. Biocell 2023, 47, 1509–1518. [Google Scholar] [CrossRef]
- Voros, C.; Athanasiou, D.; Papapanagiotou, I.; Mavrogianni, D.; Varthaliti, A.; Bananis, K.; Athanasiou, A.; Athanasiou, A.; Papadimas, G.; Gkirgkinoudis, A.; et al. Cracking the Code of Oocyte Quality: The Oxidative Stress Link to IVF Success. Int. J. Mol. Sci. 2025, 26, 6377. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.R.; Xu, D.J. Mitochondrial Quality Control in Bovine Oocyte Maturation: Mechanisms, Challenges, and Prospects for Enhancing Reproductive Efficiency. Animals 2025, 15, 2000. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Jia, Y.; Meng, S.; Luo, Y.; Yang, Q.; Pan, Z. Mechanisms of and Potential Medications for Oxidative Stress in Ovarian Granulosa Cells: A Review. Int. J. Mol. Sci. 2023, 24, 9205. [Google Scholar] [CrossRef]
- Yan, F.; Zhao, Q.; Li, Y.; Zheng, Z.; Kong, X.; Shu, C.; Liu, Y.; Shi, Y. The role of oxidative stress in ovarian aging: A review. J. Ovarian Res. 2022, 15, 100. [Google Scholar] [CrossRef]
- Li, X.; Wang, C.; Zhu, J.; Lin, Q.; Yu, M.; Wen, J.; Feng, J.; Hu, C. Sodium Butyrate Ameliorates Oxidative Stress-Induced Intestinal Epithelium Barrier Injury and Mitochondrial Damage through AMPK-Mitophagy Pathway. Oxid. Med. Cell. Longev. 2022, 2022, 3745135. [Google Scholar] [CrossRef]
- Zhang, C.; Sun, X.; Wu, D.; Wang, G.; Lan, H.; Zheng, X.; Li, S. IP3R1 is required for meiotic progression and embryonic development by regulating mitochondrial calcium and oxidative damage. Theriogenology 2024, 229, 147–157. [Google Scholar] [CrossRef]
- Yuan, M.; Gong, M.; He, J.; Xie, B.; Zhang, Z.; Meng, L.; Tse, G.; Zhao, Y.; Bao, Q.; Zhang, Y.; et al. IP3R1/GRP75/VDAC1 complex mediates endoplasmic reticulum stress-mitochondrial oxidative stress in diabetic atrial remodeling. Redox Biol. 2022, 52, 102289. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Zhang, C.; Yu, S.; Liu, S.; Wang, G.; Lan, H.; Zheng, X.; Li, S. Glycine ameliorates MBP-induced meiotic abnormalities and apoptosis by regulating mitochondrial-endoplasmic reticulum interactions in porcine oocytes. Environ. Pollut. 2022, 309, 119756. [Google Scholar] [CrossRef]
- Cai, M.; Wang, J.; Sun, H.; Guo, Q.; Zhang, C.; Yao, H.; Zhao, C.; Jia, Y.; Zhu, H. Resveratrol Attenuates Hydrogen Peroxide-induced Injury of Rat Ovarian Granulosa-lutein Cells by Resisting Oxidative Stress via the SIRT1/Nrf2/ARE Signaling Pathway. Curr. Pharm. Des. 2023, 29, 947–956. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Song, H.; Xu, J.; Wang, Y.; Bai, L.; Wang, H.; Zhang, J. TIGAR relieves PCOS by inhibiting granulosa cell apoptosis and oxidative stress through activating Nrf2. Mol. Cell. Endocrinol. 2024, 594, 112381. [Google Scholar] [CrossRef]
- Li, Z.; Liang, Y.; Wang, Y.; Lin, Y.; Zeng, L.; Zhang, Y.; Zhu, L. Zuogui Pills alleviate cyclophosphamide-induced ovarian aging by reducing oxidative stress and restoring the stemness of oogonial stem cells through the Nrf2/HO-1 signaling pathway. J. Ethnopharmacol. 2024, 333, 118505. [Google Scholar] [CrossRef] [PubMed]
- Xing, X.; Jiang, Z.; Tang, X.; Wang, P.; Li, Y.; Sun, Y.; Le, G.; Zou, S. Sodium butyrate protects against oxidative stress in HepG2 cells through modulating Nrf2 pathway and mitochondrial function. J. Physiol. Biochem. 2016, 73, 405–414. [Google Scholar] [CrossRef]
- Bian, Z.; Zhang, Q.; Qin, Y.; Sun, X.; Liu, L.; Liu, H.; Mao, L.; Yan, Y.; Liao, W.; Zha, L.; et al. Sodium Butyrate Inhibits Oxidative Stress and NF-κB/NLRP3 Activation in Dextran Sulfate Sodium Salt-Induced Colitis in Mice with Involvement of the Nrf2 Signaling Pathway and Mitophagy. Dig. Dis. Sci. 2023, 68, 2981–2996. [Google Scholar]
- Lin, J.; Wang, L. Oxidative Stress in Oocytes and Embryo Development: Implications for In Vitro Systems. Antioxid. Redox Signal. 2020, 34, 1394–1406. [Google Scholar] [CrossRef] [PubMed]
- Cimadomo, D.; Capalbo, A.; Ubaldi, F.M.; Scarica, C.; Palagiano, A.; Canipari, R.; Rienzi, L. The Impact of Biopsy on Human Embryo Developmental Potential during Preimplantation Genetic Diagnosis. Biomed. Res. Int. 2016, 2016, 7193075. [Google Scholar] [CrossRef] [PubMed]
- Kono, T. Genomic imprinting is a barrier to parthenogenesis in mammals. Cytogenet. Genome Res. 2006, 113, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Le, B.A.M.; Nguyen, L.B.L.; Lam, D.T.P.; Lam, C.T.; Nguyen, N.T.; Nguyen, V.T.; Bui, H.T. Agarose-based 3D culture improved the developmental competence of oocyte-granulosa complex isolated from porcine preantral follicle. Theriogenology 2024, 223, 11–21. [Google Scholar] [CrossRef]
- Hirao, Y. Current Status of In Vitro Oocyte Growth and Development in Mammals. Reprod. Med. Biol. 2025, 24, e12669. [Google Scholar] [CrossRef]



| Gene | Gene Bank No. | Forward (5′-3′) | Reverse (5′-3′) | Product (bp) |
|---|---|---|---|---|
| CAT | NM_009804.2 | AGCGACCAGATGAAGCAGTG | TCCGCTCTCTGTCAAAGTGTG | 181 |
| SOD1 | NM_011434.2 | GGGTTCCACGTCCATCAGTA | TTTCCACCTTTGCCCAAGTC | 263 |
| SOD2 | NM_013671.3 | CCAGACCTGCCTTACGACTA | TGAAGAGCGACCTGAGTTGT | 170 |
| GSR | NM_010344.4 | GACACCTCTTCCTTCGACTACC | CACATCCAACATTCACGCAAG | 142 |
| NRF2 | NM_010902.5 | TAGATGACCATGAGTCGCTTGC | GCCAAACTTGCTCCATGTCC | 153 |
| IP3R-1 | JQ839262.1 | AGGAGAATCTCTCCCTTCTCC | GAGCCCTCTGTGCTGAAGAG | 155 |
| STAR | GQ415073.1 | ACCAACAAAGGAGCAGCAA | TCAGGGACCTCAAAGTTCATC | 103 |
| CYP11A1 | NM_214055.1 | ACCTGGACCTTGGTTCTCTG | CATCTGCCTGATGCTCTTGT | 83 |
| CYP19A1 | AF518322.1 | CTGGCAGAAAACAACCTGAACC | TGATTCTCATCAAGCAGGTCTCC | 94 |
| CYP1B1 | NM_001364889.1 | CACTATTACGGACATCTTCGG | AGGTTGGGCTGGTCACTC | 168 |
| GDF9 | NM_001439459.1 | GATGGTGGACCTGCTGTTTA | GAGGAAGAGGCAGAGTTGTTC | 99 |
| BMP15 | NM_009757.5 | AGTGTACCTCAGCCTTCCT | GGGCAATCATACCCTCATACTC | 109 |
| CX37 | NM_008120.3 | CCCACATCCGATACTGGGTG | CGAAGACGACCGTCCTCTG | 220 |
| BCL-2 | NM_009741.5 | GATGACTGAGTACCTGAACCG | CAGAGACAGCCAGGAGAAATC | 124 |
| BAX | NM_007527.4 | CGGCGAATTGGAGATGAACTG | GCAAAGTAGAAGAGGGCAACC | 161 |
| Caspase-3 | NM_001284409.1 | TGACTGGAAAGCCGAAACTC | GCAAGCCATCTCCTCATCAG | 101 |
| GAPDH | NM_001411840.1 | TGTGTCCGTCGTGGATCTGA | TTGCTGTTGAAGTCGCAGGAG | 150 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, X.; Virk, T.L.; Pi, M.; Liu, Q.; Yang, S.; Ma, Z.; Yuan, Y.; Chen, F. Sodium Butyrate Promotes In Vitro Development of Mouse Preantral Follicles and Improves Oocyte Quality by Regulating Steroidogenesis, Oxidative Stress, and Cytoskeleton Remodeling. Animals 2025, 15, 3567. https://doi.org/10.3390/ani15243567
Liu X, Virk TL, Pi M, Liu Q, Yang S, Ma Z, Yuan Y, Chen F. Sodium Butyrate Promotes In Vitro Development of Mouse Preantral Follicles and Improves Oocyte Quality by Regulating Steroidogenesis, Oxidative Stress, and Cytoskeleton Remodeling. Animals. 2025; 15(24):3567. https://doi.org/10.3390/ani15243567
Chicago/Turabian StyleLiu, Xiaohuan, Tuba Latif Virk, Mengdie Pi, Qi Liu, Sheng Yang, Zhiyu Ma, Yuguo Yuan, and Fenglei Chen. 2025. "Sodium Butyrate Promotes In Vitro Development of Mouse Preantral Follicles and Improves Oocyte Quality by Regulating Steroidogenesis, Oxidative Stress, and Cytoskeleton Remodeling" Animals 15, no. 24: 3567. https://doi.org/10.3390/ani15243567
APA StyleLiu, X., Virk, T. L., Pi, M., Liu, Q., Yang, S., Ma, Z., Yuan, Y., & Chen, F. (2025). Sodium Butyrate Promotes In Vitro Development of Mouse Preantral Follicles and Improves Oocyte Quality by Regulating Steroidogenesis, Oxidative Stress, and Cytoskeleton Remodeling. Animals, 15(24), 3567. https://doi.org/10.3390/ani15243567






