Otolith Strontium Isotope (87Sr/86Sr) Reveals Mixed Life Histories of Coilia brachygnathus in the Middle–Lower Yangtze River Floodplain
Simple Summary
Abstract
1. Introduction
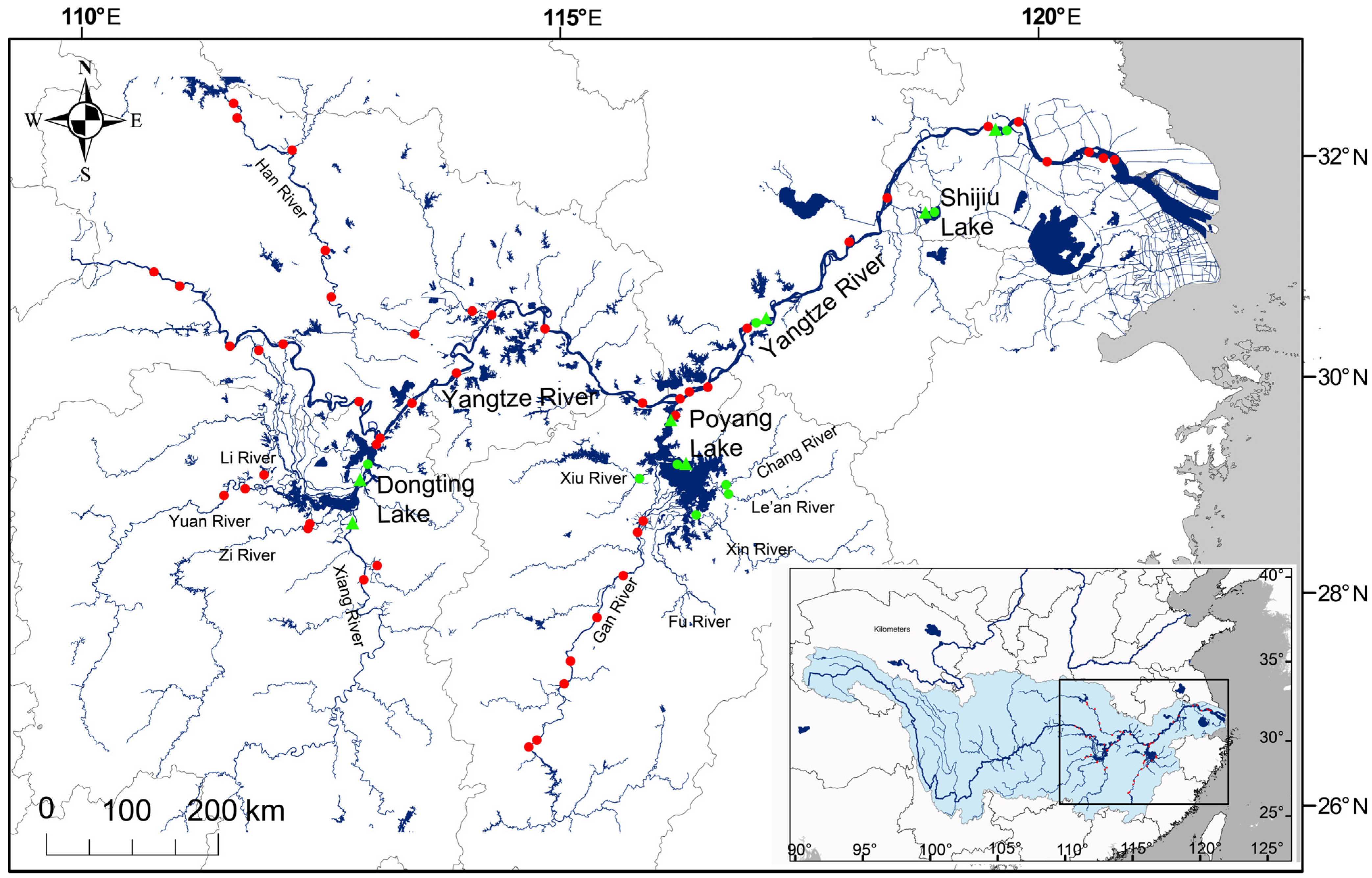
2. Materials and Methods
3. Results
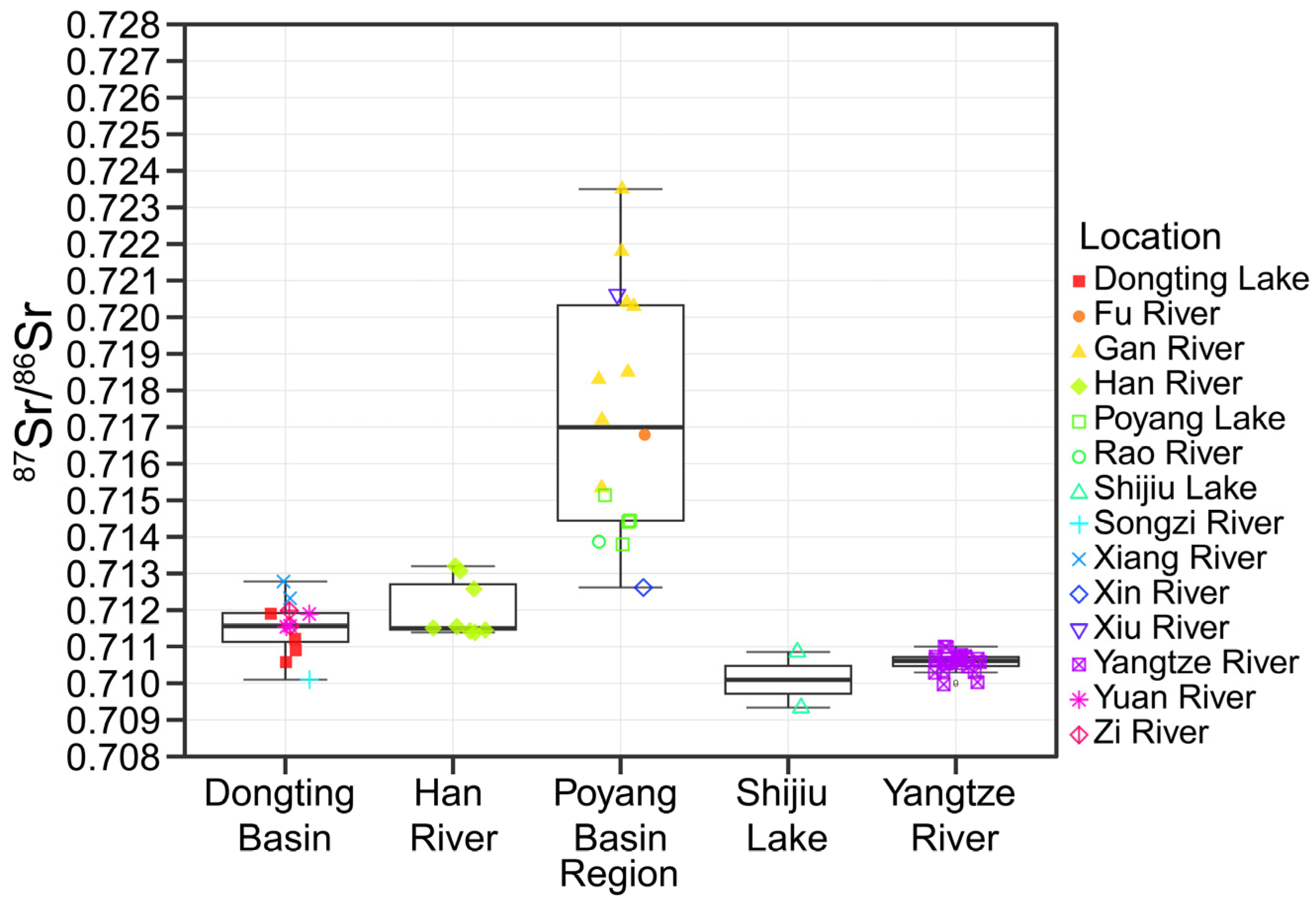
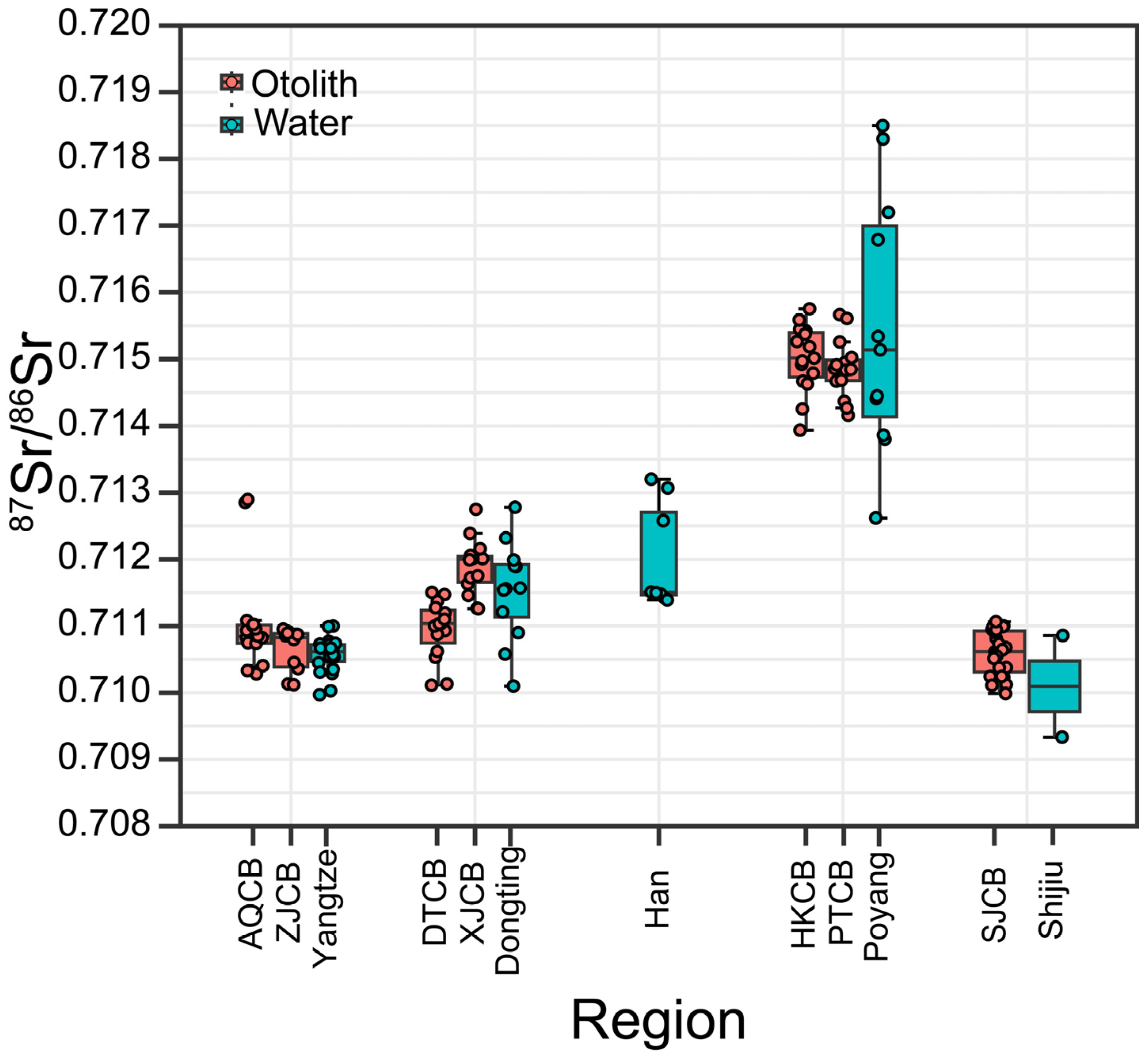
3.1. Water 87Sr/86Sr Baselines
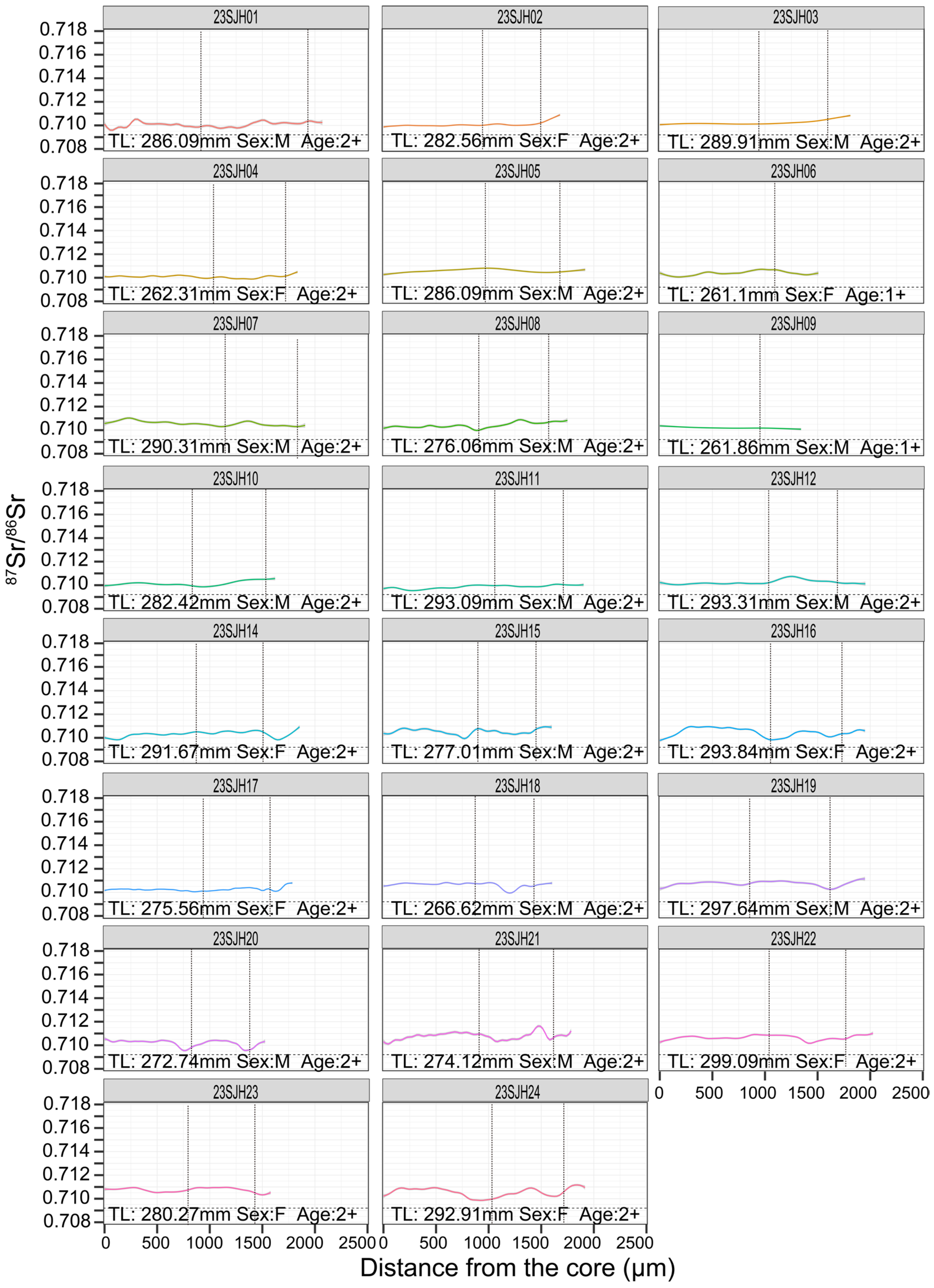
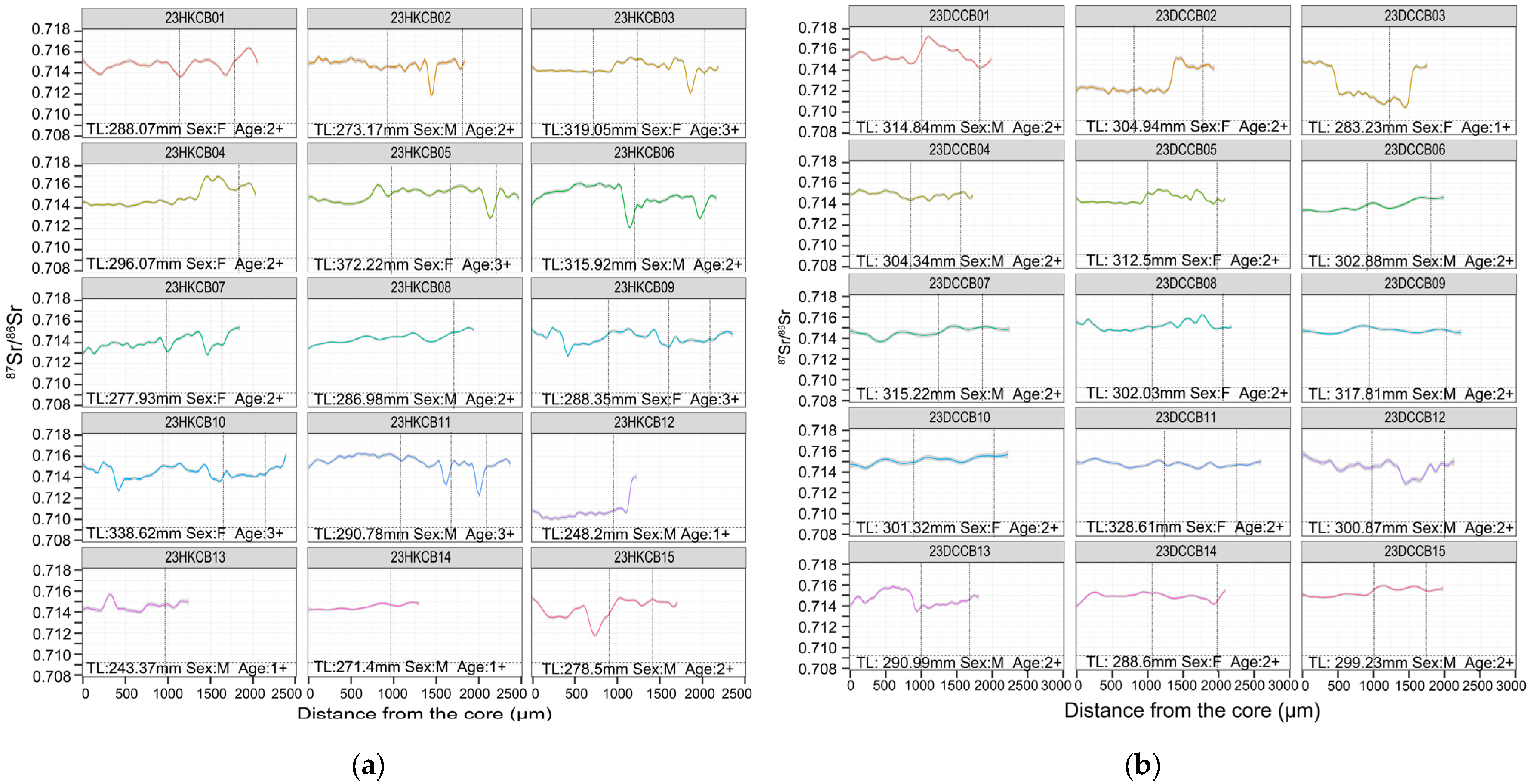
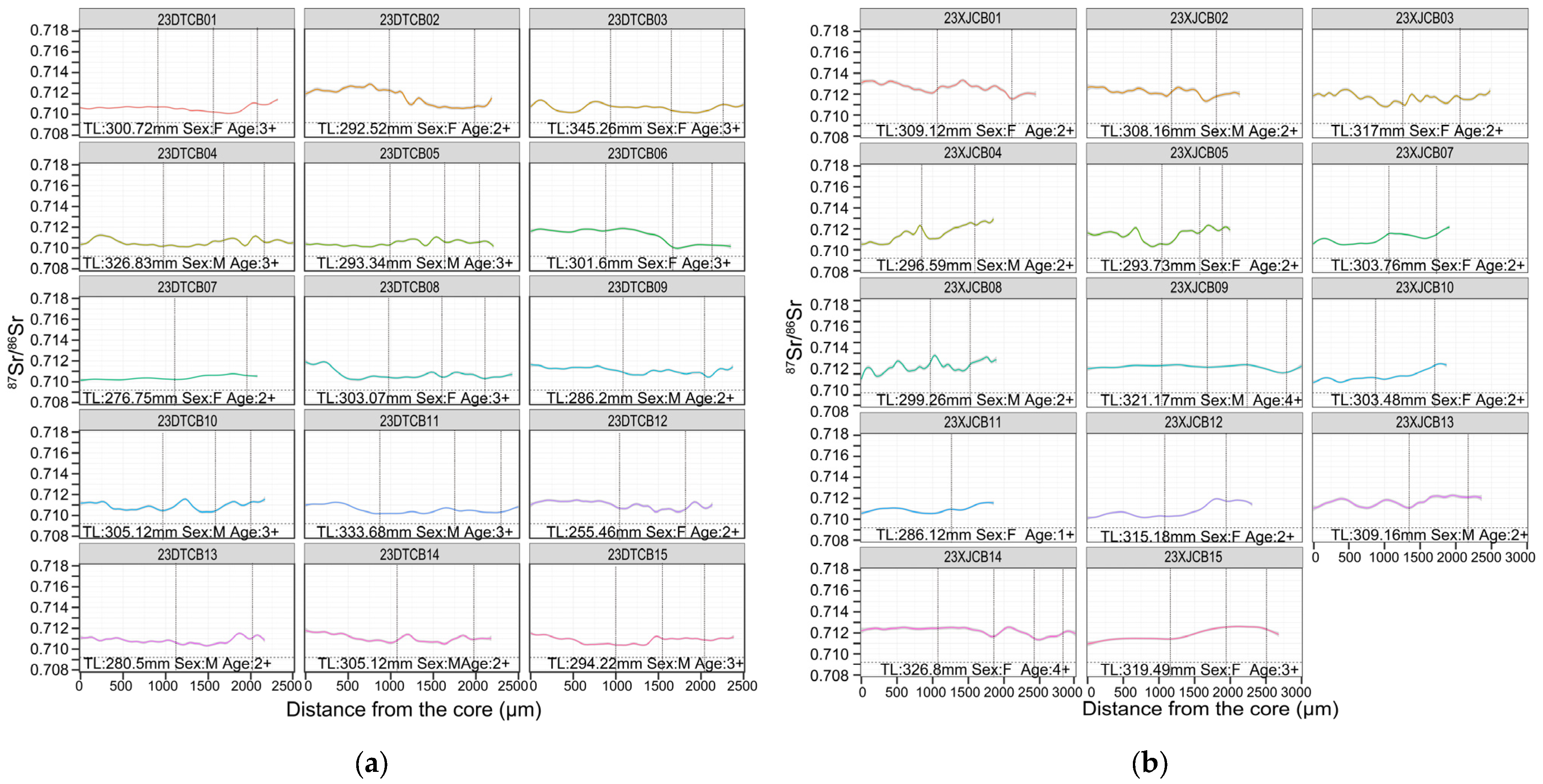
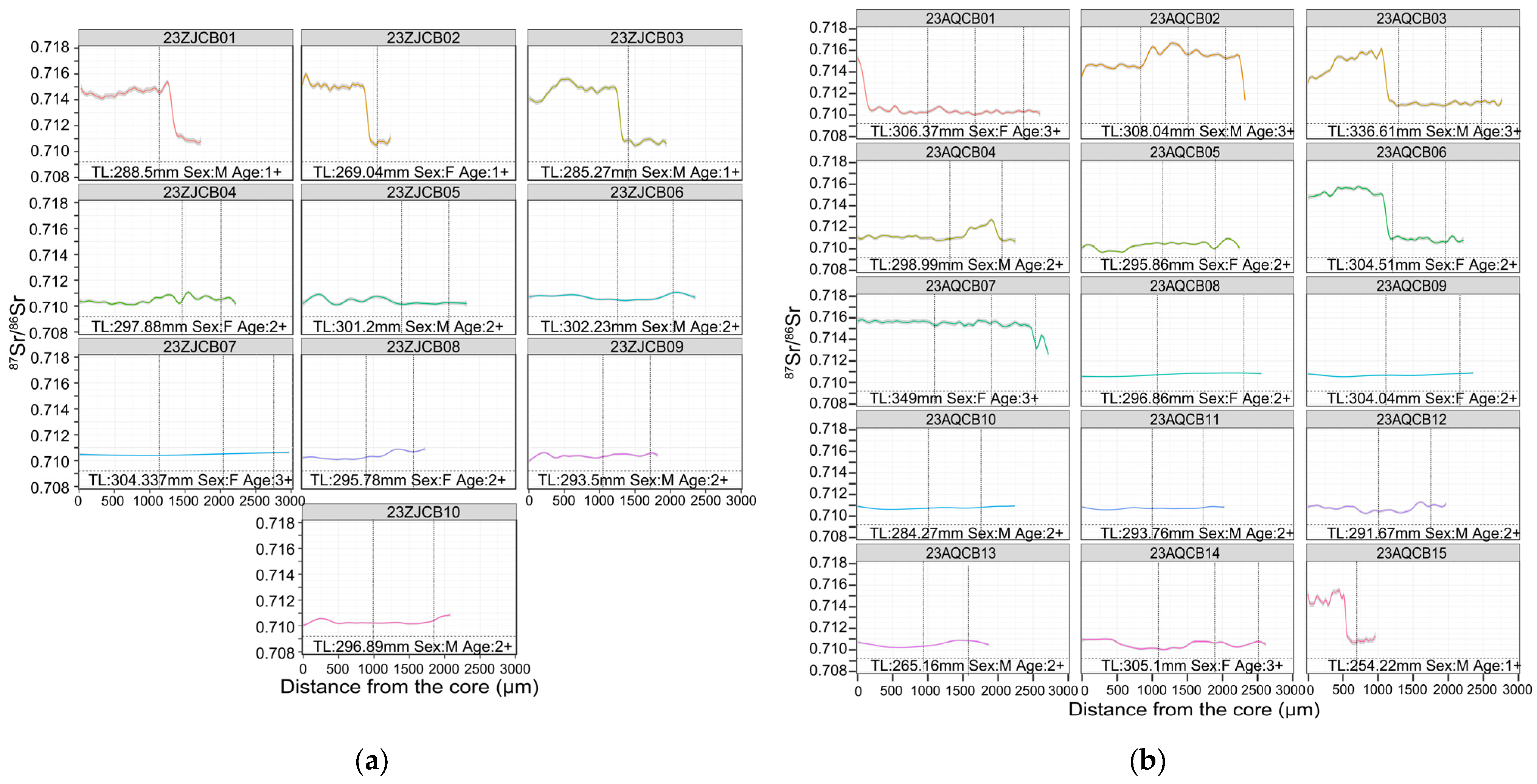
3.2. Otolith Edge 87Sr/86Sr and Recent Habitat Use
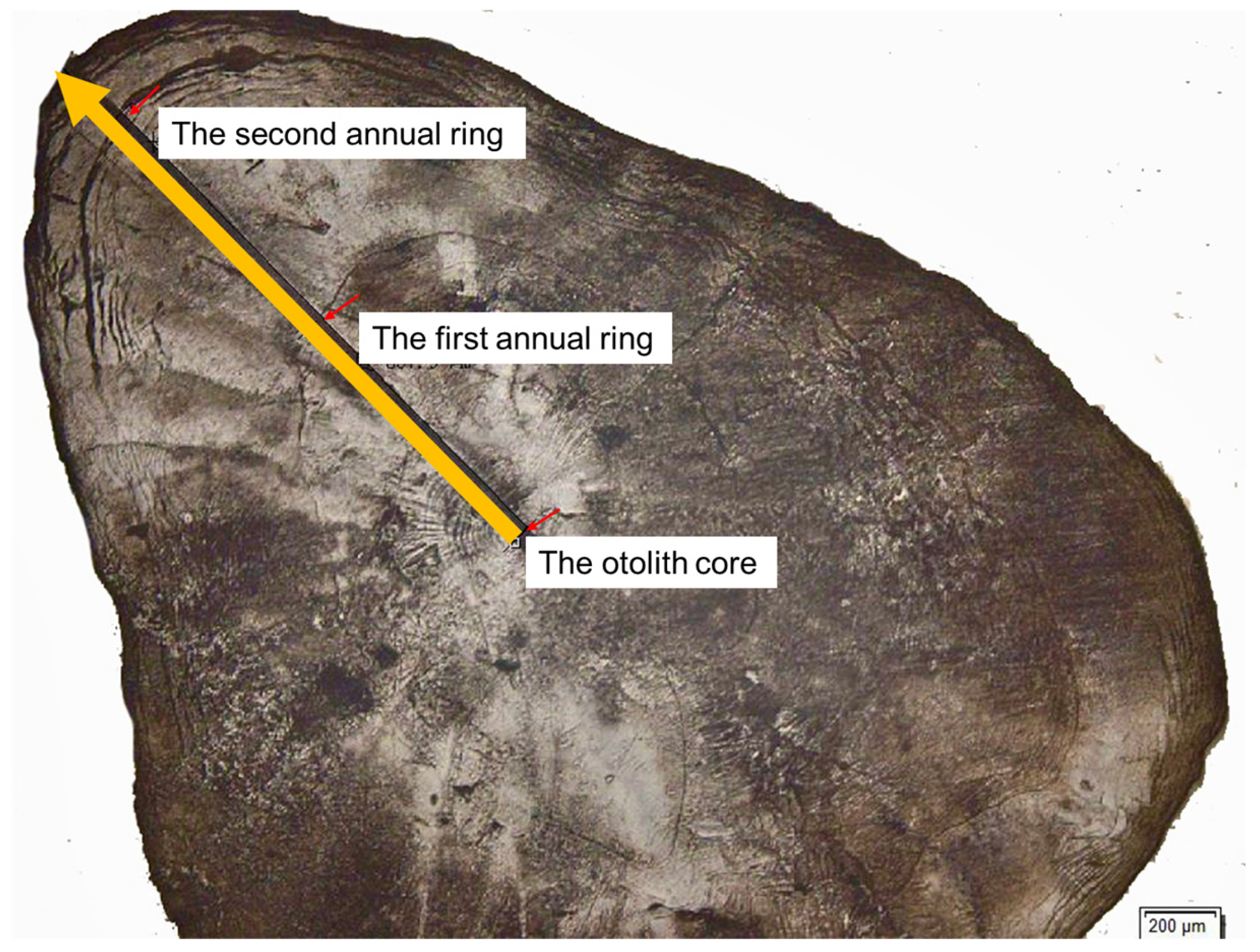
3.3. Life-History Patterns Revealed by Otolith Transects
4. Discussion
4.1. Life-History Strategies of C. brachygnathus
4.2. Biological and Ecological Implications
4.3. Implications for Ecological Research and Management in the Yangtze Basin
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MLY | the middle and lower reaches of the Yangtze River |
| GAM | Generalized Additive Models (GAM) |
Appendix A
| Sample Name | Latitude | Longitude | 87Sr/86 Sr | Sampling Site | Collection Date | References |
|---|---|---|---|---|---|---|
| CJ-59 | 29.377 | 113.079 | 0.71190 | Dongting Lake | 2006.08 | Chetelat et al. [38] |
| DTH | 29.434 | 113.118 | 0.71090 | Dongting Lake | 2011.08 | Luo et al. [37] |
| Dongting | 29.305 | 113.049 | 0.71058 | Dongting Lake | 2023.08 | This study |
| Dongting | 29.304 | 113.049 | 0.71121 | Dongting Lake | 2024.03 | This study |
| CJ-31 | 28.906 | 111.486 | 0.71189 | Yuan River | 2006.08 | Chetelat et al. [38] |
| YJ | 28.968 | 111.707 | 0.71157 | Yuan River | 2011.08 | Luo et al. [37] |
| YJ | 29.096 | 111.904 | 0.71154 | Yuan River | 2011.08 | Luo et al. [37] |
| CJ-32 | 28.599 | 112.363 | 0.71156 | Zi River | 2006.08 | Chetelat et al. [38] |
| ZJ | 28.644 | 112.382 | 0.71199 | Zi River | 2011.08 | Luo et al. [37] |
| SZ | 30.24 | 111.85 | 0.71010 | Songzi River | 2011.08 | Luo et al. [37] |
| CJ-33 | 28.123 | 112.947 | 0.71278 | Xiang River | 2006.08 | Chetelat et al. [38] |
| XJ | 28.255 | 113.087 | 0.71232 | Xiang River | 2011.08 | Luo et al. [37] |
| CJ-43 | 29.645 | 116.204 | 0.71514 | Poyang Lake | 2006.08 | Chetelat et al. [38] |
| PYH | 29.861 | 116.351 | 0.71441 | Poyang Lake | 2011.08 | Luo et al. [37] |
| Piaotou | 29.196 | 116.191 | 0.71445 | Poyang Lake | 2023.08 | This study |
| Duchang | 29.206 | 116.268 | 0.71380 | Poyang Lake | 2024.01 | This study |
| FH | 28.345 | 116.076 | 0.71679 | Fu River | 2023.08 | This study |
| RH | 29.024 | 116.629 | 0.713862 | Rao River | 2023.08 | This study |
| XJ | 28.725 | 116.422 | 0.71262 | Xin River | 2023.08 | This study |
| XS-01 | 29.061 | 115.828 | 0.72062 | Xiu River | 2023.08 | This study |
| CJ-34 | 28.67 | 115.868 | 0.71534 | Gan River | 2006.08 | Chetelat et al. [38] |
| GJ10 | 26.619 | 114.756 | 0.72030 | Gan River | 2007.11 | Wang et al. [41] |
| GJ09 | 26.555 | 114.671 | 0.72350 | Gan River | 2007.11 | Wang et al. [41] |
| GJ13 | 27.151 | 115.042 | 0.72180 | Gan River | 2007.11 | Wang et al. [41] |
| GJ15 | 27.362 | 115.108 | 0.72040 | Gan River | 2007.11 | Wang et al. [41] |
| GJ18 | 27.769 | 115.384 | 0.71850 | Gan River | 2007.11 | Wang et al. [41] |
| GJ20 | 28.16 | 115.659 | 0.71830 | Gan River | 2007.11 | Wang et al. [41] |
| GJ22 | 28.565 | 115.808 | 0.71720 | Gan River | 2007.11 | Wang et al. [41] |
| CJ-47 | 30.597 | 114.081 | 0.71151 | Han River | 2006.08 | Chetelat et al. [38] |
| HJ-49 | 32.469 | 111.586 | 0.71258 | Han River | 2007.09 | Xu et al. [40] |
| HJ-50 | 32.339 | 111.622 | 0.71307 | Han River | 2007.09 | Xu et al. [40] |
| HJ-56 | 31.148 | 112.545 | 0.71143 | Han River | 2007.09 | Xu et al. [40] |
| HJ-57 | 30.726 | 112.607 | 0.71148 | Han River | 2007.09 | Xu et al. [40] |
| HJ-60 | 30.389 | 113.479 | 0.71139 | Han River | 2007.09 | Xu et al. [40] |
| HJ-62 | 30.564 | 114.284 | 0.71150 | Han River | 2007.09 | Xu et al. [40] |
| HJ-54 | 32.051 | 112.198 | 0.71320 | Han River | 2007.09 | Xu et al. [40] |
| SJH_Guxihe | 31.529 | 118.663 | 0.709335 | Shijiu Lake | 2024.01 | This study |
| SJH_Guxihe | 31.555 | 118.466 | 0.710858 | Shijiu Lake | 2023.08 | This study |
| CJ03 | 31.964 | 120.795 | 0.71077 | Yangtze River | 1997.10 | Wang et al. [41] |
| CJ04 | 32.034 | 120.526 | 0.71071 | Yangtze River | 1997.10 | Wang et al. [41] |
| CJ05 | 31.949 | 120.089 | 0.71073 | Yangtze River | 1997.10 | Wang et al. [41] |
| CJ06 | 32.304 | 119.79 | 0.71072 | Yangtze River | 1997.10 | Wang et al. [41] |
| CJ07 | 32.178 | 118.878 | 0.71074 | Yangtze River | 1997.10 | Wang et al. [41] |
| CJ08 | 31.623 | 118.417 | 0.71065 | Yangtze River | 1997.10 | Wang et al. [41] |
| CJ09 | 31.225 | 118.021 | 0.71074 | Yangtze River | 1997.10 | Wang et al. [41] |
| CJ11 | 30.442 | 116.955 | 0.71067 | Yangtze River | 1997.10 | Wang et al. [41] |
| CJ12 | 29.796 | 116.252 | 0.71066 | Yangtze River | 1997.10 | Wang et al. [41] |
| CJ13 | 29.758 | 115.861 | 0.71054 | Yangtze River | 1997.10 | Wang et al. [41] |
| CJ14 | 30.436 | 114.841 | 0.71058 | Yangtze River | 1997.10 | Wang et al. [41] |
| CJ16 | 30.033 | 113.916 | 0.71055 | Yangtze River | 1997.10 | Wang et al. [41] |
| CJ17 | 29.757 | 113.451 | 0.71045 | Yangtze River | 1997.10 | Wang et al. [41] |
| CJ18 | 29.772 | 112.896 | 0.71029 | Yangtze River | 1997.10 | Wang et al. [41] |
| CJ19 | 30.297 | 112.103 | 0.70997 | Yangtze River | 1997.10 | Wang et al. [41] |
| CJ20 | 30.278 | 111.547 | 0.71003 | Yangtze River | 1997.10 | Wang et al. [41] |
| CJ21 | 30.825 | 111.023 | 0.71031 | Yangtze River | 1997.10 | Wang et al. [41] |
| SX5 | 30.954 | 110.754 | 0.71054 | Yangtze River | 2006.08 | Chetelat et al. [38] |
| CJ-42 | 31.98 | 120.679 | 0.71100 | Yangtze River | 2006.08 | Chetelat et al. [38] |
| CJ-44 | 29.903 | 116.542 | 0.71099 | Yangtze River | 2006.08 | Chetelat et al. [38] |
| Anqing | 30.457 | 116.946 | 0.71035 | Yangtze River | 2023.08 | This study |
| ZJ | 32.263 | 119.474 | 0.71057 | Yangtze River | 2023.08 | This study |
References
- Sturrock, A.M.; Carlson, S.M.; Wikert, J.D.; Heyne, T.; Nussle, S.; Merz, J.E.; Sturrock, H.J.W.; Johnson, R.C. Unnatural selection of salmon life histories in a modified riverscape. Glob. Change Biol. 2020, 26, 1235–1247. [Google Scholar] [CrossRef] [PubMed]
- Xuan, Z.; Wang, W.X. Trace elemental and stable isotopic signatures to reconstruct the large-scale environmental connectivity of fish populations. Mar. Ecol. Prog. Ser. 2024, 730, 95–111. [Google Scholar] [CrossRef]
- Avigliano, E.; Pouilly, M.; Silva, N.; Sánchez, S. High plasticity in short- and medium-scale movements in two catfish species from the Paraná Basin. Environ. Biol. Fishes 2023, 106, 541–552. [Google Scholar] [CrossRef]
- Greene, C.M.; Hall, J.E.; Guilbault, K.R.; Quinn, T.P. Improved viability of populations with diverse life-history portfolios. Biol. Lett. 2010, 6, 382–386. [Google Scholar] [CrossRef]
- Turko, A.J.; Rossi, G.S. Habitat choice promotes and constrains phenotypic plasticity. Biol. Lett. 2022, 18, 20210468. [Google Scholar] [CrossRef]
- Wong, B.B.M.; Candolin, U. Behavioral responses to changing environments. Behav. Ecol. 2015, 26, 665–673. [Google Scholar] [CrossRef]
- Hegg, J.C.; Giarrizzo, T.; Kennedy, B.P. Diverse early life-history strategies in migratory Amazonian catfish: Implications for conservation and management. PLoS ONE 2015, 10, e0129697. [Google Scholar] [CrossRef]
- Gillanders, B.M.; Izzo, C.; Doubleday, Z.A.; Ye, Q. Partial migration: Growth varies between resident and migratory fish. Biol. Lett. 2015, 11, 20140850. [Google Scholar] [CrossRef]
- Schindler, D.E.; Hilborn, R.; Chasco, B.; Boatright, C.P.; Quinn, T.P.; Rogers, L.A.; Webster, M.S. Population diversity and the portfolio effect in an exploited species. Nature 2010, 465, 609–612. [Google Scholar] [CrossRef] [PubMed]
- Vu, A.V.; Baumgartner, L.J.; Limburg, K.E.; Doran, G.S.; Mallen-Cooper, M.; Gillanders, B.M.; Thiem, J.D.; Howitt, J.A.; Kewish, C.M.; Reinhardt, J.; et al. Life history strategies of Mekong pangasiid catfishes revealed by otolith microchemistry. Fish. Res. 2022, 249, 106239. [Google Scholar] [CrossRef]
- Andersson, M.; Jonsson, B.; Calles, O.; Greenberg, L. Assessing movements between freshwater and saltwater by brown trout (Salmo trutta L.) based on otolith microchemistry. Animals 2024, 14, 2116. [Google Scholar] [CrossRef] [PubMed]
- Freshwater, C.; Trudel, M.; Beacham, T.D.; Gauthier, S.; Johnson, S.C.; Neville, C.E.; Juanes, F. Individual variation, population-specific behaviours and stochastic processes shape marine migration phenologies. J. Anim. Ecol. 2019, 88, 67–78. [Google Scholar] [CrossRef]
- Ohta, T.; Sueyoshi, M.; Shima, M.; Ida, S.; Iizuka, T. Estimation of tributary–mainstem migration of freshwater fishes by Sr isotope analysis of otoliths. Ecol. Res. 2024, 40, 409–421. [Google Scholar] [CrossRef]
- Xuan, Z.; Jiang, T.; Liu, H.; Yang, J. Otolith microchemistry and microsatellite DNA provide evidence for divergence between estuarine tapertail anchovy (Coilia nasus) populations from the Poyang Lake and the Yangtze River Estuary of China. Reg. Stud. Mar. Sci. 2022, 56, 102649. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, J.; Lu, M.J.; Liu, H.B.; Chen, T.T.; Gao, Y.W. Discovery of a spawning area for anadromous Coilia nasus Temminck et Schlegel, 1846 in Poyang Lake, China. J. Appl. Ichthyol. 2017, 33, 189–192. [Google Scholar] [CrossRef]
- Jiang, T.; Yang, J.; Liu, H.; Shen, X. Life history of Coilia nasus from the Yellow Sea inferred from otolith Sr:Ca ratios. Environ. Biol. Fishes 2012, 95, 503–508. [Google Scholar] [CrossRef]
- Xuan, Z.; Jiang, T.; Liu, H.; Chen, X.; Yang, J. Mitochondrial dna and microsatellite analyses reveal strong genetic differentiation between two types of estuarine tapertail anchovies (Coilia) in Yangtze River Basin, China. Hydrobiologia 2021, 848, 1409–1431. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, H.; Hu, Y.; Chen, X.; Yang, J. Revealing population connectivity of the estuarine tapertail anchovy Coilia nasus in the Changjiang River Estuary and its adjacent waters using otolith microchemistry. Fishes 2022, 7, 0147. [Google Scholar] [CrossRef]
- Ru, H.-J.; Liu, X.-Q. River-Lake migration of fishes in the Dongting Lake area of the Yangtze Floodplain. J. Appl. Ichthyol. 2013, 29, 594–601. [Google Scholar] [CrossRef]
- Qin, X.; Age, Z.G. Growth, mortality and movement patterns of shortjaw tapertail anchovy. Aquat. Living Resour. 2018, 31, 3. [Google Scholar] [CrossRef]
- Li, M.; Jiang, T.; Daniel, K.; Liu, H.; Yang, J. Reconstructing habitat history of Coilia nasus from the Hexian section of the Yangtze River in Anhui Province by otolith microchemistry. Acta Hydrobiol. Sin. 2017, 41, 1054–1061. [Google Scholar] [CrossRef]
- Liu, S.; Wu, Y.; Xu, G.; Cheng, S.; Zhong, Y.; Zhang, Y. Characterizing the 2022 extreme drought event over the Poyang Lake basin using multiple satellite remote sensing observations and in situ data. Remote Sens. 2023, 15, 5125. [Google Scholar] [CrossRef]
- Reis-Santos, P.; Gillanders, B.M.; Sturrock, A.M.; Izzo, C.; Oxman, D.S.; Lueders-Dumont, J.A.; Hüssy, K.; Tanner, S.E.; Rogers, T.; Doubleday, Z.A.; et al. Reading the biomineralized book of life: Expanding otolith biogeochemical research and applications for fisheries and ecosystem-based management. Rev. Fish Biol. Fisher. 2022, 33, 411–449. [Google Scholar] [CrossRef]
- Thomas, O.R.B.; Swearer, S.E. Otolith biochemistry—A review. Rev. Fish. Sci. Aquac. 2019, 27, 458–489. [Google Scholar] [CrossRef]
- Thomas, O.R.; Ganio, K.; Roberts, B.R.; Swearer, S.E. Trace element-protein interactions in endolymph from the inner ear of fish: Implications for environmental reconstructions using fish otolith chemistry. Metallomics 2017, 9, 239–249. [Google Scholar] [CrossRef]
- Izzo, C.; Reis-Santos, P.; Gillanders, B.M. Otolith chemistry does not just reflect environmental conditions: A meta-analytic evaluation. Fish Fish. 2018, 19, 441–454. [Google Scholar] [CrossRef]
- Karin Hüssy, K.E.L. Trace element patterns in otoliths: The role of biomineralization. Rev. Fish. Sci. Aquac. 2021, 29, 445–477. [Google Scholar] [CrossRef]
- Bareille, G.; Vignon, M.; Chappaz, A.; Fontaine, A.; Tabouret, H.; Morat, F.; Martin, J.; Aymes, J.C.; Daverat, F.; Pécheyran, C.; et al. Freshwater fish otoliths record signals from both water and physiological processes: New insights from Sr/Ca and Ba/Ca ratios. Can. J. Fish. Aquat. Sci. 2024, 81, 223–240. [Google Scholar] [CrossRef]
- Sokta, L.; Jiang, T.; Liu, H.; Xuan, Z.; Qiu, C.; Chen, X.; Yang, J. Loss of Coilia nasus habitats in Chinese freshwater lakes: An otolith microchemistry assessment. Heliyon 2020, 6, e04571. [Google Scholar] [CrossRef]
- Brennan, K.G.; Brennan, S.R.; Cline, T.; Bowen, G.J. Delineating population structure of resilient sea/river-type sockeye salmon. Limnol. Oceanogr. Lett. 2024, 10, 223–233. [Google Scholar] [CrossRef]
- Wang, X.; Bocksberger, G.; Arandjelovic, M.; Agbor, A.; Angedakin, S.; Aubert, F.; Ayimisin, E.A.; Bailey, E.; Barubiyo, D.; Bessone, M.; et al. Strontium isoscape of sub-Saharan Africa allows tracing origins of victims of the transatlantic slave trade. Nat. Commun. 2024, 15, 10891. [Google Scholar] [CrossRef]
- Cordoleani, F.; Phillis, C.C.; Sturrock, A.M.; Willmes, M.; Whitman, G.; Holmes, E.; Weber, P.K.; Jeffres, C.; Johnson, R.C. Restoring freshwater habitat mosaics to promote resilience of vulnerable salmon populations. Ecosphere 2024, 15, e4803. [Google Scholar] [CrossRef]
- Cordoleani, F.; Phillis, C.C.; Sturrock, A.M.; FitzGerald, A.M.; Malkassian, A.; Whitman, G.E.; Weber, P.K.; Johnson, R.C. Threatened salmon rely on a rare life history strategy in a warming landscape. Nat. Clim. Change 2021, 11, 982–988. [Google Scholar] [CrossRef]
- Willmes, M.; Sturrock, A.M.; Cordoleani, F.; Hugentobler, S.; Meek, M.H.; Whitman, G.; Evans, K.; Palkovacs, E.P.; Stauffer-Olsen, N.J.; Johnson, R.C. Integrating otolith and genetic tools to reveal intraspecific biodiversity in a highly impacted salmon population. J. Fish Biol. 2024, 105, 412–430. [Google Scholar] [CrossRef] [PubMed]
- Hermann, T.W.; Duponchelle, F.; Castello, L.; Limburg, K.E.; Pereira, L.A.; Hauser, M. Harnessing the potential for otolith microchemistry to foster the conservation of amazonian fishes. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 1206–1220. [Google Scholar] [CrossRef]
- Fitzpatrick, R.M.; Winkelman, D.L.; Johnson, B.M. Using isotopic data to evaluate Esox lucius (Linnaeus, 1758) natal origins in a hydrologically complex river basin. Fishes 2021, 6, 67. [Google Scholar] [CrossRef]
- Luo, C.; Zheng, H.; Tada, R.; Wu, W.; Irino, T.; Yang, S.; Saito, K. Tracing Sr isotopic composition in space and time across the Yangtze River Basin. Chem. Geol. 2014, 388, 59–70. [Google Scholar] [CrossRef]
- Chetelat, B.; Liu, C.Q.; Zhao, Z.Q.; Wang, Q.L.; Li, S.L.; Li, J.; Wang, B.L. Geochemistry of the dissolved load of the Changjiang Basin Rivers: Anthropogenic impacts and chemical weathering. Geochim. Cosmochim. Acta 2008, 72, 4254–4277. [Google Scholar] [CrossRef]
- Wang, Z.L.; Zhang, J.; Liu, C.Q. Strontium isotopic compositions of dissolved and suspended loads from the main channel of the Yangtze River. Chemosphere 2007, 69, 1081–1088. [Google Scholar] [CrossRef]
- Xu, Z.; Shi, C.; Tang, Y.; Han, H. Chemical and strontium isotopic compositions of the Hanjiang basin rivers in China: Anthropogenic impacts and chemical weathering. Aquat. Geochem. 2011, 17, 243–264. [Google Scholar] [CrossRef]
- Wang, X.; Tang, Z. The first large-scale bioavailable Sr isotope map of China and its implication for provenance studies. Earth-Sci. Rev. 2020, 210, 103353. [Google Scholar] [CrossRef]
- Yimer, M.A.; Cao, L.; Shen, J.-Z.; Zhang, E. Age, growth, maturity and mortality of the tapetail anchovy Coilia brachygnathus (Engraulidae) in Lake Honghu, China. J. Fish Biol. 2024, 104, 410–421. [Google Scholar] [CrossRef]
- Lugli, F.; Weber, M.; Giovanardi, T.; Arrighi, S.; Bortolini, E.; Figus, C.; Marciani, G.; Oxilia, G.; Romandini, M.; Sil-vestrini, S.; et al. Fast offline data reduction of laser ablation MC-ICP-MS Sr isotope measurements via an inter-active Excel-based spreadsheet ‘SrDR’. J. Anal. Atom. Spectrom. 2020, 35, 852–862. [Google Scholar] [CrossRef]
- Brennan, S.R.; Fernandez, D.P.; Zimmerman, C.E.; Cerling, T.E.; Brown, R.J.; Wooller, M.J. Strontium isotopes in otoliths of a non-migratory fish (Slimy sculpin): Implications for provenance studies. Geochim. Cosmochim. Acta 2015, 149, 32–45. [Google Scholar] [CrossRef]
- Lu, Q.; Liu, X.; Qiu, X.; Liang, T.; Chen, J.; Zhao, S.; Ouyang, S.; Jin, B.; Wu, X. Changes and drivers of zooplankton diversity patterns in the middle reach of Yangtze River floodplain lakes, China. Ecol. Evol. 2021, 11, 17885–17900. [Google Scholar] [CrossRef] [PubMed]
- Chang, T.; Li, M.; Gao, X. Dispersal-based processes as drivers of fish communities and species distributions in the Yangtze River–Poyang Lake riverine floodplain of China. Ecol. Processes 2025, 14, 48. [Google Scholar] [CrossRef]
- Jiang, T.; Liu, H.; Shen, X.; Shimasaki, Y.; Oshima, Y.; Yang, J. Life history variations among different populations of Coilia nasus along the Chinese coast inferred from otolith microchemistry. J. Fac. Agric. Kyushu Univ. 2014, 59, 383–389. [Google Scholar] [CrossRef]
- Birnie-Gauvin, K.; Larsen, M.H.; Aarestrup, K. Energetic state and the continuum of migratory tactics in brown trout (Salmo trutta). Can. J. Fish. Aquat. Sci. 2021, 78, 1435–1443. [Google Scholar] [CrossRef]
- Clark, S.R.; Kreiser, B.R.; Schaefer, J.F.; Stewart, L.K. Scale dependence of sex-specific movement in a small-bodied stream fish. Freshw. Biol. 2019, 64, 1342–1353. [Google Scholar] [CrossRef]
- Hauser, M.; Doria, C.R.C.; Santos, R.V.; García-Vasquez, A.; Pouilly, M.; Pécheyran, C.; Ponzevera, E.; Torrente-Vilara, G.; Bérail, S.; Panfili, J.; et al. Shedding light on the migratory patterns of the amazonian goliath catfish, Brachyplatystoma Platynemum, using otolith 87Sr/86Sr analyses. Aquat. Conserv. Mar. Freshw. Ecosyst. 2019, 29, 397–408. [Google Scholar] [CrossRef]
- Ren, P.; Schmidt, B.V.; Fang, D.; Xu, D. Spatial distribution patterns of fish egg and larval assemblages in the lower reach of the Yangtze River: Potential Implications for conservation and management. Aquat. Conserv. Mar. Freshw. Ecosyst. 2021, 31, 1929–1944. [Google Scholar] [CrossRef]
- Xie, C.; Huang, X.; Mu, H.; Yin, W. Impacts of land-use changes on the lakes across the Yangtze floodplain in China. Environ. Sci. Technol. 2017, 51, 3669–3677. [Google Scholar] [CrossRef]
- Liu, X.; Wang, H. Effects of loss of lateral hydrological connectivity on fish functional diversity. Conserv. Biol. 2018, 32, 1336–1345. [Google Scholar] [CrossRef] [PubMed]
- Michie, L.E.; Harrisson, K.A.; Rourke, M.L.; Crook, D.A.; Stuart, I.; Ellis, I.; Sharpe, C.P.; Butler, G.L.; Thiem, J.D. Dispersal and kinship patterns of a pelagic-spawning riverine fish highlight the value of connectivity over large spatial scales. Ecohydrology 2025, 18, e70032. [Google Scholar] [CrossRef]
- Qiu, J.; Yuan, S.; Tang, H.; Zhang, Q.; Wolter, C.; Nikora, V. Ecological connectivity of river-lake ecosystem: Evidence from fish population dynamics in a connecting channel. Water Resour. Res. 2024, 60, e2024WR037495. [Google Scholar] [CrossRef]
- Brennan Sean, R.; Schindler Daniel, E.; Cline Timothy, J.; Walsworth Timothy, E.; Buck, G.; Fernandez Diego, P. Shifting habitat mosaics and fish production across river basins. Science 2019, 364, 783–786. [Google Scholar] [CrossRef]
| Region | Location | Number | Total Length | Standard Length | Body Weight |
|---|---|---|---|---|---|
| Poyang Lake | Duchang | 15 | 305.65 ± 12.21 | 286.73 ± 11.83 | 74.09 ± 13.94 |
| Hukou | 15 | 292.58 ± 33.19 | 272.00 ± 31.68 | 69.30 ± 23.99 | |
| Dongting Lake | Yueyang | 15 | 294.09 ± 33.41 | 273.52 ± 31.60 | 75.97 ± 28.55 |
| Xiangyin | 14 | 307.79 ± 11.51 | 289.42 ± 11.62 | 83.60 ± 12.60 | |
| Shijiu Lake | Shijiu Lake | 23 | 280.81 ± 12.69 | 261.79 ± 12.63 | 58.23 ± 13.59 |
| Yangtze River | Anqing | 15 | 299.63 ± 23.30 | 281.38 ± 21.73 | 81.96 ± 20.27 |
| Zhenjiang | 10 | 293.46 ± 10.44 | 270.29 ± 13.27 | 65.08 ± 10.67 |
| Region | Location | Number | Mean | Standard Deviation | Lower | Upper |
|---|---|---|---|---|---|---|
| Dongting Basin | Dongting | 4 | 0.71115 | 0.00056 | 0.71058 | 0.71171 |
| Xiang River | 2 | 0.71255 | 0.00033 | 0.71223 | 0.71288 | |
| Yuan River | 3 | 0.71167 | 0.00019 | 0.71147 | 0.71186 | |
| Zi River | 2 | 0.71178 | 0.00030 | 0.71147 | 0.71208 | |
| Shijiu Lake | Shijiu Lake | 2 | 0.71010 | 0.00108 | 0.70902 | 0.71118 |
| Poyang Basin | Poyang | 4 | 0.71445 | 0.00055 | 0.71390 | 0.71499 |
| Gan River | 8 | 0.71942 | 0.00261 | 0.71681 | 0.72203 | |
| Han River | Han River | 8 | 0.71202 | 0.00079 | 0.71123 | 0.71281 |
| Yangtze River | Yangtze River | 22 | 0.71057 | 0.00026 | 0.71031 | 0.71082 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xuan, Z.; Wang, Y.; Wang, S.; Yang, Y.; Wang, C.; Liu, S.; Liu, K. Otolith Strontium Isotope (87Sr/86Sr) Reveals Mixed Life Histories of Coilia brachygnathus in the Middle–Lower Yangtze River Floodplain. Animals 2025, 15, 3434. https://doi.org/10.3390/ani15233434
Xuan Z, Wang Y, Wang S, Yang Y, Wang C, Liu S, Liu K. Otolith Strontium Isotope (87Sr/86Sr) Reveals Mixed Life Histories of Coilia brachygnathus in the Middle–Lower Yangtze River Floodplain. Animals. 2025; 15(23):3434. https://doi.org/10.3390/ani15233434
Chicago/Turabian StyleXuan, Zhongya, Yinping Wang, Sheng Wang, Yanping Yang, Chongrui Wang, Silei Liu, and Kai Liu. 2025. "Otolith Strontium Isotope (87Sr/86Sr) Reveals Mixed Life Histories of Coilia brachygnathus in the Middle–Lower Yangtze River Floodplain" Animals 15, no. 23: 3434. https://doi.org/10.3390/ani15233434
APA StyleXuan, Z., Wang, Y., Wang, S., Yang, Y., Wang, C., Liu, S., & Liu, K. (2025). Otolith Strontium Isotope (87Sr/86Sr) Reveals Mixed Life Histories of Coilia brachygnathus in the Middle–Lower Yangtze River Floodplain. Animals, 15(23), 3434. https://doi.org/10.3390/ani15233434







