Patent Pulmonary Infection with the Invasive Pentastomid Raillietiella orientalis in a Panther Chameleon (Furcifer pardalis) in Belgium
Simple Summary
Abstract
1. Introduction
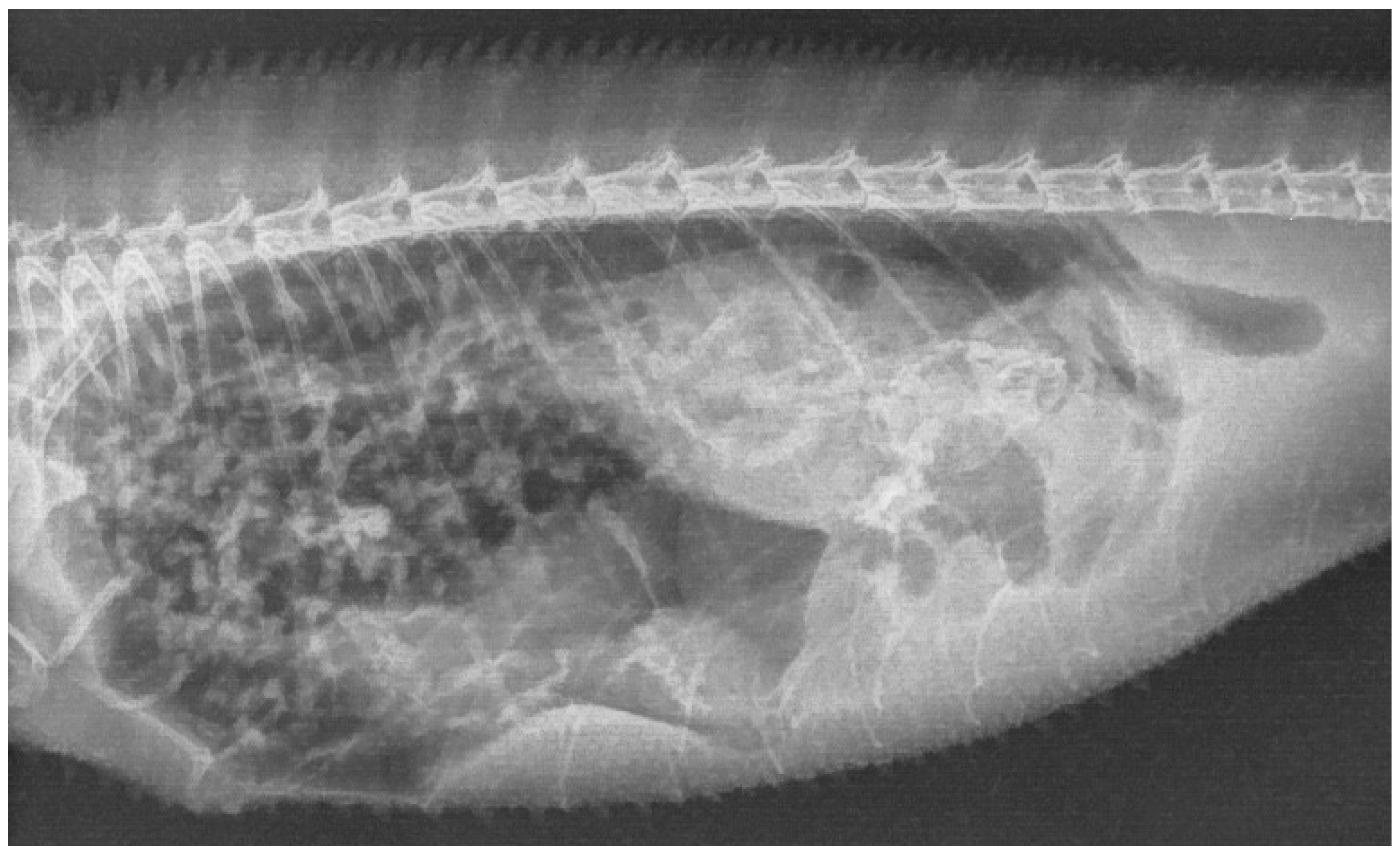
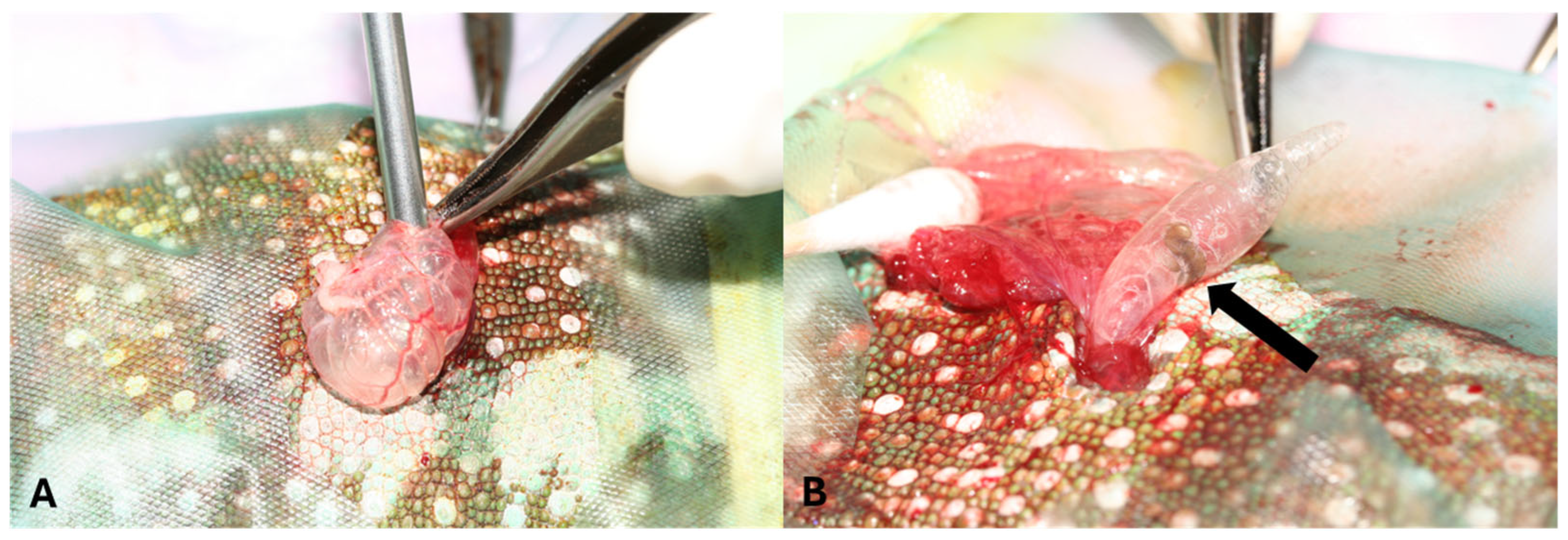
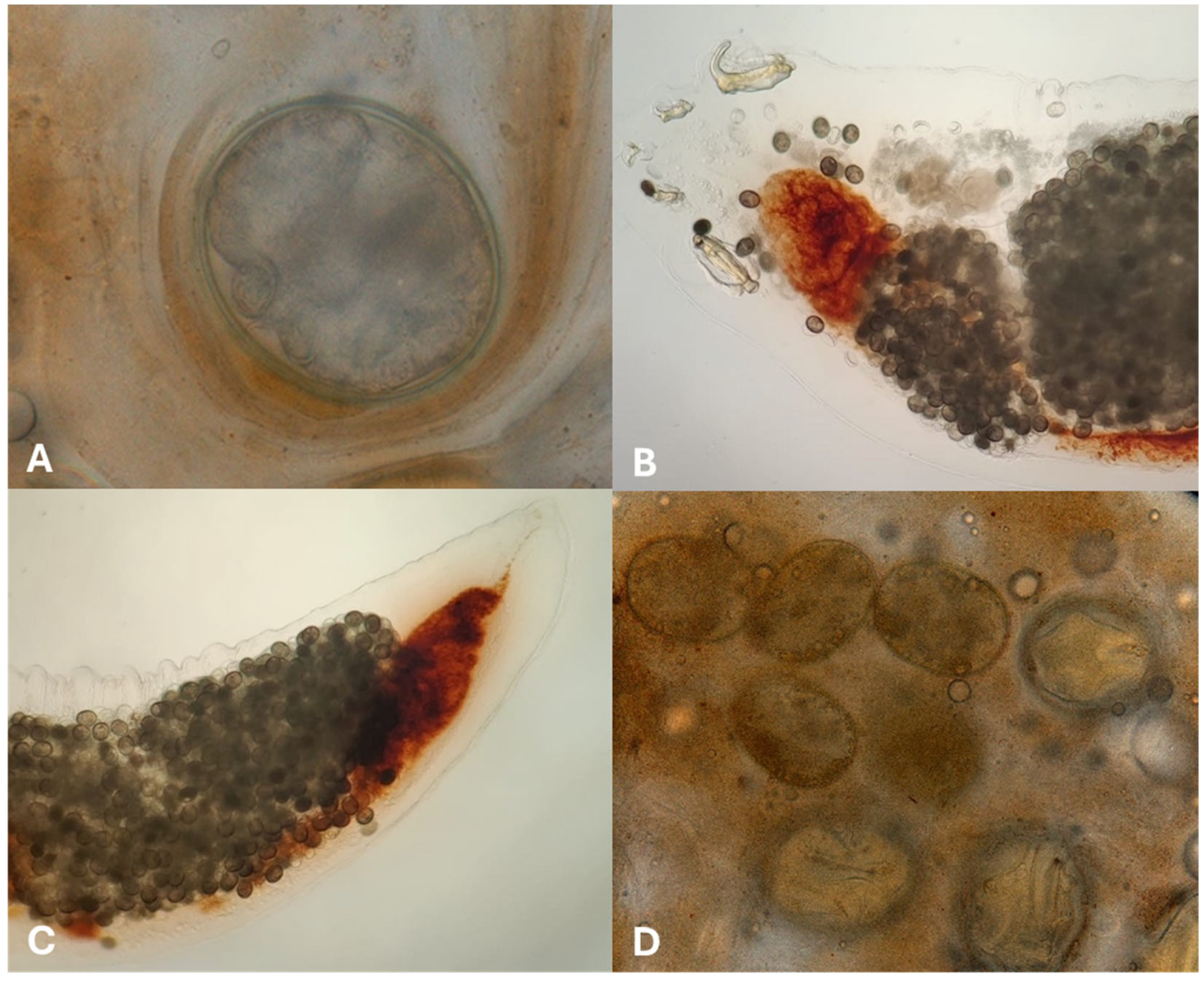
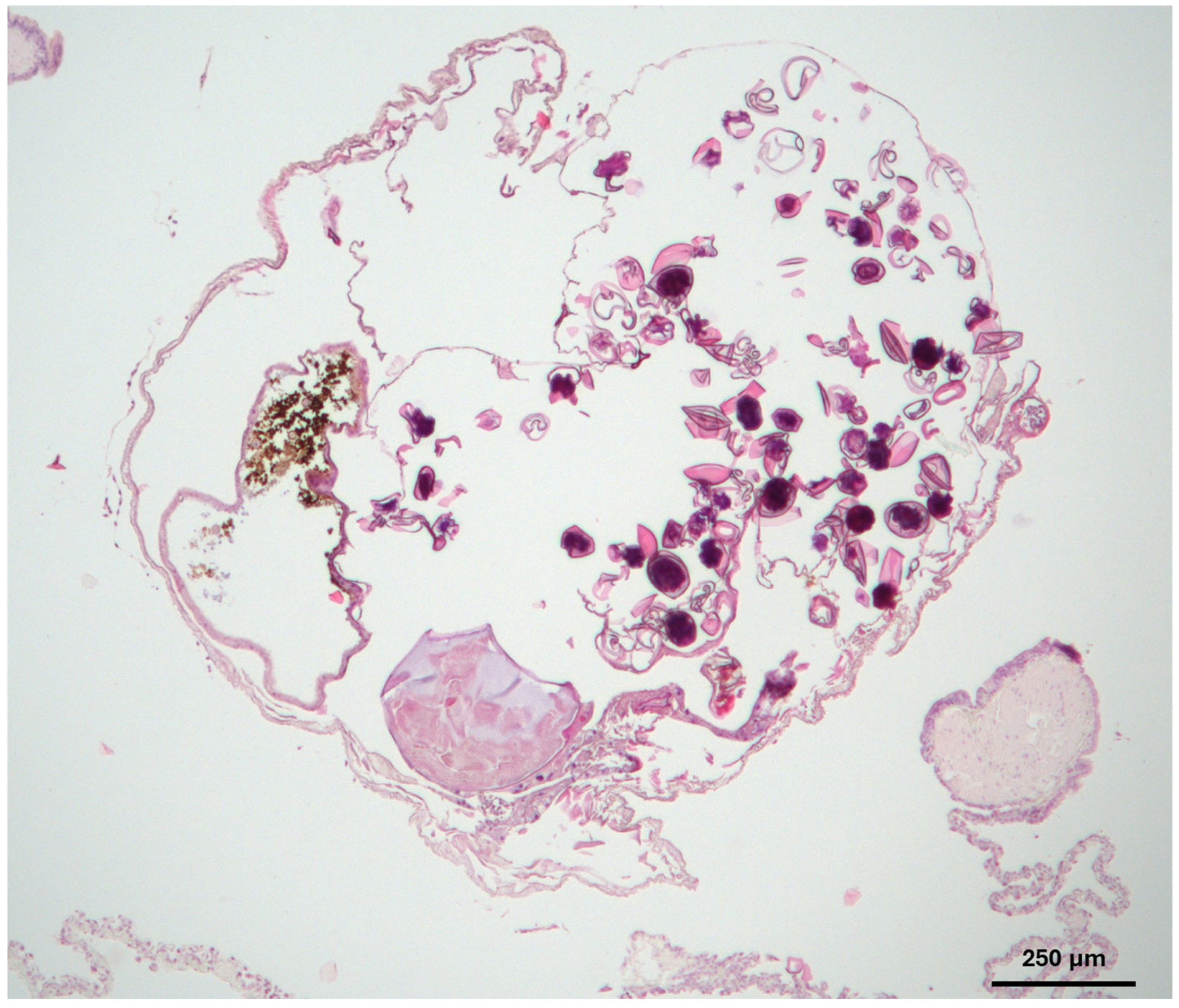
2. Case Description
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| DH | Definitive host |
| IH | Intermediate host |
| DNA | Deoxyribonucleic acid |
| rRNA | Ribosomal ribonucleic acid |
| PCR | Polymerase-chain reaction |
| IM | Intramuscular |
| IV | Intravenous |
| SC | Subcutaneous |
| PO | Per os |
| BLAST | Basic Local Alignment Search Tool |
References
- Self, J.T. Pentastomida: Tongue worms. In Roberts’ Foundations of Parasitology, 8th ed.; Roberts, L.S., Janovy, J., Schmidt, G.D., Larry, S., Eds.; McGraw-Hill: New York, NY, USA, 2009; pp. 561–568. [Google Scholar]
- Kelehear, C.; Spratt, D.M.; O’Meally, D.; Shine, R. Pentastomids of wild snakes in the Australian tropics. Int. J. Parasitol. Parasites Wildl. 2014, 3, 20–31. [Google Scholar] [CrossRef] [PubMed]
- Lavrov, D.V.; Brown, W.M.; Boore, J.L. Phylogenetic position of the Pentastomida and (pan)crustacean relationships. Proc. R. Soc. B Biol. Sci. 2004, 271, 537–544. [Google Scholar] [CrossRef] [PubMed]
- Self, J.T. Biological relationships of the Pentastomida; a bibliography on the Pentastomida. Exp. Parasitol. 1969, 24, 63–119. [Google Scholar] [CrossRef]
- Riley, J. Some observations on the life-cycle of Reighardia sternae Diesing 1864 (Pentastomida). Z. Parasitenkd. 1972, 40, 49–59. [Google Scholar] [CrossRef]
- Lavoipierre, M.M.J.; Rajamanickam, C. Experimental studies on the life cycle of a lizard pentastomid. J. Med. Entomol. 1973, 10, 301–302. [Google Scholar] [CrossRef]
- Ali, J.H.; Riley, J. Experimental life-cycle studies of Raillietiella gehyrae Bovien, 1927 and Raillietiella frenatus Ali, Riley and Self, 1981: Pentastomid parasites of geckos utilizing insects as intermediate hosts. Parasitology 1983, 86, 147–160. [Google Scholar] [CrossRef]
- Haugerud, R.E. Evolution in the pentastomids. Parasitol. Today 1989, 5, 126–132. [Google Scholar] [CrossRef]
- Spratt, D.M. Rilleyella petauri gen. nov., sp. nov. (Pentastomida: Cephalobaenida) from the lungs and nasal sinus of Petaurus breviceps (Marsupialia: Petauridae) in Australia. Parasite 2003, 10, 235–241. [Google Scholar] [CrossRef]
- Paré, J.A. An overview of pentastomiasis in reptiles and other vertebrates. J. Exot. Pet Med. 2008, 17, 285–294. [Google Scholar] [CrossRef]
- Barton, D.P.; Morgan, J.A. A morphological and genetic description of pentastomid infective nymphs belonging to the family Sebekidae Sambon, 1922 in fish in Australian waters. Folia Parasitol. 2016, 63, 1–9. [Google Scholar] [CrossRef]
- Palmisano, J.N.; Bockoven, C.; McPherson, S.M.; Ossiboff, R.J.; Walden, H.D.S.; Farrell, T.M. Infection experiments indicate that common Florida anurans and lizards may serve as intermediate hosts for the invasive pentastome parasite, Raillietiella orientalis. J. Herpetol. 2022, 56, 355–361. [Google Scholar] [CrossRef]
- Kelehear, C.; Spratt, D.M.; Dubey, S.; Brown, G.P.; Shine, R. Using combined morphological, allometric and molecular approaches to identify species of the genus Raillietiella (Pentastomida). PLoS ONE 2011, 6, e24936. [Google Scholar] [CrossRef] [PubMed]
- Christoffersen, M.L.; De Assis, J.E. A systematic monograph of the recent Pentastomida, with a compilation of their hosts. Zool. Med. Leiden 2013, 87, 29. [Google Scholar]
- Riley, J. The biology of pentastomids. In Advances in Parasitology; Baker, J.R., Muller, R., Eds.; Academic Press: London, UK, 1986; pp. 45–128. [Google Scholar]
- Riley, J.; Henderson, R.J. Pentastomids and the tetrapod lung. Parasitology 1999, 119, S89–S105. [Google Scholar] [CrossRef] [PubMed]
- Riley, J.; Spratt, D.M.; Presidente, P.J.A. Pentastomids (Arthropoda) parasitic in Australian reptiles and mammals. Aust. J. Zool. 1985, 33, 39–53. [Google Scholar] [CrossRef]
- Barton, D.; Porter, M.; Baker, A.; Zhu, X.; Jenkins, D.; Shamsi, S. First report of nymphs of the introduced pentastomid, Linguatula serrata, in red-necked wallabies (Notamacropus rufogriseus) in Australia. Aust. J. Zool. 2020, 67, 106–113. [Google Scholar] [CrossRef]
- Barton, D.; Shackelford, B.; Shamsi, S.; Jenkins, D. Are feral goats intermediate hosts for Linguatula (Pentastomida) in Australia? Int. J. Parasitol. Parasites Wildl. 2022, 18, 283–286. [Google Scholar] [CrossRef]
- Barton, D.P.; Baker, A.; Porter, M.; Zhu, X.; Jenkins, D.; Shamsi, S. Verification of rabbits as intermediate hosts for Linguatula serrata (Pentastomida) in Australia. Parasitol. Res. 2020, 119, 1553–1562. [Google Scholar] [CrossRef]
- Boyce, W.M.; Kazacos, E.A. Histopathology of nymphal pentastomid infections (Sebekia mississippiensis) in paratenic hosts. J. Parasitol. 1991, 77, 104–110. [Google Scholar] [CrossRef]
- Tappe, D.; Büttner, D.W. Diagnosis of human visceral pentastomiasis. PLoS Negl. Trop. Dis. 2009, 3, e320. [Google Scholar] [CrossRef]
- Miller, M.A.; Kinsella, J.M.; Snow, R.W.; Falk, B.G.; Reed, R.N.; Mazzotti, F.J.; Guyer, C.; Romagosa, C.M. Parasite spillover: Indirect effects of invasive Burmese pythons. Ecol. Evol. 2018, 8, 830–840. [Google Scholar] [CrossRef]
- Ali, J.H.; Riley, J.; Self, J.T. A review of the taxonomy and systematics of the pentastomid genus Raillietiella Sambon, 1910 with a description of a new species. Syst. Parasitol. 1985, 7, 111–123. [Google Scholar] [CrossRef]
- Farrell, T.M.; Agugliaro, J.; Walden, H.; Wellehan, J.; Childress, A.; Lind, C. Spillover of pentastome parasites from invasive Burmese pythons (Python bivittatus) to pygmy rattlesnakes (Sistrurus miliarius), extending parasite range in Florida, USA. Herpetol. Rev. 2019, 50, 73–76. [Google Scholar]
- Walden, H.D.S.; Iredale, M.E.; Childress, A.; Wellehan, J.F.X.; Ossiboff, R.J. Case report: Invasive pentastomes, Raillietiella orientalis (Sambon, 1922), in a free-ranging banded water snake (Nerodia fasciata) in North central Florida, USA. Front. Vet. Sci. 2020, 7, 467. [Google Scholar] [CrossRef] [PubMed]
- Wellehan, J.F.X.; Walden, H.D.S. Parasitology (including hemoparasites). In Mader’s Reptile and Amphibian Medicine and Surgery, 3rd ed.; Divers, S.J., Stahl, S.J., Eds.; Elsevier Health Sciences: St. Louis, MO, USA, 2018; pp. 281–300.e3. [Google Scholar]
- Miller, M.A.; Kinsella, J.M.; Snow, R.W.; Hayes, M.M.; Falk, B.G.; Reed, R.N.; Mazzotti, F.J.; Guyer, C.; Romagosa, C.M. Highly competent native snake hosts extend the range of an introduced parasite beyond its invasive Burmese python host. Ecosphere 2020, 11, e03153. [Google Scholar] [CrossRef]
- Metcalf, M.F.; Marsh, A.; Brosse, W.; Herman, J.E. Crotalus adamanteus: Endoparasite. Herpetol. Rev. 2019, 50, 389. [Google Scholar]
- Fonseca, M. Invasive Parasites: A Survey of Endoparasites in Salvator merianae Populations in Florida. Ph.D. Thesis, Florida Southern College, Lakeland, FL, USA, 2021. [Google Scholar]
- Barton, D.P.; Trembath, D. Pentastomes in Australian reptiles and amphibians: A review of the history and a checklist of records. J. Parasitol. 2024, 110, 428–439. [Google Scholar] [CrossRef]
- Goetz, S.M.; Steen, D.A.; Miller, M.A.; Guyer, C.; Kottwitz, J.; Roberts, J.F.; Blankenship, E.; Pearson, P.R.; Warner, D.A.; Reed, R.N. Argentine black and white tegu (Salvator merianae) can survive the winter under semi-natural conditions well beyond their current invasive range. PLoS ONE 2021, 16, e0245877. [Google Scholar] [CrossRef]
- Fieldsend, T.; Harman, M.; Miller, M. First record of an Asian tongueworm, Raillietiella orientalis (Pentastomida: Raillietiellidae), parasitizing a tokay gecko (Gekko gecko): A novel interaction between two non-native species in Florida. Reptiles Amphib. 2021, 28, 255–256. [Google Scholar] [CrossRef]
- Röhlig, D.; Dunlop, J.A.; Horacio-Grau, J.; Friederichs, A. An annotated catalogue of the tongue worms (Pentastomida) held in the Museum für Naturkunde Berlin. Zoosyst. Evol. 2010, 86, 129–154. [Google Scholar] [CrossRef]
- Sapion-Miranda, P.; Ebmer, D.; Kniha, E.; Walochnik, J.; Dreyer, S.; Fischer, D.; Grund, L.; Taubert, A.; Hermosilla, C.; Hallinger, M.J. First identification of a patent pentastomid pulmonary (Raillietiella orientalis) infection in a captive Meller’s chameleon (Trioceros melleri) in Germany. Int. J. Parasitol. Parasites Wildl. 2025, 26, 101045. [Google Scholar] [CrossRef] [PubMed]
- Laube, A.; Pendl, H.; Clauss, M.; Altherr, B.; Hatt, J.M. Plasma biochemistry and hematology reference values of captive panther chameleons (Furcifer pardalis) with special emphasis on seasonality and gender differences. J. Zoo Wildl. Med. 2016, 47, 743–753. [Google Scholar] [CrossRef] [PubMed]
- Brookins, M.D.; Wellehan, J.F.X., Jr.; Roberts, J.F.; Allison, K.; Curran, S.S.; Childress, A.L.; Greiner, E.C. Massive visceral pentastomiasis caused by Porocephalus crotali in a dog. Vet. Pathol. 2009, 46, 460–463. [Google Scholar] [CrossRef] [PubMed]
- Bosch, H. Experimental life-cycle studies of Raillietiella Sambon, 1910 (Pentastomida: Cephalobaenida): The fourth-stage larva is infective for the definitive host. Z. Parasitenkd. 1986, 72, 673–680. [Google Scholar] [CrossRef]
- Westfall, A.K.; Miller, M.A.; Murray, C.M.; Falk, B.G.; Guyer, C.; Romagosa, C.M. Host-specific phenotypic variation of a parasite co-introduced with invasive Burmese pythons. PLoS ONE 2019, 14, e0209252. [Google Scholar] [CrossRef]
- Flach, E.J.; Riley, J.; Mutlow, A.G.; McCandlish, I.A.P. Pentastomiasis in Bosc’s monitor lizards (Varanus exanthematicus) caused by an undescribed Sambonius species. J. Zoo Wildl. Med. 2000, 31, 91–95. [Google Scholar]
- Wright, K. Ivermectin for the treatment of pentastomids in the Standing’s day gecko (Phelsuma standingi). Bull. Assoc. Reptil. Amphib. Vet. 1997, 7, 5. [Google Scholar] [CrossRef]
- Gałęcki, R.; Sokół, R.; Dudek, A. Tongue worm (Pentastomida) infection in ball pythons (Python regius)—A case report. Ann. Parasitol. 2016, 62, 363–365. [Google Scholar]
- Takaki, Y.; Irie, T.; Takami, Y.; Asakawa, M.; Yoshida, A. Tongue worm (subclass: Pentastomida) infection and treatment in two domesticated reptiles—A case report. Parasitol. Int. 2022, 91, 102617. [Google Scholar] [CrossRef]
- Panayotova-Pencheva, M.S. Experience in the ivermectin treatment of internal parasites in zoo and captive wild animals: A review. Zool. Gart. 2016, 85, 280–308. [Google Scholar] [CrossRef]
- Foldenauer, U.; Pantchev, N.; Simova-Curd, S.; Jurado, O.M.; Hatt, J.-M. Pentastomidenbefall bei Abgottschlangen (Boa constrictor)—Diagnostik und endoskopische Parasitenentfernung. Tierärztl. Prax. Ausg. K Kleintiere Heimtiere 2008, 36, 443–449. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hellebuyck, T.; Solanes-Vilanova, F.; Decorte, B.; Miljković, J.; Claerebout, E. Patent Pulmonary Infection with the Invasive Pentastomid Raillietiella orientalis in a Panther Chameleon (Furcifer pardalis) in Belgium. Animals 2025, 15, 3433. https://doi.org/10.3390/ani15233433
Hellebuyck T, Solanes-Vilanova F, Decorte B, Miljković J, Claerebout E. Patent Pulmonary Infection with the Invasive Pentastomid Raillietiella orientalis in a Panther Chameleon (Furcifer pardalis) in Belgium. Animals. 2025; 15(23):3433. https://doi.org/10.3390/ani15233433
Chicago/Turabian StyleHellebuyck, Tom, Ferran Solanes-Vilanova, Bregt Decorte, Josip Miljković, and Edwin Claerebout. 2025. "Patent Pulmonary Infection with the Invasive Pentastomid Raillietiella orientalis in a Panther Chameleon (Furcifer pardalis) in Belgium" Animals 15, no. 23: 3433. https://doi.org/10.3390/ani15233433
APA StyleHellebuyck, T., Solanes-Vilanova, F., Decorte, B., Miljković, J., & Claerebout, E. (2025). Patent Pulmonary Infection with the Invasive Pentastomid Raillietiella orientalis in a Panther Chameleon (Furcifer pardalis) in Belgium. Animals, 15(23), 3433. https://doi.org/10.3390/ani15233433




