Influence of Nutritional Strategies on Performance, Gut Barrier Function and Microbiota Composition in Weaned Piglets
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design and Housing
2.2. Performance Data
2.3. Diets
2.4. Animal Sampling
2.5. Microbiota Composition Analysis—16S rRNA Isolation
- - Forward: 341f (CCTACGGGDGGCWGCAG, CCTAYGGGGYGCWGCAG).
- - Reverse: 806r (GACTACNVGGGTMTCTAATCC).
2.6. Sequencing of Intestinal Integrity Markers
2.7. Bioinformatics and Statistical Analysis
3. Results
3.1. Productive Data
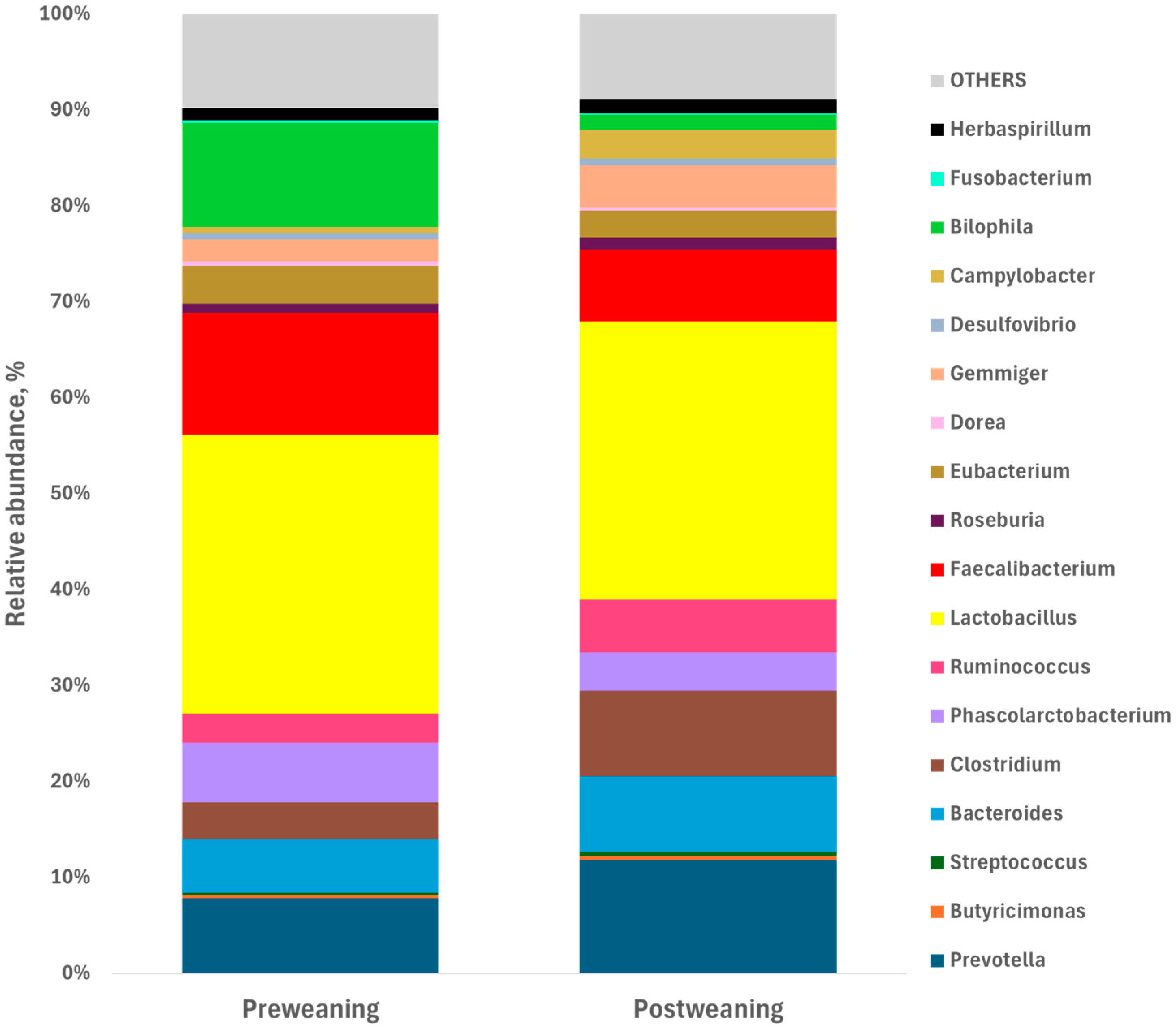
3.2. Evolution of the Fecal Microbiota After Weaning
3.3. Evolution of the Intestinal Integrity After Weaning
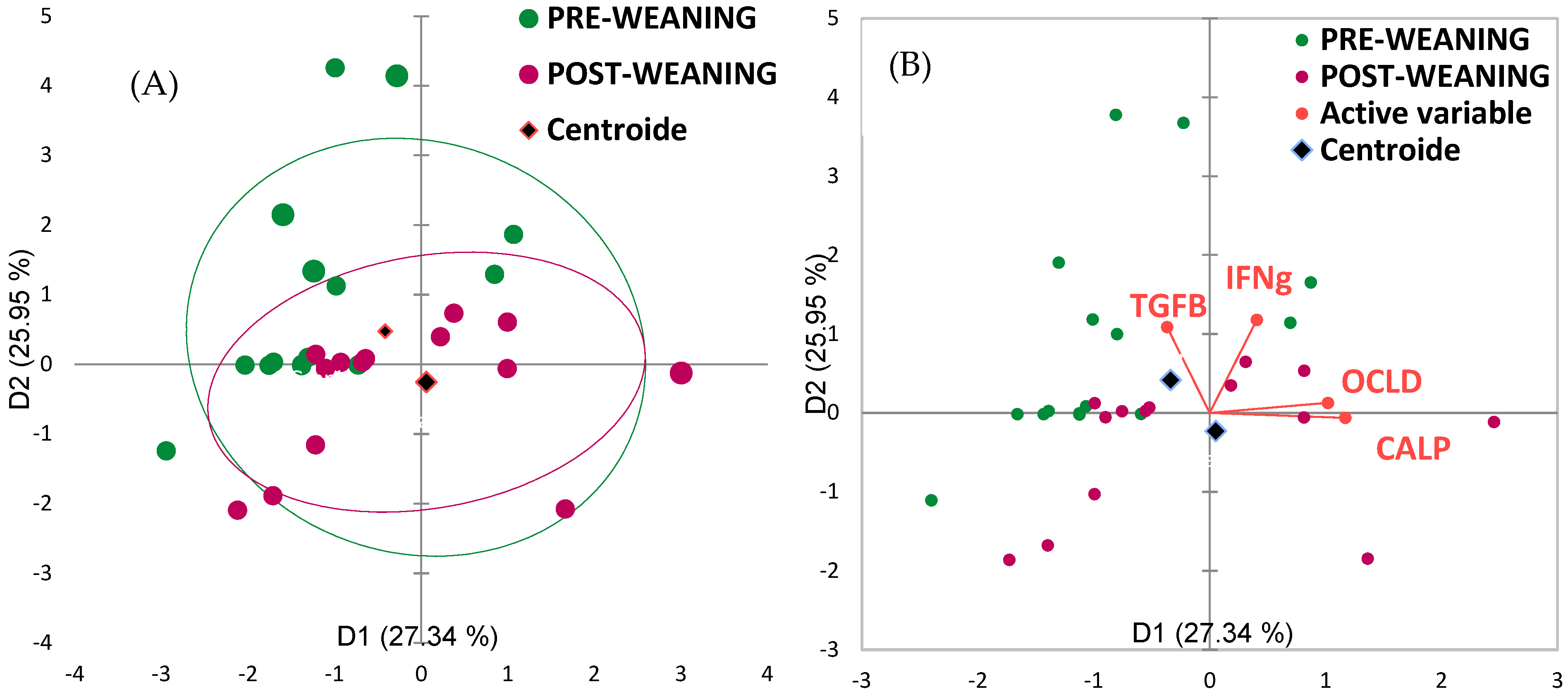
3.4. Correlation Between Intestinal Integrity and Fecal Microbiota
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, Y.; Ren, W.; Smidt, H.; Wright, A.-D.G.; Yu, B.; Schyns, G.; McCormack, U.M.; Cowieson, A.J.; Yu, J.; He, J.; et al. Dynamic distribution of gut Microbiota in pigs at different growth stages: Composition and contribution. Microbiol. Spectr. 2022, 10, e0068821. [Google Scholar] [CrossRef]
- Hu, C.H.; Xiao, K.; Luan, Z.S.; Song, J. Early weaning increases intestinal permeability, alters expression of cytokine and tight junction proteins, and activates mitogen-activated protein kinases in pigs. J. Anim. Sci. 2013, 91, 1094–1101. [Google Scholar] [CrossRef]
- Moeser, A.J.; Pohl, C.S.; Rajput, M. Weaning stress and gastrointestinal barrier development: Implications for lifelong gut health in pigs. Anim. Nutr. 2017, 3, 313–321. [Google Scholar] [CrossRef]
- Kapel, N.; Ouni, H.; Benahmed, N.A.; Barbot-Trystram, L. Fecal calprotectin for the diagnosis and management of inflammatory bowel diseases. Clin. Transl. Gastroenterol. 2023, 14, e00617. [Google Scholar] [CrossRef]
- Stadnyk, A.W. Cytokine production by epithelial cells. FASEB J. 1994, 8, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Parra, J.; Agudelo, J.; Sanín, D.; Forero, J.; Muskus, C.; López Herrera, A. Intestinal expression of pro-inflammatory cytokines induced by oral intake of lipopolysaccharide (LPS) from E. coli in weaned pigs. Rev. Colomb. Cienc. Pecu. 2013, 26, 108–118. [Google Scholar] [CrossRef]
- Shirkey, T.W.; Siggers, R.H.; Goldade, B.G.; Marshall, J.K.; Drew, M.D.; Laarveld, B.; Van Kessel, A.G. Effects of commensal bacteria on intestinal morphology and expression of proinflammatory cytokines in the gnotobiotic pig. Exp. Biol. Med. 2006, 231, 1333–1345. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Xu, J.; Ren, E.; Su, Y.; Zhu, W. Co-occurrence of early gut colonization in neonatal piglets with microbiota in the maternal and surrounding delivery environments. Anaerobe 2018, 49, 30–40. [Google Scholar] [CrossRef]
- Jacela, J.Y.; DeRouchey, J.M.; Tokach, M.D.; Goodband, R.D.; Nelssen, J.L.; Renter, D.G.; Dritz, S.S. Feed additives for swine: Fact sheets—Acidifiers and antibiotics. J. Swine Health Prod. 2009, 17, 270–275. [Google Scholar] [CrossRef]
- Cai, L.; Zhao, Y.; Chen, W.; Li, Y.; Han, Y.; Zhang, B.; Pineda, L.; Li, X.; Jiang, X. Effect of an organic acid blend as an antibiotic alternative on growth performance, antioxidant capacity, intestinal barrier function, and fecal microbiota in weaned piglets. J. Anim. Sci. 2024, 102, skae149. [Google Scholar] [CrossRef] [PubMed]
- Barba-Vidal, E.; Martín-Orúe, S.M.; Castillejos, L. Review: Are we using probiotics correctly in post-weaning piglets? Animal 2018, 12, 2489–2498. [Google Scholar] [CrossRef]
- Cao, G.; Tao, F.; Hu, Y.; Li, Z.; Zhang, Y.; Deng, B.; Zhan, X. Positive effects of a Clostridium butyricum-based compound probiotic on growth performance, immune responses, intestinal morphology, hypothalamic neurotransmitters, and colonic microbiota in weaned piglets. Food Funct. 2019, 10, 2926–2934. [Google Scholar] [CrossRef]
- Directiva 2008/120/CE del Consejo de 18 de Diciembre de 2008 Relativa a las Normas Mínimas a la Protección de Cerdos. DOUE-L-2009-80287. Available online: https://www.boe.es/buscar/doc.php?id=DOUE-L-2009-80287 (accessed on 15 October 2025).
- Real Decreto 1135/2002, de 31 de Octubre, Relativo a las Normas Mínimas Para la Protección de Cerdos. Boletin Oficial del Estado, 20 de Noviembre de 2002. Available online: https://www.boe.es/eli/es/rd/2002/10/31/1135/con (accessed on 15 October 2025).
- Real Decreto 159/2023, de 7 de Marzo, Por el Que se Establecen Disposiciones Para la Aplicación en España de la Normativa de la Unión Europea Sobre Controles Oficiales en Materia de Bienestar Animal, y se Modifican Varios Reales Decretos. Boletín Oficial del Estado, 8 de Marzo de 2023. Available online: https://www.boe.es/eli/es/rd/2023/03/07/159 (accessed on 15 October 2025).
- Real decreto 53/2013, de 1 de Febrero, por el que se Establecen las Normas Básicas Aplicables Para la Protección de los Animales Utilizados en Experimentación y Otros Fines Científicos, Incluyendo la Docencia. Boletín Oficial del Estado, 34, de 8 de febrero de 2013. Available online: https://www.boe.es/eli/es/rd/2013/02/01/53 (accessed on 15 October 2025).
- Ramis, G.; Pérez-Esteruelas, L.; Gómez-Cabrera, C.G.; de Pascual-Monreal, C.; Gonzalez-Guijarro, B.; Párraga-Ros, E.; Sánchez-Uribe, P.; Claver-Mateos, M.; Mendonça-Pascoal, L.; Martínez-Alarcón, L. Oral and parenteral vaccination against Escherichia coli in piglets results in different responses. Animals 2022, 12, 2758. [Google Scholar] [CrossRef]
- Royaee, A.R.; Husmann, R.J.; Dawson, H.D.; Calzada-Nova, G.; Schnitzlein, W.M.; Zuckermann, F.A.; Lunney, J.K. Deciphering the involvement of innate immune factors in the development of the host response to PRRSV vaccination. Vet. Immunol. Immunopathol. 2004, 102, 199–216. [Google Scholar] [CrossRef]
- Moue, M.; Tohno, M.; Shimazu, T.; Kido, T.; Aso, H.; Saito, T.; Kitazawa, H. Toll-like receptor 4 and cytokine expression involved in functional immune response in an originally established porcine intestinal epitheliocyte cell line. Biochim. Biophys. Acta 2008, 1780, 134–144. [Google Scholar] [CrossRef] [PubMed]
- European Medicines Agency (EMA). Commite for Medicinal Products for Veterinary Use (CVMP). In CVMP Meeting Highlights, 13–15 June 2017; EMA/430075/2017; European Medicines Agency: Amsterdam, The Netherlands, 2017. [Google Scholar]
- European Medicines Agency. Committee for Medicinal Products for Veterinary Use (CVMP). Meeting 6–8 December 2016; European Medicines Agency-Science Medicines Health: Amsterdam, The Netherlands, 2016; pp. 1–5. Available online: http://www.ema.europa.eu/ema/index.jsp?curl=pages/news_and_events/newa/2016/12/news_detail_002661.jsp&mid=WC0b01ac058004d5c (accessed on 22 June 2025).
- Eriksen, E.Ø.; Kudirkiene, E.; Christensen, A.E.; Agerlin, M.V.; Weber, N.R.; Nødtvedt, A.; Nielsen, J.P.; Hartmann, K.T.; Skade, L.; Larsen, L.E.; et al. Post-weaning diarrhea in pigs weaned without medicinal zinc: Risk factors, pathogen dynamics, and association to growth rate. Porc. Health Manag. 2021, 7, 54. [Google Scholar] [CrossRef] [PubMed]
- Ferrando, M.L.; Schultsz, C. A hypothetical model of host-pathogen interaction of Streptococcus suis in the gastro-intestinal tract. Gut Microbes 2016, 7, 154–162. [Google Scholar] [CrossRef]
- Júnior, D.T.V.; de Amorim Rodrigues, G.; Soares, M.H.; Silva, C.B.; Frank, E.O.; Gonzalez-Vega, J.C.; Htoo, J.K.; Brand, H.G.; Silva, B.A.N.; Saraiva, A. Supplementation of Bacillus subtilis DSM 32540 improves performance and intestinal health of weaned pigs fed diets containing different fiber sources. Livest. Sci. 2023, 270, 105202. [Google Scholar] [CrossRef]
- Fu, J.; Wang, T.; Xiao, X.; Cheng, Y.; Wang, F.; Jin, M.; Wang, Y.; Zong, X. Clostridium butyricum ZJU-F1 benefits the intestinal barrier function and immune response associated with its modulation of gut Microbiota in weaned piglets. Cells 2021, 10, 527. [Google Scholar] [CrossRef]
- Zhang, J.; Chen, X.; Liu, P.; Zhao, J.; Sun, J.; Guan, W.; Johnston, L.J.; Levesque, C.L.; Fan, P.; He, T.; et al. Dietary Clostridium butyricum induces a phased shift in fecal Microbiota structure and increases the acetic acid-producing bacteria in a weaned piglet model. J. Agric. Food Chem. 2018, 66, 5157–5166. [Google Scholar] [CrossRef]
- Frese, S.A.; Parker, K.; Calvert, C.C.; Mills, D.A. Diet shapes the gut microbiome of pigs during nursing and weaning. Microbiome 2015, 3, 28. [Google Scholar] [CrossRef] [PubMed]
- Megahed, A.; Zeineldin, M.; Evans, K.; Maradiaga, N.; Blair, B.; Aldridge, B.; Lowe, J. Impacts of environmental complexity on respiratory and gut microbiome community structure and diversity in growing pigs. Sci. Rep. 2019, 9, 13773. [Google Scholar] [CrossRef] [PubMed]
- Fan, L.; Liu, B.; Han, Z.; Ren, W. Insights into host-microbe interaction: What can we do for the swine industry? Anim. Nutr. 2021, 7, 17–23. [Google Scholar] [CrossRef]
- Marcobal, A.; Barboza, M.; Froehlich, J.W.; Block, D.E.; German, J.B.; Lebrilla, C.B.; Mills, D.A. Consumption of human milk oligosaccharides by gut-related microbes. J. Agric. Food Chem. 2010, 58, 5334–5340. [Google Scholar] [CrossRef]
- Lin, A.; Yan, X.; Wang, H.; Su, Y.; Zhu, W. Effects of lactic acid bacteria-fermented formula milk supplementation on ileal microbiota, transcriptomic profile, and mucosal immunity in weaned piglets. J. Anim. Sci. Biotechnol. 2022, 13, 113. [Google Scholar] [CrossRef]
- Kim, H.B.; Borewicz, K.; White, B.A.; Singer, R.S.; Sreevatsan, S.; Tu, Z.J.; Isaacson, R.E. Longitudinal investigation of the age-related bacterial diversity in the feces of commercial pigs. Vet. Microbiol. 2011, 153, 124–133. [Google Scholar] [CrossRef] [PubMed]
- Mahmud, M.R.; Jian, C.; Uddin, M.K.; Huhtinen, M.; Salonen, A.; Peltoniemi, O.; Venhoranta, H.; Oliviero, C. Impact of intestinal Microbiota on growth performance of suckling and weaned piglets. Microbiol. Spectr. 2023, 11, e0374422. [Google Scholar] [CrossRef]
- St-Pierre, B.; Perez Palencia, J.Y.; Samuel, R.S. Impact of early weaning on development of the swine gut microbiome. Microorganisms 2023, 11, 1753. [Google Scholar] [CrossRef]
- Niu, Q.; Pu, G.; Fan, L.; Gao, C.; Lan, T.; Liu, C.; Du, T.; Kim, S.W.; Niu, P.; Zhang, Z.; et al. Identification of gut Microbiota affecting fiber digestibility in pigs. Curr. Issues Mol. Biol. 2022, 44, 4557–4569. [Google Scholar] [CrossRef]
- Gresse, R.; Chaucheyras-Durand, F.; Garrido, J.J.; Denis, S.; Jiménez-Marín, A.; Beaumont, M.; Van de Wiele, T.; Forano, E.; Blanquet-Diot, S. Pathogen challenge and dietary shift alter Microbiota composition and activity in a mucin-associated in vitro model of the piglet colon (MPigut-IVM) simulating weaning transition. Front. Microbiol. 2021, 12, 703421. [Google Scholar] [CrossRef]
- Vasquez, R.; Oh, J.K.; Song, J.H.; Kang, D.K. Gut microbiome-produced metabolites in pigs: A review on their biological functions and the influence of probiotics. J. Anim. Sci. Technol. 2022, 64, 671–695. [Google Scholar] [CrossRef]
- Yoshimura, S.; Tsukahara, T.; Takahashi, T.; Miura, H.; Morishima, S.; Kise, M.; Shin, J.; Yahara, Y.; Inoue, R. Causal association between the mucosal and luminal microbiotas from the gastrointestinal tract of weaned piglets using Bayesian network. Microorganisms 2025, 13, 256. [Google Scholar] [CrossRef]
- Heinzel, S.; Jureczek, J.; Kainulainen, V.; Nieminen, A.I.; Suenkel, U.; von Thaler, A.-K.; Kaleta, C.; Eschweiler, G.W.; Brockmann, K.; Aho, V.T.E.; et al. Elevated fecal calprotectin is associated with gut microbial dysbiosis, altered serum markers and clinical outcomes in older individuals. Sci. Rep. 2024, 14, 13513. [Google Scholar] [CrossRef]
- Shin, J.H.; Tillotson, G.; MacKenzie, T.N.; Warren, C.A.; Wexler, H.M.; Goldstein, E.J.C. Bacteroides and related species: The keystone taxa of the human gut microbiota. Anaerobe 2024, 85, 102819. [Google Scholar] [CrossRef]
- de Groot, N.; Fariñas, F.; Cabrera-Gómez, C.G.; Pallares, F.J.; Ramis, G. Weaning causes a prolonged but transient change in immune gene expression in the intestine of piglets. J. Anim. Sci. 2021, 99, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Mei, J.; Xu, R.-J. Transient changes of transforming growth factor-β expression in the small intestine of the pig in association with weaning. Br. J. Nutr. 2005, 93, 37–45. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Wu, X.; Pan, Y.; Wang, L.; Cui, C.; Guo, Y.; Zhu, L.; Peng, J.; Wei, H. Early-life intervention using fecal Microbiota combined with probiotics promotes gut Microbiota maturation, regulates immune system development, and alleviates weaning stress in piglets. Int. J. Mol. Sci. 2020, 21, 503. [Google Scholar] [CrossRef]
- Ren, Z.; Fan, H.; Deng, H.; Yao, S.; Jia, G.; Zuo, Z.; Hu, Y.; Shen, L.; Ma, X.; Zhong, Z.; et al. Effects of dietary protein level on small intestinal morphology, occludin protein, and bacterial diversity in weaned piglets. Food Sci. Nutr. 2022, 10, 2168–2201. [Google Scholar] [CrossRef] [PubMed]
| Ingredients, % | CONTROL | TRANSITION | 0M |
|---|---|---|---|
| Wheat | 29.99 | 29.99 | 29.99 |
| Maize | 17.00 | 24.80 | 25.00 |
| Barley | 15.00 | 15.00 | 15.00 |
| Soybean 48 | 14.00 | 5.30 | 5.30 |
| Full-fat soybean | 4.00 | 4.00 | 4.00 |
| Mineral and vitamin premix | 4.00 | 4.00 | 4.00 |
| Soybean oil | 2.18 | 1.92 | 1.92 |
| Wheat bran | 2.00 | 2.00 | 2.00 |
| Blood meal | 2.00 | 2.00 | 2.00 |
| Whey powder fat-filled | 1.80 | 1.80 | 1.80 |
| Fish oil | 1.25 | 1.25 | 1.25 |
| Whey powder | 1.25 | 1.25 | 1.25 |
| Beetroot pulp | 1.00 | 1.00 | 1.00 |
| Sacarose | 1.00 | 1.00 | 1.00 |
| Monocalcium phosphate | 0.85 | 0.90 | 0.90 |
| Calcium carbonate | 0.72 | 0.76 | 0.76 |
| Salt | 0.50 | 0.50 | 0.50 |
| Sodium bicarbonate | 0.32 | 0.33 | 0.33 |
| Zinc oxide | 0.31 | 0.20 | - |
| Lysine | 0.27 | 0.54 | 0.54 |
| Threonine | 0.23 | 0.35 | 0.35 |
| Methionine | 0.20 | 0.29 | 0.29 |
| Tryptophan | 0.09 | 0.13 | 0.13 |
| Valine | 0.05 | 0.20 | 0.20 |
| Blend of organic acids | 0.30 | 0.30 | |
| Clostridium butyricum UFC/g | 12.5 × 1010 | 12.5 × 1010 | |
| Chemical composition | CONTROL | TRANSITION | 0M |
| Crude protein, % | 19.1 | 16.0 | 16.0 |
| M. E., Kcal/kg | 3342.0 | 3332.0 | 3332.0 |
| Lysine SID | 1.3 | 1.3 | 1.3 |
| LysSID/NE, g/MKcal | 5.2 | 5.1 | 5.1 |
| Gene | Forwerd Primer | Reverse Primer | Reference |
|---|---|---|---|
| Calprotectin (S100 calcium binding protein A8) | 5′-AATTACCACGCCATCTACGC-3′ | 5′-TGATGTCCAG CTCTTTGAACC-3′ | [17] |
| Occludin | 5′-TTGCTGTGAAA ACTCGAAGC-3′ | 5′-CCACTCTCTCCGCATAGTCC-3′ | [17] |
| Zonulin 1 | 5′-CACAGATGCC ACAGATGACAG-3′ | 5′-AGTGATAGCGAACCATGTGC-3′ | [17] |
| IFN-γ | 5′-TGGTAGCTCTGGGAAACTGAATG-3′ | 5′-GGCTTTGCGCTGGATCTG-3′ | [18] |
| TGF-β | 5′-CACGTGGAGCTATACCAGAA-3′ | 5′-TCCGGTGACATCA AAGGACA-3′ | [19] |
| LS Mean | SEM | p-Value | |||
|---|---|---|---|---|---|
| CONTROL (n = 174,000) | TRANSITION (n = 105,000) | 0M (n = 212,000) | |||
| BW0, kg | 5.58 | 5.47 | 5.44 | 0.050 | ns |
| BW1, kg | 17.40 a | 18.99 b | 18.72 b | 0.233 | 0.011 |
| ADG, g/day | 292.28 a | 300.09 ab | 307.45 b | 2.303 | 0.012 |
| FCR | 1.80 a | 1.59 b | 1.58 b | 0.029 | 0.005 |
| Mortality, % | 6.48% | 5.10% | 5.08% | 0.004 | 0.089 |
| Family/Genera/Species | LS Mean | SEM | p-Value | |
|---|---|---|---|---|
| PRE-WEANING | POST-WEANING | |||
| Desulfovibrionaceae/Bilophila | ||||
| B. wadsworthia | 0.135 | 0.022 | 0.016 | <0.001 |
| Desulfovibrionaceae/Desulfovibrio | ||||
| D. piger | 0.007 | 0.008 | 0.002 | 0.021 |
| Lactobacillaceae/Lactobacillus | ||||
| L. reuteri | 0.007 | 0.017 | 0.003 | 0.021 |
| Eubacteriaceae/Eubacterium | ||||
| E. coprostanoligenes | 0.042 | 0.012 | 0.007 | 0.045 |
| E. biforme | 0.005 | 0.018 | 0.003 | 0.016 |
| Oscillospiraceae/Faecalibacterium | ||||
| F. prausnitzii | 0.170 | 0.100 | 0.013 | 0.017 |
| Oscillospiraceae/Ruminoccus | ||||
| R. bromii | 0.003 | 0.024 | 0.004 | 0.009 |
| R. faecis | 0.002 | 0.000 | 0.000 | <0.001 |
| Prevotellaceae/Prevotella | ||||
| P. copri | 0.045 | 0.093 | 0.016 | 0.224 |
| Lachnospiraceae/Roseburia | ||||
| R. faecis | 0.017 | 0.021 | 0.005 | 0.425 |
| Clostridiaceae/Clostridium | ||||
| Cl. butyricum | 0.005 | 0.015 | 0.004 | 0.279 |
| Rikenellaceae/Alistipes | ||||
| A. shahii | 0.009 | 0.001 | 0.002 | 0.012 |
| Intestinal Marker | LS Mean | SEM | p-Value | |
|---|---|---|---|---|
| PRE-WEANING | POST-WEANING | |||
| CALP_LOG2 | 11.129 | 13.090 | 0.804 | 0.227 |
| OCLD_LOG2 | 8.024 | 7.250 | 0.413 | 0.430 |
| ZON1_LOG2 | 7.373 | 6.857 | 0.463 | 0.673 |
| IFNg_LOG2 | 15.507 | 10.016 | 0.716 | 0.002 |
| TGFB_LOG2 | 10.347 | 4.817 | 0.409 | <0.001 |
| Bacterial Species | Calprotectin | Occludin | Zonulin | IFNγ | TGFβ | Significance (p-Value) |
|---|---|---|---|---|---|---|
| Eubacterium_biforme | −0.289 | −0.148 | 0.075 | −0.238 | 0.639 | 0.070 |
| Alistipes_shahii | −0.232 | −0.207 | −0.047 | 0.263 | −0.274 | ns |
| Lactobacillus_reuteri | 0.161 | −0.330 | −0.052 | −0.018 | 0.001 | 0.087 |
| Prevotella_copri | −0.205 | −0.035 | 0.017 | 0.044 | 0.286 | ns |
| Bilophila_wadsworthia | −0.048 | 0.138 | 0.125 | 0.421 | 0.380 | 0.093 |
| Desulfovibrio_piger | 0.234 | 0.407 | −0.179 | 0.343 | 0.085 | 0.032 |
| Eubacterium_coprostanoligenes | 0.223 | −0.037 | −0.120 | 0.145 | −0.373 | ns |
| Roseburia_faecis | 0.291 | 0.113 | −0.236 | 0.075 | −0.064 | 0.069 |
| Faecalibacterium_prausnitzii | −0.152 | 0.270 | 0.446 | 0.250 | 0.584 | 0.033 |
| Clostridium_butyricum | 0.052 | 0.114 | 0.105 | 0.192 | - | ns |
| Ruminococcus_faecis | −0.026 | −0.239 | 0.003 | 0.240 | 0.828 | 0.006 |
| Ruminococcus_bromii | −0.249 | −0.193 | −0.052 | −0.269 | - | ns |
| Bacteroides_fragilis | 0.342 | 0.277 | 0.029 | - | −0.374 | 0.031 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Isusi, S.; Usero-Alonso, G.; Murillo, J.A.; Gonzalez-Guijarro, A.B.; Muñoz, A.; Ramis, G. Influence of Nutritional Strategies on Performance, Gut Barrier Function and Microbiota Composition in Weaned Piglets. Animals 2025, 15, 3422. https://doi.org/10.3390/ani15233422
Isusi S, Usero-Alonso G, Murillo JA, Gonzalez-Guijarro AB, Muñoz A, Ramis G. Influence of Nutritional Strategies on Performance, Gut Barrier Function and Microbiota Composition in Weaned Piglets. Animals. 2025; 15(23):3422. https://doi.org/10.3390/ani15233422
Chicago/Turabian StyleIsusi, Sara, Guillermo Usero-Alonso, Jose Alberto Murillo, Ana Belén Gonzalez-Guijarro, Antonio Muñoz, and Guillermo Ramis. 2025. "Influence of Nutritional Strategies on Performance, Gut Barrier Function and Microbiota Composition in Weaned Piglets" Animals 15, no. 23: 3422. https://doi.org/10.3390/ani15233422
APA StyleIsusi, S., Usero-Alonso, G., Murillo, J. A., Gonzalez-Guijarro, A. B., Muñoz, A., & Ramis, G. (2025). Influence of Nutritional Strategies on Performance, Gut Barrier Function and Microbiota Composition in Weaned Piglets. Animals, 15(23), 3422. https://doi.org/10.3390/ani15233422








