Degenerative Changes in MCP/MTP Joints of Working Horses Without Lameness: Integrating CT-Based Assessment and Synovial Fluid Biomarkers
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Clinical Examination
2.3. Post Mortem CT Examination
2.4. Post Mortem Collection and Routine Analysis of Synovial Fluid
2.5. Biochemical Analyses of Synovial Fluid
2.6. Statistical Analysis
3. Results
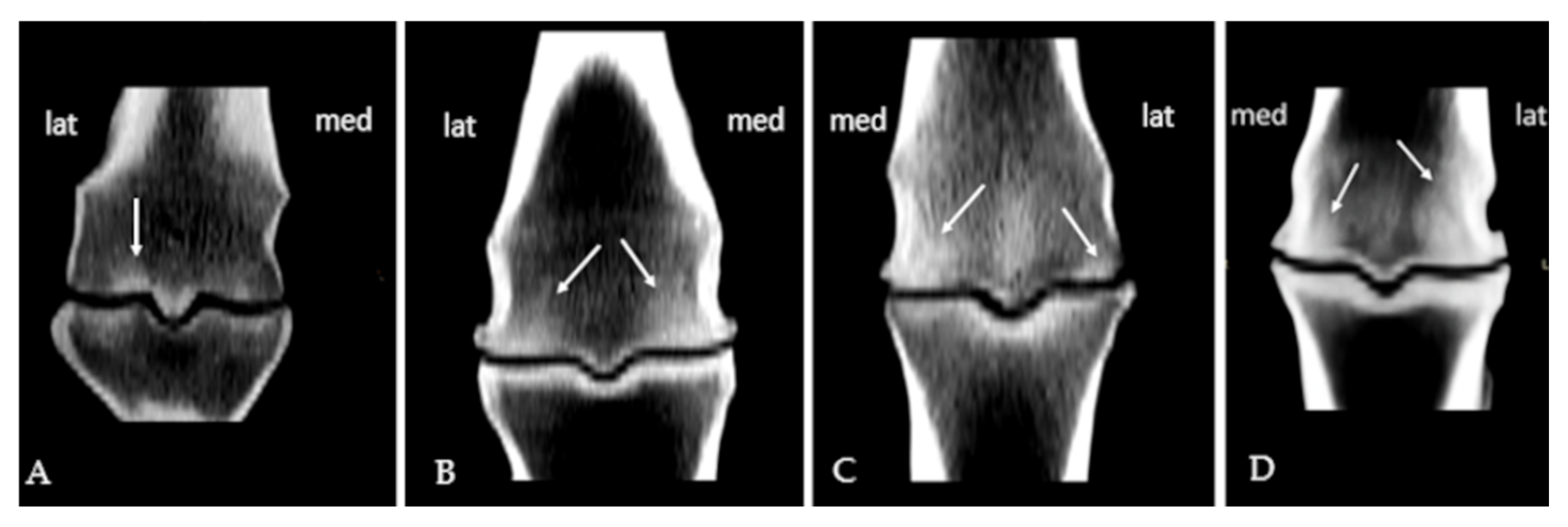
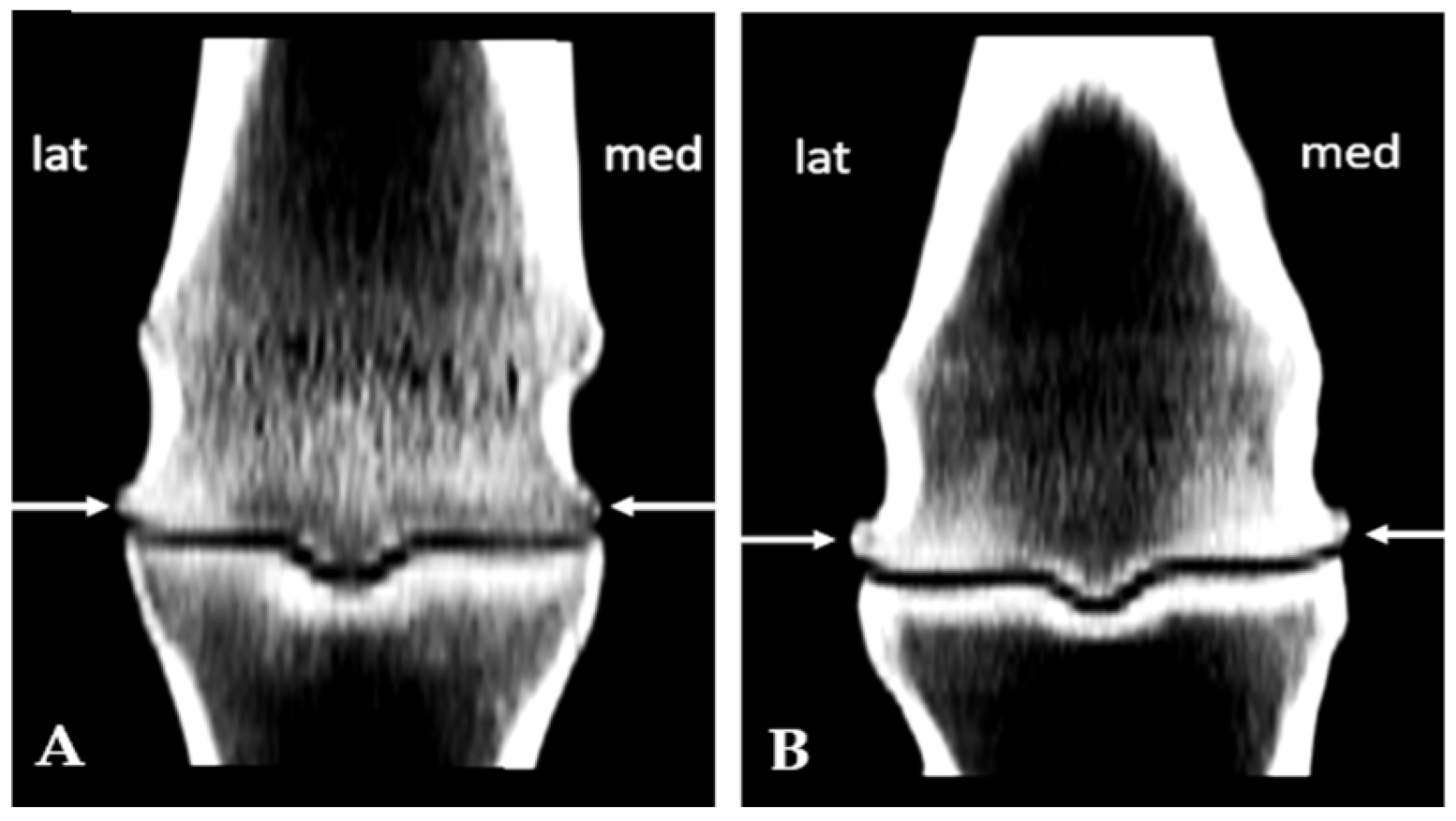
3.1. Subchondral Sclerosis Presence
3.2. Osteophytes Presence
3.3. Correlation Analysis
4. Discussion
4.1. Age and Weight Are Linked to Structural Joint Adaptations
4.2. TNCC Emerges as a Sensitive Indicator of Early Degenerative Joint Changes
4.3. Osteophytosis Is More Strongly Linked to Oxidative Stress than Subchondral Sclerosis
4.4. Synovial Fluid MMP Activity Reflects Adaptive Rather than Degenerative Joint Responses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bertoni, L.; Jacquet-Guibon, S.; Branly, T.; Legendre, F.; Desancé, M.; Mespoulhes, C.; Melin, M.; Hartmann, D.J.; Schmutz, A.; Denoix, J.M.; et al. An experimentally induced osteoarthritis model in horses performed on both metacarpophalangeal and metatarsophalangeal joints: Technical, clinical, imaging, biochemical, macroscopic and microscopic characterization. PLoS ONE 2020, 15, 235–251. [Google Scholar] [CrossRef] [PubMed]
- Stewart, H.L.; Kawcak, C.E. The importance of subchondral bone in the pathophysiology of osteoarthritis. Front. Vet. Sci. 2018, 5, 178. [Google Scholar] [CrossRef] [PubMed]
- McCoy, A.M.; Scolman, K.N. Impact of periarticular osteophytes of the distal tarsus diagnosed in nonlame yearling Standardbred horses on racing performance. Vet. Surg. 2023, 52, 1050–1056. [Google Scholar] [CrossRef]
- van der Kraan, P.M.; van den Berg, W.B. Osteophytes: Relevance and biology. Osteoarthr. Cartil. 2007, 15, 237–244. [Google Scholar] [CrossRef]
- Baccarin, R.Y.A.; Seidel, S.R.T.; Michelacci, Y.M.; Tokawa, P.K.A.; Oliveira, T.M. Osteoarthritis: A common disease that should be avoided in the athletic horse’s life. Anim. Front. 2022, 12, 25–36. [Google Scholar] [CrossRef]
- Villasante, A.; Araneda, O.F.; Behn, C.; Galleguillos, M.; Adarmes, H. Antioxidant capacity and oxidative damage determination in synovial fluid of chronically damaged equine metacarpophalangeal joint. Vet. Res. Commun. 2010, 34, 133–141. [Google Scholar] [CrossRef]
- Tsuzuki, N.; Kanbayashi, Y.; Kusano, K. Markers for oxidative stress in the synovial fluid of Thoroughbred horses with a carpal bone fracture. J. Equine Sci. 2019, 30, 13–16. [Google Scholar] [CrossRef]
- Lepetsos, P.; Papavassiliou, A.G. ROS/oxidative stress signaling in osteoarthritis. Biochim. Biophys. Acta Mol. Basis Dis. 2016, 1862, 576–591. [Google Scholar] [CrossRef]
- Liang, H.P.H.; Xu, J.; Xue, M.; Jackson, C.J. Matrix metalloproteinases in bone development and pathology. Met. Med. 2016, 3, 93–102. [Google Scholar] [CrossRef]
- Zrim, P.; Ek, V.; Mrkun, J.; Kosec, M. Diagnostic value of MMP-2 and MMP-9 in equine DIP joint OA. Acta Vet. Brno. 2007, 76, 87–95. [Google Scholar]
- Fietz, S.; Einspanier, R.; Hoppner, S.; Hertsch, B.; Bondzio, A. Determination of MMP-2 and -9 activities in synovial fluid of horses with osteoarthritic and arthritic joint diseases using gelatin zymography and immunocapture activity assays. Equine Vet. J. 2008, 40, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Buckwalter, J.A.; Felson, D.T. Post-traumatic arthritis: Definitions and burden of disease. In Post-Traumatic Arthritis: Pathogenesis, Diagnosis and Management; Springer: Berlin/Heidelberg, Germany, 2015; pp. 7–15. [Google Scholar]
- Singh, A.; Pal, Y.; Kumar, R.; Kumar, S.; Rani, K.; Prasad, J. Working equids: Their conditions, invisible earning and challenges—A review. Asian J. Agric. Ext. Econ. Sociol. 2021, 39, 357–364. [Google Scholar] [CrossRef]
- Trailović, R.; Ivanov, S.; Dimitrijević, V.; Trailović, D. Eksterijerne Karakteristike i Zdravstveno Stanje Domaćeg Magarca u Parku Prirode Stara Planina. Drugi Međunarodni Sajam Konjarstva “Horseville”. 2011, pp. 180–186. Available online: https://www.cepib.org.rs/wp-content/uploads/2011/10/horsvil1.pdf (accessed on 21 November 2025).
- Stashak, T.S. Examination for lameness. In Adams’ Lameness in Horses; E-Publishing Inc.: Philadelphia, PA, USA, 2002; pp. 113–183. [Google Scholar]
- Olive, J.; D’Anjou, M.-A.; Girard, C.; Laverty, S.; Theoret, C. Fat-suppressed spoiled gradient-recalled imaging of equine metacarpophalangeal articular cartilage. Vet. Radiol. Ultrasound. 2010, 51, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Olive, J.; D’Anjou, M.A.; Alexander, K.; Laverty, S.; Theoret, C. Comparison of MRI, CT, and radiography for noncartilaginous changes in equine MCP OA. Vet. Radiol. Ultrasound. 2010, 51, 267–279. [Google Scholar] [CrossRef]
- Steel, C.M. Equine synovial fluid analysis. Vet. Clin. N. Am. Equine Pract. 2008, 24, 437–454. [Google Scholar] [CrossRef]
- Erel, O. A new automated colorimetric method for measuring total oxidant status. Clin. Biochem. 2005, 38, 1103–1111. [Google Scholar] [CrossRef]
- Erel, O. A novel automated direct measurement method for total antioxidant capacity using a new generation, more stable ABTS radical cation. Clin. Biochem. 2004, 37, 277–285. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar] [CrossRef]
- Dantoine, T.F.; Debord, J.; Charmes, J.P.; Merle, L.; Marquet, P.; Lachatre, G.; Leroux-Robert, C. Decrease of serum paraoxonase activity in chronic renal failure. J. Am. Soc. Nephrol. 1998, 9, 2082–2088. [Google Scholar] [CrossRef]
- Hussein, H.A.; Bäumer, J.; Staufenbiel, R. Validation of an automated assay for measurement of bovine plasma ceruloplasmin. Acta Vet. Scand. 2019, 61, 34. [Google Scholar] [CrossRef]
- Kovačić, M.; Marković, D.; Maslovarić, I.; Obrenović, S.; Grujić-Milanović, J.; Arsić, A.; Milanović, Z.; Savić, O.; Fratrić, N.; Ilić, V. Serum proteins and lipids in mild form of calf bronchopneumonia: Candidates for reliable biomarkers. Acta Vet.-Beogr. 2017, 67, 201–221. [Google Scholar] [CrossRef]
- Murray, R.C.; Dyson, S.J.; Tranquille, C.; Adams, V. Association of type of sport and performance level with anatomical site of orthopaedic injury diagnosis. Equine Vet. J. 2006, 38, 411–416. [Google Scholar] [CrossRef]
- McIlwraith, C.W.; Frisbie, D.D.; Kawcak, C.E. The horse as a model of naturally occurring osteoarthritis. Bone Joint J. 2012, 1, 297–309. [Google Scholar] [CrossRef]
- Kawcak, C.E.; McIlwraith, C.W.; Norrdin, R.W.; Park, R.D.; James, S.P. The role of subchondral bone in joint disease: A review. Equine Vet. J. 2001, 33, 120–126. [Google Scholar] [CrossRef] [PubMed]
- Smith, S. Becoming horse in the duration of the moment: The trainer’s challenge. Phenom Pract. 2011, 5, 7–26. [Google Scholar] [CrossRef]
- Frisbie, D.D.; McIlwraith, C.W. Evaluation of gene therapy as a treatment for equine traumatic arthritis and osteoarthritis. Clin. Orthop. Relat. Res. 2000, 379, S273–S287. [Google Scholar] [CrossRef]
- van Weeren, P.R.; de Grauw, J.C. Pain in osteoarthritis. Vet. Clin. N. Am. Equine Pract. 2010, 26, 619–642. [Google Scholar] [CrossRef] [PubMed]
- Marković, L.; Radaković, M.; Radovanović, A.; Francuski Andrić, J.; Đoković, S.; Milošević, I.; Kovačević Filipović, M. Association of cartilage, synovial fluid and membrane pathological findings in Serbian mountain horses without signs of lameness. Acta Vet. Beogr. 2024, 74, 331–346. [Google Scholar] [CrossRef]
- Ziskoven, C.; Jäger, M.; Zilkens, C.; Bloch, W.; Brixius, K.; Krauspe, R. Oxidative stress in secondary osteoarthritis: From cartilage destruction to clinical presentation? Orthop. Rev. 2010, 2, e23. [Google Scholar] [CrossRef]
- Koike, M.; Nojiri, H.; Ozawa, Y.; Watanabe, K.; Muramatsu, Y.; Kaneko, H.; Morikawa, D.; Kobayashi, K.; Saita, Y.; Sasho, T.; et al. Mechanical overloading causes mitochondrial superoxide and SOD2 imbalance in chondrocytes resulting in cartilage degeneration. Sci. Rep. 2015, 5, 11722. [Google Scholar] [CrossRef]
- Awan, U.N.; Noor, S.S.; Siddiqui, I.A.; Nangrejo, R. Correlation of radiographic and histopathological changes with IL-17 and advanced oxidation protein products in knee osteoarthritic individuals with metabolic syndrome. Innov. Surg. Sci. 2025. [Google Scholar] [CrossRef]
- Xia, G.; Wen, Z.; Zhang, L.; Huang, J.; Wang, X.; Liang, C.; Cui, X.; Cao, X.; Wu, S. β-Hydroxybutyrate alleviates cartilage senescence through hnRNP A1-mediated up-regulation of PTEN. Exp. Gerontol. 2023, 175, 112140. [Google Scholar] [CrossRef]
- Nesterova, V.V.; Babenkova, P.I.; Brezgunova, A.A.; Samoylova, N.A.; Sadovnikova, I.S.; Semenovich, D.S.; Andrianova, N.V.; Gureev, A.P.; Plotnikov, E.Y. Differences in the effect of β-hydroxybutyrate on mitochondrial biogenesis, oxidative stress and inflammation markers in tissues from young and old rats. Biochemistry 2024, 89, 1336–1348. [Google Scholar] [CrossRef] [PubMed]
- Mora, R.; Binanti, D.; Mora, N.; Fantinato, E.; Ferrante, V.; Pedrotti, L.; Riccaboni, P. Pathological findings and IHC evaluation of MMP-2 and TIMPs in equine fetlock DJD. Am. J. Clin. Exp. Med. 2015, 3, 172–177. [Google Scholar] [CrossRef]
- Lerchbacher, C.F.; Ribitsch, I.; Kofler, J.; Fuchs-Baumgartinger, A.; Geyer, H.; Gehwolf, R.; Brehm, W.; Weinberger, T. Evaluation of MMP-2 and -9 activity in SF of horses with OA. Vet. Comp. Orthop. Traumatol. 2018, 31, 354–362. [Google Scholar]
- Rai, M.F.; Sandell, L.J.; Cheverud, J.M.; Brophy, R.H. Relationship of age and BMI to expression of obesity/OA-related genes in human meniscus. Int. J. Obes. 2013, 37, 1238–1246. [Google Scholar] [CrossRef]
| B | SE | Wald | OR | 95% CI | p Value | |
|---|---|---|---|---|---|---|
| Age | ||||||
| >9 years | - | - | - | 1.00 | - | - |
| ≤9 years | 0.76 | 0.34 | 5.05 | 2.13 | 1.10–4.14 | 0.02 |
| Localization | ||||||
| Condyle | - | - | - | 1.00 | - | - |
| Ridge | −2.14 | 0.40 | 28.27 | 0.12 | 0.05–0.26 | 0.0001 |
| Localization | ||||||
| Dorsal | - | - | - | 1.00 | - | - |
| Palmar/plantar | 1.04 | 0.34 | 9.29 | 2.82 | 1.45–5.50 | 0.002 |
| Parameter | Age | p Value | Subch. Sclerosis Grade | p Value | SEM | Interaction | ||
|---|---|---|---|---|---|---|---|---|
| ≤9 Years | >9 Years | ≤2 | >2 | |||||
| n (number of joints) | 15 | 16 | 16 | 15 | ||||
| Viscosity (cm) | 3.81 | 2.15 | 0.02 | 2.48 | 3.61 | 0.13 | 0.46 | 0.94 |
| TNCC (cells/µL) | 1070.67 | 1338.18 | 0.27 | 1001.67 | 1396.92 | 0.10 | 158.34 | 0.31 |
| Total proteins (g/L) | 10.01 | 9.11 | 0.42 | 10.82 | 8.18 | 0.02 | 0.74 | 0.51 |
| CER (mg/dL) | 3.70 | 4.43 | 0.33 | 3.81 | 4.38 | 0.46 | 0.52 | 0.16 |
| CHE (U/L) | 562.20 | 540.37 | 0.81 | 575.25 | 526.93 | 0.61 | 65.17 | 0.35 |
| BHB (mmol/L) | 0.25 | 0.15 | 0.002 | 0.22 | 0.18 | 0.20 | 0.02 | 0.01 |
| AOPP (µmol/g) | 50.61 | 42.94 | 0.10 | 42.20 | 51.56 | 0.05 | 3.23 | 0.76 |
| -SH (mmol/L) | 0.14 | 0.15 | 0.41 | 0.14 | 0.14 | 0.82 | 0.01 | 0.18 |
| PON-1 (U/mL) | 2.24 | 2.36 | 0.34 | 2.34 | 2.24 | 0.43 | 0.08 | 0.07 |
| UA (µmol/L) | 60.74 | 91.52 | 0.04 | 65.99 | 88.89 | 0.18 | 10.53 | 0.62 |
| TAC (mmol Trolox Equiv/L) | 0.28 | 0.29 | 0.79 | 0.29 | 0.27 | 0.08 | 0.01 | 0.71 |
| TOS (µM H2O2 Equiv/L) | 119.27 | 121.65 | 0.94 | 133.25 | 110.18 | 0.48 | 20.39 | 0.40 |
| OSI (AU) | 0.39 | 0.43 | 0.71 | 0.49 | 0.33 | 0.15 | 0.06 | 0.48 |
| Caseinase (AU) | 19.83 | 19.71 | 0.94 | 19.39 | 20.20 | 0.84 | 2.90 | 0.07 |
| Gelatinase (AU) | 4.15 (0.14–15.45) | 3.16 (0.32–29.16) | 0.89 | 4.35 (0.33–29.16) | 1.48 (0.14–20.17) | 0.18 | - | - |
| B | SE | Wald | OR | 95% CI | p-Value | |
|---|---|---|---|---|---|---|
| Localization | ||||||
| Dorso-palmar | - | - | - | 1.00 | - | - |
| Palmaromedial | 2.76 | 0.59 | 21.75 | 15.78 | 4.95–50.33 | 0.001 |
| Lateromedial | 3.32 | 0.61 | 29.59 | 27.87 | 8.41–92.41 | 0.001 |
| Parameter | Live Weight | p Value | Osteophytosis Grades | p Value | SEM | Interaction | ||
|---|---|---|---|---|---|---|---|---|
| ≤500 kg | >500 kg | <3 | 3 | |||||
| n (number of joints) | 15 | 16 | 20 | 11 | ||||
| Viscosity (cm) | 3.09 | 2.87 | 0.76 | 2.95 | 3.17 | 0.77 | 0.57 | 0.31 |
| TNCC (cells/µL) | 923.64 | 1347.69 | 0.09 | 975.71 | 1491.11 | 0.05 | 148.99 | 0.59 |
| Total proteins (g/L) | 10.88 | 8.19 | 0.01 | 9.65 | 9.45 | 0.87 | 0.74 | 0.20 |
| CER (mg/dL) | 3.88 | 4.27 | 0.61 | 3.63 | 5.11 | 0.07 | 0.57 | 0.55 |
| CHE (U/L) | 630.56 | 466.00 | 0.06 | 568.35 | 521.40 | 0.64 | 65.41 | 0.17 |
| BHB (mmol/L) | 0.23 | 0.16 | 0.03 | 0.23 | 0.15 | 0.04 | 0.02 | 0.03 |
| AOPP (µmol/g) | 47.09 | 47.02 | 0.99 | 46.88 | 46.41 | 0.93 | 3.51 | 0.65 |
| -SH (mmol/L) | 0.17 | 0.15 | 0.36 | 0.17 | 0.13 | 0.04 | 0.01 | 0.51 |
| PON-1 (U/mL) | 2.35 | 2.18 | 0.16 | 2.24 | 2.31 | 0.61 | 0.07 | 0.46 |
| UA (µmol/L) | 78.28 | 77.37 | 0.96 | 82.44 | 69.45 | 0.47 | 11.27 | 0.53 |
| TAC (mmol Trolox Equiv/L) | 0.28 | 0.28 | 0.62 | 0.28 | 0.27 | 0.56 | 0.01 | 0.47 |
| TOS (µM H2O2 Equiv/L) | 108.91 | 91.46 | 0.47 | 79.53 | 140.94 | 0.01 | 12.03 | 0.34 |
| OSI (AU) | 0.41 | 0.32 | 0.32 | 0.30 | 0.51 | 0.02 | 0.04 | 0.37 |
| Caseinase (AU) | 22.41 | 12.50 | 0.01 | 20.59 | 18.83 | 0.66 | 2.56 | 0.86 |
| Gelatinase (AU) | 11.16 (0.42–29.16) | 1.18 (0.14–6.49) | 0.02 | 3.62 (0.14–20.17) | 2.11 (0.35–29.16) | 0.93 | - | - |
| Parameters | Coefficient | p-Value |
|---|---|---|
| Live weight vs. age | 0.59 | 0.001 |
| Live weight vs. CHE | −0.48 | 0.006 |
| Live weight vs. BHB | −0.37 | 0.04 |
| Live weight vs. gelatinase | −0.64 | 0.001 |
| Live weight vs. total proteins | −0.60 | 0.001 |
| Live weight vs. osteophytosis grade | 0.45 | 0.01 |
| Age vs. BHB | −0.44 | 0.01 |
| Subchondral sclerosis grade vs. TNCC | 0.55 | 0.005 |
| Subchondral sclerosis vs. total proteins | −0.44 | 0.01 |
| Subchondral sclerosis presence vs. BHB | −0.72 | 0.0001 |
| Osteophytosis grade vs. BHB | −0.61 | 0.001 |
| Osteophytosis grade vs. CER | 0.42 | 0.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marković, L.; Vićić, I.; Lazarević Macanović, M.; Francuski Andrić, J.; Kovačević Filipović, M.; Radaković, M. Degenerative Changes in MCP/MTP Joints of Working Horses Without Lameness: Integrating CT-Based Assessment and Synovial Fluid Biomarkers. Animals 2025, 15, 3392. https://doi.org/10.3390/ani15233392
Marković L, Vićić I, Lazarević Macanović M, Francuski Andrić J, Kovačević Filipović M, Radaković M. Degenerative Changes in MCP/MTP Joints of Working Horses Without Lameness: Integrating CT-Based Assessment and Synovial Fluid Biomarkers. Animals. 2025; 15(23):3392. https://doi.org/10.3390/ani15233392
Chicago/Turabian StyleMarković, Lazar, Ivan Vićić, Mirjana Lazarević Macanović, Jelena Francuski Andrić, Milica Kovačević Filipović, and Milena Radaković. 2025. "Degenerative Changes in MCP/MTP Joints of Working Horses Without Lameness: Integrating CT-Based Assessment and Synovial Fluid Biomarkers" Animals 15, no. 23: 3392. https://doi.org/10.3390/ani15233392
APA StyleMarković, L., Vićić, I., Lazarević Macanović, M., Francuski Andrić, J., Kovačević Filipović, M., & Radaković, M. (2025). Degenerative Changes in MCP/MTP Joints of Working Horses Without Lameness: Integrating CT-Based Assessment and Synovial Fluid Biomarkers. Animals, 15(23), 3392. https://doi.org/10.3390/ani15233392






