1. Introduction
Avian influenza viruses (AIVs), belonging to the genus Alphainfluenzavirus (Influenza A virus) of the Orthomyxoviridae family, exhibit significant host diversity, infecting wild
aquatic birds,
humans,
pigs,
dogs, and
horses [
1]. Among AIVs,
H3-subtype influenza viruses represent a globally prevalent and evolutionarily dynamic group, phylogenetically divided into three main lineages based on host specialization: avian
H3Nx (encompassing multiple neuraminidase (
NA) subtypes such as
H3N2,
H3N8, and
H3N6), seasonal human
H3N2, and equine
H3N8 viruses [
2]. Notably,
H3-subtype viruses display substantial virulence variation across host species: most avian
H3Nx viruses are low pathogenic avian influenza viruses (LPAIVs) in wild
waterfowl (e.g.,
mallards) and poultry, but some can evolve into highly pathogenic avian influenza viruses (HPAIVs) via adaptive mutations or gene reassortment; seasonal human
H3N2 viruses typically cause mild to moderate respiratory illness in
humans, while equine
H3N8 viruses induce severe respiratory disease in
horses [
2,
3]. Key determinants of host specificity and pathogenicity for
H3-subtype viruses primarily involve the hemagglutinin (
HA) protein—especially amino acid mutations in its receptor-binding domain (RBD), such as Q226L and G228S, which shift binding affinity between SAα-2,3-Gal and SAα-2,6-Gal [
2,
4]. Influenza A viruses are classified into 19
HA and 11
NA subtypes based on the antigenic diversity of
HA and
NA, from which only two subtypes have non-avian hosts (
H17N10 and
H19N11,
bats) [
5,
6]. The spread of infectious diseases driven by global human interconnectedness has led to multiple pandemics over the previous century and past decade, with avian influenza being a case in point [
7]. In 1968, the pandemic was caused by a new reassortment of the influenza A virus
H3N2 carrying two segments (
HA and
PB1) from
H3 avian viruses [
8]. Descendants of that pandemic continue to circulate among
humans as a seasonal flu viruses to date. Recently,
H3N8 subtype avian influenza viruses have breached the species barrier, causing human infections and fatalities. The Chinese CDC reported three confirmed cases of human
H3N8 virus infection with severe respiratory symptoms in 2022–2023, all linked to live poultry exposure. The
H3 subtype AIVs are constantly evolving, exhibiting frequent reassortment and cross-species transmission [
9]. For example, first discovered in 1963,
H3N8 equine influenza viruses have recently impacted Asia and the Middle East [
10]. Surveillance data indicate increasing
H3N8 avian virus isolations annually, with
H3N8 avian viruses prevalent throughout China and becoming one of the most frequently isolated AIV subtypes [
8,
11].
The genome of all influenza A viruses, including
H3N8, comprises 8 single-stranded, negative-sense RNA segments, totaling approximately 13.6 kb, encoding at least 10 functional proteins: the RNA polymerase complex (
PB2,
PB1,
PA), surface glycoproteins
HA and
NA, nucleocapsid protein (
NP), matrix proteins (
M1,
M2), and non-structural proteins (
NS1,
NS2) [
12]. Alterations in glycosylation sites of the
HA protein, particularly those proximal to the receptor-binding domain (RBD), can significantly impact its binding affinity and specificity to host cell surface sialylated glycoprotein receptors by inducing steric hindrance or conformational changes, thereby modulating viral replication and host adaptation [
13,
14]. The frequent genome reassortment and antigenic drift of
HA and
NA drive continuous zoonotic outbreaks. Epidemiological studies on the
H3N8 virus indicate that its sporadic human infections exhibit certain observable epidemiological features, though these are based on a limited number of documented cases [
15], and while the
HA protein originates from AIVs, its affinity for SAα-2,6-Gal is significantly increased. This evolutionary adaptation suggests that changes in viral receptor-binding properties are a key driver in the emergence of influenza pandemics [
16,
17].
2. Materials and Methods
2.1. Ethics Statement
This study was approved by the Ethics Committee of the School of Animal Science and Technology, Foshan University (protocol code 20221818). All chickens were humanely handled in accordance with the protocols and principles of animal ethics. The veterinarians obtained written consent from the owners to collect samples.
2.2. Serological Survey of Poultry for the H3N8 Virus
A total of 2040
chicken and 677
duck serum samples were obtained from animal disease prevention and control centers across various prefecture-level cities in Guangdong Province (
Table 1). Serum samples were collected in October 2022. Notably, these birds did not exhibit any clinical symptoms of influenza infection at the time of sampling. Serum samples were treated with Receptor Destroying Enzyme (RDE) at a 1:3 ratio and incubated at 37 °C for 18–24 h to remove non-specific inhibitors. RDE was subsequently inactivated by incubation in a 56 °C water bath for 30 min. Following cooling to room temperature, samples were diluted in phosphate-buffered saline (PBS) and serially two-fold diluted in 96-well plates. Hemagglutination inhibition (HI) assays were performed by adding 0.5%
chicken red blood cells and 8 hemagglutinating units of antigen to each well, and the strain
A/chicken/Qingyuan/22/2022 (H3N8) was used as antigen. After a 30 min incubation at 37 °C, HI titers were determined by visual inspection [
18].
2.3. Case Sample Collection
From March to November 2022, pharyngeal swab samples were collected from diseased chicken flocks on a farm in Qingyuan City, Guangdong Province, and stored at the Veterinary Preventive Medicine Laboratory of Foshan University. Upon arrival at the laboratory, samples were mixed with phosphate-buffered saline (PBS, pH 7.4) containing 1% penicillin-streptomycin, subjected to three freeze–thaw cycles, and centrifuged at 1200 rpm for 5 min. Following sterile filtration, the supernatant was collected and stored for subsequent analysis.
2.4. Pathogen Isolation and Plaque Purification
Embryonated chicken eggs and MDCK cells were used to isolate viruses. 0-day-old specific-pathogen-free (SPF) chicken embryos, purchased from Xinxing Dahuainong Poultry and Egg Co., Ltd., were placed in an incubator at 37 °C, and their status was observed regularly daily. 10-day-old chicken embryos were selected for allantoic cavity inoculation with the sterile-filtered supernatant described in 2.3, with an inoculation dose of 200 μL per embryo. After inoculation, the inoculation sites were sealed, and the embryos were labeled with time and serial numbers before being placed in the incubator for continuous culture for 72 h. The condition of the chicken embryos was observed every 8 h. The allantoic fluid of embryos that died between 24 and 72 h was collected and labeled as the F1 generation. Hemagglutination assays (HAA) were performed on the collected allantoic fluid; those with hemagglutination activity were passaged continuously to the F3 generation. For each passage, the pathological changes and mortality of the chicken embryos were observed, and the allantoic fluid was stored in a −80 °C refrigerator for later use.
Madin-Darby Canine Kidney (MDCK) cells were cultured until they formed a dense monolayer. The culture medium was then removed, and the cells were washed twice with PBS. The treated samples were diluted in minimal essential medium (MEM), and 1 mL of the diluted sample was inoculated into a T25 culture flask (catalog number: 13112A, manufactured by Beijing Labselect Technology Co., Ltd., Beijing, China). After a 1 h incubation at 37 °C, 4 mL of MEM supplemented with 1 μg/mL TPCK-treated trypsin was added for further culture. Virus culture was harvested when the cytopathic effect (CPE) reached ≥75%, subjected to three freeze–thaw cycles, and passaged continuously until a stable CPE was observed. The resulting viral strain was then isolated and subjected to virus identification.
For plaque purification, the virus solution was serially diluted and inoculated onto MDCK cells. Following incubation, cells were overlaid with a low melting point agarose mixture (concentration: 2%; Cat. No. A8350; Manufacturer: Solarbio, Beijing, China). After 3–5 days of culture, individual plaques were selected, used to inoculate fresh MDCK cells, and the virus was amplified. This plaque purification process was repeated three times to obtain a purified viral strain. Viral nucleic acid was then extracted using a kit from Bioer Technology Co., Ltd., of Hangzhou, China, and the virus was identified.
To determine
HAA titer, the purified virus fluid was serially diluted in 96-well V-bottom plates, and 0.5%
chicken red blood cells were added. After a 30 min incubation at 37 °C, the HAA titer was determined by visual inspection, based on the highest dilution at which complete agglutination was observed [
19].
2.5. Receptor-Binding Assays
Streptavidin-coated 96-well plates were used to bind varying concentrations of glycopolymers SAα-2,3-Gal and SAα-2,6-Gal (3′SLN-C3-BP, 6′SLN-C3-PAA-biot, GlycoNZ, Auckland, New Zealand), with three technical replicates per concentration and one negative control group. Plates were blocked with 5% bovine serum albumin (BSA) in PBS. Viral samples were diluted to a concentration of 16 hemagglutinating units (HAU) per 50 μL and added to the wells as antigens. Following incubation, viral nucleic acids were extracted from each well.
A SYBR Green-based Real-Time Quantitative PCR (RT-qPCR) assay was established for the detection of the
H3N8 virus using TB Green
® Premix Ex Taq™ II (Tli RNaseH Plus) from Takara Bio Inc. (Catalog No.: RR820A; location: Kusatsu, Shiga, Japan) on the qTOWER3 G instrument manufactured by Analytik Jena. The standard curve equation was Y = −3.72lgX + 42.52 (R
2 = 0.997, E = 79.61%). The PCR conditions were initial denaturation at 94 °C for 3 min, followed by 40 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 20 s (primer sequences are listed in
Table 2).
2.6. Complete Genome Sequencing
H3N8 virus gene sequences published in GenBank were aligned and analyzed using MEGA 5.0 software. Premier 6.0 software was then used to design primers for whole-genome amplification. All primers were synthesized by Sangon Biotech (Shanghai) Co., Ltd. (primer sequences listed in
Table 3). RNA was extracted using the RNAfast200 Total RNA Rapid Extraction Kit (Cat# 220011; Shanghai Feijie Biotechnology Co. Ltd., Shanghai, China). Then reverse transcription combined with PCR in one tube was performed using the HiScript II One Step RT-PCR Kit (Dye Plus) (Cat# P612-01; Vazyme Biotech Co., Ltd., Nanjing, China) with specific primers (see
Table 3) in a thermocycler (qTOWER3 G, Analytik Jena, Jena, Thuringia, Germany) according to the following program: reverse transcription at 50 °C for 30 min, initial denaturation at 94 °C for 5 min, followed by 30 cycles of denaturation at 94 °C for 30 s, annealing at 55 °C for 30 s, and extension at 72 °C for 1 min/kb. The amplified products were purified via agarose gel electrophoresis and fragments of the expected size were sequenced by Sanger method using (BigDye™ Direct, Thermo Fisher) kit and sequence analyzer (Applied BiosystemsTM 3730XL, Thermo Fisher) (Sangon Biotech, Shanghai, China) [
20]. The complete genome sequences of the three isolated
H3N8 strains have been deposited in the GenBank database. The GenBank accession numbers for QY15, QY21, and QY31 are PX454529-PX454536, PX454537-PX454544, and PX455047-PX455054, respectively.
2.7. Alignment and Phylogenetic Analysis
To elucidate the genetic and evolutionary relationships of the isolated strain, sequencing results were compared to published
H3N8 whole-genome sequences in GenBank using the Basic Local Alignment Search Tool for Nucleotides (BLASTn) algorithm at the National Center for Biotechnology Information (NCBI, available at
https://www.ncbi.nlm.nih.gov/). Recombination analysis was performed by screening for sequences exhibiting the highest homology to each gene segment of the isolated strain. Phylogenetic trees for each gene segment were then constructed using the Maximum Likelihood method implemented in MEGA 5.0 software. The reliability of the phylogenetic trees was assessed using Bootstrap analysis with 1000 replicates.
2.8. Cleavage Site Analysis and Prediction of N-Glycosylation Sites
Potential N-glycosylation sites on the HA and NA proteins of the isolated strains were predicted using the NetNGlyc 1.0 server. The amino acid sequence at the HA protein cleavage site was also analyzed to predict the cleavage motif.
2.9. Pathological Experiments
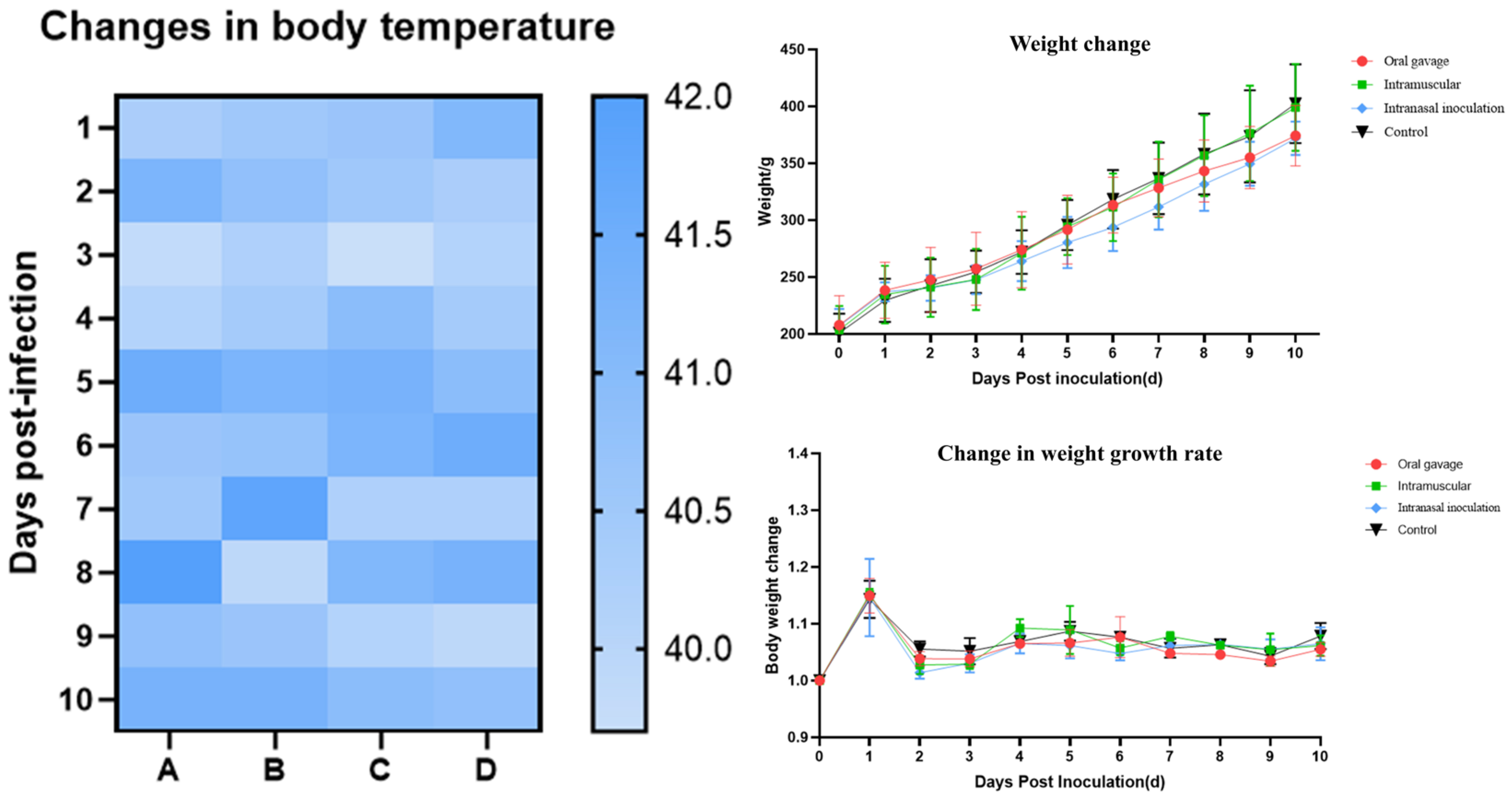
To assess viral pathogenicity, 21-day-old
specific-pathogen-free (
SPF)
chickens were randomly assigned to one of four groups.
chickens in the treatment groups were inoculated with 100 µL of the QY22 strain (6.00 lg 50% Egg Infectious Dose (EID50)/mL) per bird via intranasal, oral gavage, and intramuscular routes, respectively. The control group received an equal volume of sterile PBS via the same routes. Clinical signs and weight changes were monitored daily in all groups for 10 consecutive days post-inoculation (dpi). Pharyngeal swabs were collected from all
chickens at 1, 3, 5, 7, and 9 dpi to quantify viral shedding. At 3, 5, and 7 dpi, four
chickens from each group were randomly selected and euthanized in accordance with approved animal care protocols. Euthanasia was performed via intravenous injection of sodium pentobarbital solution (dosage: 100–150 mg/kg body weight; concentration: 60 mg/mL). After injection, the
chickens’ responses were observed: loss of consciousness and respiratory arrest occurred within 30–60 s. At 1–2 min post-injection, cardiac arrest was confirmed by palpation of the heart, and euthanasia was deemed successful. Necropsies were performed, and gross lesions were recorded. Samples of brain, nasal turbinate, trachea, lung, kidney, and bursa of Fabricius were collected for histopathology and viral load determination [
21,
22].
2.10. Histological Examination
Collected tissues and organs were fixed in 4% paraformaldehyde in PBS for 24 h at room temperature, followed by dehydration in a graded series of ethanol, clearing in xylene, and embedding in paraffin. Sections were cut at a thickness of 5 μm using a microtome. The sections were then stained with hematoxylin and eosin (H&E) and examined by light microscopy to assess pathological changes.
2.11. Viral Genome Quantity and Tissue Viral Load Detection
Nucleic acids were extracted from the collected pharyngeal/anal combined swabs and tissue/organ samples following three freeze-thaw cycles. The
H3N8 virus SYBR Green-based RT-qPCR assay, as described in
Section 2.5 above, was used to quantify viral contents in the samples. Viral contents in pharyngeal/anal combined swabs and each organ were calculated based on the Quantification Cycle (Cq) values. These data were used to analyze Viral Genome Detection Dynamics and viral distribution characteristics in various organs at different time points post-inoculation.
4. Discussion
As a major
waterfowl-breeding country, China accounts for three-quarters of the global breeding volume. Domestic
ducks are predominantly raised in southern regions through high-density and free-range farming [
23,
24]. The
H3Nx virus has the highest isolation rate in
ducks, reaching 91.76% [
25]. Previous serological surveys also highlighted the prevalence of
H3 subtype avian influenza viruses. From 2006 to 2007, Pu et al. [
26] reported an overall positive rate of 2.83% for
H3Nx in 173
chicken flocks across 18 provinces in China, with notably higher rates in the southeastern coastal provinces of Jiangsu and Guangdong. More recently, in 2018–2019, Liu et al. [
27] conducted testing on 2474 poultry samples from Jiangsu Province, revealing a 1.57% positive rate for the
H3Nx virus. Within these positive samples,
chickens exhibited a 12.47% positive rate, and
ducks showed 19.15%.
Building upon these historical trends, our current study, conducted in 2022, investigated the seroprevalence of H3N8 virus antibodies in chicken and duck sera from various cities in Guangdong Province. Among 2040 chicken serum samples collected, the overall positive rate was 10.85%. For the 677 duck serum samples, the overall positive rate stood at 7.97%. The seroprevalence of H3N8 in chickens remains comparatively high, with cities exceeding a 10% positive rate concentrated in northeastern Guangdong Province (e.g., Shaoguan, Meizhou, and Guangzhou). These findings collectively suggest that H3N8 virus infection rates in both chickens and ducks in Guangdong Province remain elevated. This sustained high infection rate is particularly noteworthy given that ducks often serve as asymptomatic carriers or exhibit only mild symptoms upon infection, while chickens, being a primary commodity in live poultry markets, appear to demonstrate a greater susceptibility to the virus, leading to higher observed infection rates.
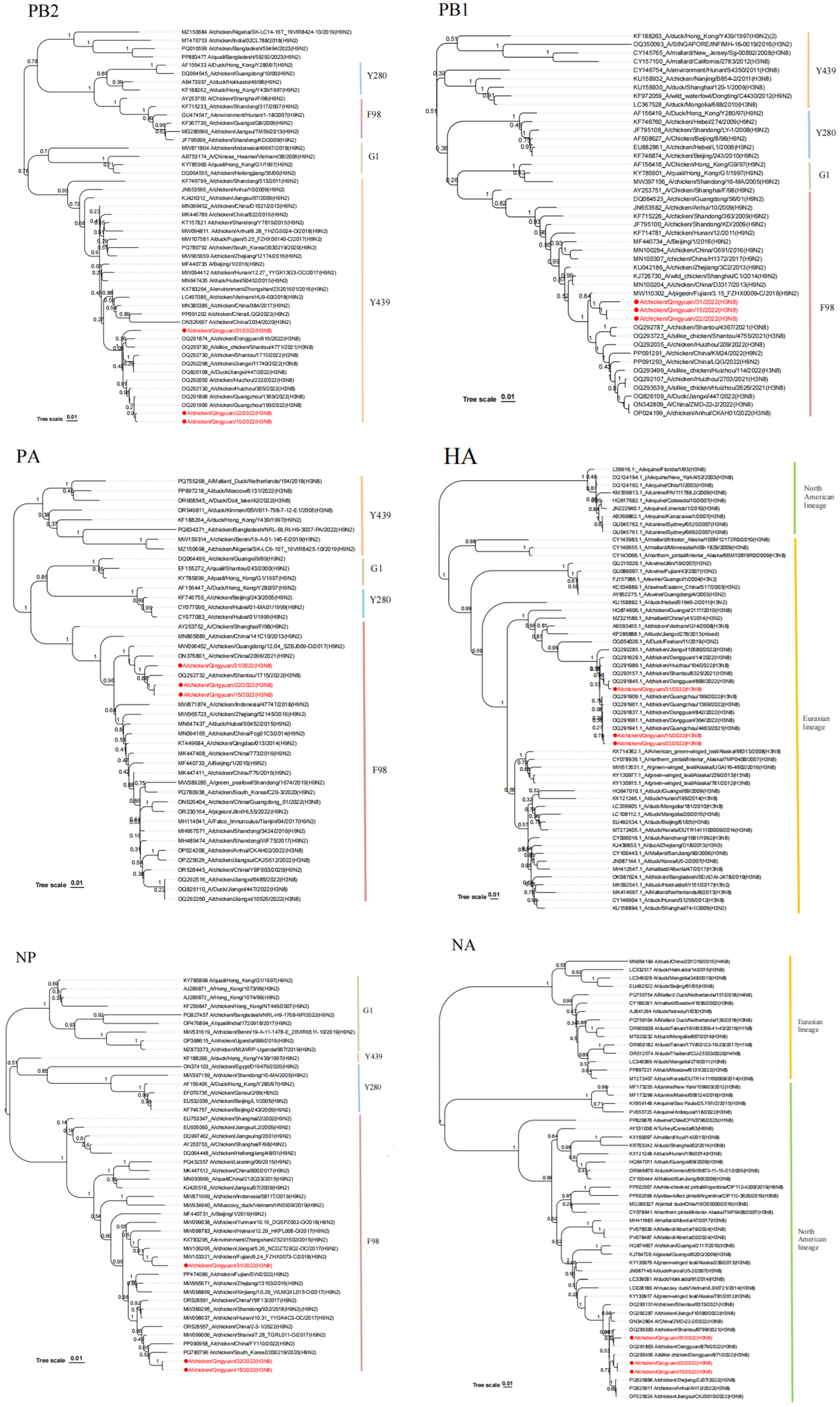
In this study, three
H3N8 viruses, designated QY15, QY22, and QY31, were isolated and investigated. The
HA gene of these isolates belonged to the Eurasian lineage, while the
NA gene was of North American origin. Furthermore, phylogenetic analysis of the internal genes revealed their classification into distinct lineages:
PB2 belonged to the Y439-like lineage,
M to the G1-like lineage, and
PB1,
PA,
NP, and
NS to the F98-like lineage [
3]. Notably, several internal genes, including
PB1, PA, and
NP, clustered with
H9N2 viruses that have been prevalent in Guangdong Province in recent years. Among these, the
PA genes of all three strains share a common ancestral origin with
chicken H9N2 viruses circulating in China in 2021 (with
A/chicken/China/2096/2021(H9N2) as a representative homologous strain). With respect to the
M and
NS genes of the three isolates, they share a common precursor with those of other
chicken-origin
H3N8 viruses prevalent in Guangdong Province in 2022. These two genes are also present in some
chicken-origin
H9N2 viruses and
duck-origin
H3N8 viruses—specifically the
M gene of
A/chicken/China/KM212/2022(H9N2), the
NS gene of
A/chicken/China/XD3/2022(H9N2), and both the
M and
NS genes of
A/duck/Jiangxi/447/2022(H3N8). The
NP genes of QY15 and QY22 exhibit identical nucleotide sequences and cluster with
chicken H9N2 viruses prevalent in South Korea and China during 2020–2022, and all three
H3N8 strains harbor
NP genes acquired from
H9N2 viruses. Additionally, the
PB1 genes of QY15, QY22, and QY31 share a common ancestor with that of the
pigeon-origin
H9N2 virus
A/pigeon/Fujian/3.15_FZHX0008-C/2018(H9N2), whose
PB1 gene is derived from early avian
H9N2 viruses. This suggests that the
H9N2 gene module may have long served as an “internal gene donor”, promoting the adaptability of recombinant viruses [
28,
29]. Notably, the three strains carry internal genes derived from
H9N2, and their genomes may have formed earlier through complex genetic reassortment events between
H3N8 viruses and
H9N2 viruses circulating among different poultry,
waterfowl, and wild or
migratory birds. This highlights the crucial role of the
waterfowl-land
fowl ecological interface in gene exchange, consistent with the viral diversity hotspot characteristics observed in the migratory bird habitats of the Pearl River Delta wetlands [
30]. Research has demonstrated that influenza A viruses (IAVs), including
H3N8 viruses, can persist in aquatic environments for extensively long periods under favorable conditions—for instance, Ramey et al. [
31] found that IAVs remained infectious for more than seven months in surface waters of northern wetlands in North America. These wetlands were identified as biologically important media for viral transmission and maintenance, even serving as environmental reservoirs for IAVs during the overwintering period of
migratory birds. This gene reassortment event significantly increases the likelihood of the virus acquiring new traits, thereby enhancing its transmission potential and pathogenicity [
25].
Analysis of the
HA receptor-binding site revealed that the QY15, QY22, and QY31 isolates collectively retain the typical avian-signature residues (Q226 and G228). This indicates that these
H3N8 isolates have not yet acquired the core genetic markers for efficient adaptation to the human host, which is a key factor limiting their direct and widespread transmission among
humans. Second, the E190 residue in the three isolates can synergize with Q226 and G228 to enhance the virus’s binding affinity for SAα-2,6-Gal [
4]. This common genetic feature suggests that despite retaining avian signatures, the
H3N8 isolates may have gained a certain capacity to bind to human-type receptors, laying a potential foundation for cross-species infection. Furthermore, the N193 residue of the isolates has the potential to form hydrogen bonds with α2-6-linked glycans, which may further strengthen the virus’s binding to human cells; the W222 and S227 residues of the isolates may alter the conformational flexibility of the 220-loop—a region critical for receptor recognition, indirectly adjusting the virus’s ability to interact with different types of sialic acid receptors [
2]. Collectively, the synergistic effects of the above amino acid residues endow the QY15, QY22, and QY31
H3N8 chicken isolates with dual receptor-binding properties. This dual-binding capability is a key intermediate phenotype in the process of influenza virus adaptation from avian to human hosts. QY15, QY22, and QY31 isolates exhibit a preference for binding to SAα-2,6-Gal but retain the ability to bind SAα-2,3-Gal. Consistent with the receptor-binding properties of the
chicken-origin
H3N8 viruses [
A/chicken/Fujian/F0101/2022(H3N8),
A/chicken/Henan/F0308/2022(H3N8),
A/chicken/Anhui/FE12/2022(H3N8),
A/chicken/Jiangsu/B314/2022(H3N8)] isolated in 2022 by Yang et al. [
2] and the
duck-origin
H3N8 viruses [
A/duck/Guangxi/446D28/2021(H3N8)] isolated in 2024 by Li et al. [
20]. These
H3N8 virus isolates exhibit the ability to bind to and potentially infect human receptors, posing a risk of crossing the species barrier to infect mammals and
humans.
SPF chickens were infected with the purified QY22 strain to assess its pathogenicity. The virus demonstrated efficient replication in the
chicken respiratory system, with lung tissue viral titers reaching 5.04 lg EID50/mL. This viral load was significantly higher than those previously observed for both the four
duck-origin
H3N8 viruses [
A/duck/Guangdong/S1286/2009(H3N8),
A/duck/Anhui/S4053/2010(H3N8),
A/duck/Jiangxi/S3855/2012(H3N8),
A/duck/Zhejiang/S4088/2013(H3N8)] isolated in 2016 by Cui et al. [
11] and the three
chicken-origin
H3N8 isolates, which were isolated in 2022 by Yang et al. [
2]. Furthermore, the infection resulted in organ damage beyond the respiratory tract, including the brain, kidneys, and bursa of Fabricius. These findings indicated that, in comparison to earlier
waterfowl-origin
H3N8 viruses, the novel
H3N8 viruses harboring the internal genes of
H9N2 exhibited elevated pathogenicity in
chickens. This suggests that the acquisition of
H9N2 internal genes is a key factor contributing to the increased infection and pathogenicity observed in
chickens.
5. Conclusions
This study systematically investigated the epidemiological characteristics, genetic evolution patterns, and virological properties of the avian influenza A H3N8 virus circulating in Guangdong Province, China, providing comprehensive evidence for an in-depth understanding of the virus’s epidemic dynamics and zoonotic risk. Serological surveillance of 2040 chicken serum samples and 677 duck serum samples from 21 prefecture-level cities in Guangdong Province in 2022 revealed an overall seroprevalence of 10.85% in chickens and 7.97% in ducks. Notably, the seroprevalence in chickens from northeastern Guangdong was significantly higher, indicating that the H3N8 virus persists and spreads widely in local avian populations, with chickens being more susceptible to the virus than ducks. Three H3N8 virus strains (QY15, QY22, QY31) were successfully isolated from diseased chicken flocks in Qingyuan City, and these viruses were confirmed to possess dual receptor-binding specificity—a key phenotypic adaptive trait that facilitates the virus’s potential cross-species transmission.
Genetic and phylogenetic analyses uncovered the complex evolutionary characteristics of the isolates: the HA gene clustered within the Eurasian lineage, while the NA gene belonged to the North American lineage. The internal genes were classified into the Y439-like lineage (PB2), G1-like lineage (M), and F98-like lineage (PB1, PA, NP, NS). Among them, internal genes such as PB1, PA, and NP clustered with H9N2 viruses prevalent in Guangdong Province in recent years. Additionally, the pigeon-origin H3N8 strain A/pigeon/Fujian/3.15_FZHX0008-C/2018(H9N2) shared a common ancestor with the PB1 gene of the isolates, and the duck-origin H3N8 virus A/duck/Jiangxi/447/2022(H3N8) shared a common precursor for the M and NS genes with the three isolates. These findings indicate that the three H3N8 isolates originated from genetic reassortment between H3N8 and H9N2 viruses circulating among poultry, waterfowl, and migratory bird-related populations, highlighting the role of the Pearl River Delta wetland ecosystem as a hotspot for viral gene exchange. Analysis of the amino acid sequence of the HA receptor-binding domain revealed the presence of both conserved avian-signature residues (Q226, G228) and adaptive mutation sites (E190, N193, W222, S227). These mutations, in synergy with conserved residues, enhance the virus’s binding affinity for human-type receptors while retaining its tropism for avian-type receptors, laying a molecular foundation for the virus’s zoonotic potential.
Pathogenicity assessment in the SPF chicken model demonstrated that the QY22 strain replicates efficiently in the respiratory tract and exhibits broad tissue tropism, with high viral loads detected in tissues such as the nasal turbinate, brain, trachea, lung, and bursa of Fabricius. chickens inoculated intranasally showed the most severe pathological lesions, including cerebral hemorrhage, tracheal hemorrhage, and alveolar edema, along with the highest viral genome load level, which peaked at 5 dpi post-inoculation (105.7 copies/μL). Compared with early waterfowl-origin H3N8 viruses, the acquisition of internal genes from H9N2 viruses is associated with increased pathogenicity of this strain in chickens, underscoring the impact of genetic reassortment on viral adaptability. Our study contributes to a better understanding of the evolutionary trajectory and zoonotic potential of the H3N8 low pathogenic avian influenza virus circulating in Guangdong and emphasizes the importance of strengthening surveillance of the H3N8 virus in poultry populations to mitigate the risk of cross-species transmission and prevent potential public health threats.