Development and Validation of a Standardised Genomic Tool for Conservation Management of the Koala (Phascolarctos cinereus)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Biological Sample Processing
2.2. Assay Design (SNP Library Preparation)
- (1)
- The Koala-fixed dataset consists of SNPs identified from three published koala datasets; (A) DArTseq [13], (B) Exon-capture [52] and (C) WGS-derived [53]. (A) The DArTseq marker set consisted of 4606 SNPs, filtered according to [13], with outliers, sex-linked, parentage and traceability markers identified, and remainder allocated putatively neutral markers [13]. The filtered dataset obtained was mapped to the reference genome using Burrow-Wheeler alignment (BWA) tool [66] and SNP positions were identified within tags with a custom bioawk script. (B) The 2167 exon-capture dataset, filtered according to [52] was obtained and mapped to the reference genome as described above. (C) The WGS-derived marker set was produced by extracting approximately 46,434,864 SNPs from the 387 genomes across the koala distribution, using a modified version of an existing pipeline previously described [67] that employs a consensus approach [68]. These SNPs were then filtered using [BCFtools] [69] with the following thresholds: removing sites with Minor Allele Frequency (MAF) < 0.05 and pruning for Linkage Disequilibrium (LD) by retaining only one SNP per 10 Mb (i.e., one per contig), then retaining only the 1000 SNPs with the highest MAF. In total 5172 SNPs across the three marker sets were kept for downstream analyses.
- (2)
- The Koala-discovery dataset was developed by identifying candidate koala fitness-related genes and annotations from the NCBI [65]. Fitness targets included genes linked to immunity and disease (e.g., Chlamydia, viral infections, tumour development (neoplasia)), stress response, metabolism and temperature regulation—reflecting the koala’s broad climatic range. Diet-related genes including the koala-expanded (Cytochrome P450) family (involved in detoxifying diverse, toxic eucalypt compounds), and reproduction-related genes particularly Y -linked (SOX genes from SRY complex) to be utilised for sex-determination. To capture all available sequences for each fitness target a Basic Local Alignment Search Tool (BLAST) v2.11.0 [70] search was performed, and sequences with over 98% similarity and E-value of <0.01 were kept. To identify SNPs for each target, all relevant sequences were aligned using Geneious Prime 20201.1 [71]. A maximum of three SNPs per target gene were retained, prioritising conserved flanking regions (≤75 bp) for probe attachment, selected markers were subsequently mapped to the reference genome as with the Koala-fixed dataset.
- (3)
- The Pathogen-discovery dataset was developed by identifying major infectious pathogens of the koala with available sequences on NCBI database [65] and that are potentially detectable within a koala biological sample. Gene and genomic sequences for each target pathogen were retrieved using BLAST [70], retaining those with >98% similarity and E-value of <0.01. SNP markers were identified by aligning all sequences to respective reference genome or gene with Geneious Prime 20201.1 [71]. To prevent cross-species contamination, each pathogen reference was mapped against the koala genome to ensure no overlap with host sequences- excluding KoRV which is known to endogenously integrate into the koala genome and was labelled accordingly.
2.3. Assay Development and Genotyping
2.4. Sample Quality Control and Performance
2.5. SNP Quality Control and Validation
2.5.1. Koala-Fixed Dataset
2.5.2. Koala-Discovery Dataset
2.5.3. Pathogen-Discovery Dataset
2.6. Outlier Detection
- (1)
- Principal Component Adaption (PCAdapt) analysis detects outliers related to local adaption and was performed in RStudio v4.4.0 [75] using the [pcadapt] package [84]. p-values were assigned to each locus and adjusted for multiple testing using a False Discovery Rate (FDR) of 0.01. SNPs were considered candidates outliers if p < 0.001.
- (2)
- Latent Factor Mixed Model (LFMM) detects associations between SNP variation and environmental predictors while accounting for unobserved population structure through latent factors. Analyses were performed in RStudio v4.4.0 [75] using the packages [adegenet] [77], [LEA] [78], [qvalue] [85], [vegan] [86], [PopGenReport] [86], and [geosphere] [86]. Five environmental variables were tested: mean annual temperature (°C), mean annual rainfall (mm), elevation (m), aridity index, and leaf area index, following [52]. LFMM was run for 5000 iterations with a 10,000 burn-in. p-values were adjusted with the Benjamini–Hochberg procedure (FDR 0.01), and loci significantly associated with one or more predictors were retained as candidate outliers.
- (3)
- Redundancy Analysis (RDA) was used to detect multivariate associations between SNP variation and environmental predictors. Analyses were performed in RStudio v4.4.0 [75] using packages [vegan] [87] and [adegenet] [77]. The same five environmental variables tested in LFMM were calculated, and strongly correlated variables were identified using pairwise Spearman’s correlations. Model significance was assessed using permutation tests (n = 999). SNP loadings on significant RDA axes were then calculated, and loci with z-scores > 3 were retained as candidate outliers.
2.7. Sex Determination
2.8. Parentage Assignment and Provenance
2.9. Population Diversity and Differentiation
3. Results
3.1. Assay Design and Development
3.2. Sample Quality Control and Performance
3.3. SNP Quality and Validation
3.3.1. Koala-Fixed Dataset
3.3.2. Koala-Discovery Dataset
3.3.3. Pathogen-Discovery Dataset
3.4. Outlier Detection
- (1)
- PCAdapt analysis only included samples from northern populations (QLD, NSW; n = 229), excluding 82 southern individuals, to minimise confounding effects of historical bottlenecks. Pairwise comparisons across the nine northern populations yielded 37 population pairs (Supplementary Figure S1). From this, PCAdapt identified 12,723 candidate outlier SNPs at p < 0.01, reduced to 6422 at p < 0.001 and finally, 1703 (1618 Koala-fixed, 85 Koala-discovery) unique candidate outliers after ranking by frequency across pairs and average p-value.
- (2)
- LFMM identified 401 candidate SNPs associated with environmental variables (386 Koala-fixed, 15 Koala-discovery). Histograms of p-value distributions showed left-skewed enrichment, indicating non-random associations with environmental factors (Supplementary Figure S1). Manhattan plots revealed several loci with high significance (−log10(p) > 6), suggesting the presence of loci under potential selection.
- (3)
- RDA revealed significant multivariate associations between SNPs and environmental gradients (permutation test: F = 3.84, p < 0.001), consistent with literature [52] (Supplementary Figure S1). A total of 536 SNPs (534 Koala-fixed, 33 Koala-discovery) were significantly correlated with predictors, most strongly with temperature (355 SNPs), followed by LAI (87), elevation (44), aridity (30), and rainfall (22).
3.5. Sex Determination
3.6. Parentage Assignment and Provenance
3.7. Population Diversity and Differentiation
4. Discussion
4.1. Limitations
4.2. Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AGRF | Australian Genome Research Facility |
| Ap | Private alleles |
| Ar | Rare Alleles |
| BLAST | Basic Local Alignment Search Tool |
| BWA | Burrow–Wheeler alignment |
| C. pecoum | Chlamydia pecorum |
| DArTseq | Diversity Arrays Technology sequencing |
| EPBC | Environmental Protection and Biodiversity Conservation |
| FDR | False Discovery Rate |
| FIS | Inbreeding coefficient |
| gDNA | Genomic DNA |
| GWAS | Genome-wide association studies |
| HEcorr | Expected heterozygosity (corrected for sample size) |
| HO | Observed heterozygosity |
| HWE | Hardy–Weinberg Equilibrium |
| IR | Internal Relatedness |
| IUCN | International Union of Conservation of Nature |
| kNN | k-nearest neighbour |
| KoAA | Koala papillomavirus |
| KoEBV | Koala Epstein–Barr Virus |
| KoRV | Koala Retrovirus |
| KoRVend | Endogenous koala retrovirus |
| KoRVexo | Exogenous koala retrovirus |
| LD | Linkage Disequilibrium |
| LFMM | Latent Factor Mixed Model |
| LOD | logarithm of the odds |
| MAF | Minor allele Frequency |
| MHC | Major Histocompatibility Complex |
| ML | Maximum likelihood |
| mtDNA | mitochondrial DNA |
| NCBI | National Centre for Biotechnology Information |
| NGS | Next-Generation Sequencing |
| NSW | New South Wales |
| PCAdapt | Principal Component Adaption |
| PhaHV | Phascolarctos gammaherpesviruses |
| PIC | Polymorphic Information Content |
| QC | Quality Control |
| QLD | Queensland |
| RAPD | Randomly Amplified Polymorphic DNA |
| RDA | Redundancy Analysis |
| SA | South Australia |
| sMLH | standardised multilocus heterozygosity |
| SNP | Single-Nucleotide Polymorphism |
| VIC | Victoria |
| WC | Weir and Cockerham’s |
| WGS | Whole-genome sequencing |
References
- ACT Government. National Koala Recovery Plan—Phascolarctos cinereus; ACT Government: Canberra, Australia, 2022; p. 136.
- Melzer, A.; Carrick, F.; Menkhorst, P.; Lunney, D.; St John, B. Overview, Critical Assessment, and Conservation Implications of Koala Distribution and Abundance. J. Conserv. Biol. 2000, 14, 9. [Google Scholar] [CrossRef]
- McAlpine, C.A.; Rhodes, J.R.; Callaghan, J.G.; Bowen, M.E.; Lunney, D.; Mitchell, D.L.; Pullar, D.V.; Possingham, H.P. The importance of forest area and configuration relative to local habitat factors for conserving forest mammals: A case study of koalas in Queensland, Australia. Biol. Conserv. 2006, 132, 12. [Google Scholar] [CrossRef]
- Gordon, G.; Hrdina, F.; Patterson, R. Decline in the distribution of the Koala Phascolarctos cinereus in Queensland. Aust. Zool. 2006, 33, 14. [Google Scholar] [CrossRef]
- Hrdina, F.; Gordon, G. The Koala and Possum Trade in Queensland, 1906–1936. Aust. Zool. 2004, 32, 43. [Google Scholar] [CrossRef]
- Menkhorst, P. Hunted, marooned, re-introduced, contracepted: A history of Koala management in Victoria. In Too Close for Comfort: Contentious Issues in Human-Wildlife Encounters; Royal Zoological Society of New South Wales: Sydney, Australia, 2008; Volume 19. [Google Scholar]
- Phillips, B. Koalas: The Little Australians We’d All Hate to Lose; Commonwealth of Australia: Canberra, Australia, 1990. [Google Scholar]
- Houlden, B.; Costello, B.H.; Sharkey, D.; Fowler, E.V.; Melzer, A.; Ellis, W.; Carrick, F.; Baverstock, P.R.; Elphinstone, M.S. Phylogeographic differentiation in the mitochondrial control region in the koala, Phascolarctos cinereus. J. Mol. Ecol. 1999, 8, 13. [Google Scholar]
- Lunney, D.; Stalenberg, E.; Santika, T.; Rhodes, J.R. Extinction in Eden: Identifying the role of climate change in the decline of the koala in south-eastern NSW. Wildl. Res. 2014, 41, 22–34. [Google Scholar] [CrossRef]
- Houlden, B.; England, P.R.; Taylor, A.C.; Greville, W.D.; Sherwin, W.B. Low genetic variability of the koala Phasclaractos cinereus in south-eastern Australia following a severe populaiton bottleneck. Mol. Ecol. 1996, 5, 13. [Google Scholar]
- Lee, K.E.; Seddon, J.M.; Corley, S.W.; Ellis, W.A.H.; Johnston, S.D.; de Villiers, D.L.; Preece, H.J.; Carrick, F.N. Genetic variation and structuring in the threatened koala populations of Southeast Queensland. Conserv. Genet. 2010, 11, 2091–2103. [Google Scholar] [CrossRef]
- Taylor, A.; Marshall Graves, J.A.; Murray, N.D.; O’Brien, S.L.; Yuhki, N.; Sherwin, W.B. Conservation genetics of koala (Phascolarctos cinereus) low mitochondrial DNA variation amongst southern Australian populations. Genet. Res. 1997, 69, 9. [Google Scholar] [CrossRef]
- Kjeldsen, S.R.; Raadsma, H.W.; Leigh, K.A.; Tobey, J.R.; Phalen, D.; Krockenberger, A.; Ellis, W.A.; Hynes, E.; Higgins, D.P.; Zenger, K.R. Genomic comparisons reveal biogeographic and anthropogenic impacts in the koala (Phascolarctos cinereus): A dietary-specialist species distributed across heterogeneous environments. Heredity 2019, 122, 525–544. [Google Scholar] [CrossRef]
- Cristescu, R.; Cahill, V.; Sherwin, W.B.; Handasyde, K.; Carlyon, K.; Whisson, D.; Herbert, C.A.; Carlsson, B.L.J.; Wilton, A.N.; Cooper, D.W. Inbreeding and testicular abnormalities in a bottlenecked population of koalas (Phascolarctos cinereus). Wildl. Res. 2009, 39, 11. [Google Scholar] [CrossRef]
- De Cahsan, B.; Sandoval Velasco, M.; Westbury, M.V.; Duchene, D.A.; Strander Sinding, M.H.; Morales, H.E.; Kalthoff, D.C.; Barnes, I.; Brace, S.; Portela Miguez, R.; et al. Road to Extinction? Past and Present Population Structure and Genomic Diversity in the Koala. Mol. Biol. Evol. 2025, 42, msaf057. [Google Scholar] [CrossRef] [PubMed]
- Lott, M.J.; Frankham, G.J.; Eldridge, M.D.B.; Alquezar-Planas, D.E.; Donnelly, L.; Zenger, K.R.; Leigh, K.A.; Kjeldsen, S.R.; Field, M.A.; Lemon, J.; et al. Reversing the decline of threatened koala (Phascolarctos cinereus) populations in New 1 South Wales: Using genomics to define meaningful conservation goals. Ecol. Evol. 2023, 14, e11700. [Google Scholar] [CrossRef] [PubMed]
- Woinarski, J.; Burbirdge, A.A. Phascolarctos cinereus. The IUCN Red List of Threatened Species 2018; IUCN: Gland, Switzerland, 2016. [Google Scholar] [CrossRef]
- The Commonwealth Government of Australia. EPBC Act Referral Guidelines for the Vulnerable Koala (Combined Populations of Queensland, New South Wales and the Australian Capital Territory; The Commonwealth Government of Australia: Canberra, Australia, 2014. [Google Scholar]
- Adams-Hosking, C.; McAlpine, C.A.; Rhodes, J.R.; Grantham, H.S.; Moss, P.T. Modelling changes in the distribution of the critical food resources of a specialist folivore in response to climate change. Divers. Distrib. 2012, 18, 13. [Google Scholar] [CrossRef]
- Briscoe, N.J.; Kearney, M.R.; Taylor, C.A.; Wintlye, B.A. Unpacking the mechanisms captured by a correlative species distribution model to improve predictions of climate refugia. Glob. Change Biol. 2016, 22, 14. [Google Scholar] [CrossRef]
- Black, K.H.; Price, G.J.; Archer, M.; Hand, S.J. Bearing up well? Understanding the past, present and future of Australia’s koalas. Gondwana Res. 2014, 25, 1186–1201. [Google Scholar] [CrossRef]
- McAlpine, C.; Lunney, D.; Melzer, A.; Menkhorst, P.; Phillips, S.; Phalen, D.; Ellis, W.; Foley, W.; Baxter, G.; de Villiers, D.; et al. Conserving koalas: A review of the contrasting regional trends, outlooks and policy challenges. Biol. Conserv. 2015, 192, 226–236. [Google Scholar] [CrossRef]
- Quigley, B.L.; Timms, P. Helping koalas battle disease—Recent advances in Chlamydia and Koala Retrovirus (KoRV) disease understanding and treatment in koalas. FEMS Microbiol. Rev. 2020, 44, 583–605. [Google Scholar] [CrossRef]
- Polkinghorne, A.; Hanger, J.; Timms, P. Recent advances in understanding the biology, epidemiology and control of chlamydial infections in koalas. Vet. Microbiol. 2013, 165, 214–223. [Google Scholar] [CrossRef]
- Sarker, N.; Fabijan, J.; Owen, H.; Seddon, J.; Simmons, G.; Speight, N.; Kaler, J.; Woolford, L.; Emes, R.D.; Hemmatzadeh, F.; et al. Koala retrovirus viral load and disease burden in distinct northern and southern koala populations. Sci. Rep. 2020, 10, 263. [Google Scholar] [CrossRef]
- Seymour, A.; Montgomery, M.E.; Costello, B.H.; Ihle, S.; Johnsson, G.; St John, B.; Taggart, D.; Houlden, B.A. High effectivty inbreeding coefficients correlate with morphological abnormalities in populations of South Australian Koalas (Phascolarctos cinereus). Anim. Conserv. 2001, 4, 9. [Google Scholar] [CrossRef]
- Spielman, D.; Brook, B.W.; Briscoe, D.A.; Frankham, R. Does Inbreeding and Loss of Genetic Diversity Decrease Disease Resistance? Conserv. Genet. 2004, 5, 9. [Google Scholar] [CrossRef]
- Allendorf, F. Genetics and the conservation of natural populations: Allozymes to genomes. Mol. Ecol. 2017, 26, 11. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R. Stress and adaptation in conservation genetics. J. Evol. Biol. 2005, 18, 750–755. [Google Scholar] [CrossRef] [PubMed]
- Frankham, R. Genetics and extinction. Biol. Conserv. 2005, 126, 131–140. [Google Scholar] [CrossRef]
- Frankham, R.; Ballou, J.D.; Ralls, K.; Eldridge, M.D.B.; Dudash, M.R.; Fenester, C.B.; Lacy, R.C.; Sunnuck, P. Genetic Management of Fragmented Animal and Plant Populations; Oxford University Press: Oxford, UK, 2017. [Google Scholar]
- Allendorf, F.; Hohenlohe, P.A.; Luikart, G. Genomics and the future of conservation genetics. Nat. Rev. Genet. 2010, 11, 697–709. [Google Scholar] [CrossRef]
- Harrison, K.A.; Pavlova, A.; Telonis-Scott, M.; Sunnucks, P. Using genomics to characterise evolutionary potential for conservation of wild populations. Evol. Appl. 2014, 7, 1008–1025. [Google Scholar] [CrossRef]
- Kohn, M.H.; Murphy, W.J.; Ostrander, E.A.; Wayne, R.K. Genomics and conservation genetics. Trends Ecol. Evol. 2006, 21, 629–637. [Google Scholar] [CrossRef]
- Laikre, L. Genetic diversity is overlooked in international conservation policy implementation. Conserv. Genet. 2010, 11, 349–354. [Google Scholar] [CrossRef]
- Kleinman-Ruiz, D.; Martinez-Cruz, B.; Soriano, L.; Lucena-Perez, M.; Cruz, F.; Villanueva, B.; Fernandez, J.; Godoy, J.A. Novel efficient genome-wide SNP panels for the conservation of the highly endangered Iberian lynx. BMC Genom. 2017, 18, 556. [Google Scholar] [CrossRef]
- Takach, B.V.; Cameron, S.F.; Cremona, T.; Eldridge, M.D.B.; Fisher, D.O.; Hohnen, R.; Jolly, C.J.; Kelly, E.; Phillips, B.L.; Radford, I.J.; et al. Conservation prioritisation of genomic diversity to inform management of a declining mammal species. Biol. Conserv. 2024, 291, 110467. [Google Scholar] [CrossRef]
- Chang, Y.; Bertola, L.V.; Zenger, K.R.; Hoskin, C.J. Conservation genetics of Mahogany Gliders and their complex evolutionary relationship with Squirrel Gliders. Conserv. Genet. 2025, 26, 731–750. [Google Scholar] [CrossRef]
- Cocciolone, R.; Timms, P. DNA profiling of Queensland Koalas reveales Sufficient Variability for individual Identification and Parentage Determine. Wildl. Res. 1992, 19, 9. [Google Scholar] [CrossRef]
- Taylor, A.; Marshall Graves, J.A.; Murray, N.D.; Sherwin, W.B. Conservation Genetics of the Koala (Phascolarctos cinereus) II. Limited Variability in Minisatellite DNA sequences. Biochem. Genet. 1991, 29, 9. [Google Scholar] [CrossRef] [PubMed]
- Timms, P.; Kato, J.; Maugeri, M.; White, N. DNA fingerprint analysis of a free-range koala population. Biochem. Genet. 1993, 31, 363–374. [Google Scholar] [CrossRef]
- Fowler, E.; Houlden, B.A.; Sherwin, W.B.; Hoeben, P.; Timms, P. Genetic variation in Captive Koalas (Phascolarctos cinereus): Parentage Determination and Individual Identification. Biochem. Genet. 1998, 36, 15. [Google Scholar] [CrossRef]
- Fowler, E.; Hoeben, P.; Timms, P. Randomly Amplified Polymorphic DNA Variation in Populations of Eastern Australian Koalas, Phascolarctos cinereus. Biochem. Genet. 1998, 36, 14. [Google Scholar] [CrossRef]
- Neaves, L.E.; Frankham, G.J.; Dennison, S.; FitzGibbon, S.; Flannagan, C.; Gillett, A.; Hynes, E.; Handasyde, K.; Helgen, K.M.; Tsangaras, K.; et al. Phylogeography of the Koala, (Phascolarctos cinereus), and Harmonising Data to Inform Conservation. PLoS ONE 2016, 11, e0162207. [Google Scholar] [CrossRef]
- Tsangaras, K.; Avila-Arcos, M.C.; Ishida, Y.; Helgen, K.M.; Roca, A.L.; Greenwood, A.D. Historically low mitochondrial DNA diversity in koalas (Phascolarctos cinereues). BMC Genet. 2012, 13, 11. [Google Scholar] [CrossRef]
- Houlden, B.; England, P.; Sherwin, W.B. Paternity Exclusion in Koalas using Hypervariable Microsatellites. J. Hered. 1996, 87, 4. [Google Scholar] [CrossRef]
- Lee, T.; Zenger, K.R.; Close, R.L.; Jones, M.; Phalen, D.N. Defining spatial genetic structure and managemnet unit for vulnerable koala (Phascolarctos cinereus) populations in hte Sydney region, Australia. J. Wildl. Res. 2010, 37, 10. [Google Scholar] [CrossRef]
- Lee, T.; Zenger, K.R.; Close, R.L.; Phalen, D.N. Genetic analysis reveals a distinct and highly diverse koala (Phascolarctos cinereus) population in South Gippsland, Victoria, Australia. Aust. Mammal. 2012, 34, 68–74. [Google Scholar] [CrossRef]
- Ruiz-Rodriguez, C.T.; Ishida, Y.; Greenwood, A.D.; Roca, A.L. Development of 14 microsatellite markers in the Queensland koala (Phascolarctos cinereus adustus) using next generation sequencing technology. Conserv. Genet. Resour. 2014, 6, 429–431. [Google Scholar] [CrossRef]
- Ruiz-Rodriguez, C.T.; Ishida, Y.; Murray, N.D.; O’Brien, S.J.; Graves, J.A.; Greenwood, A.D.; Roca, A.L. Koalas (Phascolarctos cinereus) from Queensland Are Genetically Distinct from 2 Populations in Victoria. J. Hered. 2016, 107, 573–580. [Google Scholar] [CrossRef]
- Kjeldsen, S.R.; Zenger, K.R.; Leigh, K.A.; Ellis, W.A.; Tobey, J.R.; Phalen, D.; Melzer, A.; FitzGibbon, S.; Raadsma, H. Genome-wide SNP loci reveal novel insights into koala (Phascolarctos cinereus) population variability across its range. Conserv. Genet. 2015, 17, 337–353. [Google Scholar] [CrossRef]
- Lott, M.J.; Wright, B.R.; Neaves, L.E.; Frankham, G.J.; Dennison, S.; Eldridge, M.D.B.; Potter, S.; Alquezar-Planas, D.E.; Hogg, C.J.; Belov, K.; et al. Future-proofing the koala: Synergising genomic and environmental data for effective species management. Mol. Ecol. 2022, 31, 3035–3055. [Google Scholar] [CrossRef]
- Hogg, C.J.; Silver, L.; McLennan, E.A.; Belov, K. Koala Genome Survey: An Open Data Resource to Improve Conservation Planning. Genes 2023, 14, 546. [Google Scholar] [CrossRef]
- McLennan, E.A.; Kovacs, T.G.L.; Silver, L.W.; Chen, Z.; Jaya, F.R.; Ho, S.Y.W.; Belov, K.; Hogg, C.J. Genomics identifies koala populations at risk across eastern Australia. Ecol. Appl. 2025, 35, e3062. [Google Scholar] [CrossRef]
- Silver, L.W.; McLennan, E.A.; Beaman, J.; da Silva, K.B.; Timms, P.; Hogg, C.J.; Belov, K. Using bioinformatics to investigate functional diversity: A case study of MHC diversity in koalas. Immunogenetics 2024, 76, 381–395. [Google Scholar] [CrossRef]
- Frankham, R.; Ballou, J.D.; Briscoe, D.A. Introduction to Conservation Genetics, 2nd ed.; Cambridge University Press: Cambridge, UK, 2009; p. 618. [Google Scholar]
- Breed, M.F.; Harrison, P.A.; Blyth, C.; Byrne, M.; Gaget, V.; Gellie, N.J.C.; Groom, S.V.C.; Hodgson, R.; Mills, J.G.; Prowse, T.A.A.; et al. The Potential of genomics for restoring ecosystems and biodiversity. Nat. Rev. Genet. 2019, 20, 13. [Google Scholar] [CrossRef]
- Ekblom, R.; Galindo, J. Applications of next generation sequencing in molecular ecology of non-model organisms. Heredity 2011, 107, 15. [Google Scholar] [CrossRef] [PubMed]
- Ekblom, R.; Saether, S.A.; Jacobsson, P.; Fiske, P.; Sahlman, T.; Grahn, M.; Kalas, J.A.; Hoglund, J. Spatial pattern of MHC class II variation in the great snipe (Gallinago media). Mol. Ecol. 2007, 16, 1439–1451. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, Y.; Wang, W.; Zou, F.; Yang, J.; Gao, W.; Feng, S.; Chen, G.; Shi, C.; Cai, Y.; et al. Integrating pathogen- and host-derived blood biomarkers for enhanced tuberculosis diagnosis: A comprehensive review. Front. Immunol. 2024, 15, 1438989. [Google Scholar] [CrossRef]
- Adamkewicz, S.; Harawych, M.G. Systematics and biogeography of the genus Donax (Bivalvia: Donacidae) in eastern North America. Am. Malacol. Bull. 1996, 13, 6. [Google Scholar]
- Bioline. ISOLATE II Fecal DNA Kit Product Manual/Protocol. Available online: https://www.bioline.com/mwdownloads/download/link/id/7455//i/s/isolate_ii_fecal_productmanual_digital_web.pdf (accessed on 1 March 2020).
- Promega Corporation. Quantifluor (R) dsDNA Technical Manual. 2018. Available online: https://www.promega.com/-/media/files/resources/protocols/technical-manuals/101/tm405-quantifluor-one-dsdna-system.pdf?rev=5b55c606b6f84d49b4fd2238f9dc350d (accessed on 1 April 2020).
- TECAN Allegro® Targeted Genotyping V2. Available online: https://lifesciences.tecan.com/allegro-targeted-genotyping-v2?p=tab--5 (accessed on 1 August 2020).
- Coordinators, N.R. Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2018, 46, D8–D13. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 2009, 25, 6. [Google Scholar] [CrossRef]
- Field, M.A. Detecting pathogenic variants in autoimmune diseases using high-throughput sequencing. Immunol. Cell Biol. 2021, 99, 146–156. [Google Scholar] [CrossRef]
- Waardenberg, A.J.; Field, M.A. consensusDE: An R package for assessing consensus of multiple RNA-seq algorithms with RUV correction. PeerJ 2019, 7, e8206. [Google Scholar] [CrossRef]
- Danecek, P.; Bonfield, J.K.; Liddle, J.; Marshall, J.; Ohan, V.; Pollard, M.O.; Whitwham, A.; Keane, T.; McCarthy, S.A.; Davies, R.M.; et al. Twelve years of SAMtools and BCFtools. GigaScience 2021, 10, giab008. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Myers, E.W.; Lipman, D.J. BLAST: Basic Local Alignment search tool. J. Microbiol. 1990, 215, 7. [Google Scholar] [CrossRef]
- Biomatters Ltd. Geneious Prime, Version 2025.1; Geneious: Auckland, New Zealand, 2025.
- AGRF. Bioinformatics; Bioplatforms Australia: Sydney, Australia, 2021. [Google Scholar]
- Purcell, S.; Neale, B.; Todd-Brown, K.; Thomas, L.; Ferreira, M.A.R.; Bender, D.; Maller, J.; Sklar, P.; de Bakker, P.I.W.; Daly, M.J.; et al. PLINK: A tooset for whole-genome association and population-based linkage analysis. Am. J. Hum. Genet. 2007, 81, 559–575. [Google Scholar] [CrossRef]
- Danecek, P.; Auton, A.; Abecasis, G.; Albers, C.A.; Banks, E.; DePristo, M.A.; Handsaker, R.E.; Lunter, G.; Marth, G.T.; Sherry, S.T.; et al. The variant call format and VCFtools. Bioinformatics 2011, 27, 2156–2158. [Google Scholar] [CrossRef] [PubMed]
- RStudio Team. RStudio: Integrated Development for R; PBC: Boston, MA, USA, 2020. [Google Scholar]
- Knaus, B.J.; Grunwald, N.J. VCFR: A package to manipulate and visualize variant call format data in R. Mol. Ecol. Resour. 2017, 17, 9. [Google Scholar] [CrossRef] [PubMed]
- Jombart, T.; Ahmed, I. adegenet 1.3-1: New tools for the analysis of genome-wide SNP data. Bioinformatics 2011, 27, 3070–3071. [Google Scholar] [CrossRef] [PubMed]
- Frichot, E.; François, O.; O’Meara, B. LEA: An R package for landscape and ecological association studies. Methods Ecol. Evol. 2015, 6, 925–929. [Google Scholar] [CrossRef]
- Wickham, H. ggplot2: Elegant Grapihcs for Data Analysis; Springer: New York, NY, USA, 2016. [Google Scholar]
- Wickham, H. Reshaping data with reshae package. J. Stat. Softw. 2007, 21, 20. [Google Scholar] [CrossRef]
- Lau, Q.; Jaratlerdsiri, W.; Griffith, J.E.; Gongora, J.; Higgins, D.P. MHC class II diversity of koala (Phascolarctos cinereus) populations across their range. Heredity 2014, 113, 287–296. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA Sequecing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 4. [Google Scholar] [CrossRef]
- Olivry, T.; Fontao, A.M.; Ivanovoa, N.; Mitterer, G.; Harwanegg, C. Validation of multiplex molecular macroarray for the determination of allergen-specific IgE sensitisation in dogs. Vet. Sci. 2024, 11, 482. [Google Scholar]
- Luu, K.; Bazin, E.; Blum, M.G.B. pcadapt: An R package to perform genome scans for selection based on principal component analysis. Mol. Ecol. Resour. 2017, 17, 10. [Google Scholar] [CrossRef]
- Storey, J.D.; Bass, A.J.; Dabney, A.; Robinson, D. qvalue: Q-Value Estimation for False Discovery Rate Control, Version 2.40.0; Bioconductor: Boston, MA, USA, 2025.
- Hijmans, R.J. geosphere: Spherical Trigonometry, Version 1.5-20; Github: San Francisco, CA, USA, 2024.
- Dixon, P. VEGAN, a package of R functoins for community ecology. J. Veg. Sci. 2003, 14, 3. [Google Scholar] [CrossRef]
- Marshall, T.C.; Slate JKruuk, L.E.B.; Pemberton, J.M. Statistical confidence for likelihood-based paternity inference in natural populaitons. Mol. Ecol. 1998, 7, 16. [Google Scholar] [CrossRef] [PubMed]
- Steinig, E.J.; Neuditschko, M.; Khatkar, M.S.; Raadsma, H.W. Netview P: A network visualisation tool to unravel complex population structure using genome-wide SNPs. Molecluar Ecol. Resour. 2016, 16, 11. [Google Scholar] [CrossRef] [PubMed]
- Keenan, K.; McGinnity, P.; Cross, T.F.; Crozier, W.W.; Prodohl, P.A. diveRsity: An R package for the estimation and exploration of popultion genetics parameteres and their associated errors. Methods Ecol. Evol. 2013, 4, 6. [Google Scholar] [CrossRef]
- Amos, W.; Blamford, A. When does conservation genetics matter? Heredity 2001, 87, 9. [Google Scholar] [CrossRef]
- Kalinowski, S.; Wagner, A.P.; Taper, M.L. ML-Relate: A computer program for maximum likelihood estimation of relatedness and relationship. Mol. Ecol. 2006, 6, 3. [Google Scholar] [CrossRef]
- Weir, B.S.; Cockerham, C.C. Estimating F-Statistics for the Analysis of Population Structure. Evolution 1984, 38, 1358–1370. [Google Scholar] [CrossRef]
- Nei, M. Estimation of average heterozygosity and genetic distance from a small number of individuals. Genetics 1978, 89, 7. [Google Scholar] [CrossRef]
- Goudet, J. Heirfstat: Estimation and Tests of Hierarchial F-Statistics, Version 0.5-11; Github: San Francisco, CA, USA, 2022.
- Wickham, H.; Francois, R.; Henry, L.; Muller, K.; Vaughan, D. dplyr: A Grammar of Data Manipulation; Version 1.1.4; Tidyverse. 2025. Available online: https://dplyr.tidyverse.org/ (accessed on 1 April 2024).
- Lee, K.E.; Seddon, J.M.; Johnston, S.; FitzGibbon, S.I.; Carrick, F.; Melzer, A.; Bercovitch, F.; Ellis, W. Genetic diversity in natural and introduced island populations of koalas in Queensland. Aust. J. Zool. 2012, 60, 303–310. [Google Scholar] [CrossRef]
- Funk, W.C.; Forester, B.R.; Converse, S.J.; Darst, C.; Morey, S. Improving conservation policy with genomics: A guide to integrating adaptive potential into U.S. Endangered Species Act decisions. Conserv. Genet. 2019, 20, 19. [Google Scholar] [CrossRef]
- McMahon, B.J.; Teeling, E.C.; Höglund, J. How and why should we implement genomics into conservation? Evol. Appl. 2014, 7, 8. [Google Scholar] [CrossRef]
- Wedrowicz, F.; Mosse, J.; Wright, W.; Hogan, F.E. Isolating DNA sourced non-invasively from koala scats: A comparison of four commercial DNA stool kits. Conserv. Genet. Resour. 2018, 11, 219–229. [Google Scholar] [CrossRef]
- Wedrowicz, F.; Saxton, T.; Mosse, J.; Wright, W.; Hogan, F.E. A non-invasive tool for assessing pathogen prevalence in koala (Phascolarctos cinereus) populations: Detection of Chlamydia pecorum and koala retrovirus (KoRV) DNA in genetic material sourced from scats. Conserv. Genet. Resour. 2016, 8, 511–521. [Google Scholar] [CrossRef]
- Bulla, A.; de Witt, B.; Ammerlaan, W.; Betsou, F.; Lescuyer, P. Blood DNA yield but not integrity or methylation is impacted after long-term storage. Biopreservation Biobanking 2016, 14, 9. [Google Scholar] [CrossRef] [PubMed]
- Taberlet, P.; Luikart, G. Non-invasive genetic sampling: Look before you leap. Trends Ecol. Evol. 1999, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Schultz, A.J.; Cristescu, R.H.; Littleford-Colquhoun, B.L.; Jaccoud, D.; Frere, C.H. Fresh is best: Accurate SNP genotyping from koala scats. Ecol. Evol. 2018, 8, 3139–3151. [Google Scholar] [CrossRef]
- Beja-Pererira, A.; Oliveira, R.P.; Alves, P.C.; Schwartz, M.K.; Luikart, G. Advancing ecological understandings through technological transformations in noninvasive genetics. Mol. Ecol. Resour. 2009, 9, 22. [Google Scholar] [CrossRef]
- Waits, L.P.; Paetkau, D. Noninvasive genetic sampling tools for wildlife biologists: A review of applications and recommendations for accurate data collection. J. Od Wildl. Manag. 2005, 69, 15. [Google Scholar] [CrossRef]
- Cui, J.; Frankham, G.J.; Johnson, R.N.; Polkinghorne, A.; Timms, P.; O’Meally, D.; Cheng, Y.; Belov, K. SNP marker discovery in koala TLR genes. PLoS ONE 2015, 10, e0121068. [Google Scholar] [CrossRef]
- Johnson, R.N.; O’Meally, D.; Chen, Z.; Etherington, G.J.; Ho, S.Y.W.; Nash, W.J.; Grueber, C.E.; Cheng, Y.; Whittington, C.M.; Dennison, S.; et al. Adaptation and conservation insights from the koala genome. Nat. Genet. 2018, 50, 1102–1111. [Google Scholar] [CrossRef]
- Davies, N.A.; Gramotnev, G.; McAlpine, C.; Seabrook, L.; Baxter, G.; Lunney, D.; Rhodes, J.R.; Bradley, A. Physiological Stress in Koala Populations near the Arid Edge of Their Distribution. PLoS ONE 2013, 8, e79136. [Google Scholar] [CrossRef]
- Chappell, K.; Brealey, J.C.; Amarilla, A.A.; Watterson, D.; Hulse, L.; Palmieri, C.; Johnston, S.D.; Holmes, E.C.; Meers, J.; Young, P.R. Phylogenetic Diversity of Koala Retrovirus within a Wild Koala Population. J. Virol. 2017, 91, e01820-16. [Google Scholar] [CrossRef] [PubMed]
- Narayan, E. Physiological stress levels in wild koala sub-populations facing anthropogenic induced environmental trauma and disease. Sci. Rep. 2019, 9, 6031. [Google Scholar] [CrossRef] [PubMed]
- Titcomb, G.C.; Jerde, C.L.; Young, H.S. High-Throughput Sequencing for Understanding the Ecology of Emerging Infectious Diseases at the Wildlife-Human Interface. Front. Ecol. Evol. 2019, 7, 126. [Google Scholar] [CrossRef]
- De Cario, R.; Kura, A.; Suraci, S.; Magi, A.; Volta, A.; Marcucci, R.; Gori, A.M.; Pepe, G.; Giusti, B.; Sticchi, E. Sanger Validation of High-Throughput Sequencing in Genetic Diagnosis: Still the Best Practice? Front. Genet. 2020, 11, 592588. [Google Scholar] [CrossRef]
- Robbins, A.; Hanger, J.; Jelocnik, M.; Quigley, B.L.; Timms, P. Koala immunogenetics and chlamydial strain type are more directly involved in chlamydial disease progression in koalas from two south east Queensland koala populations than koala retrovirus subtypes. Sci. Rep. 2020, 10, 15013. [Google Scholar] [CrossRef]
- Tarlinton, R.; Meers, J.; Hanger, J.; Young, P. Real-time reverse transcriptase PCR for the endogenous koala retrovirus reveals an association between plasma viral load and neoplastic disease in koalas. J. Gen. Virol. 2005, 86, 783–787. [Google Scholar] [CrossRef]
- Waugh, C.A.; Hanger, J.; Loader, J.; King, A.; Hobbs, M.; Johnson, R.; Timms, P. Infection with koala retrovirus subgroup B (KoRV-B), but not KoRV-A, is associated with chlamydial disease in free-ranging koalas (Phascolarctos cinereus). Sci. Rep. 2017, 7, 134. [Google Scholar] [CrossRef]
- Blanchong, J.A.; Robinson, S.J.; Samuel, M.D.; Foster, J.T. Application of genetics and genomics to wildlife epidemiology. J. Wildl. Manag. 2016, 80, 593–608. [Google Scholar] [CrossRef]
- Vander Wal, E.; Garant, D.; Calme, S.; Chapman, C.A.; Festa-Bianchet, M.; Millien, V.; Rioux-Paquette, S.; Pelletier, F. Applying evolutionary concepts to wildlife disease ecology and management. Evol. Appl. 2014, 7, 856–868. [Google Scholar] [CrossRef]
- Zarzoso-Lacoste, D.; Jan, P.L.; Lehnen, L.; Girard, T.; Besnard, A.L.; Puechmaille, S.J.; Petit, E.J. Combining noninvasive genetics and a new mammalian sex-linked marker provides new tools to investigate population size, structure and individual behaviour: An application to bats. Mol. Ecol. Resour. 2018, 18, 217–228. [Google Scholar] [CrossRef] [PubMed]
- Tarlinton, R.E.; Legione, A.R.; Sarker, N.; Fabijan, J.; Meers, J.; McMichael, L.; Simmons, G.; Owen, H.; Seddon, J.M.; Dick, G.; et al. Differential and defective expression of Koala Retrovirus indicate complexity of host and virus evolution. bioRxiv 2021, 103. [Google Scholar] [CrossRef]
- Hauser, L.; Baird, M.; Hilborn, R.; Seeb, L.W.; Seeb, J.E. An empirical comparison of SNPs and microsatellites for parentage and kinship assignment in a wild sockeye salmon (Oncorhynchus nerka) population. Mol. Ecol. Resour. 2011, 11 (Suppl. S1), 150–161. [Google Scholar] [CrossRef]
- Ivy, J.A.; Miller, A.; Lacy, R.C.; DeWoody, J.A. Methods and prospects for using molecular data in captive breeding programs: An empiricial example using parma wallabies (Macropus parma). J. Hered. 2009, 104, 13. [Google Scholar] [CrossRef] [PubMed]
- Ogden, R.; Linacre, A. Wildlife forensic science: A review of genetic geographic origin assignment. Forensic Sci. Int. Genet. 2015, 18, 7. [Google Scholar] [CrossRef] [PubMed]
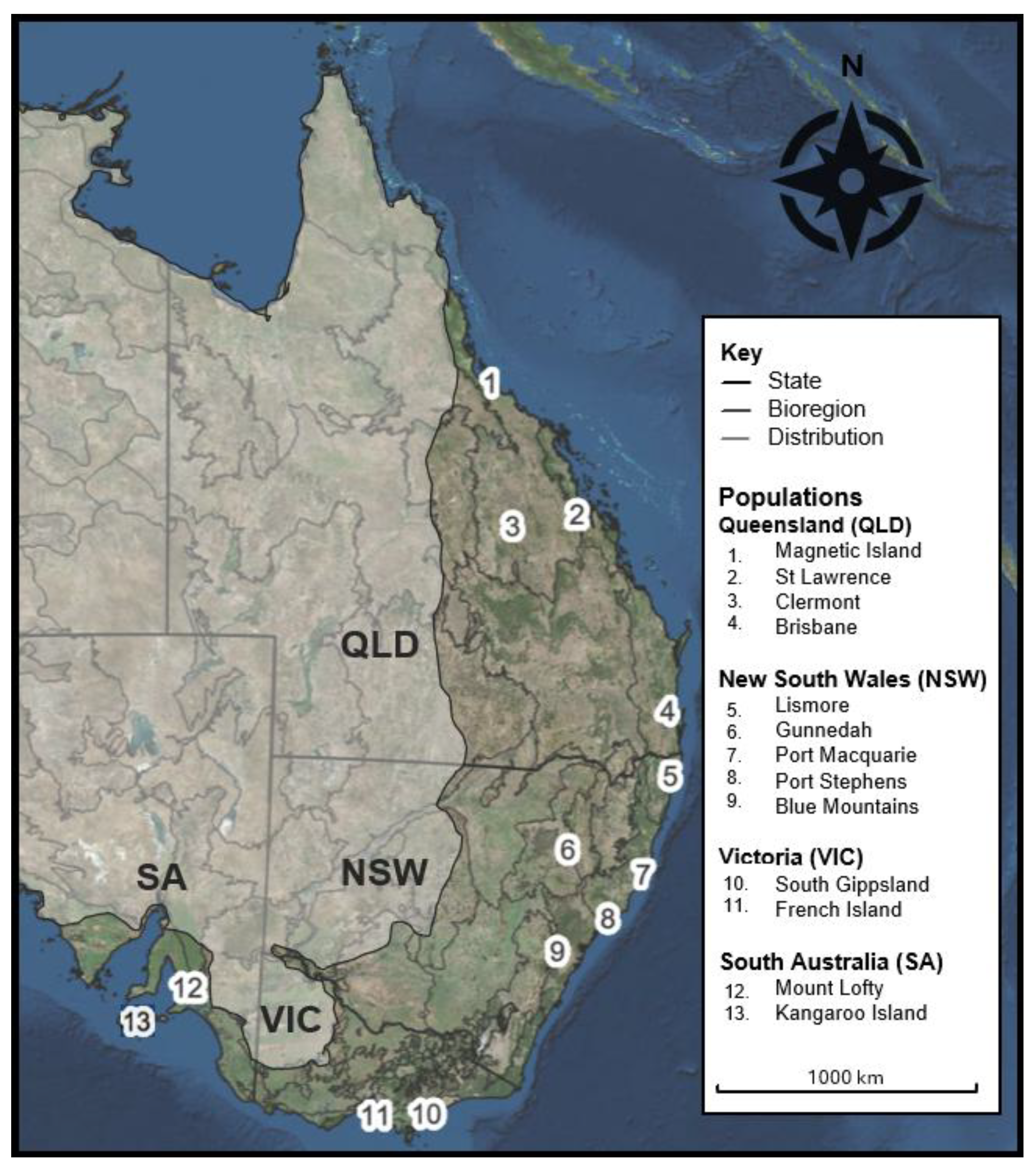




| State | No. | Population Name | Bioregion | n | HO | Hecorr | % PL | FIS | AvFST | Ap | rare A | Ar | sMLH | IR |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| QLD | 1 | Magnetic Island | Q_MAI | 31 | 0.14 | 0.20 | 55.05 | 0.06 | 0.22 | 0 | 0.04 | 1.37 | 1.02 | 0.60 |
| 2 | St Lawrence | Q_CMC | 33 | 0.15 | 0.19 | 56.42 | 0.03 | 0.18 | 0 | 0.02 | 1.44 | 1.01 | 0.60 | |
| 3 | Clermont | Q_BBN | 28 | 0.16 | 0.21 | 57.55 | 0.02 | 0.18 | 0 | 0.03 | 1.39 | 1.01 | 0.61 | |
| 4 | Southeast QLD | Q_SEQ | 19 | 0.16 | 0.19 | 60.32 | 0.05 | 0.15 | 0 | 0.00 | 1.50 | 1.01 | 0.60 | |
| NSW | 5 | Lismore | N_FNC | 34 | 0.14 | 0.19 | 59.01 | 0.09 | 0.20 | 0 | 0.02 | 1.40 | 1.00 | 0.61 |
| 6 | Gunnedah | N_BBS | 19 | 0.15 | 0.21 | 53.26 | 0.07 | 0.18 | 0 | 0.04 | 1.39 | 1.00 | 0.62 | |
| 7 | Port Macquarie | N_NNC | 25 | 0.15 | 0.20 | 55.94 | 0.02 | 0.18 | 0 | 0.02 | 1.43 | 0.99 | 0.61 | |
| 8 | Port Stephens | N_SBPS | 21 | 0.15 | 0.20 | 56.56 | 0.03 | 0.17 | 0 | 0.03 | 1.43 | 1.00 | 0.62 | |
| 9 | Blue Mountains | N_SBBM | 19 | 0.15 | 0.20 | 52.46 | 0.03 | 0.18 | 0 | 0.02 | 1.42 | 0.99 | 0.61 | |
| VIC | 10 | South Gippsland | V_SCPE | 17 | 0.11 | 0.16 | 35.67 | −0.03 | 0.24 | 0 | 0.04 | 1.24 | 0.98 | 0.63 |
| 11 | French Island | V_FRI | 20 | 0.10 | 0.16 | 48.73 | 0.22 | 0.20 | 0 | 0.12 | 1.24 | 0.99 | 0.63 | |
| SA | 12 | Mount Lofty | S_FLB | 30 | 0.10 | 0.16 | 55.22 | 0.19 | 0.22 | 0 | 0.02 | 1.36 | 0.99 | 0.63 |
| 13 | Kangaroo Island | S_KAI | 15 | 0.09 | 0.13 | 29.09 | −0.07 | 0.26 | 0 | 0.02 | 1.21 | 0.99 | 0.65 | |
| TOTAL | 311 |
| Q_MAI | Q_CMC | Q_BBN | Q_SEQ | N_FNC | N_BBS | N_NNC | N_SBPS | N_SBBM | V_SCPE | V_FRI | S_FLB | S_KAI | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Q_MAI | 0.05 | 0.08 | 0.12 | 0.18 | 0.20 | 0.18 | 0.18 | 0.23 | 0.37 | 0.32 | 0.33 | 0.39 | |
| Q_CMC | 0.06 | 0.04 | 0.07 | 0.13 | 0.16 | 0.14 | 0.14 | 0.19 | 0.32 | 0.28 | 0.29 | 0.35 | |
| Q_BBN | 0.09 | 0.05 | 0.07 | 0.13 | 0.15 | 0.15 | 0.13 | 0.18 | 0.32 | 0.27 | 0.29 | 0.35 | |
| Q_SEQ | 0.13 | 0.07 | 0.07 | 0.08 | 0.11 | 0.10 | 0.08 | 0.14 | 0.28 | 0.23 | 0.24 | 0.31 | |
| N_FNC | 0.20 | 0.15 | 0.15 | 0.08 | 0.16 | 0.15 | 0.14 | 0.18 | 0.33 | 0.29 | 0.30 | 0.36 | |
| N_BBS | 0.22 | 0.17 | 0.17 | 0.13 | 0.17 | 0.09 | 0.09 | 0.10 | 0.29 | 0.24 | 0.26 | 0.32 | |
| N_NNC | 0.20 | 0.16 | 0.16 | 0.11 | 0.16 | 0.10 | 0.07 | 0.13 | 0.30 | 0.26 | 0.28 | 0.34 | |
| N_SBPS | 0.20 | 0.15 | 0.15 | 0.09 | 0.16 | 0.10 | 0.08 | 0.11 | 0.27 | 0.23 | 0.24 | 0.30 | |
| N_SBBM | 0.25 | 0.21 | 0.20 | 0.15 | 0.20 | 0.12 | 0.14 | 0.13 | 0.24 | 0.19 | 0.22 | 0.28 | |
| V_SCPE | 0.40 | 0.37 | 0.36 | 0.31 | 0.37 | 0.32 | 0.34 | 0.30 | 0.26 | 0.05 | 0.07 | 0.10 | |
| V_FRI | 0.36 | 0.32 | 0.31 | 0.25 | 0.32 | 0.27 | 0.29 | 0.25 | 0.21 | 0.05 | 0.03 | 0.05 | |
| S_FLB | 0.36 | 0.32 | 0.32 | 0.26 | 0.32 | 0.28 | 0.30 | 0.26 | 0.23 | 0.08 | 0.03 | 0.04 | |
| S_KAI | 0.45 | 0.41 | 0.40 | 0.34 | 0.41 | 0.36 | 0.38 | 0.34 | 0.31 | 0.11 | 0.05 | 0.04 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Donnelly, L.F.; Kjeldsen, S.R.; Lott, M.J.; Leigh, K.; Field, M.A.; Cooke, I.R.; Wright, B.R.; Zenger, K.R. Development and Validation of a Standardised Genomic Tool for Conservation Management of the Koala (Phascolarctos cinereus). Animals 2025, 15, 3375. https://doi.org/10.3390/ani15233375
Donnelly LF, Kjeldsen SR, Lott MJ, Leigh K, Field MA, Cooke IR, Wright BR, Zenger KR. Development and Validation of a Standardised Genomic Tool for Conservation Management of the Koala (Phascolarctos cinereus). Animals. 2025; 15(23):3375. https://doi.org/10.3390/ani15233375
Chicago/Turabian StyleDonnelly, Lily F., Shannon R. Kjeldsen, Matthew J. Lott, Kellie Leigh, Matthew A. Field, Ira R. Cooke, Belinda R. Wright, and Kyall R. Zenger. 2025. "Development and Validation of a Standardised Genomic Tool for Conservation Management of the Koala (Phascolarctos cinereus)" Animals 15, no. 23: 3375. https://doi.org/10.3390/ani15233375
APA StyleDonnelly, L. F., Kjeldsen, S. R., Lott, M. J., Leigh, K., Field, M. A., Cooke, I. R., Wright, B. R., & Zenger, K. R. (2025). Development and Validation of a Standardised Genomic Tool for Conservation Management of the Koala (Phascolarctos cinereus). Animals, 15(23), 3375. https://doi.org/10.3390/ani15233375






