Comprehensive Assessment of Silver Bioaccumulation and DNA Damage Effects in Coturnix coturnix japonica via Blood, Feather, and Egg Using Two Different Sources
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Silver Nanoparticles
2.2. Trial Birds and Research Methodology
2.3. Blood and Feather Sample Collection
2.4. Collection of Eggs
2.5. Silver Bioaccumulation and Screening Sample Digestion
2.6. Chemical Analysis of Samples
2.7. Calculation of Silver Concentration
2.8. DNA Damage Examination
2.9. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, Y.; Liu, Q.Y.; Bu, Z.Q.; Quan, M.X.; Lu, J.Y.; Huang, W.T. Colorimetric multi-channel sensing of metal ions and advanced molecular information protection based on fish scale-derived carbon nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2023, 290, 122291. [Google Scholar] [CrossRef]
- Mwafy, A.; Youssef, D.Y.; Mohamed, M.M. Antibacterial activity of zinc oxide nanoparticles against some multidrug-resistant strains of Escherichia coli and Staphylococcus aureus. Int. J. Vet. Sci. 2023, 12, 284–289. [Google Scholar]
- Aslam, N.; Ali, A.; Sial, B.E.; Maqsood, R.; Mahmood, Y.; Mustafa, G.; Sana, A. Assessing the dual impact of zinc oxide nanoparticles on living organisms: Beneficial and noxious effects. Int. J. Agric. Biosci. 2023, 12, 267–276. [Google Scholar] [CrossRef]
- Mahmood, Q.; Shaheen, S.; Azeem, M. Nanobiosensors: Application in healthcare, environmental monitoring and food safety. Asian J. Agric. Biol. 2024, 2024, 2023157. [Google Scholar]
- El-Hamaky, A.M.A.; Hassan, A.A.; Wahba, A.K.A.; El Mosalamy, M.M.E.A. Influence of copper and zinc nanoparticles on genotyping characterizations of multi-drug resistance genes for some calf pathogens. Int. J. Vet. Sci. 2023, 12, 309–317. [Google Scholar] [CrossRef]
- Ibrahim, E.S.; Abdalhamed, A.M.; Arafa, A.A.; Eid, R.H.; Khalil, H.M.A.; Hedia, R.H.; Dorgham, S.M.; Hozyen, H.F. In vitro and in vivo antibacterial and antibiofilm efficacy of selenium nanoparticles against Staphylococcus aureus supported with toxicopathological and behavioral studies in rats. Int. J. Vet. Sci. 2024, 13, 490–500. [Google Scholar] [CrossRef]
- Anjum, R.; Hamid, M.; Khalil, R.; Ajmal, A. Possible effect of ascorbic acid against zinc oxide nanoparticles induced hepatotoxicity in Swiss albino mice. Int. J. Agric. Biosci. 2023, 12, 193–198. [Google Scholar] [CrossRef]
- Mehwish; Azam, S.E.; Muzamail, S. Unveiling the future: Nanotechnology’s role in advanced food packaging. Agrobiol. Rec. 2024, 15, 24–33. [Google Scholar] [CrossRef]
- Afzal, G.; Ullah, M.I.; Ali, N.; Afzal, M.; Hussain, R.; Alhakamy, N.A.; Rajeh, N.; Rehan, S.; Iqbal, R.; Iqbal, M.S.; et al. Mechanistic approach to investigate the induction of toxicity by magnesium oxide nanoparticles on testicular, nervous and muscular tissues of albino rats. Asian J. Agric. Biol. 2024, 4, 2024152. [Google Scholar] [CrossRef]
- Ahsan, H.; Ayub, M.; Gul, M.; Qureshi, A.; Asfour, H.Z.; Bilal, H.M.; Azeem, M.; Wahid, A.; Ali, N.; Naveed, R.; et al. Efficacy of silver oxide nanoparticles against multi-drug resistant Pseudomonas aeruginosa and methicillin-resistant Staphylococcus aureus in burn wound infections. Asian J. Agric. Biol. 2024, 4, 2024096. [Google Scholar] [CrossRef]
- Mahmood, Y.; Ijaz, N.; Maheen, A.; Mustafa, G.; Bafail, D.A.; Qamar, M.R.; Ahsan, M.A.; Masood, N.; Rajeh, N.; Mohiuddin, M. Multi-biomarker approach to assess oxidative stress and antioxidant profile in male albino rats exposed to ZnO nanoparticles. Asian J. Agric. Biol. 2024, 4, 2024115. [Google Scholar] [CrossRef]
- El-Dawy, K.; Saad, S.; Hussein, M.M.A.; Yahia, R.; Al-Gamal, M. Naturally based nano formulation in metabolic and reproductive disorders: A review. Int. J. Vet. Sci. 2023, 12, 7–17. [Google Scholar] [CrossRef]
- Altaf, S.; Iqbal, T.; Salma, U.; Sajid, M.; Basit, I.; Sabir, M.Z.; Riaz, K.; Rasheed, R.; Umair, M.; Talha, R. Gold nanoparticles for the detection of organophosphate. Agrobiol. Rec. 2024, 16, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Hendrawan, V.F.; Agustina, G.C.; Residiwati, G.; Amir, M.A.M. The effect of nano-curcumin on vascular endothelial growth factor (VEGF) expression and fetal length in stressed pregnant mice. Int. J. Vet. Sci. 2024, 13, 903–907. [Google Scholar] [CrossRef]
- Azam, S.E.; Yasmeen, F.; Rashid, M.S.; Ahmad, U.; Hussain, S.; Perveez, A.; Sarib, M. Silver nanoparticles loaded LDPE packaging: Antibacterial study against Listeria monocytogenes, Bacillus subtilis, and Staphylococcus aureus. Int. J. Agric. Biosci. 2023, 12, 165–171. [Google Scholar] [CrossRef]
- Rasool, M.; Rasool, M.H.; Khurshid, M.; Aslam, B. Biogenic synthesis and characterization of silver nanoparticles: Antioxidant, anti-inflammatory, and antimicrobial potential. Asian J. Agric. Biol. 2024, 3, 2023364. [Google Scholar]
- Xu, L.; Wang, Y.Y.; Huang, J.; Chen, C.Y.; Wang, Z.X.; Xie, H. Silver nanoparticles: Synthesis, medical applications, and biosafety. Theranostics 2020, 10, 8996–9005. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, H.N.; Abood, S.S.; Jawad, H.S. A review on the effects of neem (Azadirachta indica) as feed additive in poultry production. J. Entomol. Zool. Stud. 2018, 6, 1331–1333. [Google Scholar]
- Landy, N.; Ghalamkari, G.H.; Toghyani, M. Performance, carcass characteristics, and immunity in broiler chickens fed dietary neem (Azadirachta indica) as alternative for an antibiotic growth promoter. Livest. Sci. 2011, 142, 305–309. [Google Scholar] [CrossRef]
- Ali, E.; Islam, M.S.; Hossen, M.I.; Khatun, M.M.; Islam, M.A. Extract of neem (Azadirachta indica) leaf exhibits bactericidal effect against multidrug resistant pathogenic bacteria of poultry. Vet. Med. Sci. 2021, 7, 1921–1927. [Google Scholar] [CrossRef]
- Tortella, G.R.; Rubilar, O.; Durán, N.; Diez, M.C.; Martínez, M.; Parada, J.; Seabra, A.B. Silver nanoparticles: Toxicity in model organisms and hazards for human health and the environment. J. Hazard. Mater. 2020, 390, 121974. [Google Scholar] [CrossRef]
- Liaqat, I.; Omer, M.O.; Rasheed, M.A.; Raza, S. Preparation and anticancer evaluation of albendazole-loaded zinc oxide nanoparticles. Pak. Vet. J. 2024, 44, 1338–1344. [Google Scholar]
- Naz, S.; Bibi, G.; Nadeem, R.; Alhidary, I.; Dai, S.; Israr, M.; Khan, R.U. Evaluation of biological selenium nanoparticles in Japanese quails. Vet. Q. 2024, 44, 1–10. [Google Scholar] [CrossRef]
- David, L.; Moldovan, B.; Vulcu, A.; Olenic, L.; Perde-Schrepler, M.; Fischer-Fodor, E.; Florea, A.; Crisan, M.; Chiorean, I.; Clichici, S.; et al. Green synthesis, characterization and anti-inflammatory activity of silver nanopar-ticles using European black elderberry fruits extract. Colloids Surf. B Biointerfaces 2014, 122, 767–777. [Google Scholar] [CrossRef]
- Sahayaraj, K.; Rajesh, S.; Rathi, J.M. Silver nanoparticles biosynthesis using marine alga Padina pavonica and its microbicidal activity. Dig. J. Nanomater. Biostruct. 2012, 7, 1842–3582. [Google Scholar]
- Ugwuoke, K.C. Effects of green-synthesized silver nanoparticles from Azadirachta indica on growth and liver function in rats. Cell Biol. Dev. 2023, 7, 1–10. [Google Scholar] [CrossRef]
- Oliveira, M.J.A.; Silva, R.A.; Pereira, L.C.; Villegas, G.M.E.; Otubo, L.; García, A.F.; Gonzalez-Pérez, G.; Vasquez, P.A.S. Assessment of long-term stability in silver nanoparticlesgenerated by gamma radiation. Mater. Lett. 2025, 371, 135285. [Google Scholar] [CrossRef]
- Izak-Nau, E.; Huk, A.; Reidy, B.; Uggerud, H.; Vadset, M.; Eiden, S.; Voetz, M.; Himly, M.; Duschl, A.; Dusinska, M.; et al. Impact of storage conditions and storage time on silver nanoparticles’ physicochemical properties and implications for their biological effects. RSC Adv. 2015, 5, 84172–84185. [Google Scholar] [CrossRef]
- Pineda, L.; Chwalibog, A.; Sawosz, E.; Lauridsen, C.; Engberg, R.M.; Elnif, J.; Hotowy, A.; Sawosz, F.; Gao, Y.; Ali, A.; et al. Effect of silver nanoparticles on growth performance, metabolism and microbial profile of broiler chickens. Arch. Anim. Nutr. 2012, 66, 416–429. [Google Scholar] [CrossRef]
- Kumar, I.; Bhattacharya, J.; Das, B.K.; Lahiri, P. Growth, serum biochemical and histopathological responses of broilers administered with silver nanoparticles as a drinking-water disinfectant. 3 Biotech 2020, 10, 94. [Google Scholar] [CrossRef]
- Gallocchio, F.; Biancotto, G.; Cibin, V.; Losasso, C.; Belluco, S.; Peters, R.; van Bemmel, G.; Cascio, C.; Weigel, S.; Tromp, P.; et al. Transfer study of silver nanoparticles in poultry production. J. Agric. Food Chem. 2017, 65, 1234–1242. [Google Scholar] [CrossRef]
- Zaoui, Y.; Belanche, A.; Ben-Jeddou, K.; Jiménez, M.S.; Fondevila, G.; Fondevila, M. Effect of the dietary administration pattern of silver nanoparticles on broiler performance. Feed Res. J. 2024, 309, 115888. [Google Scholar]
- Saeb, A.T.M.; Alshammari, A.S.; Al-Brahim, H.; Al-Rubeaan, K.A. Production of silver nanoparticles with strong and stable colloidal properties: Zeta potential and stability considerations. J. Nanomater. 2014, 2014, 704708. [Google Scholar] [CrossRef]
- Khalid, A.; Satti, S.; Ayyub, A.; Nawab, F.; Tahir, M.; Naz, S.; Ibiwoye, D.I. Blood, feathers, and eggs as bioindicators of selenium sources and DNA damage in Japanese quails (Coturnix japonica). Res. Vet. Sci. 2025, 185, 105556. [Google Scholar] [CrossRef]
- Rutkowska, M.; Płotka-Wasylka, J.; Lubinska-Szczygeł, M.; Różańska, A.; Możejko-Ciesielska, J.; Namieśnik, J. Birds’ feathers—Suitable samples for environmental pollutant analysis. TrAC Trends Anal. Chem. 2018, 109, 97–115. [Google Scholar] [CrossRef]
- Ashkoo, A.; Amininasab, S.M.; Zamani-Ahmadmahmoodi, R. Bioaccumulation of heavy metals in eggshells and egg contents of seabirds. Mar. Pollut. Bull. 2020, 154, 111126. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Chand, N.; Naz, S.; Rehman, Z.; Khan, R.U. Blood biochemical profile of four fast-growing broiler strains under heat stress. Appl. Biol. Chem. 2018, 61, 273–279. [Google Scholar] [CrossRef]
- Bhattarai, B.; Zaker, Y.; Bigioni, T.P. Green synthesis of gold and silver nanoparticles: Challenges and op-portunities. Curr. Opin. Green Sustain. Chem. 2018, 12, 91–100. [Google Scholar] [CrossRef]
- Khan, Y.; Sadia, H.; Ali Shah, S.Z.; Khan, M.N.; Shah, A.A.; Ullah, N.; Khan, M.I. Classification and applications of nanoparticles in nanotechnology. Catalysts 2022, 12, 1386. [Google Scholar] [CrossRef]
- Behra, R.; Sigg, L.; Clift, M.J.; Herzog, F.; Minghetti, M.; Johnston, B.; Rothen-Rutishauser, B. Bioavailability of silver nanoparticles and ions: A biochemical perspective. J. R. Soc. Interface 2013, 10, 20130396. [Google Scholar] [CrossRef]
- Luo, X.; Xie, X.; Meng, Y.; Sun, T.; Ding, J.; Zhou, W. Ligands dissociation induced gold nanoparticles aggregation for colorimetric Al3+ detection. Anal. Chim. Acta 2019, 1087, 76–85. [Google Scholar] [CrossRef]
- Ansari, M.; Darvishi, A. A review of the current state of natural biomaterials in wound healing. Front. Bioeng. Biotechnol. 2024, 12, 1309541. [Google Scholar] [CrossRef] [PubMed]
- Einoder, L.D.; MacLeod, C.K.; Coughanowr, C. Metal and isotope analysis of bird feathers in a contaminated estuary reveals bioaccumulation, biomagnification, and potential toxic effects. Arch. Environ. Contam. Toxicol. 2018, 75, 96–110. [Google Scholar] [CrossRef] [PubMed]
- Adeogun, A.O.; Chukwuka, A.V.; Fadahunsi, A.A.; Okali, K.D.; Oluwakotanmi, P.G.; Ibor, O.R.; Emasoga, P.; Egware, T.U. Bird feathers as a non-invasive method for ecotoxicological monitoring; a rapid review. Zoologist 2022, 20, 26–40. [Google Scholar] [CrossRef]
- Borghesi, F.; Migani, F.; Andreotti, A.; Baccetti, N.; Bianchi, N.; Birke, M.; Dinelli, E. Metals and trace elements in feathers: A geochemical approach. Sci. Total Environ. 2016, 544, 476–494. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, B.B.; Silva, P.L.A.P.A.; Silva, A.C.A.; Dantas, N.O.; De Paula, A.T.; Olivieri, O.C.L.; Goulart, L.R. Nanocomposite of Ag-doped ZnO and AgO nanocrystals to control biofilm formation in eggshells. Front. Microbiol. 2019, 10, 217. [Google Scholar] [CrossRef]
- Shevchenko, L.V.; Dovbnia, Y.Y.; Zheltonozhskaya, T.B.; Permyakova, N.M.; Shulyak, S.V. Influence of preparation of silver nanoparticles in carriers based on polymer/inorganic hybrids on the mineral composition of chicken eggs. Regul. Mech. Biosyst. 2021, 12, 608–613. [Google Scholar] [CrossRef]
- Farzinpour, P.; Roudsari, A.T.; Nazari, M.; Samadi, F. Effects of silver nanoparticles on egg quality traits in laying Japanese quail. Appl. Nanosci. 2013, 3, 13–18. [Google Scholar] [CrossRef]
- Chen, J.; Chen, F.; Peng, S.; Ou, Y.; He, B.; Li, Y.; Lin, Q. Effects of Artemisia argyi powder on egg quality, antioxidant capacity, and intestinal development of roman laying hens. Front. Physiol. 2022, 13, 902568. [Google Scholar] [CrossRef]
- Hincke, M.T.; Nys, Y.; Gautron, J.; Mann, K.; Rodriguez-Navarro, A.B.; McKee, M.D. The eggshell: Structure, composition and mineralization. Front. Biosci. 2012, 17, 1266–1280. [Google Scholar] [CrossRef]
- Rosaiah, P.; Yue, D.; Dayanidhi, K.; Ramachandran, K.; Vadivel, P.; Eusuff, N.S.; Kim, W.K. Eggshells and membranes: Sustainable resources for energy applications. Adv. Colloid Interface Sci. 2024, 327, 103144. [Google Scholar] [CrossRef]
- Wilson, P.B. Recent advances in avian egg science: A review. Poult. Sci. J. 2017, 96, 3747–3754. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Huang, R.; Chen, C.; Yi, J.; Kai, T.; Zhan, Y.; Wei, X.; Wang, D.; Zhang, J.; Ding, P. Aptamer-AuNP-conjugated carboxymethyl chitosan–functionalized graphene oxide for colorimetric identification of Salmonella typhimurium. Microchim. Acta 2022, 189, 408. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Li, Z.H.; Liu, T.H.; Li, C.; Xie, Y.L.; Ge, L.X.; Tang, J.; Li, F.F.; Luo, X.; Fu, L.J.; et al. Evaluating the role of DNA methyltransferases in trophoblast fusion and preeclampsia development: Insights from methylation-regulated genes. FASEB J. 2024, 38, e23706. [Google Scholar] [CrossRef] [PubMed]
- Croteau, M.N.; Misra, S.K.; Luoma, S.N.; Valsami-Jones, E. Silver bioaccumulation dynamics in freshwater invertebrates. Environ. Sci. Technol. 2011, 45, 6600–6607. [Google Scholar] [CrossRef]
- Mahjoubian, M.; Naeemi, A.S.; Moradi-Shoeili, Z.; Tyler, C.R.; Mansouri, B. Oxidative stress and genotoxic effects of Ag and ZnO nanoparticles in zebrafish. Toxicol. Appl. Pharmacol. 2023, 472, 116569. [Google Scholar] [CrossRef]
- Kumar, S.; Basumatary, I.B.; Sudhani, H.P.; Bajpai, V.K.; Chen, L.; Shukla, S.; Mukherjee, A. Plant extract-mediated silver nanoparticles and their antimicrobial applications. Trends Food Sci. Technol. 2021, 112, 651–666. [Google Scholar] [CrossRef]
- Bashir, F.; Sharif, S.; Manzoor, F.; Naz, S.; Rashid, F. Protective effects of Moringa oleifera leaf extract against AgNP- and arsenic-induced hepatotoxicity in rats. Pak. Vet. J. 2024, 44, 377–383. [Google Scholar]
- Wang, E.C.; Wang, A.Z. Nanoparticles and their applications in cell and molecular biology. Integr. Biol. 2014, 6, 9–26. [Google Scholar] [CrossRef]

| Ingredients | Percentage (%) |
|---|---|
| Soyabean oil | 0.50 |
| Soyabean meal | 21.60 |
| Wheat | 4.10 |
| Calcium phosphate (GR) | 1.00 |
| Calcium carbonate (GR) | 9.50 |
| Cottonseed meal | 2.00 |
| Corn | 60.50 |
| Phytases | 0.015 |
| Sodium chloride | 0.30 |
| DL-methionine | 0.08 |
| Vitamin premix | 0.15 |
| Mineral premix | 0.035 |
| 50% Choline chloride | 0.10 |
| Experimental additives | 0.12 |
| Total | 100.0 |
| Nutrient analysis | |
| Methionine + Cysteine (%) | 0.55 |
| Apparent metabolizable energy (AME), Mcal/kg | 2.57 |
| Sec (mg/kg) | 0.056 |
| Crude protein (%) | 24.0 |
| Lysine (%) | 0.74 |
| Total phosphorus (%) | 0.54 |
| Available phosphorus (%) | 0.31 |
| Calcium (%) | 3.7 |
| Methionine (%) | 0.3 |
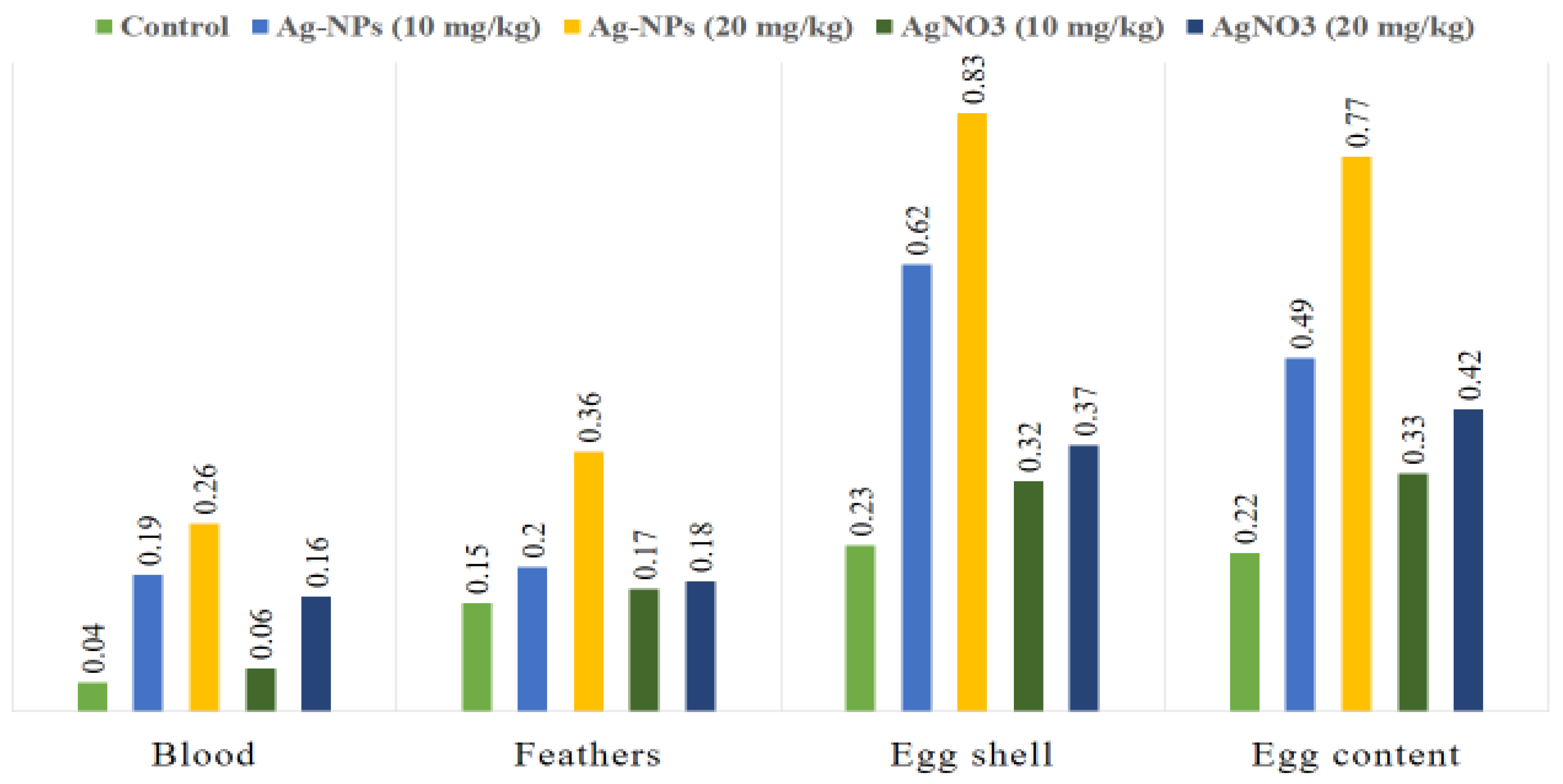
| Samples | Control | Ag-NPs (10 mg/kg) | Ag-NPs (20 mg/kg) | Ag-NO3 (10 mg/kg) | Ag-NO3 (20 mg/kg) | p Value |
|---|---|---|---|---|---|---|
| Blood | 0.04 ± 0.01 d (SE = 0.004; 95% CI: 0.03–0.05) | 0.19 ± 0.01 b (SE = 0.004; CI: 0.18–0.20) | 0.26 ± 0.01 a (SE = 0.004; CI: 0.25–0.27) | 0.06 ± 0.02 d (SE = 0.004; CI: 0.25–0.27) | 0.16 ± 0.02 c (SE = 0.008; CI: 0.04–0.08 | 0.001 |
| Feathers | 0.15 ± 0.00 d (SE = 0.002; CI: 0.15–0.16) | 0.20 ± 0.02 b (SE = 0.008; CI: 0.18–0.22) | 0.36 ± 0.01 a (SE = 0.004; CI: 0.35–0.37) | 0.17 ± 0.00 cd (SE = 0.012; CI: 0.29–0.35) | 0.18 ± 0.01 c (SE = 0.002; CI: 0.16–0.18) | 0.001 |
| Egg shell | 0.23 ± 0.02 e (SE = 0.008; CI: 0.21–0.25) | 0.62 ± 0.04 b (SE = 0.016; CI: 0.59–0.65) | 0.83 ± 0.02 a (SE = 0.008; CI: 0.81–0.85) | 0.32 ± 0.03 d (SE = 0.012; CI: 0.29–0.35) | 0.37 ± 0.04 c (SE = 0.012; CI: 0.29–0.35) | 0.001 |
| Egg content | 0.22 ±0.04 e (SE = 0.016; CI: 0.18–0.26) | 0.49 ± 0.07 b (SE = 0.028; CI: 0.42–0.56) | 0.77 ± 0.04 a (SE = 0.016; CI: 0.73–0.81) | 0.33 ± 0.04 d (SE = 0.016; CI: 0.29–0.37) | 0.42 ± 0.04 c (SE = 0.016; CI: 0.29–0.37) | 0.001 |
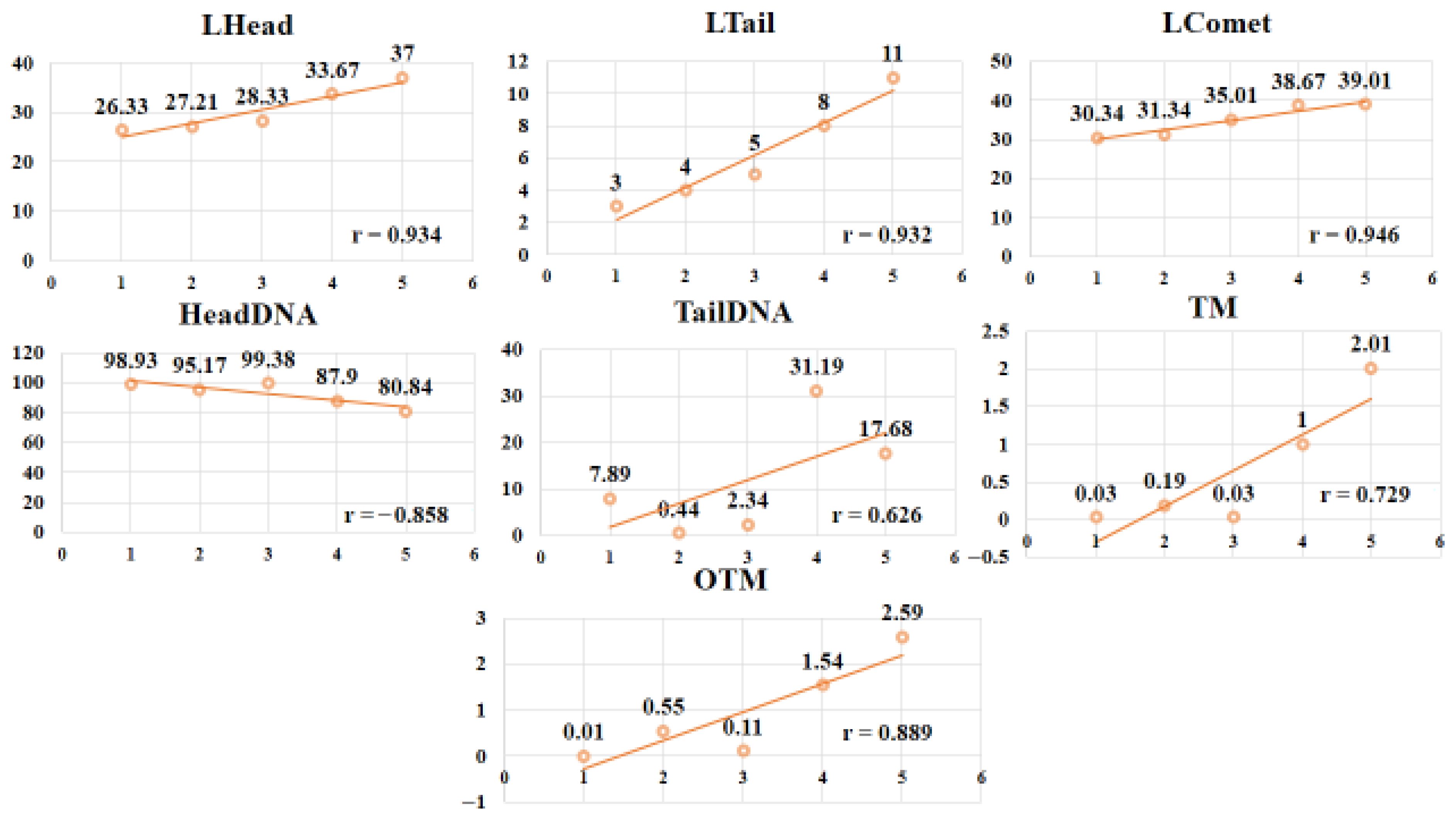
| Parameters | Control | Ag-NPs (10 mg/kg) | Ag-NPs (20 mg/kg) | AgNO3 (10 mg/kg) | AgNO3 (20 mg/kg) | p Value |
|---|---|---|---|---|---|---|
| LHead | 26.33 ± 2.31 c (SE = 0.94; CI: 24.3–28.3) | 27.21 ± 2.31 c (SE = 0.94; CI: 25.2–29.2) | 28.33 ± 8.33 c (SE = 3.40; CI: 21.0–35.7) | 33.67 ± 4.16 b (SE = 1.70; CI: 30.1–37.2) | 37.00 ± 3.46 a (SE = 1.41; CI: 33.9–40.1) | 0.000 |
| LTail | 3.00 ± 0.00 b (SE = 0.00; CI: 3.0–3.0) | 4.00 ± 0.00 b (SE = 0.00; CI: 4.0–4.0) | 5.00 ± 0.00 b (SE = 0.00; CI: 5.0–5.0) | 8.00 ± 1.00 a (SE = 0.41; CI: 7.1–8.9) | 10.67 ± 1.15 a (SE = 0.47; CI: 9.5–11.8) | 0.000 |
| LComet | 30.34 ± 2.30 c (SE = 0.94; CI: 28.3–32.3) | 31.34 ± 2.31 c (SE = 0.94; CI: 29.3–33.3) | 35.00 ± 3.61 b (SE = 1.47; CI: 32.0–38.0) | 38.67 ± 4.16 a (SE = 1.70; CI: 35.1–42.2) | 39.00 ± 8.71 a (SE = 3.56; CI: 31.4–46.6) | 0.000 |
| HeadDNA | 98.93 ± 0.94 a (SE = 1.63; CI: 4.3–11.5) | 95.17 ± 2.82 b (SE = 1.15; CI: 92.9–97.4) | 99.38 ± 0.26 a (SE = 0.11; CI: 99.1–99.7) | 87.90 ± 7.87 c (SE = 3.21; CI: 80.9–94.9) | 80.84 ± 5.44 d (SE = 2.22; CI: 75.8–85.8) | 0.000 |
| TailDNA | 7.89 ± 4.00 c (SE = 0.01; CI: 0.02–0.04) | 0.44 ± 0.41 d (SE = 0.17; CI: 0.0–0.8) | 2.34 ± 2.04 d (SE = 0.83; CI: 0.7–4.0) | 31.19 ± 24.37 a (SE = 9.95; CI: 11.6–50.8) | 17.68 ± 27.42 b (SE = 11.2; CI: −6.0–41.3) | 0.000 |
| TM | 0.03 ± 0.02 b (SE = 0.01; CI: 0.02–0.04) | 0.19 ± 0.11 b (SE = 0.04; CI: 0.09–0.29) | 0.03 ± 0.01 b (SE = 0.00; CI: 0.02–0.04) | 1.00 ± 0.67 ab (SE = 0.27; CI: 0.3–1.7) | 2.01 ± 0.45 a (SE = 0.18; CI: 1.5–2.5) | 0.013 |
| OTM | 0.01 ± 0.11 e (SE = 0.05; CI: −0.09–0.11) | 0.55 ± 0.32 c (SE = 0.13; CI: 0.3–0.8) | 0.11 ± 0.53 d (SE = 0.22; CI: −0.4–0.6) | 1.54 ± 0.89 b (SE = 0.36; CI: 0.7–2.4) | 2.59 ± 0.48 a (SE = 0.19; CI: 2.0–3.2) | 0.000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Khalaifah, H.; Fatima, N.; Naz, S.; Maqbool, B.; Khan, R.U.; Ayyub, A.; Usama, M.; Ashfaq, S.; Shehzadi, H.; Satti, S.; et al. Comprehensive Assessment of Silver Bioaccumulation and DNA Damage Effects in Coturnix coturnix japonica via Blood, Feather, and Egg Using Two Different Sources. Animals 2025, 15, 3370. https://doi.org/10.3390/ani15233370
Al-Khalaifah H, Fatima N, Naz S, Maqbool B, Khan RU, Ayyub A, Usama M, Ashfaq S, Shehzadi H, Satti S, et al. Comprehensive Assessment of Silver Bioaccumulation and DNA Damage Effects in Coturnix coturnix japonica via Blood, Feather, and Egg Using Two Different Sources. Animals. 2025; 15(23):3370. https://doi.org/10.3390/ani15233370
Chicago/Turabian StyleAl-Khalaifah, Hanan, Nudrat Fatima, Shabana Naz, Babar Maqbool, Rifat Ullah Khan, Ankqash Ayyub, Muhammad Usama, Swaira Ashfaq, Hifza Shehzadi, Sania Satti, and et al. 2025. "Comprehensive Assessment of Silver Bioaccumulation and DNA Damage Effects in Coturnix coturnix japonica via Blood, Feather, and Egg Using Two Different Sources" Animals 15, no. 23: 3370. https://doi.org/10.3390/ani15233370
APA StyleAl-Khalaifah, H., Fatima, N., Naz, S., Maqbool, B., Khan, R. U., Ayyub, A., Usama, M., Ashfaq, S., Shehzadi, H., Satti, S., Abudabos, A., & Alhidary, I. A. (2025). Comprehensive Assessment of Silver Bioaccumulation and DNA Damage Effects in Coturnix coturnix japonica via Blood, Feather, and Egg Using Two Different Sources. Animals, 15(23), 3370. https://doi.org/10.3390/ani15233370









