M-Mode and Tissue Doppler Ultrasonographic Assessment of Diaphragmatic Function in Dogs with and Without Respiratory Distress
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Diaphragm Ultrasound Technique and Diaphragmatic Measurements
2.3. Interobserver Variability
2.4. Statistical Analysis
3. Results
3.1. Clinical Demographic Data
3.2. M-Mode Results
3.3. Tissue Doppler Results
3.4. Interobserver Variability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AUC | Area under the curve |
| CI | Confidence interval |
| DE | Diaphragmatic excursion |
| ICC | Intraclass correlation coefficient |
| ICU | Intensive care unit |
| MRR | Maximal relaxation rate |
| PCV | Peak contraction velocity |
| PRV | Peak relaxation velocity |
| RD | Respiratory distress |
| ROC | Receiver operating characteristic |
| RR | Respiratory rate |
| TDI | Tissue Doppler Imaging |
| Te | Expiratory time |
| Ti | Inspiratory time |
| US | Ultrasound |
| Ve | Diaphragmatic contraction velocity |
References
- May, L.A.; Epelman, M.; Navarro, O.M. Ultrasound imaging of diaphragmatic motion. Pediatr. Radiol. 2022, 52, 2051–2061. [Google Scholar] [CrossRef] [PubMed]
- Rozanski, E.; Chan, D.L. Approach to the patient with respiratory distress. Vet. Clin. N. Am. Small Anim. Pract. 2005, 35, 307–317. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.; Park, S.; Lee, S.K.; Cheon, B.; Choi, J. Fluoroscopic evaluation of diaphragmatic excursion during spontaneous breathing in healthy Beagles. Am. J. Vet. Res. 2017, 78, 1043–1048. [Google Scholar] [CrossRef] [PubMed]
- Qian, Z.; Yang, M.; Li, L.; Chen, Y. Ultrasound assessment of diaphragmatic dysfunction as a predictor of weaning outcome from mechanical ventilation: A systematic review and meta-analysis. BMJ Open 2018, 8, e021189. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.R.; Pereira, J.M.; Paiva, J.A.; de Casasola-Sánchez, G.G.; Tung-Chen, Y. Ultrasonography to Access Diaphragm Dysfunction and Predict the Success of Mechanical Ventilation Weaning in Critical Care: A Narrative Review. J. Ultrasound Med. 2024, 43, 223–236. [Google Scholar] [CrossRef]
- Zhao, H.; Long, L.; Liu, Y.; Yan, Y.; Wang, X.; Zhao, H.; Li, L. Multimodal diaphragmatic ultrasound indicators in healthy adults: Reliability and consistency observation by ultrasound physician and critical care physician. Front. Med. 2025, 12, 1542979. [Google Scholar] [CrossRef]
- Soilemezi, E.; Savvidou, S.; Sotiriou, P.; Smyrniotis, D.; Tsagourias, M.; Matamis, D. Tissue Doppler Imaging of the Diaphragm in Healthy Subjects and Critical Ill Patients. Am. J. Respir. Crit. Care Med. 2020, 202, 1005–10212. [Google Scholar] [CrossRef]
- Liu, R.; Liu, Y.; Liang, Y.; He, C.; Liu, X.; Xin, S. Prediction of Weaning Outcomes in Mechanically Ventilated Patients Using Diaphragmatic Excursion with Tissue Doppler Imaging Variables of the Diaphragm. Respir. Care 2025, 70, 408–416. [Google Scholar] [CrossRef]
- Carrillo, D.; Ibatá, D.; Lyons, L.; Silva, N.; Pulido-Medellin, M.; Balaguera, H.; Romero-Núñez, C.; Roque-Rodríguez, A. Evaluación del movimiento diafragmático por ultrasonografía mediante la medición del índice de excursión en perros. Orinoquia 2019, 23, 54–62. [Google Scholar] [CrossRef]
- Saisawart, P.; Sutthigran, S.; Soontornvipart, K.; Thanaboonnipat, C.; Darawiroj, D.; Choisunirachon, N. The Feasibility of Ultrasonographic Diaphragmatic Excursion in Healthy Dogs: Effect of Positioning, Diaphragmatic Location and Body Weight of Dogs. Front. Vet. Sci. 2021, 8, 763556. [Google Scholar] [CrossRef]
- Choi, M.; Lee, N.; Kim, A.; Keh, S.; Lee, J.; Kim, H.; Choi, M. Evaluation of diaphragmatic motion in normal and diaphragmatic paralyzed dogs using M-Mode ultrasonography. Vet. Radiol. Ultrasound 2014, 55, 102–108. [Google Scholar] [CrossRef]
- Drury, B.L.; Brinkman, E.L.; Gambino, J.M.; Lee, A.M.; Wills, R.W.; Beasley, M.J. Diaphragmatic dysfunction in dogs with cervical spinal disorders before and after surgery using fluoroscopy, motion-mode ultrasound and radiography was not different than a group of control dogs. Vet. Radiol. Ultrasound 2020, 61, 353–363. [Google Scholar] [CrossRef] [PubMed]
- Saisawart, P.; Sutthigran, S.; Kasemsuwan, T.; Sakulsirajit, R.; Ritthikulprasert, S.; Tachampa, K.; Thanaboonnipat, C.; Choisunirachon, N. Efficacy of ultrasonographic diaphragmatic parameters in distinguishing diaphragmatic dysfunction in cats. J. Feline Med. Surg. 2024, 26, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Marrugat, J. GRANMO Sample Size Calculator. Available online: https://www.datarus.eu/aplicaciones/granmo/ (accessed on 17 November 2025).
- Greene, C.E.; Basinger, R.R.; Whitfield, J.B. Surgical management of bilateral diaphragmatic paralysis in a dog. J. Am. Vet. Med. Assoc. 1988, 193, 1542–1544. [Google Scholar] [CrossRef] [PubMed]
- Amory, H.; Lomba, F.; Lekeux, P.M.; Solal, A.N.; Jauniaux, T.P.; Desmecht, D.J. Bilateral diaphragmatic paralysis in a pony. J. Am. Vet. Med. Assoc. 1994, 205, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Bedenice, D.; Mazan, M.R.; Kuehn, H.; Hoffman, A.M. Diaphragmatic paralysis due to phrenic nerve degeneration in a llama. J. Vet. Intern. Med. 2002, 16, 603–606. [Google Scholar] [CrossRef]
- Vignoli, M.; Toniato, M.; Rossi, F.; Terragni, R.; Manzini, M.; Franchi, A.; Pozzi, L. Transient post-traumatic hemidiaphragmatic paralysis in two cats. J. Small Anim. Pract. 2002, 43, 312–316. [Google Scholar] [CrossRef]
- Park, R.D. The diaphragm. In Textbook of Veterinary Diagnostic Radiology, 5th ed.; Thrall, D.E., Ed.; WB Saunders: Philadelphia, PA, USA, 2007; p. 537. [Google Scholar]
- Lori, L.L.; Simpson, A.M.; Han, E. Pleural and extrapleural disease. In Textbook of Veterinary Internal Medicine, 7th ed.; Ettinger, S.J., Feldman, E.C., Eds.; WB Saunders: Philadelphia, PA, USA, 2010; p. 1125. [Google Scholar]
- Byers, S.; Barrington, G.; Nelson, D.; Haldorson, G.; Holt, T.; Callan, R. Neurological causes of diaphragmatic paralysis in 11 alpacas (Vicugna pacos). J. Vet. Intern. Med. 2011, 25, 380–385. [Google Scholar] [CrossRef]
- Lloyd, T.; Tang, Y.M.; Benson, M.D.; King, S. Diaphragmatic paralysis: The use of M mode ultrasound for diagnosis in adults. Spinal Cord. 2006, 44, 505–508. [Google Scholar] [CrossRef]
- Scarlata, S.; Di Matteo, E.; Finamore, P.; Perri, G.; Mancini, D.; Sogaro, L.; Grandi, T.; Brando, E.; Travaglino, F.; Sambuco, F.; et al. Diaphragmatic ultrasound evaluation in acute heart failure: Clinical and functional associations. Intern. Emerg. Med. 2024, 19, 705–711. [Google Scholar] [CrossRef]
- Ricoy, J.; Rodríguez-Núñez, N.; Álvarez-Dobaño, J.M.; Toubes, M.E.; Riveiro, V.; Valdés, L. Diaphragmatic dysfunction. Pulmonology 2019, 25, 223–235. [Google Scholar] [CrossRef] [PubMed]
- Saisawart, P.; Sutthigran, S.; Suksangvoravong, H.; Thanaboonnipat, C.; Ritthikulprasert, S.; Tachampa, K.; Choisunirachon, N. Computed tomographic diaphragmatic thickness: A promising method for the evaluation of diaphragmatic muscle in cardiopulmonary diseased cats. Front. Vet. Sci. 2023, 10, 1247531. [Google Scholar] [CrossRef] [PubMed]
- Boussuges, A.; Brégeon, F.; Blanc, P.; Gil, J.M.; Poirette, L. Characteristics of the paralysed diaphragm studied by M-mode ultrasonography. Clin. Physiol. Funct. Imaging 2019, 39, 143–149. [Google Scholar] [CrossRef] [PubMed]
- Boussuges, A.; Gole, Y.; Blanc, P. Diaphragmatic motion studied by m-mode ultrasonography: Methods, reproducibility, and normal values. Chest 2009, 135, 391–400. [Google Scholar] [CrossRef]
- Matamis, D.; Soilemezi, E.; Tsagourias, M.; Akoumianaki, E.; Dimassi, S.; Boroli, F.; Richard, J.C.; Brochard, L. Sonographic evaluation of the diaphragm in critically ill patients. Technique and clinical applications. Intensive Care Med. 2013, 39, 801–810. [Google Scholar] [CrossRef]
- Grandage, J. The radiology of the dog’s diaphragm. J. Small Anim. Pract. 1974, 15, 1–18. [Google Scholar] [CrossRef]
- Fayssoil, A.; Nguyen, L.S.; Ogna, A.; Stojkovic, T.; Meng, P.; Mompoint, D.; Carlier, R.; Prigent, H.; Clair, B.; Behin, A.; et al. Diaphragm sniff ultrasound: Normal values, relationship with sniff nasal pressure and accuracy for predicting respiratory involvement in patients with neuromuscular disorders. PLoS ONE. 2019, 14, e0214288. [Google Scholar] [CrossRef]
- Edward Robinson, N. Control of Ventilation. In Cunningham’s Textbook of Veterinary Physiology, 5th ed.; Section VIII: Respiratory Function. Chapter 49; Elsevier Saunder: St. Louis, MI, USA, 2013; pp. 529–535. [Google Scholar]
- Fonfara, S.; de la Heras Alegret, L.; German, A.J.; Blackwood, L.; Dukes-McEwan, J.; MNoble, P.J.; Burrow, R.D. Underlying diseases in dogs referred to a veterinary teaching hospital because of dyspnea: 229 cases (2003–2007). J. Am. Vet. Med. Assoc. 2011, 239, 1219–1224. [Google Scholar] [CrossRef]
- Sigrist, N.E.; Adamik, K.N.; Doherr, M.G.; Spreng, D.E. Evaluation of respiratory parameters at presentation as clinical indicators of the respiratory localization in dogs and cats with respiratory distress. J. Vet. Emerg. Crit. Care 2011, 21, 13–23. [Google Scholar] [CrossRef]
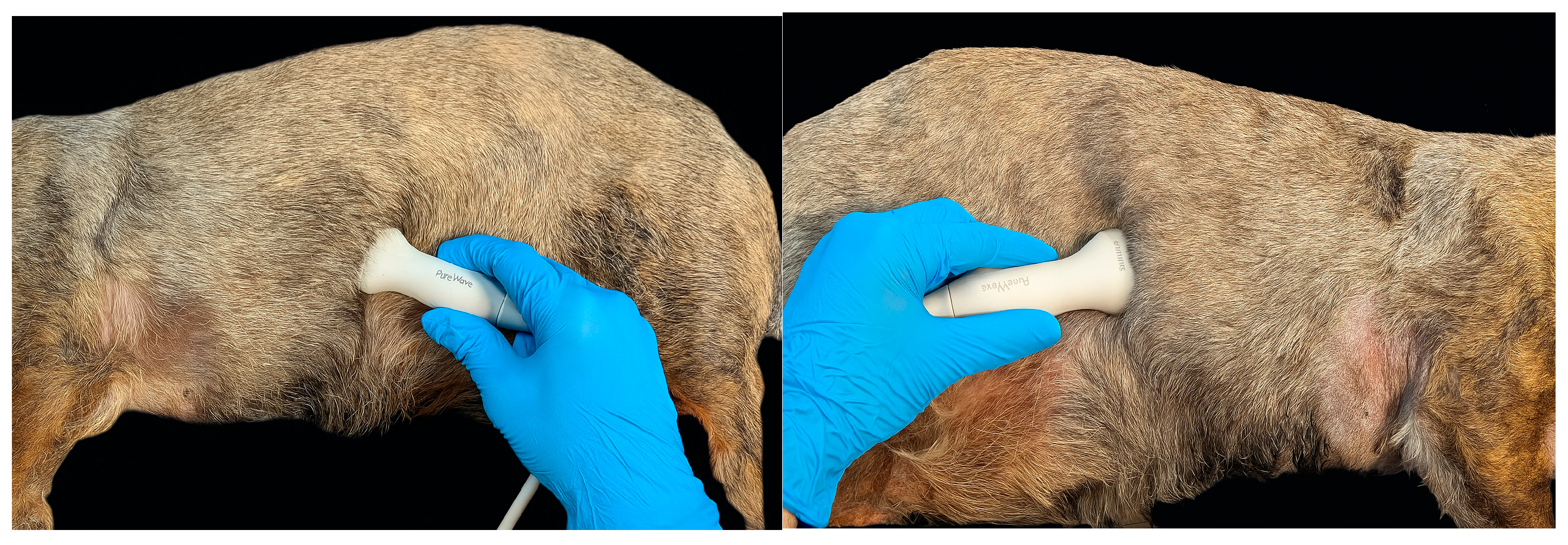
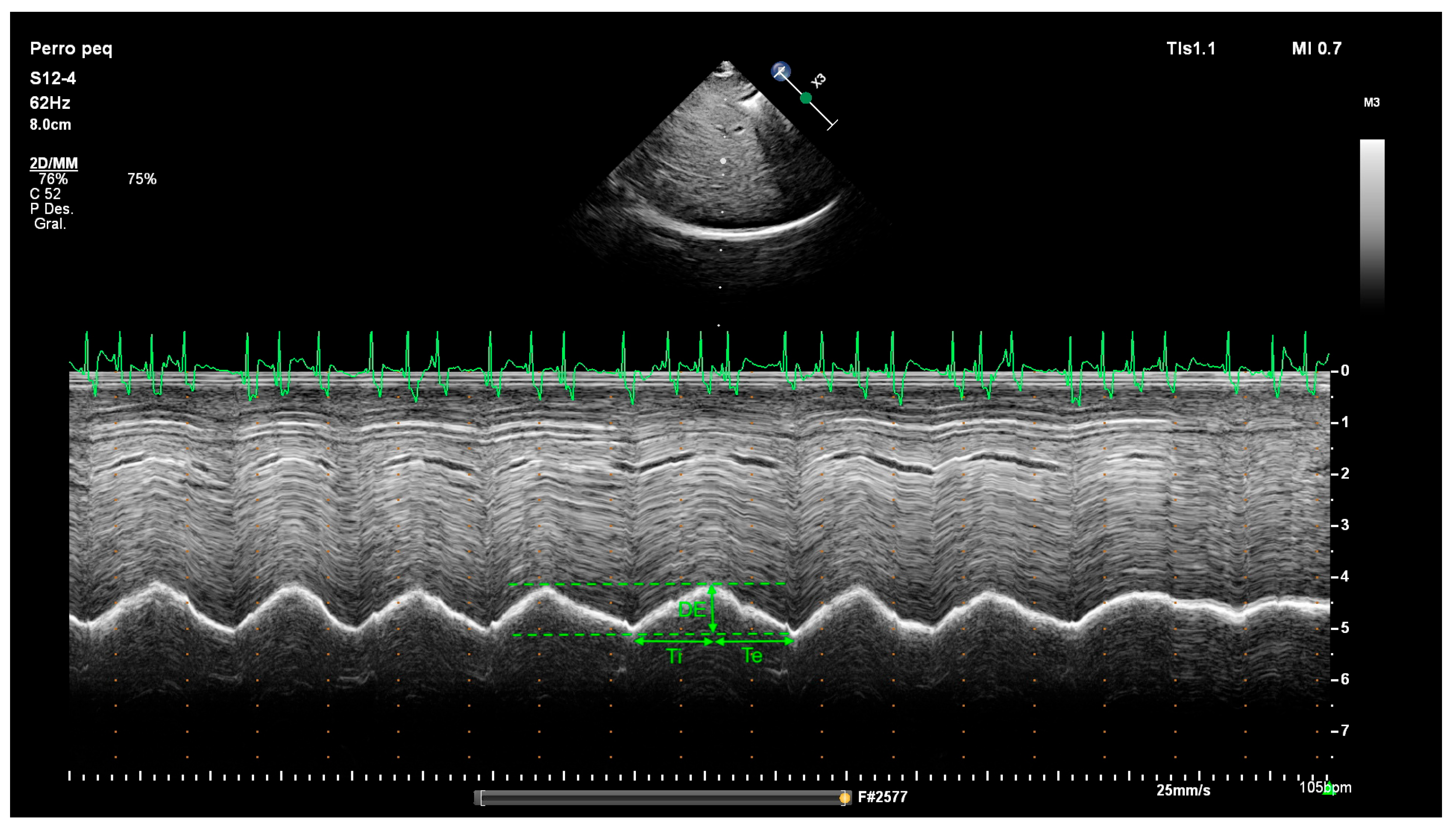
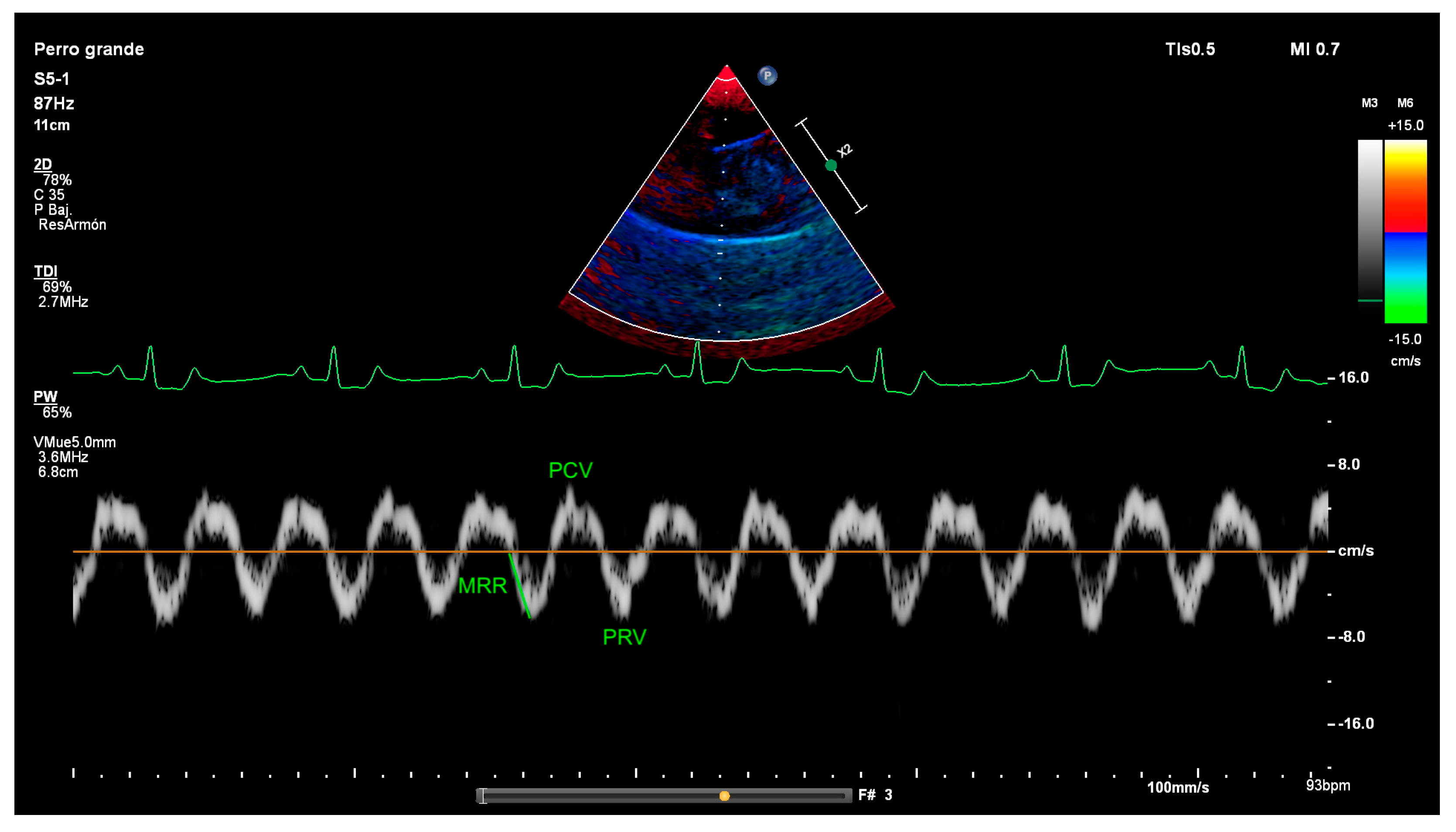
| Clinical Parameter (Resting Dogs in Consultation Room) | Score |
|---|---|
| Behaviour attitude towards breathing | Relaxed, no explicit breathing attention = 0 Glazed expression, anxious breathing = 1 |
| Body posture | Comfortable, natural = 0 Persistent standing or sitting = 1 Stretched neck, nasal flare, orthopnoea = 2 |
| Rib movement | Subtle craniolateral movement = 0 Rib marking, intercostal spaces sink = 1 |
| Abdominal movement | Slightly outward = 0 Accentuated, in opposition to the costal movement = 1 |
| Activation of the abdominal muscles during exhalation (abdominal palpation) | No activation is perceived = 0 Active and prolonged contraction of abdominal muscles during exhalation = 1 |
| Respiratory rhythmicity | Short and compensated respiratory phases = 0 Prolonged inhalation/exhalation = 1 |
| Synchronization of thoracic and abdominal movement | Synchronous = 0 Asynchronous = 1 |
| Perception of respiratory effort | Smooth and barely noticeable = 0 Difficult or laboured breathing = 1 |
| Tolerance to breathing with the mouth closed (either spontaneous or manually closed) | Complete = 0 It causes discomfort and manifests as effort = 1 |
| Presence of stridor, crackles, snoring (pre-auscultation) | Missing = 0 Present = 1 |
| Wheezing or crackles (pulmonary auscultation) | Missing = 0 Present = 1 |
| All n (%) | Respiratory Distress n (%) | p-Value (No vs. Yes) | ||
|---|---|---|---|---|
| No | Yes | |||
| 58 (100) | 31 (53.4) | 27 (46.6) | ||
| Age and size ^ | ||||
| Age (years) | 10.64 ± 3.94 (9.6–11.67) | 10.65 ± 3.99 (9.18–12.11) | 10.63 ± 3.96 (9.06–12.19) | 0.802 |
| Body weight (kg) | 7.50 ± 10.03 (8.50–13.77) | 9.67 ± 7.88 (6.78–12.56) | 12.82 ± 11.98 (8.08–17.56) | 0.322 |
| Body condition (1–9 scale) | 5.43 ± 1.34 (5.08–5.78) | 5.42 ± 1.02 (5.04–5.8) | 5.44 ± 1.65 (4.79–6.10) | 0.417 |
| Sex | ||||
| Male | 33 (56.9) | 18 (58.1) | 15 (55.6) | 0.847 |
| Female | 25 (43.1) | 13 (41.9) | 12 (44.4) | |
| p-Value (column) | 0.294 | 0.369 | 0.564 | |
| Sexual Condition | ||||
| Intact males | 18 (31) | 9 (29) | 9 (33.3) | 0.501 |
| Neutered males | 15 (25.9) | 9 (29) | 6 (22.2) | |
| Intact females | 13 (22.4) | 5 (16.1) | 8 (29.6) | |
| Neutered females | 12 (20.7) | 8 (25.8) | 4 (14.8) | |
| p-Value (column) | 0.694 | 0.709 | 0.535 | |
| Grade of respiratory distress | ||||
| No distress | 31 (53.5) | 31 (100) | - | <0.001 |
| Mild | 13 (22.41) | - | 13 (48.1) | |
| Moderate | 9 (15.5) | - | 9 (33.3) | |
| Severe | 5 (8.6) | - | 5 (18.5) | |
| p-Value (column) | <0.001 | - | 0.017 | |
| Disease group | ||||
| Asymptomatic | 8 (13.8) | 8 (25.8) | - | 0.002 |
| Cardiac disease | 31 (53.4) | 19 (61.3) | 12 (44.4) | |
| Pulmonary disease | 7 (12.1) | - | 7 (25.9) | |
| Upper airway disease | 8 (13.8) | 3 (9.7) | 5 (18.5) | |
| Lower airway disease | 2 (3.4) | 1 (3.2) | 1 (3.7) | |
| Pleural disease | 2 (3.4) | - | 2 (7.4) | |
| p-Value (column) | <0.001 | <0.001 | 0.006 | |
| Hemi-Diaphragm | All | Respiratory Distress | p-Value (No vs. Yes) | ||
|---|---|---|---|---|---|
| No | Yes | ||||
| M-mode | |||||
| DE (mm) | Right | 8.89 ± 4.40 (7.70–10.08) | 9.06 ± 4.85 (7.25–10.87) | 8.69 ± 3.89 (7.09–10.30) | 0.548 |
| Left | 7.31 ± 3.76 (6.32–8.31) | 7.41 ± 3.27 (6.19–8.63) | 7.21 ± 4.31 (5.5–8.91) | 0.472 | |
| p -Value (column) | 0.008 | 0.074 | 0.048 | ||
| Inspiration time (ms) | Right | 785.69 ± 478.91 (656.22–915.15) | 885.50 ± 472.72 (708.98–1062.02) | 665.91 ± 467.57 (472.91–858.91) | 0.033 |
| Left | 712.44 ± 377.14 (612.37–812.51) | 814.87 ± 372.13 (675.91–853.82) | 598.63 ± 355.45 (458.02–739.24) | 0.031 | |
| p -Value (column) | 0.094 | 0.214 | 0.209 | ||
| Expiration time (ms) | Right | 977.36 ± 674.30 (795.08–1159.65) | 1127.27 ± 763.05 (842.34–1412.19) | 797.48 ± 507.62 (587.94–1007.02) | 0.080 |
| Left | 878.72 ± 605.16 (718.15–1039.29) | 1026.30 ± 682.68 (771.38–1281.22) | 714.74 ± 464.66 (530.93–898.55) | 0.048 | |
| p -Value (column) | 0.137 | 0.245 | 0.288 | ||
| Ve (cm/s) | Right | 13.74 ± 9.98 (11.04–16.44) | 12.12 ± 7.20 (9.43–14.81) | 15.69 ± 12.42 (10.56–20.82) | 0.151 |
| Left | 12.30 ± 7.83 (10.21–14.40) | 10.44 ± 5.35 (8.45–12.44) | 14.45 ± 9.63 (10.57–18.34) | 0.097 | |
| p -Value (column) | 0.080 | 0.218 | 0.209 | ||
| RR (rpm) | Right | 53.15 ± 46.63 (40.55–65.76) | 47.01 ± 51.82 (27.66–66.36) | 60.53 ± 39.28 (44.32–76.65) | 0.043 |
| Left | 58.17 ± 45.91 (45.99–70.35) | 46.37 ± 36.27 (32.82–59.91) | 71.28 ± 52.28 (50.60–91.96) | 0.039 | |
| p -Value (column) | 0.116 | 0.538 | 0.115 | ||
| Tissue Doppler | |||||
| PCV (cm/s) | Right | 4.30 ± 2.06 (3.73–4.87) | 4.20 ± 1.68 (3.53–4.86) | 4.41 ± 2.44 (3.41–5.42) | 0.855 |
| Left | 4.05 ± 1.92 (3.53–4.57) | 3.53 ± 1.37 (3.01–4.05) | 4.63 ± 2.28 (3.71–5.56) | 0.019 | |
| p -Value (column) | 0.052 | 0.011 | 0.797 | ||
| PRV (cm/s) | Right | 5.44 ± 3.08 (4.58–6.29) | 4.87 ± 2.17 (4.01–5.73) | 6.04 ± 3.78 (4.48–7.60) | 0.268 |
| Left | 4.64 ± 2.32 (4.02–5.27) | 3.88 ± 1.37 (3.36–4.40) | 5.49 ± 2.85 (4.34–6.64) | 0.010 | |
| p -Value (column) | 0.012 | 0.033 | 0.179 | ||
| MRR (cm/s2) | Right | 61.39 ± 58.86 (43.91–78.87) | 45.36 ± 32.72 (30.85–59.87) | 76.09 ± 73.00 (45.26–106.91) | 0.141 |
| Left | 56.97 ± 36.80 (46.29–67.66) | 46.71 ± 26.34 (35.59–57.83) | 67.23 ± 43.05 (49.05–85.41) | 0.124 | |
| p -Value (column) | 0.871 | 0.421 | 0.390 | ||
| BW | RRe | R-DE | L-DE | R-Ve | L-Ve | R-PCV | L-PCV | R-PRV | L-PRV | |
|---|---|---|---|---|---|---|---|---|---|---|
| All dogs | ||||||||||
| BW | 0.194 | 0.442 ** | 0.287 * | 0.591 ** | 0.432 ** | 0.242 | 0.248 | 0.350 * | 0.326 * | |
| RRe | 0.194 | −0.305 * | −0.490 ** | 0.454 ** | 0.472 ** | 0.505 ** | 0.477 ** | 0.421 ** | 0.408 ** | |
| R-DE | 0.442 ** | −0.305 * | 0.608 ** | 0.387 ** | 0.177 | 0.143 | 0.121 | 0.269 * | 0.321 ** | |
| L-DE | 0.287 * | −0.490 ** | 0.608 ** | 0.117 | 0.383 ** | 0.128 | 0.108 | 0.192 | 0.211 | |
| R-Ve | 0.591 ** | 0.454 ** | 0.387 ** | 0.117 | 0.612 ** | 0.619 ** | 0.410 ** | 0.606 ** | 0.514 ** | |
| L-Ve | 0.432 ** | 0.472 ** | 0.177 | 0.383 ** | 0.612 ** | 0.558 ** | 0.500 ** | 0.630 ** | 0.565 ** | |
| R-PCV | 0.242 | 0.505 ** | 0.143 | 0.128 | 0.619 ** | 0.558 ** | 0.511 ** | 0.795 ** | 0.684 ** | |
| L-PCV | 0.248 | 0.477 ** | 0.121 | 0.108 | 0.410 ** | 0.500 ** | 0.511 ** | 0.447 ** | 0.735 ** | |
| R-PRV | 0.350 * | 0.421 ** | 0.269 * | 0.192 | 0.606 ** | 0.630 ** | 0.795 ** | 0.447 ** | 0.722 ** | |
| L-PRV | 0.326 * | 0.408 ** | 0.321 ** | 0.211 | 0.514 ** | 0.565 ** | 0.684 ** | 0.735 ** | 0.722 ** | |
| Dogs with normal respiratory pattern | ||||||||||
| BW | 0.110 | 0.645 ** | 0.321 | 0.639 ** | 0.306 | 0.144 | 0.211 | 0.229 | 0.277 | |
| RRe | 0.110 | −0.284 | −0.495 ** | 0.398 * | 0.543 ** | 0.544 ** | 0.495 ** | 0.493 * | 0.403 * | |
| R-DE | 0.645 ** | −0.284 | 0.564 ** | 0.514 ** | 0.065 | 0.098 | 0.025 | 0.195 | 0.228 | |
| L-DE | 0.321 | −0.495 ** | 0.564 ** | 0.143 | 0.346 | −0.101 | −0.010 | 0.091 | 0.036 | |
| R-Ve | 0.639 ** | 0.398 * | 0.514 ** | 0.143 | 0.542 ** | 0.520 ** | 0.377 * | 0.558 ** | 0.481 ** | |
| L-Ve | 0.306 | 0.543 ** | 0.065 | 0.346 | 0.542 ** | 0.433 * | 0.369 | 0.621 ** | 0.321 | |
| R-PCV | 0.144 | 0.544 ** | 0.098 | −0.101 | 0.520 ** | 0.433 * | 0.548 ** | 0.734 ** | 0.637 ** | |
| L-PCV | 0.211 | 0.495 ** | 0.025 | −0.010 | 0.377 * | 0.369 | 0.548 ** | 0.339 | 0.708 ** | |
| R-PRV | 0.229 | 0.493 * | 0.195 | 0.091 | 0.558 ** | 0.621 ** | 0.734 ** | 0.339 | 0.389 * | |
| L-PRV | 0.277 | 0.403 * | 0.228 | 0.036 | 0.481 ** | 0.321 | 0.637 ** | 0.708 ** | 0.389 * | |
| Dogs with respiratory distress | ||||||||||
| BW | 0.237 | 0.204 | 0.258 | 0.440 * | 0.467 * | 0.323 | 0.112 | 0.435 * | 0.314 | |
| RRe | 0.237 | −0.376 | −0.549 ** | 0.476 * | 0.393 * | 0.503 * | 0.396 * | 0.338 | 0.330 | |
| R-DE | 0.204 | −0.376 | 0.778 ** | 0.297 | 0.326 | 0.127 | 0.187 | 0.288 | 0.398 | |
| L-DE | 0.258 | −0.549 ** | 0.778 ** | 0.183 | 0.475 * | 0.160 | 0.149 | 0.315 | 0.371 | |
| R-Ve | 0.440 * | 0.476 * | 0.297 | 0.183 | 0.695 ** | 0.732 ** | 0.358 | 0.654 ** | 0.545 ** | |
| L-Ve | 0.467 * | 0.393 * | 0.326 | 0.475 * | 0.695 ** | 0.710 ** | 0.524 ** | 0.647 ** | 0.671 ** | |
| R-PCV | 0.323 | 0.503 * | 0.127 | 0.160 | 0.732 ** | 0.710 ** | 0.398 | 0.805 ** | 0.691 ** | |
| L-PCV | 0.112 | 0.396 * | 0.187 | 0.149 | 0.358 | 0.524 ** | 0.398 | 0.363 | 0.685 ** | |
| R-PRV | 0.435 * | 0.338 | 0.288 | 0.315 | 0.654 ** | 0.647 ** | 0.805 ** | 0.363 | 0.813 ** | |
| L-PRV | 0.314 | 0.330 | 0.398 | 0.371 | 0.545 ** | 0.671 ** | 0.691 ** | 0.685 ** | 0.813 ** | |
| Hemi-Diaphragm | AUC | p-Value | Youden Index | Cut-Off Value | Sensibility | Specificity | |
|---|---|---|---|---|---|---|---|
| M-mode | |||||||
| DE | Right | 0.486 | 0.859 | 0.100 | 2.73 | 1.0 | 0.1 |
| Left | 0.444 | 0.482 | 0.122 | 11.16 | 0.22 | 0.9 | |
| Both | 0.469 | 0.575 | 0.100 | 12.19 | 0.25 | 0.85 | |
| Ti | Right | 0.332 | 0.027 | 0.027 | 190.5 | 0.96 | 0.07 |
| Left | 0.346 | 0.037 | 0 | 1702 | 0 | 1 | |
| Both | 0.349 | 0.004 | 0.017 | 147 | 1.0 | 0.02 | |
| Te | Right | 0.362 | 0.074 | 0.067 | 147 | 1.0 | 0.07 |
| Left | 0.348 | 0.037 | 0 | 3624 | 0 | 1 | |
| All | 0.358 | 0.007 | 0.017 | 113.5 | 1.0 | 0.07 | |
| Ve | Right | 0.613 | 0.137 | 0.293 | 9.82 | 0.76 | 0.53 |
| Left | 0.629 | 0.085 | 0.246 | 16.55 | 0.35 | 0.90 | |
| Both | 0.624 | 0.019 | 0.256 | 9.66 | 0.71 | 0.55 | |
| RR | Right | 0.660 | 0.037 | 0.347 | 35.18 | 0.68 | 0.77 |
| Left | 0.659 | 0.029 | 0.278 | 61.89 | 0.44 | 0.83 | |
| Both | 0.659 | 0.002 | 0.304 | 37.62 | 0.65 | 0.65 | |
| Tissue Doppler | |||||||
| PCV | Right | 0.515 | 0.856 | 0.133 | 2.94 | 0.80 | 0.33 |
| Left | 0.685 | 0.012 | 0.358 | 4.41 | 0.46 | 0.90 | |
| Both | 0.600 | 0.068 | 0.211 | 3.12 | 0.77 | 0.45 | |
| PRV | Right | 0.590 | 0.259 | 0.200 | 9.25 | 0.20 | 1.0 |
| Left | 0.702 | 0.005 | 0.366 | 5.16 | 0.54 | 0.83 | |
| Both | 0.644 | 0.007 | 0.240 | 5.34 | 0.49 | 0.75 | |
| MRR | Right | 0.627 | 0.127 | 0.250 | 31.80 | 0.75 | 0.50 |
| Left | 0.629 | 0.111 | 0.292 | 55.65 | 0.54 | 0.75 | |
| Both | 0.629 | 0.024 | 0.197 | 22.70 | 0.96 | 0.24 |
| Odds Ratio (95% CI) | p-Value | |
|---|---|---|
| BW | 0.996 (0.925–1.073) | 0.921 |
| RRe | 0.979 (0.941–1.019) | 0.304 |
| R-Ti | 1.0 (0.997–1.003) | 0.998 |
| L-Ti | 0.998 (0.993–1.003) | 0.470 |
| L-Te | 1.0 (0.998–1.001) | 0.678 |
| L-PCV | 1.109 (0.630–1.952) | 0.721 |
| L-PRV | 1.576 (0.902–2.756) | 0.110 |
| Hemi-Diaphragm | ICC | 95% CI | p-Value | |
|---|---|---|---|---|
| M-mode | ||||
| DE | Right | 0.956 | 0.862–0.986 | <0.001 |
| Left | 0.886 | 0.662–0.962 | <0.001 | |
| Ti | Right | 0.989 | 0.966–0.996 | <0.001 |
| Left | 0.976 | 0.927–0.992 | <0.001 | |
| Te | Right | 0.987 | 0.960–0.996 | <0.001 |
| Left | 0.981 | 0.943–0.994 | <0.001 | |
| Tissue Doppler | ||||
| PCV | Right | 0.970 | 0.906–0.990 | <0.001 |
| Left | 0.952 | 0.856–0.984 | <0.001 | |
| PRV | Right | 0.996 | 0.986–0.999 | <0.001 |
| Left | 0.972 | 0.917–0.991 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Talavera-López, J.; Tur-Martín, A. M-Mode and Tissue Doppler Ultrasonographic Assessment of Diaphragmatic Function in Dogs with and Without Respiratory Distress. Animals 2025, 15, 3371. https://doi.org/10.3390/ani15233371
Talavera-López J, Tur-Martín A. M-Mode and Tissue Doppler Ultrasonographic Assessment of Diaphragmatic Function in Dogs with and Without Respiratory Distress. Animals. 2025; 15(23):3371. https://doi.org/10.3390/ani15233371
Chicago/Turabian StyleTalavera-López, Jesús, and Ariana Tur-Martín. 2025. "M-Mode and Tissue Doppler Ultrasonographic Assessment of Diaphragmatic Function in Dogs with and Without Respiratory Distress" Animals 15, no. 23: 3371. https://doi.org/10.3390/ani15233371
APA StyleTalavera-López, J., & Tur-Martín, A. (2025). M-Mode and Tissue Doppler Ultrasonographic Assessment of Diaphragmatic Function in Dogs with and Without Respiratory Distress. Animals, 15(23), 3371. https://doi.org/10.3390/ani15233371







