Low-Protein-Fed Chickens Benefit from Probiotic L. salivarius and L. johnsonii on Performance and Microbiota
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Bacterial Isolation and Identification
2.2. In Vitro Evaluation of Probiotic Properties
2.3. Experiment Design
2.4. Animal Experiment Design and Management
2.5. Bacterial Culture Preparation
2.6. Sampling Procedure
2.7. Plasma Markers and Pancreatic Trypsin Assay
2.8. Intestinal Histology and Microbial Diversity Analysis
2.9. Statistical Analysis
3. Results
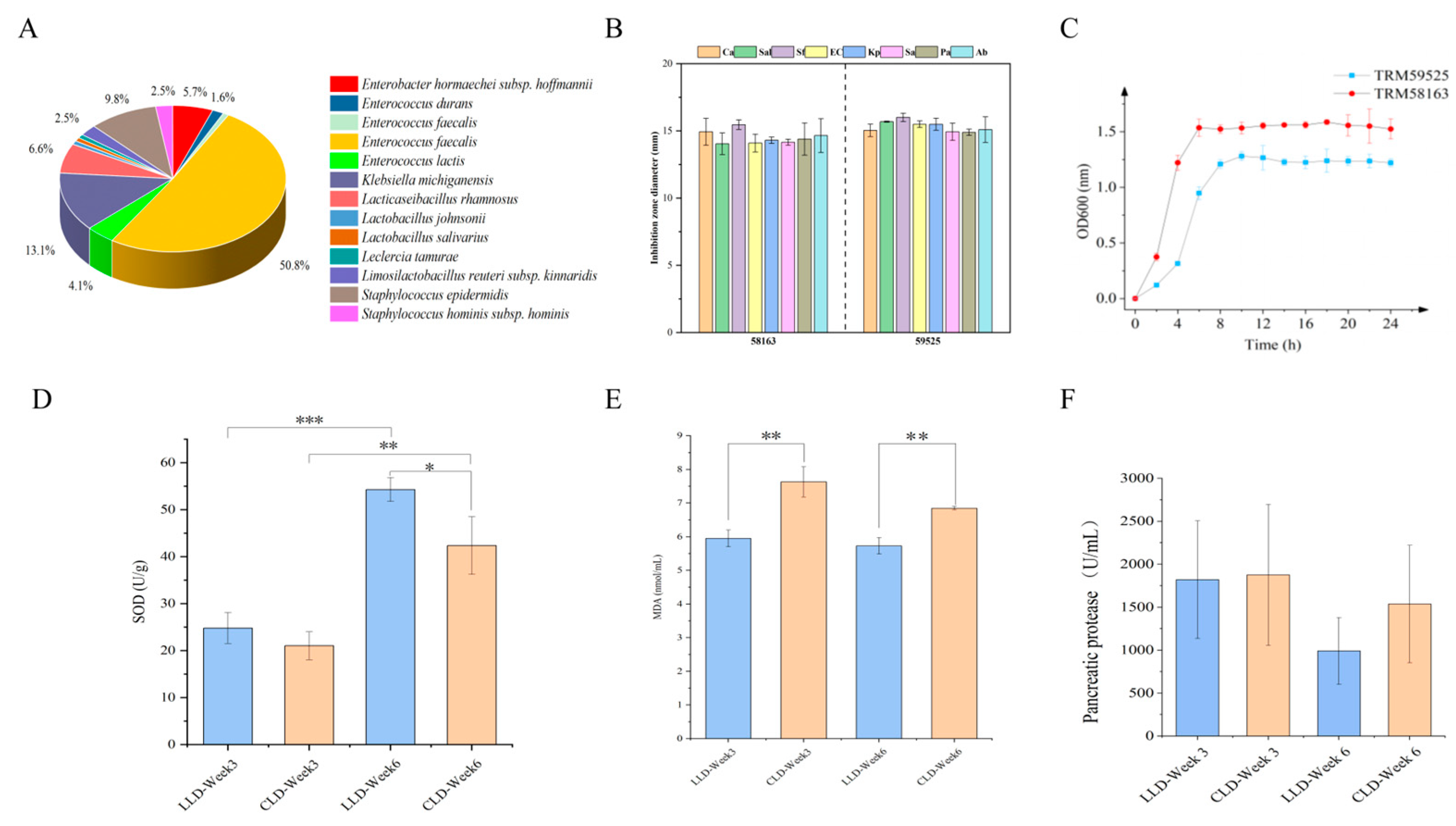
3.1. Composition of Intestinal Bacteria and Candidate Probiotic
3.2. In Vitro Characterization of Probiotic Properties of the Isolates
3.3. Growth Performance
3.4. Plasma Markers, Antioxidant Capacity and Trypsin
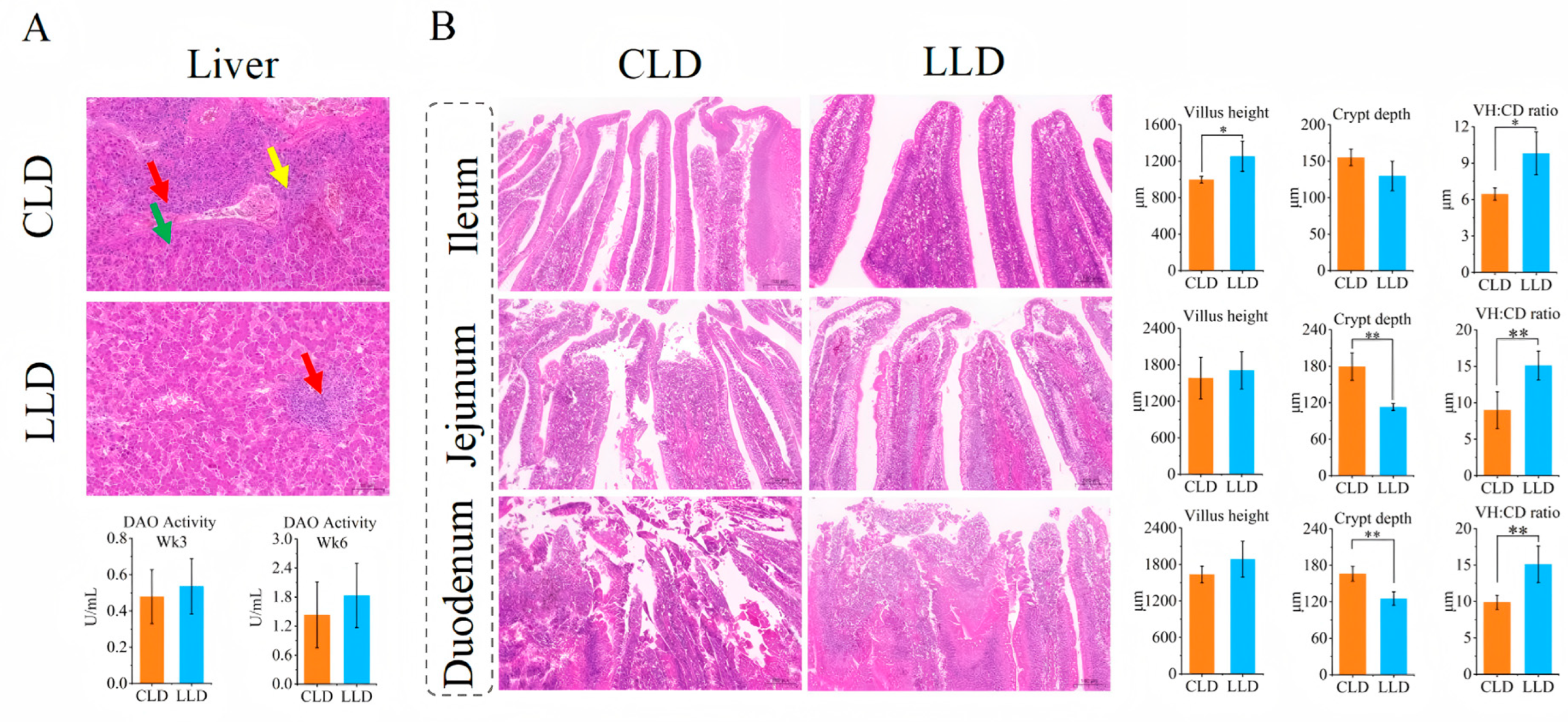
3.5. Relative Weight of Digestive Organs and Histomorphology
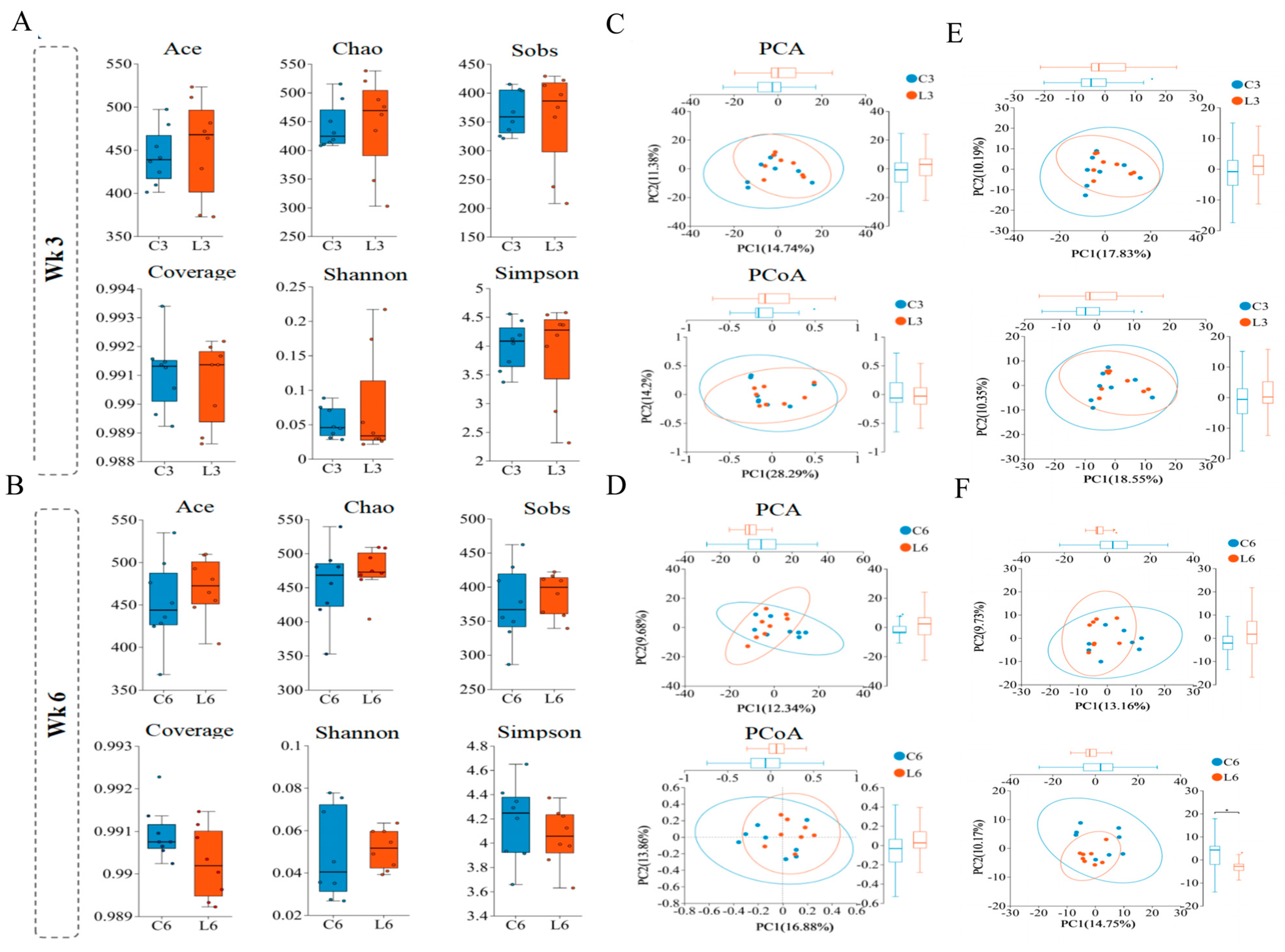
3.6. Effects on the Cecal Microbiota
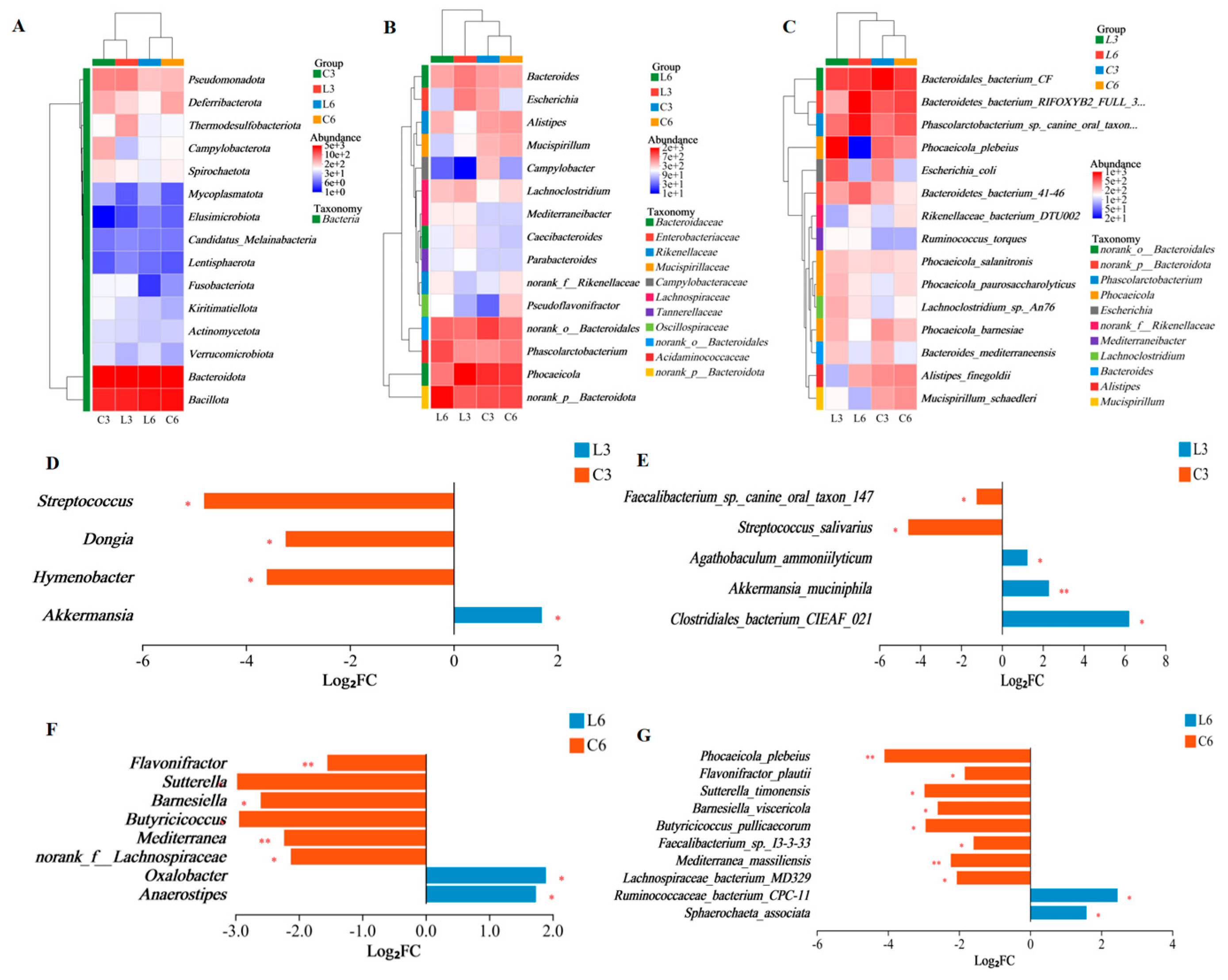
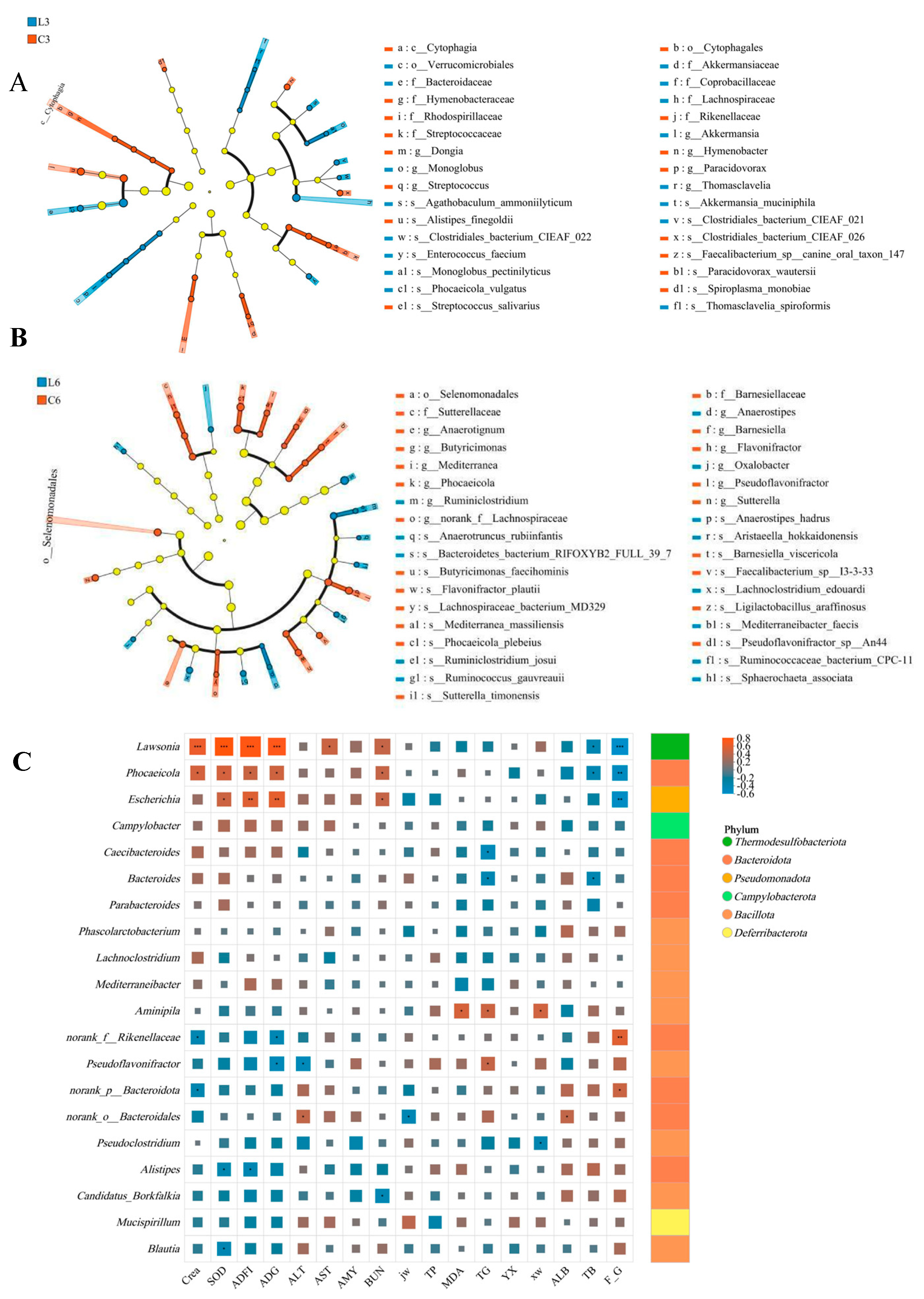
3.7. Microbial Composition Analysis
3.8. Significant Differential Microbial Analysis
3.9. Correlation Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CLD | The Control group was fed a Low-protein Diet |
| LLD | The group had Lactobacillus salivarius TRM58163 and Lactobacillus johnsoni TRM59525 added to the Low-protein Diet |
| LP | Low-Protein |
| ADG | Footnote explaining Average Daily Feed Intake |
| ADFI | Footnote explaining Average Daily Gain |
| FCR | Footnote explaining Feed to Gain Ratio |
| L3 | The LLD group at Weeks 3 |
| L6 | The LLD group at Weeks 6 |
| C3 | The CLD group at Weeks 3 |
| C6 | The CLD group at Weeks 6 |
References
- Bhatia, A.; Sharma, D.; Mehta, J.; Kumarasamy, V.; Begum, M.Y.; Siddiqua, A.; Sekar, M.; Subramaniyan, V.; Wong, L.S.; Rani, N.N.I.M. Probiotics and Synbiotics: Applications, Benefits, and Mechanisms for the Improvement of Human and Ecological Health. J. Multidiscip. Healthc. 2025, 18, 1493–1510. [Google Scholar] [CrossRef]
- Yadav, M.K.; Kumari, I.; Singh, B.; Sharma, K.K.; Tiwari, S.K. Probiotics, prebiotics and synbiotics: Safe options for next-generation therapeutics. Appl. Microbiol. Biotechnol. 2022, 106, 505–521. [Google Scholar] [CrossRef]
- Shehata, A.A.; Yalçın, S.; Latorre, J.D.; Basiouni, S.; Attia, Y.A.; El-Wahab, A.A.; Visscher, C.; El-Seedi, H.R.; Huber, C.; Hafez, H.M.; et al. Probiotics, Prebiotics, and Phytogenic Substances for Optimizing Gut Health in Poultry. Microorganisms 2022, 10, 395. [Google Scholar] [CrossRef]
- Hashemitabar, S.H.; Hosseinian, S.A. The comparative effects of probiotics on growth, antioxidant indices and intestinal histomorphology of broilers under heat stress condition. Sci. Rep. 2024, 14, 23471. [Google Scholar] [CrossRef] [PubMed]
- Kers, J.G.; Velkers, F.C.; Fischer, E.A.J.; Hermes, G.D.A.; Stegeman, J.A.; Smidt, H. Host and Environmental Factors Affecting the Intestinal Microbiota in Chickens. Front. Microbiol. 2018, 9, 235. [Google Scholar] [CrossRef]
- Bajagai, Y.S.; Van, T.T.H.; Joat, N.; Chousalkar, K.; Moore, R.J.; Stanley, D. Layer chicken microbiota: A comprehensive analysis of spatial and temporal dynamics across all major gut sections. J. Anim. Sci. Biotechnol. 2024, 15, 20. [Google Scholar] [CrossRef]
- Liu, S.Y.; Macelline, S.P.; Chrystal, P.V.; Selle, P.H. Progress towards reduced-crude protein diets for broiler chickens and sustainable chicken-meat production. J. Anim. Sci. Biotechnol. 2021, 12, 20. [Google Scholar] [CrossRef]
- Oluwabiyi, C.T.; Song, Z. Branched-chain amino acids supplementation in low-protein broiler diets: A review. Anim. Feed. Sci. Technol. 2024, 318, 116114. [Google Scholar] [CrossRef]
- Rozenboim, I.; Mahato, J.; Cohen, N.A.; Tirosh, O. Low protein and high-energy diet: A possible natural cause of fatty liver hemorrhagic syndrome in caged White Leghorn laying hens. Poult. Sci. 2016, 95, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Parenteau, I.A.; Stevenson, M.; Kiarie, E.G. Egg production and quality responses to increasing isoleucine supplementation in Shaver white hens fed a low crude protein corn-soybean meal diet fortified with synthetic amino acids between 20 and 46 weeks of age. Poult. Sci. 2020, 99, 1444–1453. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-K.; Su, S.-C.; Chang, L.-C.; Shao, S.-C.; Yang, K.-J.; Chen, C.-Y.; Chen, Y.-T.; Wu, I.-W. Effects of Low Protein Diet on Modulating Gut Microbiota in Patients with Chronic Kidney Disease: A Systematic Review and Meta-analysis of International Studies. Int. J. Med. Sci. 2021, 18, 3839–3850. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-M.; Qiu, K.; Wang, J.; Zhang, H.-J.; Qi, G.-H.; Wu, S.-G. Effect of dietary serine supplementation on performance, egg quality, serum indices, and ileal mucosal immunity in laying hens fed a low crude protein diet. Poult. Sci. 2021, 100, 101465. [Google Scholar] [CrossRef]
- Iio, W.; Shimada, R.; Nonaka, I.; Ogino, A. Effects of a low-protein diet supplemented with essential amino acids on egg production performance and environmental gas emissions from layer-manure composting in laying hens in the later laying period. Anim. Sci. J. 2023, 94, e13853. [Google Scholar] [CrossRef] [PubMed]
- Yalçınkaya, H.; Yalçın, S.; Ramay, M.S.; Onbaşılar, E.E.; Bakır, B.; Elibol, F.K.E.; Yalçın, S.; Shehata, A.A.; Basiouni, S. Evaluation of Spirulina platensis as a Feed Additive in Low-Protein Diets of Broilers. Int. J. Mol. Sci. 2024, 26, 24. [Google Scholar] [CrossRef]
- Pender, C.M.; Kim, S.; Potter, T.D.; Ritzi, M.M.; Young, M.; Dalloul, R.A. In ovo supplementation of probiotics and its effects on performance and immune-related gene expression in broiler chicks. Poult. Sci. 2017, 96, 1052–1062. [Google Scholar] [CrossRef]
- Yang, J.; Wang, J.; Liu, Z.; Chen, J.; Jiang, J.; Zhao, M.; Gong, D. Ligilactobacillus salivarius improve body growth and anti-oxidation capacity of broiler chickens via regulation of the microbiota-gut-brain axis. BMC Microbiol. 2023, 23, 395. [Google Scholar] [CrossRef]
- Martín, R.; Jiménez, E.; Olivares, M.; Marín, M.; Fernández, L.; Xaus, J.; Rodríguez, J. Lactobacillus salivarius CECT 5713, a potential probiotic strain isolated from infant feces and breast milk of a mother-child pair. Int. J. Food Microbiol. 2006, 112, 35–43. [Google Scholar] [CrossRef]
- Messaoudi, S.; Manai, M.; Kergourlay, G.; Prévost, H.; Connil, N.; Chobert, J.-M.; Dousset, X. Lactobacillus salivarius: Bacteriocin and probiotic activity. Food Microbiol. 2013, 36, 296–304. [Google Scholar] [CrossRef]
- Wang, Z.; Zhou, Y.; Luo, A.; Heng, X.; Liu, J.; Wang, H.; Chu, W. Lactobacillus salivarius CPU-01 Ameliorates Temozolomide-Induced Intestinal Mucositis by Modulating Gut Microbiota, Maintaining Intestinal Barrier, and Blocking Pro-inflammatory Cytokines. Probiotics Antimicrob. Proteins 2022, 15, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Zeng, D.; Wang, H.; Sun, N.; Zhao, Y.; Dan, Y.; Pan, K.; Jing, B.; Ni, X. Probiotic Lactobacillus johnsonii BS15 Promotes Growth Performance, Intestinal Immunity, and Gut Microbiota in Piglets. Probiotics Antimicrob. Proteins 2020, 12, 184–193. [Google Scholar] [CrossRef]
- Jiang, Y.; Yang, J.; Wei, M.; Shou, J.; Shen, S.; Yu, Z.; Zhang, Z.; Cai, J.; Lyu, Y.; Yang, D.; et al. Probiotics alleviate painful diabetic neuropathy by modulating the microbiota-gut-nerve axis in rats. J. Neuroinflammation 2025, 22, 30. [Google Scholar] [CrossRef] [PubMed]
- Kazemi, S.A.; Ahmadi, H.; Torshizi, M.A.K. Evaluating two multistrain probiotics on growth performance, intestinal morphology, lipid oxidation and ileal microflora in chickens. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1399–1407. [Google Scholar] [CrossRef]
- Zhong, Y.L.; Zhong, L.; Yin, J.Z. The Breeding Effects of “Baicheng You Chicken” in Xinjiang. Xinjiang Anim. Husb. 2008, 1, 28–30. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, W.; Hang, C.; Du, Y.; Chen, Y.; Xing, J.; Gao, J.; Qiu, D. Association of A-FABP gene polymorphism and mRNA expression with intramuscular fat content (IMF) in Baicheng-You chicken. J. Anim. Physiol. Anim. Nutr. 2019, 103, 1447–1452. [Google Scholar] [CrossRef]
- Sarsenbek, A.; Wang, T.; Zhao, J.K.; Jiang, W. Comparison of carcass yields and meat quality between Baicheng-You chickens and Arbor Acres broilers. Poult. Sci. 2013, 92, 2776–2782. [Google Scholar] [CrossRef]
- Wu, X.S.; Wei, X.L.; Li, Y.S.; Adilijiang, K.; Zikeyaer, M.; Shaershanbieke, A. Effects of Dietary Energy and Protein Levels on Apparent Nutrient Digestibility in Growing Baying You Chickens. Xinjiang Anim. Husb. 2021, 36, 27–30. [Google Scholar] [CrossRef]
- Endo, A.; Tanizawa, Y.; Maeno, S.; Arita, M. Isolation and Identification of Lactic Acid Bacteria from Environmental Samples. Methods Mol. Biol. 2024, 2851, 3–14. [Google Scholar] [CrossRef]
- Dou, X.; Zeng, X.; Tang, H.; Mei, Y.; Jiao, H.; Ren, M. Improving compost nutrient composition and microbial community structure through Bacillus licheniformis. Int. Biodeterior. Biodegradation 2025, 205, 106164. [Google Scholar] [CrossRef]
- Humphries, R.; Bobenchik, A.M.; Hindler, J.A.; Schuetz, A.N. Overview of Changes to the Clinical and Laboratory Standards Institute Performance Standards for Antimicrobial Susceptibility Testing, M100, 31st Edition. J. Clin. Microbiol. 2021, 59, e0021321. [Google Scholar] [CrossRef] [PubMed]
- Adetoye, A.; Pinloche, E.; Adeniyi, B.A.; Ayeni, F.A. Characterization and anti-salmonella activities of lactic acid bacteria isolated from cattle faeces. BMC Microbiol. 2018, 18, 96. [Google Scholar] [CrossRef]
- Youn, H.-Y.; Kim, D.-H.; Kim, H.-J.; Bae, D.; Song, K.-Y.; Kim, H.; Seo, K.-H. Survivability of Kluyveromyces marxianus Isolated from Korean Kefir in a Simulated Gastrointestinal Environment. Front. Microbiol. 2022, 13, 842097. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Zhou, K.; Xie, F.; Zhao, Q. Screening and identification of lactic acid bacteria with antimicrobial abilities for aquaculture pathogens in vitro. Arch. Microbiol. 2022, 204, 689. [Google Scholar] [CrossRef]
- Liu, L.; Liu, C.; Wang, H.; Tang, H.; Chen, Z.; Dou, X.; Chang, J.; Li, Z.; Wang, Z.; Mei, Y.; et al. Probiotic potential of enterococcus lactis in improving egg production and quality in quails during late egg-laying period. Poult. Sci. 2025, 104, 104765. [Google Scholar] [CrossRef]
- Li, H.-W.; Xiang, Y.-Z.; Zhang, M.; Jiang, Y.-H.; Zhang, Y.; Liu, Y.-Y.; Lin, L.-B.; Zhang, Q.-L. A novel bacteriocin from Lactobacillus salivarius against Staphylococcus aureus: Isolation, purification, identification, antibacterial and antibiofilm activity. LWT 2021, 140, 110826. [Google Scholar] [CrossRef]
- Hosseindoust, A.; Lee, S.; Nho, W.G.; Song, Y.H.; Shin, J.S.; Ingale, S.L.; Rathi, P.C.; Choi, J.; Chae, B.; Kim, J. A dose–response study to evaluate the effects of pH-stable β-mannanase derived from Trichoderma citrinoviride on growth performance, nutrient retention, and intestine morphology in broiler chickens. Ital. J. Anim. Sci. 2018, 18, 147–154. [Google Scholar] [CrossRef]
- Deng, Z.; Hou, K.; Zhao, J.; Wang, H. The Probiotic Properties of Lactic Acid Bacteria and Their Applications in Animal Husbandry. Curr. Microbiol. 2021, 79, 22. [Google Scholar] [CrossRef]
- Yao, Y.; Dong, W.; Li, H.; Zhao, X.; Liao, H.; Wu, Y.; Wang, G.; Huang, G. Growth and development of different feathers and fitting analysis of growth curves of Baicheng oily chicken. Xinjiang Agric. Sci. 2024, 61, 1259–1267. [Google Scholar] [CrossRef]
- Jin, L.Z.; Ho, Y.W.; Abdullah, N.; Jalaludin, S. Digestive and bacterial enzyme activities in broilers fed diets supplemented with Lactobacillus cultures. Poult. Sci. 2000, 79, 886–891. [Google Scholar] [CrossRef]
- Chen, K.-L.; Kho, W.-L.; You, S.-H.; Yeh, R.-H.; Tang, S.-W.; Hsieh, C.-W. Effects of Bacillus subtilis var. natto and Saccharomyces cerevisiae mixed fermented feed on the enhanced growth performance of broilers. Poult. Sci. 2009, 88, 309–315. [Google Scholar] [CrossRef]
- Zavelinski, V.; Vieira, V.; Bassi, L.; de Almeida, L.; Schramm, V.; Maiorka, A.; de Oliveira, S. The effect of protease supplementation in broiler chicken diets containing maize from different batches on growth performance and nutrient digestibility. Animal 2024, 18, 101363. [Google Scholar] [CrossRef] [PubMed]
- Dou, X.; Zhang, G.; Tang, H.; Chen, X.; Chen, B.; Mei, Y.; Jiao, H.; Ren, M. Carotenoids from Halophilic Archaea: A Novel Approach to Improve Egg Quality and Cecal Microbiota in Laying Hens. Animals 2024, 14, 3470. [Google Scholar] [CrossRef]
- Aziz, M.; Hemeda, S.A.; Albadrani, G.M.; Fadl, S.E.; Elgendey, F. Ameliorating effect of probiotic on nonalcoholic fatty liver disease and lipolytic gene expression in rabbits. Sci. Rep. 2023, 13, 6312. [Google Scholar] [CrossRef]
- Halliwell, B. Understanding mechanisms of antioxidant action in health and disease. Nat. Rev. Mol. Cell Biol. 2024, 25, 13–33. [Google Scholar] [CrossRef] [PubMed]
- Eissa, M.E.; Alaryani, F.S.; Elbahnaswy, S.; Khattab, M.S.; Elfeky, A.; AbouelFadl, K.Y.; Eissa, E.-S.H.; Ahmed, R.A.; Van Doan, H.; El-Haroun, E. Dietary inclusion of Pediococcus acidilactici probiotic promoted the growth indices, hemato-biochemical indices, enzymatic profile, intestinal and liver histomorphology, and resistance of Nile Tilapia against Aspergillus flavus. Anim. Feed. Sci. Technol. 2023, 306, 115814. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, Q.; Sun, J.; Wu, X.; Song, Y.; Xu, Y.; Nie, H.; Huang, J.; Mu, G. Probiotic potential of lactic acid bacteria with antioxidant properties in modulating health: Mechanisms, applications, and future directions. Food Biosci. 2025, 66, 106181. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Z.; Li, J.; Li, H.; Liu, Z.; Wang, J.; Tan, B. Ningxiang pig-derived Enterococcus hirae HNAU0516 ameliorates postweaning diarrhoea by promoting intestinal health and modulating the gut microbiota in piglets. Animal 2024, 18, 101220. [Google Scholar] [CrossRef]
- Chichlowski, M.; Croom, W.J.; Edens, F.W.; McBride, B.W.; Qiu, R.; Chiang, C.C.; Daniel, L.R.; Havenstein, G.B.; Koci, M.D.; Koci, M.D. Microarchitecture and spatial relationship between bacteria and ileal, cecal, and colonic epithelium in chicks fed a direct-fed microbial, PrimaLac, and salinomycin. Poult. Sci. 2007, 86, 1121–1132. [Google Scholar] [CrossRef]
- Chen, Y.-P.; Chen, M.-J. Effects of Lactobacillus kefiranofaciens M1 isolated from kefir grains on germ-free mice. PLoS ONE 2013, 8, e78789. [Google Scholar] [CrossRef]
- Campos, P.M.; Schreier, L.L.; Proszkowiec-Weglarz, M.; Dridi, S. Cecal microbiota composition differs under normal and high ambient temperatures in genetically distinct chicken lines. Sci. Rep. 2023, 13, 16037. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Xiang, Y.; Zhou, W.; Chen, J.; Li, K.; Yang, H. Microbial community mapping in intestinal tract of broiler chicken. Poult. Sci. 2017, 96, 1387–1393. [Google Scholar] [CrossRef]
- Yang, Y.; Song, X.; Wang, G.; Xia, Y.; Xiong, Z.; Ai, L. Understanding Ligilactobacillus salivarius from Probiotic Properties to Omics Technology. Foods 2024, 13, 895. [Google Scholar] [CrossRef]
- Charitos, I.A.; Inchingolo, A.M.; Ferrante, L.; Inchingolo, F.; Inchingolo, A.D.; Castellaneta, F.; Cotoia, A.; Palermo, A.; Scacco, S.; Dipalma, G. The Gut Microbiota’s Role in Neurological, Psychiatric, and Neurodevelopmental Disorders. Nutrients 2024, 16, 4404. [Google Scholar] [CrossRef]
- Salgado, J.F.M.; Hervé, V.; Vera, M.A.G.; Tokuda, G.; Brune, A. Unveiling lignocellulolytic potential: A genomic exploration of bacterial lineages within the termite gut. Microbiome 2024, 12, 201. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Tai, Y.; Ma, Y.; Xu, Z.; Hao, J.; Han, D.; Li, J.; Deng, X. Cecum microbiome and metabolism characteristics of Silky Fowl and White Leghorn chicken in late laying stages. Front. Microbiol. 2022, 13, 984654. [Google Scholar] [CrossRef]
- Segura-Wang, M.; Grabner, N.; Koestelbauer, A.; Klose, V.; Ghanbari, M. Genome-Resolved Metagenomics of the Chicken Gut Microbiome. Front. Microbiol. 2021, 12, 726923. [Google Scholar] [CrossRef]
- Kaakoush, N.O. Sutterella Species, IgA-degrading Bacteria in Ulcerative Colitis. Trends Microbiol. 2020, 28, 519–522. [Google Scholar] [CrossRef]
- Davoody, S.; Halimi, H.; Zali, A.; Houri, H.; Brand, S. Double-edged sword effect of Sutterella in neurological disorders: Implications for the gut-brain axis and neuroimmune interactions. Neurobiol. Dis. 2025, 214, 107032. [Google Scholar] [CrossRef]
- Wang, W.; Li, N.; Xu, H.; Wei, S.; Li, Y.; Ou, J.; Hao, J.; Zhang, J.; Dong, L.; Qiu, Y.; et al. ILC3s regulate the gut microbiota via host intestinal galactosylation to limit pathogen infection in mice. Nat. Microbiol. 2025, 10, 654–666. [Google Scholar] [CrossRef] [PubMed]
- Monika, M.; Tyagi, J.S.; Sonale, N.; Biswas, A.; Murali, D.; Tiwari, A.K.; Rokade, J.J. Evaluating the efficacy of Lactobacillus acidophilus derived postbiotics on growth metrics, Health, and Gut Integrity in broiler chickens. Sci. Rep. 2024, 14, 24768. [Google Scholar] [CrossRef] [PubMed]
- Marsh, A.J.; Yaya, A.-M.A.; Ng, S.; Chandrashekhar, K.; Roach, J.; Magness, S.T.; Azcarate-Peril, M.A. Lumen and mucosa-associated Lactobacillus rhamnosus from the intestinal tract of organ donors. Gut Microbiome 2020, 1, e4. [Google Scholar] [CrossRef]
- Virtue, A.T.; McCright, S.J.; Wright, J.M.; Jimenez, M.T.; Mowel, W.K.; Kotzin, J.J.; Joannas, L.; Basavappa, M.G.; Spencer, S.P.; Clark, M.L.; et al. The gut microbiota regulates white adipose tissue inflammation and obesity via a family of microRNAs. Sci. Transl. Med. 2019, 11, eaav1892. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Fillier, T.; Pham, T.H.; Thomas, R.; Cheema, S.K. Intraperitoneal Administration of Short-Chain Fatty Acids Improves Lipid Metabolism of Long-Evans Rats in a Sex-Specific Manner. Nutrients 2021, 13, 892. [Google Scholar] [CrossRef] [PubMed]
- Neff, A.; Lück, R.; Hövels, M.; Deppenmeier, U. Expanding the repertoire of counterselection markers for markerless gene deletion in the human gut bacterium Phocaeicola vulgatus. Anaerobe 2023, 81, 102742. [Google Scholar] [CrossRef]
- de Vos, W.M.; Tilg, H.; Van Hul, M.; Cani, P.D. Gut microbiome and health: Mechanistic insights. Gut 2022, 71, 1020–1032. [Google Scholar] [CrossRef]
- Zeng, M.; Inohara, N.; Nuñez, G. Mechanisms of inflammation-driven bacterial dysbiosis in the gut. Mucosal Immunol. 2017, 10, 18–26. [Google Scholar] [CrossRef] [PubMed]
| Item | |
|---|---|
| Ingredients, % 1 | |
| Corn | 70 |
| Soybean meal | 15 |
| Wheat bran | 10 |
| Premix1 | 5 |
| Total | 100 |
| Nutritional level 2 | |
| ME (MJ/Kg) | 12.60 |
| CP (%) | 13.21 |
| EE (%) | 3.31 |
| CF (%) | 2.40 |
| Ca (%) | 1.09 |
| TP (%) | 0.55 |
| Items | Treatment | Starter | Grower | Finisher | Overall |
|---|---|---|---|---|---|
| 0–7 d | 8–21 d | 22–42 d | 0–42 d | ||
| ADFI (g) | LLD | 48.32 ± 1.37 | 70.19 ± 2.87 | 81.57 ± 1.60 | 66.69 ± 1.76 |
| CLD | 37.24 ± 3.64 | 46.92 ± 1.95 | 56.67 ± 0.31 | 46.94 ± 1.82 | |
| p-value | 0.046 | 0.003 | 0.003 | 0.001 | |
| ADG (g) | LLD | 11.67 ± 0.86 | 16.74 ± 1.26 | 19.86 ± 0.90 | 16.09 ± 0.82 |
| CLD | 7.62 ± 1.74 | 8.79 ± 0.76 | 10.57 ± 0.17 | 8.99 ± 0.85 | |
| p-value | 0.022 | 0.002 | <0.001 | <0.001 | |
| FCR | LLD | 4.15 ± 0.14 | 4.20 ± 0.16 | 4.11 ± 0.08 | 4.15 ± 0.03 |
| CLD | 4.93 ± 0.34 | 5.35 ± 0.12 | 5.36 ± 0.07 | 5.22 ± 0.15 | |
| p-value | 0.020 | 0.001 | <0.001 | 0.005 |
| Items | LLD | CLD | p-Value | ||
|---|---|---|---|---|---|
| LLD (Week 3 vs. Week 6) | CLD (Week 3 vs. Week 6) | LLD vs. CLD | |||
| Pancreas | |||||
| Week 3 | 2.88 ± 0.78 | 2.73 ± 0.68 | 0.44 | 0.846 | 0.670 |
| Week 6 | 3.52 ± 2.07 | 2.65 ± 0.92 | 0.303 | ||
| Proventriculus | |||||
| Week 3 | 4.99 ± 1.19 | 4.93 ± 0.86 | 0.270 | 0.308 | 0.913 |
| Week 6 | 5.57 ± 1.64 | 4.43 ± 0.90 | 0.072 | ||
| Gizzard | |||||
| Week 3 | 37.49 ± 8.03 | 34.92 ± 6.44 | 0.232 | 0.741 | 0.492 |
| Week 6 | 40.05 ± 9.67 | 36.12 ± 8.24 | 0.396 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Dou, X.; Tang, H.; Huang, Y.; Wu, G.; Dong, W.; Wang, H.; Jiao, H.; Mei, Y.; Ren, M. Low-Protein-Fed Chickens Benefit from Probiotic L. salivarius and L. johnsonii on Performance and Microbiota. Animals 2025, 15, 3346. https://doi.org/10.3390/ani15223346
Dong X, Dou X, Tang H, Huang Y, Wu G, Dong W, Wang H, Jiao H, Mei Y, Ren M. Low-Protein-Fed Chickens Benefit from Probiotic L. salivarius and L. johnsonii on Performance and Microbiota. Animals. 2025; 15(22):3346. https://doi.org/10.3390/ani15223346
Chicago/Turabian StyleDong, Xiaomei, Xufeng Dou, Hao Tang, Yuanyuan Huang, Guiling Wu, Wei Dong, Hui’e Wang, Haihong Jiao, Yuxia Mei, and Min Ren. 2025. "Low-Protein-Fed Chickens Benefit from Probiotic L. salivarius and L. johnsonii on Performance and Microbiota" Animals 15, no. 22: 3346. https://doi.org/10.3390/ani15223346
APA StyleDong, X., Dou, X., Tang, H., Huang, Y., Wu, G., Dong, W., Wang, H., Jiao, H., Mei, Y., & Ren, M. (2025). Low-Protein-Fed Chickens Benefit from Probiotic L. salivarius and L. johnsonii on Performance and Microbiota. Animals, 15(22), 3346. https://doi.org/10.3390/ani15223346





