The Effects of Light on Vertebrate Welfare: A Review
Simple Summary
Abstract
1. Introduction
2. The Physical Characteristics of Light and Their Relevance to Animal Welfare
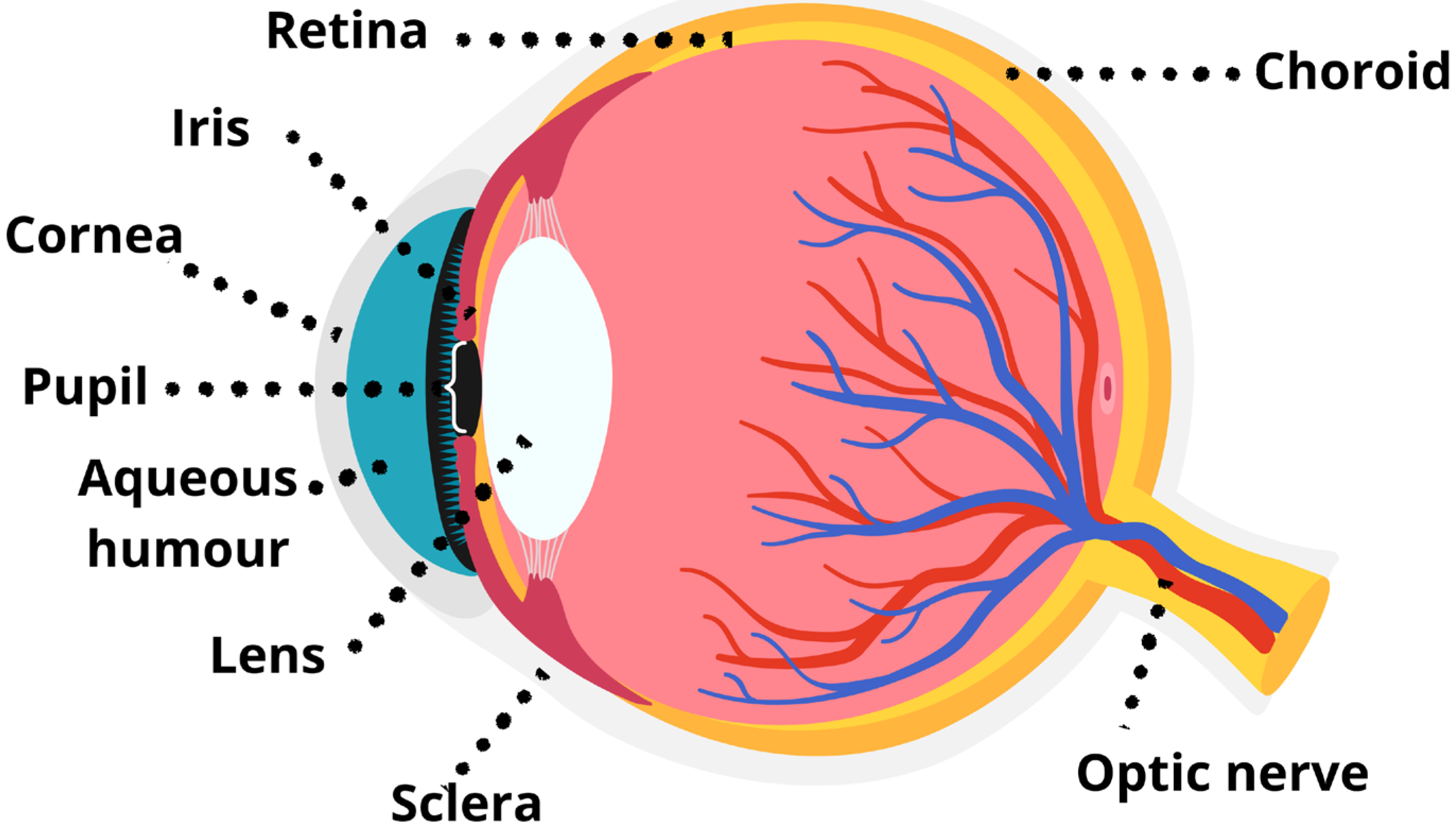
3. Light Perception in Vertebrate Animals: Eye Structure and Vision Evolution
The Evolution of Visual Systems in Vertebrates
- Non-directional photoreception: Detects light versus darkness.
- Directional detection: Identifies the direction of light.
- Low-resolution vision: Capable of detecting crude shapes or movements.
- High-resolution vision: Allows for the perception of detailed images.
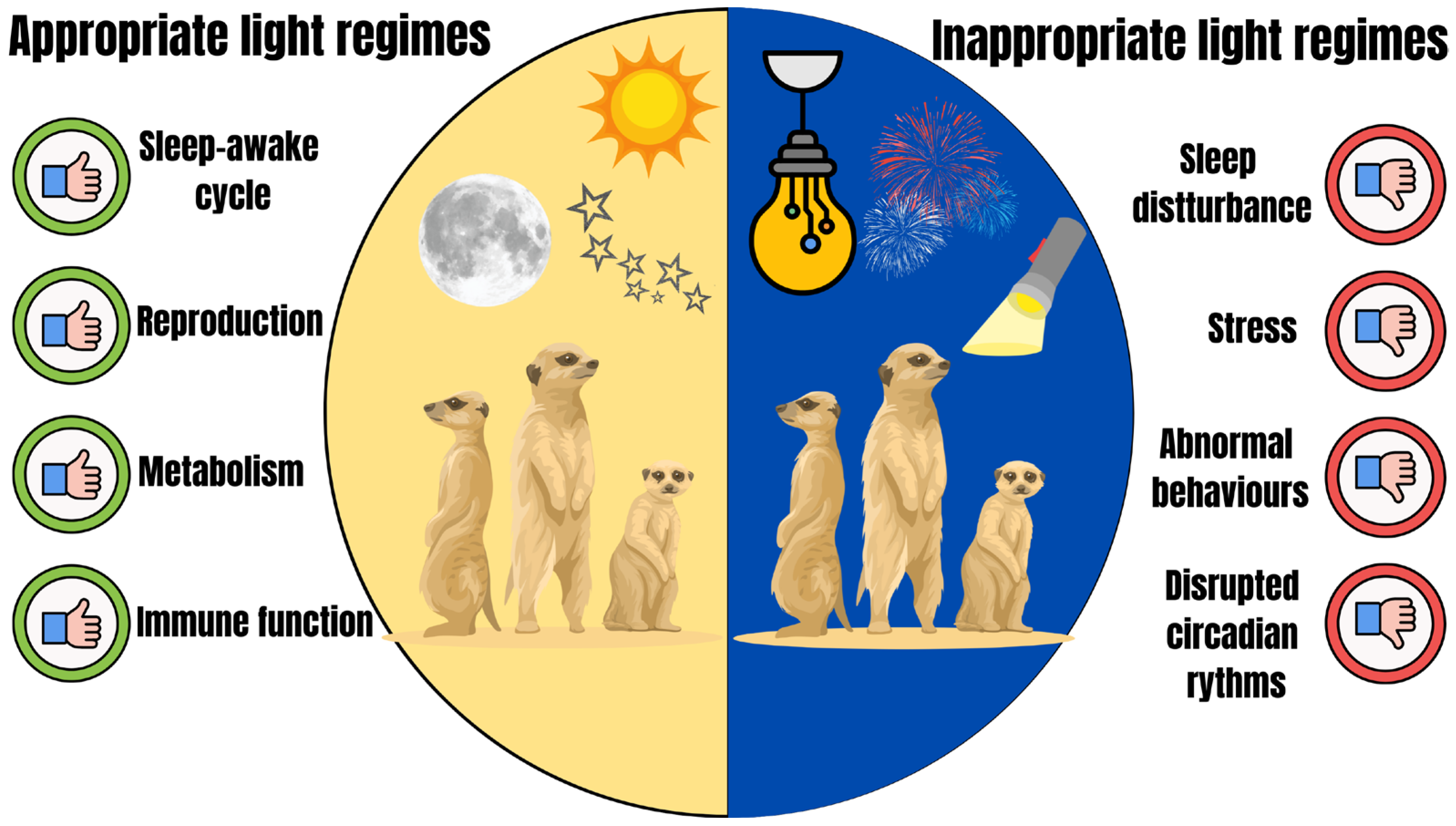
4. How Light Can Influence Animal Welfare
4.1. Nutrition
4.2. Health
4.3. Environment
4.4. Behaviour
4.5. Mental State
5. How Zoos Manage Light
5.1. How Are Animals Exposed to Artificial Light in Zoos?
5.2. What Is the Source of Artificial Light?
5.3. Animal Welfare in the “Light” of the Five Domains
6. Future Studies: The Necessity of More Studies Evaluating the Light Impact on Captive Vertebrates and How to Mitigate Them
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenwell, P.J.; Riley, L.M.; Lemos de Figueiredo, R.; Brereton, J.E.; Mooney, A.; Rose, P.E. The Societal Value of the Modern Zoo: A Commentary on How Zoos Can Positively Impact on Human Populations Locally and Globally. J. Zool. Bot. Gard. 2023, 4, 53–69. [Google Scholar] [CrossRef]
- Beer, H.N.; Shrader, T.C.; Schmidt, T.B.; Yates, D.T. The Evolution of Zoos as Conservation Institutions: A Summary of the Transition from Menageries to Zoological Gardens and Parallel Improvement of Mammalian Welfare Management. J. Zool. Bot. Gard. 2023, 4, 648–664. [Google Scholar] [CrossRef]
- Miller, L.J.; Chinnadurai, S.K. Beyond the Five Freedoms: Animal Welfare at Modern Zoological Facilities. Animals 2023, 13, 1818. [Google Scholar] [CrossRef]
- Gray, J. (Ed.) Zoo Ethics; CSIRO Publishing: Victoria, Australia, 2017; ISBN 9781486306992. [Google Scholar]
- Wolfensohn, S.; Shotton, J.; Bowley, H.; Davies, S.; Thompson, S.; Justice, W.S.M. Assessment of Welfare in Zoo Animals: Towards Optimum Quality of Life. Animals 2018, 8, 110. [Google Scholar] [CrossRef] [PubMed]
- Linstädt, J.; Thöne-Reineke, C.; Merle, R. Animal-Based Welfare Indicators for Dairy Cows and Their Validity and Practicality: A Systematic Review of the Existing Literature. Front. Vet. Sci. 2024, 11, 429097. [Google Scholar] [CrossRef]
- Cole, J.; Fraser, D. Zoo Animal Welfare: The Human Dimension. J. Appl. Anim. Welf. Sci. 2018, 21, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Rault, J.-L.; Waiblinger, S.; Boivin, X.; Hemsworth, P. The Power of a Positive Human–Animal Relationship for Animal Welfare. Front. Vet. Sci. 2020, 7, 590867. [Google Scholar] [CrossRef] [PubMed]
- Greggor, A.L.; Vicino, G.A.; Swaisgood, R.R.; Fidgett, A.; Brenner, D.; Kinney, M.E.; Farabaugh, S.; Masuda, B.; Lamberski, N. Animal Welfare in Conservation Breeding: Applications and Challenges. Front. Vet. Sci. 2018, 5, 323. [Google Scholar] [CrossRef]
- DiVincenti, L.; McDowell, A.; Herrelko, E.S. Integrating Individual Animal and Population Welfare in Zoos and Aquariums. Animals 2023, 13, 1577. [Google Scholar] [CrossRef]
- Campbell-Ward, M. Quality-of-Life Assessments in Zoo Animals: Not Just for the Aged and Charismatic. Animals 2023, 13, 3394. [Google Scholar] [CrossRef]
- Mellor, D.J. Operational Details of the Five Domains Model and Its Key Applications to the Assessment and Management of Animal Welfare. Animals 2017, 7, 60. [Google Scholar] [CrossRef]
- Mellor, D.J.; Beausoleil, N.J.; Littlewood, K.E.; McLean, A.N.; McGreevy, P.D.; Jones, B.; Wilkins, C. The 2020 Five Domains Model: Including Human–Animal Interactions in Assessments of Animal Welfare. Animals 2020, 10, 1870. [Google Scholar] [CrossRef]
- Mellor, D.J. Moving beyond the “Five Freedoms” by Updating the “Five Provisions” and Introducing Aligned “Animal Welfare Aims”. Animals 2016, 6, 59. [Google Scholar] [CrossRef] [PubMed]
- Hampton, J.O.; Hemsworth, L.M.; Hemsworth, P.H.; Hyndman, T.H.; Sandøe, P. Rethinking the Utility of the Five Domains Model. Anim. Welf. 2023, 32, e62. [Google Scholar] [CrossRef] [PubMed]
- de Azevedo, C.S.; Cipreste, C.F.; Pizzutto, C.S.; Young, R.J. Review of the Effects of Enclosure Complexity and Design on the Behaviour and Physiology of Zoo Animals. Animals 2023, 13, 1277. [Google Scholar] [CrossRef]
- Smith, A.; Rose, P.; Mettke-Hofmann, C. Effects of Enclosure Complexity and Design on Behaviour and Physiology in Captive Animals. Animals 2024, 14, 2028. [Google Scholar] [CrossRef]
- Jensvold, M.L.A.; Sanz, C.M.; Fouts, R.S.; Fouts, D.H. Effect of Enclosure Size and Complexity on the Behaviors of Captive Chimpanzees (Pan troglodytes). J. Appl. Anim. Welf. Sci. 2001, 4, 53–69. [Google Scholar] [CrossRef]
- Miller, L.; Vicino, G.; Sheftel, J.; Lauderdale, L. Behavioral Diversity as a Potential Indicator of Positive Animal Welfare. Animals 2020, 10, 1211. [Google Scholar] [CrossRef]
- Goswami, S.; Tyagi, P.C.; Malik, P.K.; Gupta, B.K. Effects of Enclosure Complexity and Visitor Presence on the Welfare of Asiatic Lions. Appl. Anim. Behav. Sci. 2023, 260, 105853. [Google Scholar] [CrossRef]
- Karan, S.; Saraswat, S.; Anusha, B.S. Light Pollution and the Impacts on Biodiversity: The Dark Side of Light. Biodiversity 2023, 24, 194–199. [Google Scholar] [CrossRef]
- Candolin, U.; Filippini, T. Light Pollution and Its Impact on Human Health and Wildlife. BMC Environ. Sci. 2025, 2, 1. [Google Scholar] [CrossRef]
- Li, X.; Xu, Y. How Lights Affect the Circadian Rhythm in Sleep-Awake Circle. Chin. J. Phys. 2024, 91, 719–733. [Google Scholar] [CrossRef]
- Said, M.H. What Is Light? J. Phys. Opt. Sci. 2023, 5, 1–3. [Google Scholar] [CrossRef]
- Sliney, D. What Is Light? The Visible Spectrum and Beyond. Eye 2016, 30, 222–229. [Google Scholar] [CrossRef]
- Allen, K. Inter-Species Variation in Colour Perception. Philos. Stud. 2009, 142, 197–220. [Google Scholar] [CrossRef]
- Longcore, T. A Compendium of Photopigment Peak Sensitivities and Visual Spectral Response Curves of Terrestrial Wildlife to Guide Design of Outdoor Nighttime Lighting. Basic Appl. Ecol. 2023, 73, 40–50. [Google Scholar] [CrossRef]
- Grunst, M.L.; Grunst, A.S. Endocrine Effects of Exposure to Artificial Light at Night: A Review and Synthesis of Knowledge Gaps. Mol. Cell. Endocrinol. 2023, 568, 111927. [Google Scholar] [CrossRef]
- Singh, S.V.; Kumar, S. Circadian Rhythm and Their Significance in Relation to Physiological Functions of Animals: A Review. J. Entomol. Zool. Stud. 2018, 6, 1861–1866. [Google Scholar]
- Raap, T.; Pinxten, R.; Eens, M. Light Pollution Disrupts Sleep in Free-Living Animals. Sci. Rep. 2015, 5, 13557. [Google Scholar] [CrossRef]
- Bumgarner, J.R.; Nelson, R.J. Light at Night and Disrupted Circadian Rhythms Alter Physiology and Behavior. Integr. Comp. Biol. 2021, 61, 1160–1169. [Google Scholar] [CrossRef]
- Burt, C.S.; Kelly, J.F.; Trankina, G.E.; Silva, C.L.; Khalighifar, A.; Jenkins-Smith, H.C.; Fox, A.S.; Fristrup, K.M.; Horton, K.G. The Effects of Light Pollution on Migratory Animal Behavior. Trends Ecol. Evol. 2023, 38, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Vassão, P.G.; Balão, A.B.; da Campos, R.M.S.; Sales, A.F.S.; Parisi, J.R.; de Andrade, A.L.M.; Tucci, H.T.; Garcia, L.A.; Renno, A.C.M. Efficacy of Acute and Long-Term Photobiomodulation Therapy on Fatigue and Muscle Performance in Different Animal Models: A Systematic Review. Braz. Arch. Biol. Technol. 2024, 67, e24220678. [Google Scholar] [CrossRef]
- Bunch, J. Photobiomodulation (Therapeutic Lasers). Vet. Clin. North Am. Small Anim. Pract. 2023, 53, 783–799. [Google Scholar] [CrossRef] [PubMed]
- Fuller, G.; Raghanti, M.A.; Dennis, P.M.; Kuhar, C.W.; Willis, M.A.; Schook, M.W.; Lukas, K.E. A Comparison of Nocturnal Primate Behavior in Exhibits Illuminated with Red and Blue Light. Appl. Anim. Behav. Sci. 2016, 184, 126–134. [Google Scholar] [CrossRef]
- Finley, R.B. Observation of Nocturnal Animals by Red Light. J. Mammal. 1959, 40, 591. [Google Scholar] [CrossRef]
- Kurvers, R.H.J.M.; Drägestein, J.; Hölker, F.; Jechow, A.; Krause, J.; Bierbach, D. Artificial Light at Night Affects Emergence from a Refuge and Space Use in Guppies. Sci. Rep. 2018, 8, 14131. [Google Scholar] [CrossRef]
- Shier, D.M.; Bird, A.K.; Wang, T.B. Effects of Artificial Light at Night on the Foraging Behavior of an Endangered Nocturnal Mammal. Environ. Pollut. 2020, 263, 114566. [Google Scholar] [CrossRef]
- Shankar, A.; Williams, C.T. The Darkness and the Light: Diurnal Rodent Models for Seasonal Affective Disorder. Dis. Model. Mech. 2021, 14, dmm047217. [Google Scholar] [CrossRef]
- Dauchy, R.T.; Hanifin, J.P.; Brainard, G.C.; Blask, D.E. Light: An Extrinsic Factor Influencing Animal-Based Research. J. Am. Assoc. Lab. Anim. Sci. 2024, 63, 116–147. [Google Scholar] [CrossRef]
- Zwinkels, J. Light, Electromagnetic Spectrum. In Encyclopedia of Color Science and Technology; Luo, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 1–8. [Google Scholar]
- Haglund, R. The Properties of Light. In Springer Handbook of Lasers and Optics; Träger, F., Ed.; Springer: New York, NY, USA, 2007; pp. 3–32. [Google Scholar]
- Bradbury, J.W.; Vehrencamp, S.L. Principles of Animal Communication, 2nd ed.; Sinauer Associates: Sunderland, UK, 2011; ISBN 0878930450. [Google Scholar]
- Hecht, E. Optics, 5th ed.; Pearson Education: Essex, UK, 2019; ISBN 9353439590. [Google Scholar]
- Halliday, D.; Resnick, R.; Walker, J. Principles of Physics, 12th ed.; John Wiley & Sons Inc: Oxford, UK, 2023. [Google Scholar]
- Hamouda, S.; Ibrahim, M.; Mohamed, M. The Electromagnetic Spectrum: Knowledge and Experimental Techniques. Eng. Sci. Int. J. 2021, 8, 156–159. [Google Scholar] [CrossRef]
- Young, H.D.; Feedman, J. University Physics with Modern Physics, 14th ed.; Pearson: Hoboken, NJ, USA, 2015; ISBN 0321982584. [Google Scholar]
- Cuthill, I.C.; Allen, W.L.; Arbuckle, K.; Caspers, B.; Chaplin, G.; Hauber, M.E.; Hill, G.E.; Jablonski, N.G.; Jiggins, C.D.; Kelber, A.; et al. The Biology of Color. Science (1979) 2017, 357, eaan0221. [Google Scholar] [CrossRef]
- Wiltschko, R.; Wiltschko, W. Animal Navigation: How Animals Use Environmental Factors to Find Their Way. Eur. Phys. J. Spec. Top. 2023, 232, 237–252. [Google Scholar] [CrossRef]
- Flamarique, I.N.; Browman, H.I. Foraging and Prey-Search Behaviour of Small Juvenile Rainbow Trout (Oncorhynchus mykiss) under Polarized Light. J. Exp. Biol. 2001, 204, 2415–2422. [Google Scholar] [CrossRef]
- Dwyer, R.G.; Bearhop, S.; Campbell, H.A.; Bryant, D.M. Shedding Light on Light: Benefits of Anthropogenic Illumination to a Nocturnally Foraging Shorebird. J. Anim. Ecol. 2013, 82, 478–485. [Google Scholar] [CrossRef]
- Trapp, R.; Fernández-Juricic, E. How Visual System Configuration Can Play a Role in Individual Recognition: A Visual Modeling Study. Anim. Cogn. 2022, 25, 205–216. [Google Scholar] [CrossRef]
- Greer, D.; Lei, T.; Kryshtal, A.; Jessen, Z.F.; Schwartz, G.W. Visual Identification of Conspecifics Shapes Social Behavior in Mice. Curr. Biol. 2025, 35, 287–299.e4. [Google Scholar] [CrossRef] [PubMed]
- Guthrie, D.M. Role of Vision in Fish Behaviour. In The Behaviour of Teleost Fishes; Springer: Boston, MA, USA, 1986; pp. 75–113. [Google Scholar]
- Lythgoe, J.N. Light and Vision in the Aquatic Environment. In Sensory Biology of Aquatic Animals; Springer: New York, NY, USA, 1988; pp. 57–82. [Google Scholar]
- Shaughnessy, A.; Cortesi, F. Coral Reef Fish Visual Adaptations to a Changing World. Funct. Ecol. 2024, 39, 2561–2572. [Google Scholar] [CrossRef]
- Wang, K.; Arrenberg, B.; Hinz, J.; Arrenberg, A.B. Reduction of Visual Stimulus Artifacts Using a Spherical Tank for Small, Aquatic Animals. Sci. Rep. 2021, 11, 3204. [Google Scholar] [CrossRef] [PubMed]
- Pearce, E.; Dunbar, R. Latitudinal Variation in Light Levels Drives Human Visual System Size. Biol. Lett. 2012, 8, 90–93. [Google Scholar] [CrossRef]
- Lutgens, F.; Tarbuck, E. Foundations of Earth Science, 9th ed.; Pearson: Haboken, NJ, USA, 2021. [Google Scholar]
- Hut, R.A.; Beersma, D.G.M. Evolution of Time-Keeping Mechanisms: Early Emergence and Adaptation to Photoperiod. Philos. Trans. R. Soc. B Biol. Sci. 2011, 366, 2141–2154. [Google Scholar] [CrossRef]
- Stockman, A.; Sharpe, L.T. Into the Twilight Zone: The Complexities of Mesopic Vision and Luminous Efficiency. Ophthalmic Physiol. Opt. 2006, 26, 225–239. [Google Scholar] [CrossRef]
- Kardong, K.V. Vertebrates: Comparative Anatomy, Function, Evolution; McGraw-Hill Education: New York, NY, USA, 2019; ISBN 1260398560. [Google Scholar]
- Land, M.F.; Nilsson, D.-E. Animal Eyes, 2nd ed.; Oxford University Press: Oxford, UK, 2012; ISBN 9780199581139. [Google Scholar]
- Brach, V. The Functional Significance of the Avian Pecten: A Review. Condor 1977, 79, 321–327. [Google Scholar] [CrossRef]
- Kusakabe, K.T.; Seto, M.; Harada, Y.; Kusakabe, A.; Yustinasari, L.R.; Hyoto, M.; Nakahara, C.; Gondo, A.; Kondo, T.; Kano, K.; et al. Characteristics of Pectens in Diurnal and Nocturnal Birds and a New Functional Proposal Relating to Non-visual Opsins. Anat. Histol. Embryol. 2024, 53, 13071. [Google Scholar] [CrossRef] [PubMed]
- Andrade, P. Highlight: More Than Meets the Eye—Nonvisual Opsins Are Crucial for Light-Sensing in Vertebrates. Genome Biol. Evol. 2025, 17, evaf075. [Google Scholar] [CrossRef]
- Poletini, M.O.; Ramos, B.C.; Moraes, M.N.; Castrucci, A.M.L. Nonvisual Opsins and the Regulation of Peripheral Clocks by Light and Hormones. Photochem. Photobiol. 2015, 91, 1046–1055. [Google Scholar] [CrossRef]
- Perez, L.N.; Lorena, J.; Costa, C.M.; Araujo, M.S.; Frota-Lima, G.N.; Matos-Rodrigues, G.E.; Martins, R.A.P.; Mattox, G.M.T.; Schneider, P.N. Eye Development in the Four-Eyed Fish Anableps anableps: Cranial and Retinal Adaptations to Simultaneous Aerial and Aquatic Vision. Proc. R. Soc. B Biol. Sci. 2017, 284, 20170157. [Google Scholar] [CrossRef]
- Walls, G.L. The Vertebrate Eye and Its Adaptive Radiation; Cranbrook Institute of Science: Bloomfield Hills, MI, USA, 1942. [Google Scholar]
- Bowmaker, J.K. Evolution of the Vertebrate Eye. In How Animals See the WorldComparative Behavior, Biology, and Evolution of Vision; Oxford University Press: Oxford, UK, 2012; pp. 441–472. [Google Scholar]
- Refinetti, R. The Diversity of Temporal Niches in Mammals. Biol. Rhythm. Res. 2008, 39, 173–192. [Google Scholar] [CrossRef]
- Devarajan, K.; Fidino, M.; Farris, Z.J.; Adalsteinsson, S.A.; Andrade-Ponce, G.; Angstmann, J.L.; Anthonysamy, W.; Aquino, J.; Asefa, A.; Avila, B.; et al. When the Wild Things Are: Defining Mammalian Diel Activity and Plasticity. Sci. Adv. 2025, 11, eado3843. [Google Scholar] [CrossRef]
- Hall, M.I. The Anatomical Relationships between the Avian Eye, Orbit and Sclerotic Ring: Implications for Inferring Activity Patterns in Extinct Birds. J. Anat. 2008, 212, 781–794. [Google Scholar] [CrossRef]
- Schmitz, L.; Wainwright, P.C. Nocturnality Constrains Morphological and Functional Diversity in the Eyes of Reef Fishes. BMC Evol. Biol. 2011, 11, 338. [Google Scholar] [CrossRef]
- Fleishman, L.J. Lizard Visual Ecology. Front. Amphib. Reptile Sci. 2024, 2, 1426675. [Google Scholar] [CrossRef]
- Mass, A.M.; Supin, A.Y. Adaptive Features of Aquatic Mammals’ Eye. Anat. Rec. 2007, 290, 701–715. [Google Scholar] [CrossRef]
- Baker, J.; Venditti, C. Rapid Change in Mammalian Eye Shape Is Explained by Activity Pattern. Curr. Biol. 2019, 29, 1082–1088.e3. [Google Scholar] [CrossRef]
- Veilleux, C.C.; Lewis, R.J. Effects of Habitat Light Intensity on Mammalian Eye Shape. Anat. Rec. 2011, 294, 905–914. [Google Scholar] [CrossRef] [PubMed]
- Baden, T.; Angueyra, J.M.; Bosten, J.M.; Collin, S.P.; Conway, B.R.; Cortesi, F.; Dedek, K.; Euler, T.; Novales Flamarique, I.; Franklin, A.; et al. A Standardized Nomenclature for the Rods and Cones of the Vertebrate Retina. PLoS Biol. 2025, 23, e3003157. [Google Scholar] [CrossRef] [PubMed]
- Muth, T.; Schipke, J.D.; Brebeck, A.K.; Dreyer, S. Assessing Critical Flicker Fusion Frequency: Which Confounders? A Narrative Review. Medicina 2023, 59, 800. [Google Scholar] [CrossRef] [PubMed]
- Mankowska, N.D.; Marcinkowska, A.B.; Waskow, M.; Sharma, R.I.; Kot, J.; Winklewski, P.J. Critical Flicker Fusion Frequency: A Narrative Review. Medicina 2021, 57, 1096. [Google Scholar] [CrossRef]
- Lafitte, A.; Sordello, R.; Legrand, M.; Nicolas, V.; Obein, G.; Reyjol, Y. A Flashing Light May Not Be That Flashy: A Systematic Review on Critical Fusion Frequencies. PLoS ONE 2022, 17, e0279718. [Google Scholar] [CrossRef]
- Cobo, R.; Navarro-Sempere, A.; Segovia, Y.; García, M. Adaptations of the Vertebrate Retina to Low-Light Conditions: A Review. Anat. Histol. Embryol. 2025, 54, e70042. [Google Scholar] [CrossRef]
- Douglas, R.H.; Jeffery, G. The Spectral Transmission of Ocular Media Suggests Ultraviolet Sensitivity Is Widespread among Mammals. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132995. [Google Scholar] [CrossRef]
- O’Carroll, D.C.; Warrant, E.J. Vision in Dim Light: Highlights and Challenges. Philos. Trans. R. Soc. B: Biol. Sci. 2017, 372, 20160062. [Google Scholar] [CrossRef]
- Bowmaker, J.K. Evolution of Colour Vision in Vertebrates. Eye 1998, 12, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Musilova, Z.; Cortesi, F.; Matschiner, M.; Davies, W.I.L.; Patel, J.S.; Stieb, S.M.; de Busserolles, F.; Malmstrøm, M.; Tørresen, O.K.; Brown, C.J.; et al. Vision Using Multiple Distinct Rod Opsins in Deep-Sea Fishes. Science (1979) 2019, 364, 588–592. [Google Scholar] [CrossRef] [PubMed]
- Boyette, J.L.; Bell, R.C.; Fujita, M.K.; Thomas, K.N.; Streicher, J.W.; Gower, D.J.; Schott, R.K. Diversity and Molecular Evolution of Nonvisual Opsin Genes across Environmental, Developmental, and Morphological Adaptations in Frogs. Mol. Biol. Evol. 2024, 41, msae090. [Google Scholar] [CrossRef] [PubMed]
- Schott, R.K.; Fujita, M.K.; Streicher, J.W.; Gower, D.J.; Thomas, K.N.; Loew, E.R.; Bamba Kaya, A.G.; Bittencourt-Silva, G.B.; Guillherme Becker, C.; Cisneros-Heredia, D.; et al. Diversity and Evolution of Frog Visual Opsins: Spectral Tuning and Adaptation to Distinct Light Environments. Mol. Biol. Evol. 2024, 41, msae049. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, J.; Xue, T. Light, Opsins, and Life: Mammalian Photophysiological Functions beyond Image Perception. Neuron 2025, 19, 3108–3128. [Google Scholar] [CrossRef]
- Lamb, T.D.; Collin, S.P.; Pugh, E.N. Evolution of the Vertebrate Eye: Opsins, Photoreceptors, Retina and Eye Cup. Nat. Rev. Neurosci. 2007, 8, 960–976. [Google Scholar] [CrossRef]
- Hunt, D.M.; Carvalho, L.S.; Cowing, J.A.; Davies, W.L. Evolution and Spectral Tuning of Visual Pigments in Birds and Mammals. Philos. Trans. R. Soc. B Biol. Sci. 2009, 364, 2941–2955. [Google Scholar] [CrossRef]
- Smith, S.O. Mechanism of Activation of the Visual Receptor Rhodopsin. Annu. Rev. Biophys. 2023, 52, 301–317. [Google Scholar] [CrossRef]
- Hagen, J.F.D.; Roberts, N.S.; Johnston, R.J. The Evolutionary History and Spectral Tuning of Vertebrate Visual Opsins. Dev. Biol. 2023, 493, 40–66. [Google Scholar] [CrossRef]
- Bloomfield, S.A.; Dacheux, R.F. Rod Vision: Pathways and Processing in the Mammalian Retina. Prog. Retin. Eye Res. 2001, 20, 351–384. [Google Scholar] [CrossRef] [PubMed]
- Tikidji-Hamburyan, A.; Reinhard, K.; Storchi, R.; Dietter, J.; Seitter, H.; Davis, K.E.; Idrees, S.; Mutter, M.; Walmsley, L.; Bedford, R.A.; et al. Rods Progressively Escape Saturation to Drive Visual Responses in Daylight Conditions. Nat. Commun. 2017, 8, 1813. [Google Scholar] [CrossRef]
- Irazábal-González, L.; Wright, D.S.; Maan, M.E. Developmental and Environmental Plasticity in Opsin Gene Expression in Lake Victoria Cichlid Fish. Evol. Dev. 2024, 26, e12465. [Google Scholar] [CrossRef] [PubMed]
- Lupše, N.; Cortesi, F.; Freese, M.; Marohn, L.; Pohlmann, J.-D.; Wysujack, K.; Hanel, R.; Musilova, Z. Visual Gene Expression Reveals a Cone-to-Rod Developmental Progression in Deep-Sea Fishes. Mol. Biol. Evol. 2021, 38, 5664–5677. [Google Scholar] [CrossRef] [PubMed]
- Fogg, L.G.; Isari, S.; Barnes, J.E.; Patel, J.S.; Marshall, N.J.; Salzburger, W.; Cortesi, F.; de Busserolles, F. Deep-Sea Fish Reveal Alternative Pathway for Vertebrate Visual Development. bioRxiv 2024. [Google Scholar] [CrossRef]
- Andrabi, M.; Upton, B.A.; Lang, R.A.; Vemaraju, S. An Expanding Role for Nonvisual Opsins in Extraocular Light Sensing Physiology. Annu. Rev. Vis. Sci. 2023, 9, 245–267. [Google Scholar] [CrossRef]
- Mitchell, L.J.; Cheney, K.L.; Lührmann, M.; Marshall, J.; Michie, K.; Cortesi, F. Molecular Evolution of Ultraviolet Visual Opsins and Spectral Tuning of Photoreceptors in Anemonefishes (Amphiprioninae). Genome Biol. Evol. 2021, 13, evab184. [Google Scholar] [CrossRef]
- Pérez i de Lanuza, G.; Font, E. Ultraviolet Vision in Lacertid Lizards: Evidence from Retinal Structure, Eye Transmittance, SWS1 Visual Pigment Genes, and Behaviour. J. Exp. Biol. 2014, 217 Pt 16, 2899–2909. [Google Scholar] [CrossRef]
- Koncicki, A.; Pietruszyńska, M.; Mieszczak, M.; Stępniewska, J.; Tykałowski, B.; Stenzel, T. Selected Issues in the Anatomy and Physiology of the Avian Organ of Vision and Eye Disorders in Farmed Poultry. J. Vet. Res. 2025, 69, 241–247. [Google Scholar] [CrossRef]
- Ausprey, I.J. Adaptations to Light Contribute to the Ecological Niches and Evolution of the Terrestrial Avifauna. Proc. R. Soc. B Biol. Sci. 2021, 288, rspb.2021.0853. [Google Scholar] [CrossRef]
- Wilby, D.; Toomey, M.B.; Olsson, P.; Frederiksen, R.; Cornwall, M.C.; Oulton, R.; Kelber, A.; Corbo, J.C.; Roberts, N.W. Optics of Cone Photoreceptors in the Chicken (Gallus gallus domesticus). J. R. Soc. Interface. 2015, 12, 20150591. [Google Scholar] [CrossRef] [PubMed]
- Field, G.D.; Sampath, A.P. Behavioural and Physiological Limits to Vision in Mammals. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160072. [Google Scholar] [CrossRef] [PubMed]
- Kaskan, P.M.; Franco, E.C.S.; Yamada, E.S.; de Lima Silveira, L.C.; Darlington, R.B.; Finlay, B.L. Peripheral Variability and Central Constancy in Mammalian Visual System Evolution. Proc. R. Soc. B Biol. Sci. 2005, 272, 91–100. [Google Scholar] [CrossRef] [PubMed]
- Peichl, L. Diversity of Mammalian Photoreceptor Properties: Adaptations to Habitat and Lifestyle? Anat. Rec. A Discov. Mol. Cell Evol. Biol. 2005, 287A, 1001–1012. [Google Scholar] [CrossRef]
- Gerl, E.J.; Morris, M.R. The Causes and Consequences of Color Vision. Evol. Educ. Outreach 2008, 1, 476–486. [Google Scholar] [CrossRef]
- Gai, Y.; Tian, R.; Liu, F.; Mu, Y.; Shan, L.; Irwin, D.M.; Liu, Y.; Xu, S.; Yang, G. Diversified Mammalian Visual Adaptations to Bright- or Dim-Light Environments. Mol. Biol. Evol. 2023, 40, msad063. [Google Scholar] [CrossRef]
- Emerling, C.A.; Springer, M.S. Genomic Evidence for Rod Monochromacy in Sloths and Armadillos Suggests Early Subterranean History for Xenarthra. Proc. R. Soc. B Biol. Sci. 2015, 282, 20142192. [Google Scholar] [CrossRef]
- Nilsson, D.-E. Eye Evolution and Its Functional Basis. Vis. Neurosci. 2013, 30, 5–20. [Google Scholar] [CrossRef]
- Nilsson, D.-E. The Diversity of Eyes and Vision. Annu. Rev. Vis. Sci. 2021, 7, 19–41. [Google Scholar] [CrossRef]
- Gehring, W.J. New Perspectives on Eye Development and the Evolution of Eyes and Photoreceptors. J. Hered. 2005, 96, 171–184. [Google Scholar] [CrossRef]
- Collin, S.P. Early Evolution of Vertebrate Photoreception: Lessons from Lampreys and Lungfishes. Integr. Zool. 2009, 4, 87–98. [Google Scholar] [CrossRef] [PubMed]
- Baldanzi, E. Physiological and Psychological Effect of Light. In Sustainable Indoor Lighting; Sansoni, P., Mercatelli, L., Farini, A., Eds.; Springer: London, UK, 2015; pp. 285–301. [Google Scholar]
- Zeitzer, J.M. Light at Night and Mental Health, Cause or Consequence? Nat. Ment. Health 2023, 1, 822–823. [Google Scholar] [CrossRef]
- Lucas, R.J.; Allen, A.E.; Brainard, G.C.; Brown, T.M.; Dauchy, R.T.; Didikoglu, A.; Do, M.T.H.; Gaskill, B.N.; Hattar, S.; Hawkins, P.; et al. Recommendations for Measuring and Standardizing Light for Laboratory Mammals to Improve Welfare and Reproducibility in Animal Research. PLoS Biol. 2024, 22, e3002535. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.Q.; Davies, S.; Dominoni, D. Hormonally Mediated Effects of Artificial Light at Night on Behavior and Fitness: Linking Endocrine Mechanisms with Function. J. Exp. Biol. 2018, 221, jeb.156893. [Google Scholar] [CrossRef]
- Rao, F.; Xue, T. Circadian-Independent Light Regulation of Mammalian Metabolism. Nat. Metab. 2024, 6, 1000–1007. [Google Scholar] [CrossRef]
- Crespo, M.T.; Trebucq, L.L.; Senna, C.A.; Hokama, G.; Paladino, N.; Agostino, P.V.; Chiesa, J.J. Circadian Disruption of Feeding-Fasting Rhythm and Its Consequences for Metabolic, Immune, Cancer, and Cognitive Processes. Biomed. J. 2025, 48, 100827. [Google Scholar] [CrossRef]
- Grosjean, E.; Simonneaux, V.; Challet, E. Reciprocal Interactions between Circadian Clocks, Food Intake, and Energy Metabolism. Biology 2023, 12, 539. [Google Scholar] [CrossRef]
- Pickel, L.; Sung, H.K. Feeding Rhythms and the Circadian Regulation of Metabolism. Front. Nutr. 2020, 7, 39. [Google Scholar] [CrossRef]
- Dardente, H.; Hazlerigg, D.G.; Ebling, F.J.P. Thyroid Hormone and Seasonal Rhythmicity. Front. Endocrinol. 2014, 5, 19. [Google Scholar] [CrossRef]
- Helm, B.; Ben-Shlomo, R.; Sheriff, M.J.; Hut, R.A.; Foster, R.; Barnes, B.M.; Dominoni, D. Annual Rhythms That Underlie Phenology: Biological Time-Keeping Meets Environmental Change. Proc. R. Soc. B Biol. Sci. 2013, 280. [Google Scholar] [CrossRef]
- Cao, D.; Barrionuevo, P.A. The Importance of Intrinsically Photosensitive Retinal Ganglion Cells and Implications for Lighting Design. J. Solid State Light. 2015, 2, 10. [Google Scholar] [CrossRef]
- Weaver, D.R. Introduction to Circadian Rhythms and Mechanisms of Circadian Oscillations. In Circadian Clocks: Role in Health and Disease; Springer: New York, NY, USA, 2016; pp. 1–55. [Google Scholar]
- Li, A.; Wei, X.; Xie, Y.; Ren, Y.; Zhu, X.; Liu, M.; Liu, S. Light Exposure and Its Applications in Human Health. J. Biophoton. 2024, 17, e202400023. [Google Scholar] [CrossRef]
- Itzhacki, J.; Clesse, D.; Goumon, Y.; Van Someren, E.J.; Mendoza, J. Light Rescues Circadian Behavior and Brain Dopamine Abnormalities in Diurnal Rodents Exposed to a Winter-like Photoperiod. Brain. Struct. Funct. 2018, 223, 2641–2652. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Xu, R.; Li, Y.; Zhou, W.; Wang, W.; Gao, H.; Wang, Z.; Deng, Y.; Liu, Y.; Feng, J. Artificial Light Reduces Foraging Opportunities in Wild Least Horseshoe Bats. Environ. Pollut. 2021, 288, 117765. [Google Scholar] [CrossRef] [PubMed]
- Gaston, K.J.; Bennie, J.; Davies, T.W.; Hopkins, J. The Ecological Impacts of Nighttime Light Pollution: A Mechanistic Appraisal. Biol. Rev. 2013, 88, 912–927. [Google Scholar] [CrossRef]
- Pal, P.; Dey, D.; Sharma, B.; Sahu, J.; Kumar, S.; Choudhary, S.; Ghosh, S. Effect of Light Management in Broiler Production: A Review. J. Entomol. Zool. Stud. 2019, 7, 437–441. [Google Scholar]
- Li, C.; Shu, H.; Gu, X. Photoperiod Management in Farm Animal Husbandry: A Review. Animals 2025, 15, 591. [Google Scholar] [CrossRef]
- Boeuf, G.; Le Bail, P.Y. Does Light Have an Influence on Fish Growth? Aquaculture 1999, 177, 129–152. [Google Scholar] [CrossRef]
- Cuthill, I.C.; Partridge, J.C.; Benneit, A.T.D.; Church, S.C.; Hart, N.S.; Hunt, S. Ultraviolet Vision in Birds. Adv. Study Behav. 2000, 29, 159–214. [Google Scholar]
- Kelber, A.; Vorobyev, M.; Osorio, D. Animal Colour Vision - Behavioural Tests and Physiological Concepts. Biol. Rev. Camb. Philos. Soc. 2003, 78, 81–118. [Google Scholar] [CrossRef]
- Schaefer, H.M.; Schmidt, V. Detectability and Content as Opposing Signal Characteristics in Fruits. Proc. R. Soc. B Biol. Sci. 2004, 271, S370–S373. [Google Scholar] [CrossRef]
- Maddocks, S.A.; Church, S.C.; Cuthill, I.C. The Effects of the Light Environment on Prey Choice by Zebra Finches. J. Exp. Biol. 2001, 204, 2509–2515. [Google Scholar] [CrossRef]
- Church, S.C.; Bennett, A.T.D.; Cuthill, I.C.; Partridge, J.C. Ultraviolet Cues Affect the Foraging Behaviour of Blue Tits. Proc. R. Soc. Lond. B 1998, 265, 1509–1514. [Google Scholar] [CrossRef]
- Browman, H.I.; Hawryshyn, W.C. The Developmental Trajectory of Ultraviolet Photosensitivity in Rainbow Trout Is Altered by Thyroxine. Vison Res. 1994, 34, 1397–1406. [Google Scholar] [CrossRef]
- Rickel, S.; Genin, A. Twilight Transitions in Coral Reef Fish: The Input of Light-Induced Changes in Foraging Behaviour. Anim. Behav. 2005, 70, 133–144. [Google Scholar] [CrossRef]
- Kozaki, T.; Kubokawa, A.; Taketomi, R.; Hatae, K. Effects of Day-Time Exposure to Different Light Intensities on Light-Induced Melatonin Suppression at Night. J. Physiol. Anthr. 2015, 34, 27. [Google Scholar] [CrossRef] [PubMed]
- Comai, S.; Gobbi, G. Melatonin, Melatonin Receptors and Sleep: Moving Beyond Traditional Views. J. Pineal Res. 2024, 76, e13011. [Google Scholar] [CrossRef] [PubMed]
- Wetterberg, L. Light and Biological Rhythms. J. Intern. Med. 1994, 235, 5–19. [Google Scholar] [CrossRef] [PubMed]
- Dauchy, R.T.; Blask, D.E.; Hoffman, A.E.; Xiang, S.; Hanifin, J.P.; Warfield, B.; Brainard, G.C.; Anbalagan, M.; Dupepe, L.M.; Dobek, G.L.; et al. Influence of Daytime LED Light Exposure on Circadian Regulatory Dynamics of Metabolism and Physiology in Mice. Comp. Med. 2019, 69, 350–373. [Google Scholar] [CrossRef]
- Nelson, R.J.; Demas, G.E. Seasonal Changes in Immune Function. Q. Rev. Biol. 1996, 71, 511–548. [Google Scholar] [CrossRef]
- Garimano, N.; Aguayo Frías, T.; González Maglio, D.H. Beyond Ultraviolet Radiation: Immune System Modulation through Skin Exposure to Visible Light and Infrared Radiation. Photochem. Photobiol. 2025, 101, 846–857. [Google Scholar] [CrossRef]
- Goldman, B.D. Mammalian Photoperiodic System: Formal Properties and Neuroendocrine Mechanisms of Photoperiodic Time Measurement. J. Biol. Rhythm. 2001, 16, 283–301. [Google Scholar] [CrossRef]
- Hazlerigg, D.G.; Simonneaux, V.; Dardente, H. Melatonin and Seasonal Synchrony in Mammals. J. Pineal Res. 2024, 76, e12996. [Google Scholar] [CrossRef] [PubMed]
- Barrett, P.; Bolborea, M. Molecular Pathways Involved in Seasonal Body Weight and Reproductive Responses Governed by Melatonin. J. Pineal Res. 2012, 52, 376–388. [Google Scholar] [CrossRef]
- Brando, S.; Buchanan-Smith, H.M. The 24/7 Approach to Promoting Optimal Welfare for Captive Wild Animals. Behav. Process. 2018, 156, 83–95. [Google Scholar] [CrossRef] [PubMed]
- Schiffmann, C.; Hoby, S.; Wenker, C.; Hård, T.; Scholz, R.; Clauss, M.; Hatt, J. When Elephants Fall Asleep: A Literature Review on Elephant Rest with Case Studies on Elephant Falling Bouts, and Practical Solutions for Zoo Elephants. Zoo Biol. 2018, 37, 133–145. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.M.; Brainard, G.C.; Cajochen, C.; Czeisler, C.A.; Hanifin, J.P.; Lockley, S.W.; Lucas, R.J.; Münch, M.; O’Hagan, J.B.; Peirson, S.N.; et al. Recommendations for Daytime, Evening, and Nighttime Indoor Light Exposure to Best Support Physiology, Sleep, and Wakefulness in Healthy Adults. PLoS Biol. 2022, 20, e3001571. [Google Scholar] [CrossRef]
- McGuire, A.; Kienlen, M.; Emory, R.; LaDue, C.A. Overnight Monitoring Reveals the Behavioral Rhythms of a Geriatric Male Elephant: An Animal-Centered Case Study of Rest and Stereotypy. Front. Conserv. Sci. 2024, 5, 1362313. [Google Scholar] [CrossRef]
- Brando, S.; Vitale, A.; Bacon, M. Promoting Good Nonhuman Primate Welfare Outside Regular Working Hours. Animals 2023, 13, 1423. [Google Scholar] [CrossRef]
- Refinetti, R. Circadian Physiology, 3rd ed.; CRC Press: Boca Raton, FL, USA, 2016; ISBN 9780429096228. [Google Scholar]
- Aulsebrook, A.E.; Jones, T.M.; Rattenborg, N.C.; Roth, T.C.; Lesku, J.A. Sleep Ecophysiology: Integrating Neuroscience and Ecology. Trends Ecol. Evol. 2016, 31, 590–599. [Google Scholar] [CrossRef]
- Ayuso, P.R.; Feliu, O.; Riba, D.; Crailsheim, D. Listening to Their Nights: Sleep Disruptions in Captive Housed Chimpanzees Affect Their Daytime Behavior. Animals 2023, 13, 696. [Google Scholar] [CrossRef]
- Gandia, K.M.; Kessler, S.E.; Buchanan-Smith, H.M. Latitudinal and Zoo Specific Zeitgebers Influence Circadian and Circannual Rhythmicity of Behavior in Captive Giant Pandas (Ailuropoda melanoleuca). Front. Psychol. 2023, 14, 1188566. [Google Scholar] [CrossRef]
- Le Tallec, T.; Perret, M.; Théry, M. Light Pollution Modifies the Expression of Daily Rhythms and Behavior Patterns in a Nocturnal Primate. PLoS ONE 2013, 8, e79250. [Google Scholar] [CrossRef] [PubMed]
- Steiger, S.S.; Valcu, M.; Spoelstra, K.; Helm, B.; Wikelski, M.; Kempenaers, B. When the Sun Never Sets: Diverse Activity Rhythms under Continuous Daylight in Free-Living Arctic-Breeding Birds. Proc. R. Soc. B Biol. Sci. 2013, 280, 20131016. [Google Scholar] [CrossRef] [PubMed]
- Brzezinski, A.; Rai, S.; Purohit, A.; Pandi-Perumal, S.R. Melatonin, Clock Genes, and Mammalian Reproduction: What Is the Link? Int. J. Mol. Sci. 2021, 22, 13240. [Google Scholar] [CrossRef] [PubMed]
- Aulsebrook, L.C.; Bertram, M.G.; Martin, J.M.; Aulsebrook, A.E.; Brodin, T.; Evans, J.P.; Hall, M.D.; O’Bryan, M.K.; Pask, A.J.; Tyler, C.R.; et al. Reproduction in a Polluted World: Implications for Wildlife. Reproduction 2020, 160, R13–R23. [Google Scholar] [CrossRef]
- Dominoni, D.M.; Quetting, M.; Partecke, J. Long-Term Effects of Chronic Light Pollution on Seasonal Functions of European Blackbirds (Turdus merula). PLoS ONE 2013, 8, e85069. [Google Scholar] [CrossRef]
- Dominoni, D.M.; Kjellberg Jensen, J.; de Jong, M.; Visser, M.E.; Spoelstra, K. Artificial Light at Night, in Interaction with Spring Temperature, Modulates Timing of Reproduction in a Passerine Bird. Ecol. Appl. 2020, 30, e02062. [Google Scholar] [CrossRef]
- Russart, K.L.G.; Nelson, R.J. Artificial Light at Night Alters Behavior in Laboratory and Wild Animals. J. Exp. Zool. A Ecol. Integr. Physiol. 2018, 329, 401–408. [Google Scholar] [CrossRef]
- Blehar, M.C. Seasonal Affective Disorders and Phototherapy. Arch. Gen. Psychiatry 1989, 46, 469. [Google Scholar] [CrossRef]
- Leach, G.; Adidharma, W.; Yan, L. Depression-Like Responses Induced by Daytime Light Deficiency in the Diurnal Grass Rat (Arvicanthis niloticus). PLoS ONE 2013, 8, e57115. [Google Scholar] [CrossRef]
- Adkins, E.; Driggers, T.; Ferguson, G.; Gehrmann, W.; Gyimesi, Z.; May, E.; Ogle, M.; Owens, T.; Klaphake, E. Ultraviolet Light and Reptiles, Amphibians. J. Herpetol. Med. Surg. 2003, 13, 27–37. [Google Scholar] [CrossRef]
- Petrović, T.G.; Gavrić-Čampar, J.P.; Burraco, P.; Gavrilović, B.R.; Despotović, S.G.; Radovanović, T.B.; Radmilović, A.; Mirč, M.; Tomašević Kolarov, N.; Prokić, M.D. Developing under Artificial Light Is Not so Bright: Oxidative Stress as a Physiological Response to Light Pollution across Amphibian Life Stages. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2025, 302, 111819. [Google Scholar] [CrossRef]
- Yamamoto, T.; Trathan, P.N. Evidences of Moon-related Effects on Animal Behaviour. Clin. Obes. 2015, 5, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Mandal, F.B. Lunar Cycle-Mediated Behaviours in Animals, Including Humans. Isr. J. Ecol. Evol. 2023, 69, 169–179. [Google Scholar] [CrossRef]
- Raible, F.; Takekata, H.; Tessmar-Raible, K. An Overview of Monthly Rhythms and Clocks. Front. Neurol. 2017, 8, 189. [Google Scholar] [CrossRef] [PubMed]
- Bischof, R.; Vallejo-Vargas, A.F.; Semper-Pascual, A.; Schowanek, S.D.; Beaudrot, L.; Turek, D.; Jansen, P.A.; Rovero, F.; Johnson, S.E.; Guimarães Moreira Lima, M.; et al. The Moon’s Influence on the Activity of Tropical Forest Mammals. Proc. R. Soc. B Biol. Sci. 2024, 291, 20240683. [Google Scholar] [CrossRef]
- Brigham, R.M. Lunar Influence on Foraging and Nesting Activity of Common Poorwills (Phalaenoptilus nuttallii). Auk 1992, 109, 315–320. [Google Scholar] [CrossRef]
- Kronfeld-Schor, N.; Dominoni, D.; de la Iglesia, H.; Levy, O.; Herzog, E.D.; Dayan, T.; Helfrich-Forster, C. Chronobiology by Moonlight. Proc. R. Soc. B Biol. Sci. 2013, 280, 20123088. [Google Scholar] [CrossRef]
- Korpach, A.M.; Davy, C.M.; Mills, A.M.; Fraser, K.C. Lunar Synchrony, Geography, and Individual Clocks Shape Autumn Migration Timing in an Avian Migrant. Behav. Ecol. 2024, 35. [Google Scholar] [CrossRef]
- Sergeyev, M.; Lombardi, J.V.; Tewes, M.E.; Campbell, T.A. Ocelots in the Moonlight: Influence of Lunar Phase on Habitat Selection and Movement of Two Sympatric Felids. PLoS ONE 2023, 18, e0286393. [Google Scholar] [CrossRef]
- Underhill, V.A.; Höbel, G. Mate Choice Behavior of Female Eastern Gray Treefrogs (Hyla Versicolor) Is Robust to Anthropogenic Light Pollution. Ethology 2018, 124, 537–548. [Google Scholar] [CrossRef]
- Grant, R.; Halliday, T.; Chadwick, E. Amphibians’ Response to the Lunar Synodic Cycle—A Review of Current Knowledge, Recommendations, and Implications for Conservation. Behav. Ecol. 2013, 24, 53–62. [Google Scholar] [CrossRef]
- Kurvers, R.H.J.M.; Hölker, F. Bright Nights and Social Interactions: A Neglected Issue. Behav. Ecol. 2015, 26, 334–339. [Google Scholar] [CrossRef]
- Taylor, L.A.; Thawley, C.J.; Pertuit, O.R.; Dennis, A.J.; Carson, I.R.; Tang, C.; Johnson, M.A. Artificial Light at Night Alters Diurnal and Nocturnal Behavior and Physiology in Green Anole Lizards. Physiol. Behav. 2022, 257, 113992. [Google Scholar] [CrossRef] [PubMed]
- Stanton, D.L.; Cowart, J.R. The Effects of Artificial Light at Night (ALAN) on the Circadian Biology of Marine Animals. Front. Mar. Sci. 2024, 11, 1372889. [Google Scholar] [CrossRef]
- Sanders, D.; Frago, E.; Kehoe, R.; Patterson, C.; Gaston, K.J. A Meta-Analysis of Biological Impacts of Artificial Light at Night. Nat. Ecol. Evol. 2020, 5, 74–81. [Google Scholar] [CrossRef]
- Scaillierez, A.J.; de van Nieuwamerongen-Koning, S.E.; Boumans, I.J.M.M.; van der Tol, P.P.J.; Bokkers, E.A.M. Review: The Influence of Light on Pig Welfare. Animal 2024, 18, 101313. [Google Scholar] [CrossRef]
- Wichman, A.; De Groot, R.; Håstad, O.; Wall, H.; Rubene, D. Influence of Different Light Spectrums on Behaviour and Welfare in Laying Hens. Animals 2021, 11, 924. [Google Scholar] [CrossRef]
- Burger, A.L.; Hartig, J.; Dierkes, P.W. Shedding Light into the Dark: Age and Light Shape Nocturnal Activity and Sleep Behaviour of Giraffe. Appl. Anim. Behav. Sci. 2020, 229, 105012. [Google Scholar] [CrossRef]
- Mellor, D.J.; Reid, C.S.W. Concepts of Animal Well-Being and Predicting the Impact of Procedures on Experimental Animals. Improving the Weel-Being of Animals in the Research Environment. 1994. Available online: https://www.wellbeingintlstudiesrepository.org/cgi/viewcontent.cgi?article=1006&context=exprawel/ (accessed on 2 November 2025).
- Bracke, M.B.M.; Hopster, H. Assessing the Importance of Natural Behavior for Animal Welfare. J. Agric. Environ. Ethics 2006, 19, 77–89. [Google Scholar] [CrossRef]
- Kalajian, T.A.; Aldoukhi, A.; Veronikis, A.J.; Persons, K.; Holick, M.F. Ultraviolet B Light Emitting Diodes (LEDs) Are More Efficient and Effective in Producing Vitamin D3 in Human Skin Compared to Natural Sunlight. Sci. Rep. 2017, 7, 11489. [Google Scholar] [CrossRef] [PubMed]
- Colwill, R.M.; Creton, R. Imaging Escape and Avoidance Behavior in Zebrafish Larvae. Rev. Neurosci. 2011, 22, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Golla, A.; Østby, H.; Kermen, F. Chronic Unpredictable Stress Induces Anxiety-like Behaviors in Young Zebrafish. Sci. Rep. 2020, 10, 10339. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, Y.J.; Ho, Y.S.; Wang, Y.S. Illumination of Different Light Wavelengths on Growth Performance and Physiological Response of Juvenile Sweetfish, Plecoglossus Altivelis. Aquac. Rep. 2023, 30, 101569. [Google Scholar] [CrossRef]
- Fukuda, H.; Torisawa, S.; Sawada, Y.; Takagi, T. Developmental Changes in Behavioral and Retinomotor Responses of Pacific Bluefin Tuna on Exposure to Sudden Changes in Illumination. Aquaculture 2010, 305, 73–78. [Google Scholar] [CrossRef]
- Gaston, K.J.; Sánchez de Miguel, A. Environmental Impacts of Artificial Light at Night. Annu. Rev. Environ. Resour. 2022, 47, 373–398. [Google Scholar] [CrossRef]
- Yan, L.; Lonstein, J.S.; Nunez, A.A. Light as a Modulator of Emotion and Cognition: Lessons Learned from Studying a Diurnal Rodent. Horm. Behav. 2019, 111, 78–86. [Google Scholar] [CrossRef]
- UK Government UK Statutory Instruments 1992, No. 3004, Regulation 8. Available online: https://www.legislation.gov.uk/uksi/1992/3004/regulation/8 (accessed on 2 November 2025).
- Morgan, K.N.; Tromborg, C.T. Sources of Stress in Captivity. Appl. Anim. Behav. Sci. 2007, 102, 262–302. [Google Scholar] [CrossRef]
- Bennie, J.J.; Duffy, J.P.; Inger, R.; Gaston, K.J. Biogeography of Time Partitioning in Mammals. Proc. Natl. Acad. Sci. USA 2014, 111, 13727–13732. [Google Scholar] [CrossRef]
- Fuller, A.; Mitchell, D.; Maloney, S.K.; Hetem, R.S. Towards a Mechanistic Understanding of the Responses of Large Terrestrial Mammals to Heat and Aridity Associated with Climate Change. Clim. Change Responses 2016, 3, 10. [Google Scholar] [CrossRef]
- Fuller, G.; Kuhar, C.W.; Dennis, P.M.; Lukas, K.E. A Survey of Husbandry Practices for Lorisid Primates in N Orth A Merican Zoos and Related Facilities. Zoo Biol. 2013, 32, 88–100. [Google Scholar] [CrossRef]
- Moreno, O.; Fuentes-Hernandez, C.; Kippelen, B. Redefining Artificial Lighting through Spectral Engineering of Light Sources for Well-Being. Sci. Rep. 2024, 14, 26298. [Google Scholar] [CrossRef]
- French, F.; Bwye, P.; Carrigan, L.; Coe, J.C.; Kelly, R.; Leek, T.; Lynch, E.C.; Mahan, E.; Mingee, C. Welfare and Enrichment of Managed Nocturnal Species, Supported by Technology. Animals 2024, 14, 2378. [Google Scholar] [CrossRef]
- Byczyk, K.; Dunn, N.; Pribrsky, F.; Tang, C. EAZA Best Practice Guidelines Nycticebus Species; European Association of Zoos and Aquaria (EAZA): Amsterdam, The Netherlands, 2022. [Google Scholar]
- Alejandro, J.; Yamanashi, Y.; Nemoto, K.; Bercovitch, F.B.; Huffman, M.A. Behavioral Changes of Solitary Housed Female Pygmy Slow Lorises (Nycticebus Pygmeaus) after Introduction into Group Enclosures. Animals 2021, 11, 2751. [Google Scholar] [CrossRef]
- Institution of Lighting Professionals & Bat Conservation Trust Guidance Note 08/18: Bats and Artificial Lighting in the UK; Rugby, 2018. Available online: https://cdn.bats.org.uk/uploads/pdf/Resources/ilp-guidance-note-8-bats-and-artificial-lighting-compressed.pdf?v=1542109349 (accessed on 2 November 2025).
- Bastian, M.L.; Glendinning, D.R.; Brown, J.L.; Boisseau, N.P.; Edwards, K.L. Effects of a Recurring Late-Night Event on the Behavior and Welfare of a Population of Zoo-Housed Gorillas. Zoo Biol. 2020, 39, 217–229. [Google Scholar] [CrossRef] [PubMed]
- Readyhough, T.S.; Joseph, S.; Vyas, K.; Schreier, A.L. The Effects of Zoo Lights on Animal Welfare: A Case Study of Great Indian Hornbills at Denver Zoo. Zoo Biol. 2022, 41, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Davey, G. Visitors’ Effects on the Welfare of Animals in the Zoo: A Review. J. Appl. Anim. Welf. Sci. 2007, 10, 169–183. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.; Fulwell, T.; Walsh, N.D.; Harley, J.J.; Johnson, B. The Impacts of Evening Events in Zoos: A Christmas Event at Knowsley Safari. J. Zool. Bot. Gard. 2023, 4, 21–38. [Google Scholar] [CrossRef]
- Queiroz, M.B.; Young, R.J. The Different Physical and Behavioural Characteristics of Zoo Mammals That Influence Their Response to Visitors. Animals 2018, 8, 139. [Google Scholar] [CrossRef]
- Harley, J.J.; Rowden, L.J.; Clifforde, L.M.; Power, A.; Stanley, C.R. Preliminary Investigation of the Effects of a Concert on the Behavior of Zoo Animals. Zoo Biol. 2022, 41, 308–327. [Google Scholar] [CrossRef]
- Schell, C.J.; Young, J.K.; Lonsdorf, E.V.; Santymire, R.M. Anthropogenic and Physiologically Induced Stress Responses in Captive Coyotes. J. Mammal. 2013, 94, 1131–1140. [Google Scholar] [CrossRef][Green Version]
- AV Magazine. Available online: https://www.avinteractive.com/markets-news/visitor-attractions/pangolin-trail-night-safari-lights-mimic-effect-of-moonlight-19-03-2025/ (accessed on 19 March 2025).
- Bat Friendly Lighting for Outdoor Spaces. Available online: https://madebylandmark.com/blog/bat-friendly-lighting-for-outdoor-spaces?utm_source=chatgpt.com (accessed on 25 October 2025).
- Peña-García, A.; Hurtado, A.; Aguilar-Luzón, M.C. Impact of Public Lighting on Pedestrians’ Perception of Safety and Well-Being. Saf. Sci. 2015, 78, 142–148. [Google Scholar] [CrossRef]
- Devereux, E.A.; Ejezie, A.V.; Lynch, A.M.; Gruen, M.E.; LaJuett, S.J.; Robertson, J.B.; Scharf, V.F. Factors Affecting Sleep Among Dogs and Cats in a Veterinary Intensive Care Unit. J. Vet. Emerg. Crit. Care 2025, 35, 225–232. [Google Scholar] [CrossRef] [PubMed]
- Bedrosian, T.A.; Nelson, R.J. Influence of the Modern Light Environment on Mood. Mol. Psychiatry 2013, 18, 751–757. [Google Scholar] [CrossRef]
- Fonken, L.K.; Nelson, R.J. The Effects of Light at Night on Circadian Clocks and Metabolism. Endocr. Rev. 2014, 35, 648–670. [Google Scholar] [CrossRef]
- Friese, R.S. Sleep and Recovery from Critical Illness and Injury: A Review of Theory, Current Practice, and Future Directions. Crit. Care Med. 2008, 36, 697–705. [Google Scholar] [CrossRef]
- Azizi, M.; Golmohammadi, R.; Aliabadi, M. Comparative Analysis of Lighting Characteristics and Ultraviolet Emissions from Commercial Compact Fluorescent and Incandescent Lamps. J. Res. Health Sci. 2016, 16, 200–205. [Google Scholar]
- Evans, J.E.; Smith, E.L.; Bennett, A.T.D.; Cuthill, I.C.; Buchanan, K.L. Short-Term Physiological and Behavioural Effects of High- versus Low-Frequency Fluorescent Light on Captive Birds. Anim. Behav. 2012, 83, 25–33. [Google Scholar] [CrossRef]
- Sales, G.D.; Wilson, K.J.; Spencer, K.E.V.; Milligan, S.R. Environmental Ultrasound in Laboratories and Animal Houses: A Possible Cause for Concern in the Welfare and Use of Laboratory Animals. Lab. Anim. 1988, 22, 369–375. [Google Scholar] [CrossRef]
- Moore-Ede, M.; Blask, D.E.; Cain, S.W.; Heitmann, A.; Nelson, R.J. Lights Should Support Circadian Rhythms: Evidence-Based Scientific Consensus. Front. Photonics 2023, 4, 1272934. [Google Scholar] [CrossRef]
- Wunderlich, S.; Griffiths, T.; Baines, F. UVB-emitting LEDs for Reptile Lighting: Identifying the Risks of Nonsolar UV Spectra. Zoo Biol. 2024, 43, 61–74. [Google Scholar] [CrossRef] [PubMed]
- Davies, T.W.; Smyth, T. Why Artificial Light at Night Should Be a Focus for Global Change Research in the 21st Century. Glob. Change Biol. 2018, 24, 872–882. [Google Scholar] [CrossRef] [PubMed]
- Wakefield, A.; Broyles, M.; Stone, E.L.; Jones, G.; Harris, S. Experimentally Comparing the Attractiveness of Domestic Lights to Insects: Do LEDs Attract Fewer Insects than Conventional Light Types? Ecol. Evol. 2016, 6, 8028–8036. [Google Scholar] [CrossRef] [PubMed]
- Grandin, T.; Johnson, C. Animals in Translation: Using the Mysteries of Autism to Decode Animal Behaviour; Scribner/Simon & Schuster: New York, NY, USA, 2005. [Google Scholar]
- Emmer, K.M.; Russart, K.L.G.; Walker, W.H.; Nelson, R.J.; DeVries, A.C. Effects of Light at Night on Laboratory Animals and Research Outcomes. Behav. Neurosci. 2018, 132, 302–314. [Google Scholar] [CrossRef]
- Longcore, T.; Villanueva, S.A.M.B.; Nguyen-Ngo, K.; Ghiani, C.A.; Harrison, B.; Colwell, C.S. Relative Importance of Intensity and Spectrum of Artificial Light at Night in Disrupting Behavior of a Nocturnal Rodent. J. Exp. Biol. 2024, 227, jeb247235. [Google Scholar] [CrossRef]
- Moinard, C.; Lewis, P.D.; Perry, G.C.; Sherwin, C.M. The Effects of Light Intensity and Light Source on Injuries Due to Pecking of Male Domestic Turkeys (Meleagris gallopavo). Anim. Welf. 2001, 10, 131–139. [Google Scholar] [CrossRef]
- Pollard, J.C.; Littlejohn, R.P. Behavioural Effects of Light Conditions on Red Deer in a Holding Pen. Appl. Anim. Behav. Sci. 1994, 41, 127–134. [Google Scholar] [CrossRef]
- Baines, F.M.; Chattell, J.; Dale, J.; Garrick, D.; Gill, I.; Goetz, M.; Skelton, T.; Swatman, M. How Much UVB Does My Reptile Need? The UV-Tool, a Guide to the Selection of UV Lighting for Reptiles and Amphibians in Captivity. J. Zoo Aquar. Res. 2016, 4, 42–63. [Google Scholar]
- López, J.; Wormell, D.; Rodríguez, A. Preliminary Evaluation of the Efficacy and Safety of a UVB Lamp Used to Prevent Metabolic Bone Disease in Pied Tamarins Saguinus Bicolor at Jersey Zoo. Dodo 2003, 37, 41–49. [Google Scholar]
- Aubé, M.; Roby, J.; Kocifaj, M. Evaluating Potential Spectral Impacts of Various Artificial Lights on Melatonin Suppression, Photosynthesis, and Star Visibility. PLoS ONE 2013, 8, e67798. [Google Scholar] [CrossRef] [PubMed]
- Gayral, B. LEDs for Lighting: Basic Physics and Prospects for Energy Savings. Comptes Rendus Phys. 2017, 18, 453–461. [Google Scholar] [CrossRef]
- Mellor, D.J.; Beausoleil, N.J. Extending the “Five Domains” Model for Animal Welfare Assessment to Incorporate Positive Welfare States. Anim Welf. 2015, 24, 241–253. [Google Scholar] [CrossRef]
- Browning, H. The Natural Behavior Debate: Two Conceptions of Animal Welfare. J. Appl. Anim. Welf. Sci. 2020, 23, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Poon, L.; Jenks, I.T.; Crampton, W.G.R. MoonShine: A Software-hardware System for Simulating Moonlight Ground Illuminance and Re-creating Artificial Moonlight Cycles in a Laboratory Environment. Methods Ecol. Evol. 2024, 15, 701–715. [Google Scholar] [CrossRef]
- Starlight Safari at Disney’s Animal Kingdom Lodge. Available online: https://www.disneyworld.co.uk/events-tours/animal-kingdom/night-safari/ (accessed on 22 September 2025).
- Ardoin, N.M.; Bowers, A.W.; Gaillard, E. Environmental Education Outcomes for Conservation: A Systematic Review. Biol. Conserv. 2020, 241, 108224. [Google Scholar] [CrossRef]
- Rose, P.E.; Lewton, J. Key Concepts for Enhancing Zoo Animal Welfare: Coping, Comfort, Choice, Control, Challenge, and Compassion. J. Appl. Anim. Welf. Sci. 2025, 28, 497–514. [Google Scholar] [CrossRef]
- Kagan, R.; Carter, S.; Allard, S. A Universal Animal Welfare Framework for Zoos. J. Appl. Anim. Welf. Sci. 2015, 18, S1–S10. [Google Scholar] [CrossRef]
- WAZA—World Association of Zoos and Aquariums. WAZA—World Association of Zoos and Aquariums; World Press: Gland, Switzerland, 2016; pp. 1–27. [Google Scholar]
- Cipreste, C.F.; Pizzutto, C.S.; Azevedo, C.S. Breve História Das Coleções de Animais e a Evolução Do Enriquecimento Ambiental. In Fundamentos do Enriquecimento Ambiental; Azevedo, C.S., Cipreste, C.F., Pizzutto, C.S., Eds.; Editora Payá: São Paulo, Brazil, 2022; pp. 1–8. [Google Scholar]
- Gaston, K.J.; Davies, T.W.; Nedelec, S.L.; Holt, L.A. Impacts of Artificial Light at Night on Biological Timings. Annu. Rev. Ecol. Evol. Syst. 2017, 48, 49–68. [Google Scholar] [CrossRef]
- Chen, J.; Okimura, K.; Yoshimura, T. Light and Hormones in Seasonal Regulation of Reproduction and Mood. Endocrinology 2020, 161, bqaa130. [Google Scholar] [CrossRef]
- Bará, S.; Falchi, F. Artificial Light at Night: A Global Disruptor of the Night-Time Environment. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220352. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, Q.; Pan, C.; Chen, J.; Xu, B.; Liu, K.; Pan, J.; Lagisz, M.; Nakagawa, S. Species Sensitivities to Artificial Light at Night: A Phylogenetically Controlled Multilevel Meta-analysis on Melatonin Suppression. Ecol. Lett. 2024, 27, e14387. [Google Scholar] [CrossRef] [PubMed]
- Young, R.J. Environmental Enrichment for Captive Animals, 1st ed.; Blackwell Science Ltda: Oxford, UK, 2003; Available online: https://www.federalcircusbill.org/wp-content/uploads/2014/04/Young20030001.pdf (accessed on 22 September 2025).
- Improve Health & Condition With The Equilume Stable Lighting System. Available online: https://equilume.com/improve-health-condition-equilume-stable-lighting-system/ (accessed on 22 September 2025).
- Seyrling, I.; Dierkes, P.W.; Burger, A.L. Diurnal and Nocturnal Behaviour of Cheetahs (Acinonyx jubatus) and Lions (Panthera Leo) in Zoos. Animals 2022, 12, 2367. [Google Scholar] [CrossRef] [PubMed]
- Russart, K.L.G.; Nelson, R.J. Light at Night as an Environmental Endocrine Disruptor. Physiol. Behav. 2018, 190, 82–89. [Google Scholar] [CrossRef]
- Gaston, K.J.; Visser, M.E.; Hölker, F. The Biological Impacts of Artificial Light at Night: The Research Challenge. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140133. [Google Scholar] [CrossRef]
- Meyer, B.; Hüppe, L.; Payton, L. Timing Requires the Right Amount and Type of Light. Nat. Ecol. Evol. 2021, 5, 153–154. [Google Scholar] [CrossRef]
- Longcore, T.; Rich, C. Ecological Light Pollution. Front. Ecol. Environ. 2004, 2, 191–198. [Google Scholar] [CrossRef]
- Desouhant, E.; Gomes, E.; Mondy, N.; Amat, I. Mechanistic, Ecological, and Evolutionary Consequences of Artificial Light at Night for Insects: Review and Prospective. Entomol. Exp. Appl. 2019, 167, 37–58. [Google Scholar] [CrossRef]
- Fobert, E.K.; Miller, C.R.; Swearer, S.E.; Mayer-Pinto, M. The Impacts of Artificial Light at Night on the Ecology of Temperate and Tropical Reefs. Philos. Trans. R. Soc. B Biol. Sci. 2023, 378, 20220362. [Google Scholar] [CrossRef]
- Rybnikova, N.A.; Haim, A.; Portnov, B.A. Does Artificial Light-at-Night Exposure Contribute to the Worldwide Obesity Pandemic? Int. J. Obes. 2016, 40, 815–823. [Google Scholar] [CrossRef]
- Fleury, G.; Masís-Vargas, A.; Kalsbeek, A. Metabolic Implications of Exposure to Light at Night: Lessons from Animal and Human Studies. Obesity 2020, 28, S18–S28. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.; Shi, D.; Li, X.; Ma, N.; Liu, Y.; Zhong, P.; Yan, X.; Zhang, J.; Lau, P.W.C.; Dong, Y.; et al. Artificial Light-at-Night Exposure and Overweight and Obesity across GDP Levels among Chinese Children and Adolescents. Nutrients 2023, 15, 939. [Google Scholar] [CrossRef] [PubMed]
- Williams, E.; Hunton, V.; Hosey, G.; Ward, S.J. The Impact of Visitors on Non-Primate Species in Zoos: A Quantitative Review. Animals 2023, 13, 1178. [Google Scholar] [CrossRef] [PubMed]
- Fischer, C.P.; Romero, L.M. Chronic Captivity Stress in Wild Animals Is Highly Species-Specific. Conserv. Physiol. 2019, 7, coz093. [Google Scholar] [CrossRef]
- Shen, J.; Tower, J. Effects of Light on Aging and Longevity. Ageing Res. Rev. 2019, 53, 100913. [Google Scholar] [CrossRef]
- Powell, D.M.; Watters, J.V. The Evolution of the Animal Welfare Movement in U.S. Zoos and Aquariums. Zool. Gart. 2017, 86, 219–234. [Google Scholar] [CrossRef]
- Mason, G.; Clubb, R.; Latham, N.; Vickery, S. Why and How Should We Use Environmental Enrichment to Tackle Stereotypic Behaviour? Appl. Anim. Behav. Sci. 2007, 102, 163–188. [Google Scholar] [CrossRef]
- Sherwen, S.L.; Hemsworth, P.H. The Visitor Effect on Zoo Animals: Implications and Opportunities for Zoo Animal Welfare. Animals 2019, 9, 366. [Google Scholar] [CrossRef]
- Binding, S.; Farmer, H.; Krusin, L.; Cronin, K. Status of Animal Welfare Research in Zoos and Aquariums: Where Are We, Where to Next? J. Zoo Aquar. Res. 2020, 8, 166–174. [Google Scholar]
- Mathis, M.W.; Mathis, A. Deep Learning Tools for the Measurement of Animal Behavior in Neuroscience. Curr. Opin. Neurobiol. 2020, 60, 1–11. [Google Scholar] [CrossRef]
- Fuchs, M.; Genty, E.; Bangerter, A.; Zuberbühler, K.; Odobez, J.-M.; Cotofrei, P. From Forest to Zoo: Great Ape Behavior Recognition with ChimpBehave. Int. J. Comput. Vis. 2025, 133, 6668–6688. [Google Scholar] [CrossRef]
- Kelber, A. Colour in the Eye of the Beholder: Receptor Sensitivities and Neural Circuits Underlying Colour Opponency and Colour Perception. Curr. Opin. Neurobiol. 2016, 41, 106–112. [Google Scholar] [CrossRef]
- Yokoyama, S. Evolution of Dim-Light and Color Vision Pigments. Annu. Rev. Genom. Hum. Genet. 2008, 9, 259–282. [Google Scholar] [CrossRef]
- Brando, S.; Coe, J. Confronting Back-of-House Traditions: Primates as a Case Study. J. Zool. Bot. Gard. 2022, 3, 366–397. [Google Scholar] [CrossRef]
- Rust, K.; Clegg, I.; Fernandez, E.J. The Voice of Choice: A Scoping Review of Choice-Based Animal Welfare Studies. Appl. Anim. Behav. Sci. 2024, 275, 106270. [Google Scholar] [CrossRef]
- Browning, H.; Veit, W. Animal Welfare, Agency, and Animal–Computer Interaction. Animals 2025, 15, 219. [Google Scholar] [CrossRef]

| Vertebrate Group | CFF (Hz) (Mean ± SD; Range) | Notes/Implications for Flicker Risk |
|---|---|---|
| Elasmobranchii | 26.4 ± 11.7 (32–45) | Relatively low temporal resolution: most artificial lights are above the perceptual threshold. |
| (sharks, rays) | ||
| Actinopterygii | 49.9 ± 23.8 (8.8–117) | Wide variability; fast-swimming or diurnal species may detect flicker at lower frequencies. |
| (ray-finned fish) | ||
| Amphibia | 19 (19; one study) | Minimal data; likely low sensitivity to flicker. |
| Reptilia | 45.9 ± 17.8 (21–80) | Moderate temporal resolution; some species may perceive flicker in older lighting systems. |
| Aves | 86.7 ± 31.9 (28–143) | High temporal resolution; likely to perceive flicker from low-frequency lights (e.g., fluorescent lamps). |
| Mammalia | 46.0 ± 18.1 (5–84) | Moderate resolution; flicker perception varies across species. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azevedo, C.S.d.; Goulart, V.D.L.R.; Pizzutto, C.S.; Cipreste, C.F.; Teixeira, C.P.; Young, R.J. The Effects of Light on Vertebrate Welfare: A Review. Animals 2025, 15, 3329. https://doi.org/10.3390/ani15223329
Azevedo CSd, Goulart VDLR, Pizzutto CS, Cipreste CF, Teixeira CP, Young RJ. The Effects of Light on Vertebrate Welfare: A Review. Animals. 2025; 15(22):3329. https://doi.org/10.3390/ani15223329
Chicago/Turabian StyleAzevedo, Cristiano Schetini de, Vinícius Donisete Lima Rodrigues Goulart, Cristiane Schilbach Pizzutto, Cynthia Fernandes Cipreste, Camila Palhares Teixeira, and Robert John Young. 2025. "The Effects of Light on Vertebrate Welfare: A Review" Animals 15, no. 22: 3329. https://doi.org/10.3390/ani15223329
APA StyleAzevedo, C. S. d., Goulart, V. D. L. R., Pizzutto, C. S., Cipreste, C. F., Teixeira, C. P., & Young, R. J. (2025). The Effects of Light on Vertebrate Welfare: A Review. Animals, 15(22), 3329. https://doi.org/10.3390/ani15223329








