Ethanol Hormesis in Honeybees (Apis mellifera L.) Infected with Vairimorpha (Nosema) spp.
Simple Summary
Abstract
1. Introduction
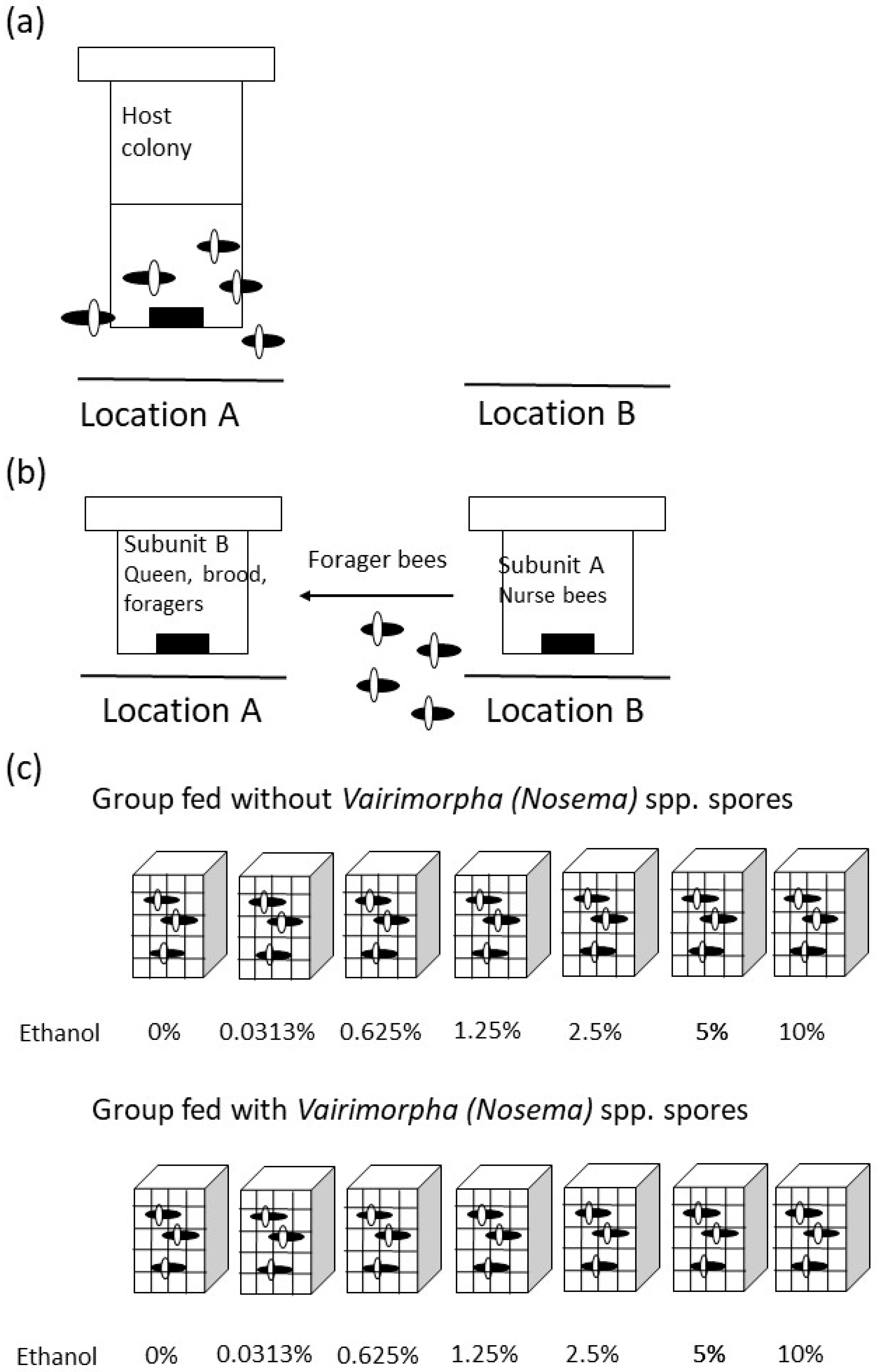
2. Methods
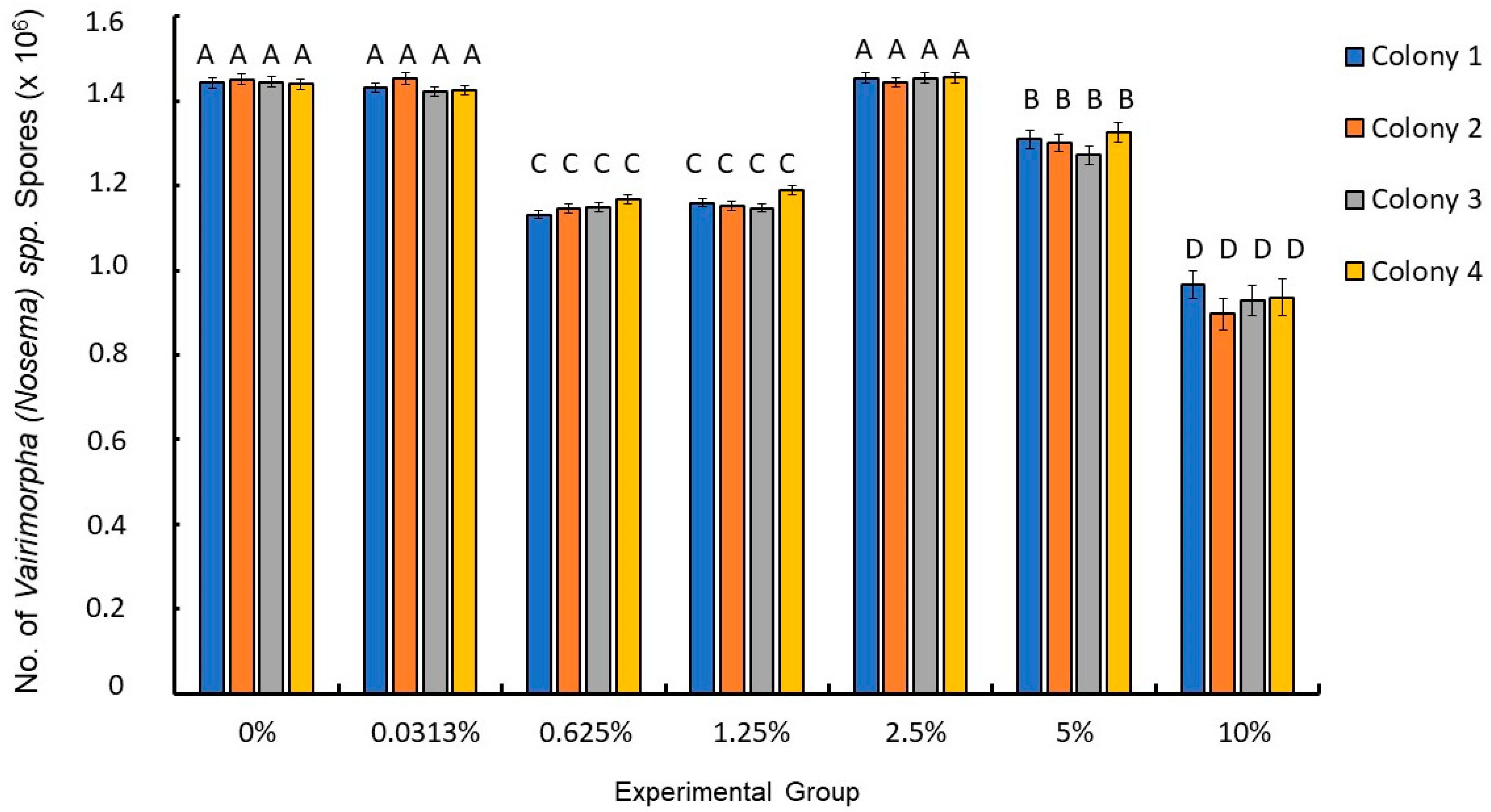
3. Results
4. Discussion
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calabrese, E.J.; Baldwin, L.A. Defining Hormesis. Hum. Exp. Toxicol. 2002, 21, 91–97. [Google Scholar] [CrossRef]
- Calabrese, E.J. The Hormetic Dose-Response Model Is More Common than the Threshold Model in Toxicology. Toxicol. Sci. 2003, 71, 246–250. [Google Scholar] [CrossRef]
- Martin, B.; Ji, S.; White, C.M.; Maudsley, S.; Mattson, M.P. Dietary Energy Intake, Hormesis, and Health. In Hormesis; Mattson, M.P., Calabrese, E.J., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 123–137. ISBN 978-1-60761-494-4. [Google Scholar]
- Calabrese, E.J. Hormesis and Medicine. Br. J. Clin. Pharmacol. 2008, 66, 594–617. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J.; Barceló, D. Environmental Hormesis: New Developments. Sci. Total Environ. 2024, 906, 167450. [Google Scholar] [CrossRef]
- McPhee, J.C.; Charles, J.B. (Eds.) Human Health and Performance Risks of Space Exploration Missions; NASA SP-2009-3405; National Aeronautics and Space Administration: Houston, TX, USA, 2009. [Google Scholar]
- Georgieva, M.; Vassileva, V. Stress Management in Plants: Examining Provisional and Unique Dose-Dependent Responses. Int. J. Mol. Sci. 2023, 24, 5105. [Google Scholar] [CrossRef]
- Godínez-Mendoza, P.L.; Rico-Chávez, A.K.; Ferrusquía-Jimenez, N.I.; Carbajal-Valenzuela, I.A.; Villagómez-Aranda, A.L.; Torres-Pacheco, I.; Guevara-González, R.G. Plant Hormesis: Revising of the Concepts of Biostimulation, Elicitation and Their Application in a Sustainable Agricultural Production. Sci. Total Environ. 2023, 894, 164883. [Google Scholar] [CrossRef]
- Rix, R.R.; Guedes, R.N.C.; Christopher Cutler, G. Hormesis Dose–Response Contaminant-Induced Hormesis in Animals. Curr. Opin. Toxicol. 2022, 30, 100336. [Google Scholar] [CrossRef]
- Nassar, M.; Dargham, A.; Jamleh, A.; Tamura, Y.; Hiraishi, N.; Tagami, J. The Hormetic Effect of Arsenic Trioxide on Rat Pulpal Cells: An In Vitro Preliminary Study. Eur. J. Dent. 2021, 15, 222–227. [Google Scholar] [CrossRef] [PubMed]
- Heinz, G.H.; Hoffman, D.J.; Klimstra, J.D.; Stebbins, K.R.; Kondrad, S.L.; Erwin, C.A. Hormesis Associated with a Low Dose of Methylmercury Injected into Mallard Eggs. Arch. Environ. Contam. Toxicol. 2012, 62, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Lumniczky, K.; Impens, N.; Armengol, G.; Candéias, S.; Georgakilas, A.G.; Hornhardt, S.; Martin, O.A.; Rödel, F.; Schaue, D. Low Dose Ionizing Radiation Effects on the Immune System. Environ. Int. 2021, 149, 106212. [Google Scholar] [CrossRef]
- Paunesku, T.; Stevanović, A.; Popović, J.; Woloschak, G.E. Effects of Low Dose and Low Dose Rate Low Linear Energy Transfer Radiation on Animals—Review of Recent Studies Relevant for Carcinogenesis. Int. J. Radiat. Biol. 2021, 97, 757–768. [Google Scholar] [CrossRef]
- Correia, A.D.; Costa, F.O.; Neuparth, T.; Diniz, M.E.; Costa, M.H. Sub-Lethal Effects of Copper-Spiked Sediments on the Marine Amphipod Gammarus Locusta: Evidence of Hormesis? Ecotoxicol. Environ. Saf. 2001, 4, 32–38. [Google Scholar]
- Lefcort, H.; Freedman, Z.; House, S.; Pendleton, M. Hormetic Effects of Heavy Metals in Aquatic Snails: Is a Little Bit of Pollution Good? EcoHealth 2008, 5, 10–17. [Google Scholar] [CrossRef]
- Moskalev, A.A.; Plyusnina, E.N.; Shaposhnikov, M.V. Radiation Hormesis and Radioadaptive Response in Drosophila Melanogaster Flies with Different Genetic Backgrounds: The Role of Cellular Stress-Resistance Mechanisms. Biogerontology 2011, 12, 253–263. [Google Scholar] [CrossRef]
- Chattopadhyay, D.; Chitnis, A.; Talekar, A.; Mulay, P.; Makkar, M.; James, J.; Thirumurugan, K. Hormetic Efficacy of Rutin to Promote Longevity in Drosophila Melanogaster. Biogerontology 2017, 18, 397–411. [Google Scholar] [CrossRef] [PubMed]
- Amsalem, E.; Derstine, N.; Murray, C. Hormetic Response to Pesticides in Diapausing Bees. Biol. Lett. 2025, 21, 20240612. [Google Scholar] [CrossRef] [PubMed]
- Cutler, G.C.; Rix, R.R. Can Poisons Stimulate Bees? Appreciating the Potential of Hormesis in Bee-Pesticide Research: Appreciating the Potential of Hormesis in Bee-Pesticide Research. Pest. Manag. Sci. 2015, 71, 1368–1370. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Kim, J.H.; Cho, S.; Lee, D.E.; Clark, J.M.; Lee, S.H. Chronic Exposure to Field-Realistic Doses of Imidacloprid Resulted in Biphasic Negative Effects on Honey Bee Physiology. Insect Biochem. Mol. Biol. 2022, 144, 103759. [Google Scholar] [CrossRef]
- Stuligross, C.; Williams, N.M. Past Insecticide Exposure Reduces Bee Reproduction and Population Growth Rate. Proc. Natl. Acad. Sci. USA 2021, 118, e2109909118. [Google Scholar] [CrossRef]
- Detzel, A.; Wink, M. Attraction, Deterrence or Intoxication of Bees (Apis mellifera) by Plant aUelochemicals. Chemoecology 1993, 4, 8–18. [Google Scholar] [CrossRef]
- Singaravelan, N.; Inbar, M.; Ne’eman, G.; Distl, M.; Wink, M.; Izhaki, I. The Effects of Nectar–Nicotine on Colony Fitness of Caged Honeybees. J. Chem. Ecol. 2006, 32, 49–59. [Google Scholar] [CrossRef]
- Köhler, A.; Pirk, C.W.W.; Nicolson, S.W. Honeybees and Nectar Nicotine: Deterrence and Reduced Survival Versus Potential Health Benefits. J. Insect Physiol. 2012, 58, 286–292. [Google Scholar] [CrossRef]
- Wright, G.A.; Baker, D.D.; Palmer, M.J.; Stabler, D.; Mustard, J.A.; Power, E.F.; Borland, A.M.; Stevenson, P.C. Caffeine in Floral Nectar Enhances a Pollinator’s Memory of Reward. Science 2013, 339, 1202–1204. [Google Scholar] [CrossRef]
- Galante, H.; De Agrò, M.; Koch, A.; Kau, S.; Czaczkes, T.J. Acute Exposure to Caffeine Improves Foraging in an Invasive Ant. iScience 2024, 27, 109935. [Google Scholar] [CrossRef]
- Thany, S.H.; Gauthier, M. Nicotine Injected into the Antennal Lobes Induces a Rapid Modulation of Sucrose Threshold and Improves Short-Term Memory in the Honeybee Apis mellifera. Brain Res. 2005, 1039, 216–219. [Google Scholar] [CrossRef]
- Cutler, G.C.; Amichot, M.; Benelli, G.; Guedes, R.N.C.; Qu, Y.; Rix, R.R.; Ullah, F.; Desneux, N. Hormesis and Insects: Effects and Interactions in Agroecosystems. Sci. Total Environ. 2022, 825, 153899. [Google Scholar] [CrossRef]
- Jones, P.; Agrawal, A.A. Caffeine and Ethanol in Nectar Interact with Flower Color Impacting Bumblebee Behavior. Behav. Ecol. Sociobiol. 2022, 76, 103. [Google Scholar] [CrossRef]
- Kevan, P.G.; Eisikowitch, D.; Fowle, S.; Thomas, K. Yeast-Contaminated Nectar and Its Effects on Bee Foraging. J. Apic. Res. 1988, 27, 26–29. [Google Scholar] [CrossRef]
- Sobhy, I.S.; Baets, D.; Goelen, T.; Herrera-Malaver, B.; Bosmans, L.; Van Den Ende, W.; Verstrepen, K.J.; Wäckers, F.; Jacquemyn, H.; Lievens, B. Sweet Scents: Nectar Specialist Yeasts Enhance Nectar Attraction of a Generalist Aphid Parasitoid Without Affecting Survival. Front. Plant Sci. 2018, 9, 1009. [Google Scholar] [CrossRef] [PubMed]
- Abramson, C.I.; d’Isa, R.; Wells, H. Physiological and Behavioral Pharmacology of Ethanol in Honey Bees. J. Comp. Physiol. A 2025, 211, 483–504. [Google Scholar] [CrossRef] [PubMed]
- Starmer, W.T.; Heed, W.B.; Rockwood-Sluss, E.S. Extension of Longevity in Drosophila Mojavensis by Environmental Ethanol: Differences Between Subraces. Proc. Natl. Acad. Sci. USA 1977, 74, 387–391. [Google Scholar] [CrossRef]
- Moneke, A.N.; Okolo, B.N.; Nweke, A.I.; Ezeogu, L.I.; Ire, F.S. Selection and Characterisation of High Ethanol Tolerant Saccharomyces Yeasts from Orchard Soil. Afr. J. Biotechnol. 2008, 7, 4567–4575. [Google Scholar]
- Ostap-Chec, M.; Bajorek, D.; Antoł, W.; Stec, D.; Miler, K. Occasional and Constant Exposure to Dietary Ethanol Shortens the Lifespan of Worker Honey Bees. J. Comp. Physiol. B 2024, 194, 403–410. [Google Scholar] [CrossRef]
- Bozic, J.; DiCesare, J.; Wells, H.; Abramson, C.I. Ethanol Levels in Honeybee Hemolymph Resulting from Alcohol Ingestion. Alcohol 2007, 41, 281–284. [Google Scholar] [CrossRef]
- Maze, I.S.; Wright, G.A.; Mustard, J.A. Acute Ethanol Ingestion Produces Dose-Dependent Effects on Motor Behavior in the Honey Bee (Apis mellifera). J. Insect Physiol. 2006, 52, 1243–1253. [Google Scholar] [CrossRef] [PubMed]
- Abramson, C.I.; Craig, D.P.A.; Varnon, C.A.; Wells, H. The Effect of Ethanol on Reversal Learning in Honey Bees (Apis mellifera Anatolica): Response Inhibition in a Social Insect Model. Alcohol 2015, 49, 245–258. [Google Scholar] [CrossRef] [PubMed]
- Abramson, C.I.; Stone, S.M.; Ortez, R.A.; Luccardi, A.; Vann, K.L.; Hanig, K.D.; Rice, J. The Development of an Ethanol Model Using Social Insects I: Behavior Studies of the Honey Bee (Apis mellifera L.). Alcohol Clin. Exp. Res. 2000, 24, 1153–1166. [Google Scholar] [CrossRef]
- Abramson, C.I.; Sanderson, C.; Painter, J.; Barnett, S.; Wells, H. Development of an Ethanol Model Using Social Insects: V. Honeybee Foraging Decisions Under the Influence of Alcohol. Alcohol 2005, 36, 187–193. [Google Scholar] [CrossRef]
- Bozic, J.; Abramson, C.I.; Bedencic, M. Reduced Ability of Ethanol Drinkers for Social Communication in Honeybees (Apis mellifera Carnica Poll.). Alcohol 2006, 38, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Mustard, J.A.; Oquita, R.; Garza, P.; Stoker, A. Honey Bees (Apis mellifera) Show a Preference for the Consumption of Ethanol. Alcohol Clin. Exp. Res. 2019, 43, 26–35. [Google Scholar] [CrossRef]
- Ptaszyńska, A.A.; Borsuk, G.; Mułenko, W.; Olszewski, K. Impact of Ethanol on Nosema spp. Infected Bees. Med. Weter. 2013, 69, 736–740. [Google Scholar]
- Abramson, C.I.; Sheridan, A.; Donohue, D.; Kandolf, A.; Božič, J.; Meyers, J.E.; Benbassat, D. Development of an Ethanol Model Using Social Insects: III. Preferences for Ethanol Solutions. Psychol. Rep. 2004, 94, 227–239. [Google Scholar] [CrossRef]
- Bojko, J.; Becnel, J.; Bessette, E.; Edwards, S.; Gao, J.; Huang, W.-F.; Katanić, N.; Khalaf, A.; Li, T.; Snow, J.W.; et al. Nosema or Vairimorpha: Genomic/Proteomic Support to a Complex Socio-Economic Issue Rooted in Taxonomic Change. J. Invertebr. Pathol. 2025, 212, 108376. [Google Scholar] [CrossRef]
- Prouty, C.; Jack, C.; Sagili, R.; Ellis, J.D. Evaluating the Efficacy of Common Treatments Used for Vairimorpha (Nosema) spp. Control. Appl. Sci. 2023, 13, 1303. [Google Scholar] [CrossRef]
- Fries, I.; Feng, F.; da Silva, A.; Slemenda, S.B.; Pieniazek, N.J. Nosema ceranae n. Sp. (Microspora, Nosematidae), Morphological and Molecular Characterization of a Microsporidian Parasite of the Asian Honey Bee Apis Cerana (Hymenoptera, Apidae). Eur. J. Protistol. 1996, 32, 356–365. [Google Scholar] [CrossRef]
- Huang, W.F.; Jiang, J.H.; Chen, Y.W.; Wang, C.H. A Nosema ceranae Isolate from the Honeybee Apis mellifera. Apidologie 2007, 38, 30–37. [Google Scholar] [CrossRef]
- Paşca, C.; Mărghitaş, L.A.; Șonea, C.; Bobiş, O.; Buzura-Matei, I.A.; Dezmirean, D.S. A Review of Nosema Cerane and Nosema Apis: Caracterization and Impact for Beekeeping. BUASVMCN-ASB 2019, 76, 77–87. [Google Scholar] [CrossRef]
- Galajda, R.; Valenčáková, A.; Sučik, M.; Kandráčová, P. Nosema Disease of European Honey Bees. J. Fungi 2021, 7, 714. [Google Scholar] [CrossRef]
- Hges, M.; Martín-Hernández, R.; Meana, A. Nosema ceranae in Europe: An Emergent Type C Nosemosis. Apidologie 2010, 41, 375–392. [Google Scholar] [CrossRef]
- Burnham, A.J. Scientific Advances in Controlling Nosema ceranae (Microsporidia) Infections in Honey Bees (Apis mellifera). Front. Vet. Sci. 2019, 6, 79. [Google Scholar] [CrossRef]
- Mayack, C.; Naug, D. Energetic Stress in the Honeybee Apis mellifera from Nosema ceranae Infection. J. Invertebr. Pathol. 2009, 100, 185–188. [Google Scholar] [CrossRef]
- Martín-Hernández, R.; Botías, C.; Barrios, L.; Martínez-Salvador, A.; Meana, A.; Mayack, C.; Higes, M. Comparison of the Energetic Stress Associated with Experimental Nosema ceranae and Nosema Apis Infection of Honeybees (Apis mellifera). Parasitol. Res. 2011, 109, 605–612. [Google Scholar] [CrossRef]
- Castelli, L.; Branchiccela, B.; Garrido, M.; Invernizzi, C.; Porrini, M.; Romero, H.; Santos, E.; Zunino, P.; Antúnez, K. Impact of Nutritional Stress on Honeybee Gut Microbiota, Immunity, and Nosema ceranae Infection. Microb. Ecol. 2020, 80, 908–919. [Google Scholar] [CrossRef]
- Kuszewska, K.; Woyciechowski, M. Reversion in Honeybee, Apis mellifera, Workers with Different Life Expectancies. Anim. Behav. 2013, 85, 247–253. [Google Scholar] [CrossRef]
- Kuszewska, K.; Woyciechowski, M. Risky Robbing Is a Job for Short-Lived and Infected Worker Honeybees. Apidologie 2014, 45, 537–544. [Google Scholar] [CrossRef]
- Woyciechowski, M.; Moroń, D. Life Expectancy and Onset of Foraging in the Honeybee (Apis mellifera). Insect. Soc. 2009, 56, 193–201. [Google Scholar] [CrossRef]
- Ostap-Chec, M.; Bajorek, D.; Antoł, W.; Stec, D.; Miler, K. Honey Bees Are Resilient to the Long-Term Presence of Alcohol in Their Diet. Ecotoxicology 2025, 34, 1158–1168. [Google Scholar] [CrossRef]
- Ostap-Chec, M.; Cait, J.; Scott, R.W.; Arct, A.; Moroń, D.; Rapacz, M.; Miler, K. Nosemosis Negatively Affects Honeybee Survival: Experimental and Meta-Analytic Evidence. Parasitology 2024, 151, 1530–1542. [Google Scholar] [CrossRef] [PubMed]
- Erofeeva, E.A. Environmental Hormesis: From Cell to Ecosystem. Curr. Opin. Environ. Sci. Health 2022, 29, 100378. [Google Scholar] [CrossRef]
- Czekońska, K. Influence of Carbon Dioxide on Nosema Apis Infection of Honeybees (Apis mellifera). J. Invertebr. Pathol. 2007, 95, 84–86. [Google Scholar] [CrossRef]
- Hornitzky, M. Nosema Disease—Literature Review and Three Surveys of Beekeepers—Part 2. Rural. Ind. Res. Dev. Corporation. 2008, 8, 3507–3516. [Google Scholar]
- Li, W.; Evans, J.D.; Li, J.; Su, S.; Hamilton, M.; Chen, Y. Spore Load and Immune Response of Honey Bees Naturally Infected by Nosema ceranae. Parasitol. Res. 2017, 116, 3265–3274. [Google Scholar] [CrossRef] [PubMed]

| % EtOH | Vairimorpha Spores Feeding | Mean | SE | −95.00% | +95.00% | N |
|---|---|---|---|---|---|---|
| 0% | No | 0 | 3447.06 | −6761 | 6761 | 177 |
| 0.0313% | No | 0 | 3456.84 | −6780 | 6780 | 176 |
| 0.625% | No | 0 | 3447.06 | −6761 | 6761 | 177 |
| 1.25% | No | 0 | 3476.65 | −6819 | 6819 | 174 |
| 2.5% | No | 0 | 4118.36 | −8078 | 8078 | 124 |
| 5% | No | 0 | 5226.25 | −10,251 | 10,251 | 77 |
| 10% | No | 0 | 7439.50 | −14,592 | 14,592 | 38 |
| 0% | Yes | 1,445,064 | 4392.61 | 1,436,448 | 1,453,680 | 109 |
| 0.0313% | Yes | 1,432,755 | 4454.33 | 1,424,018 | 1,441,492 | 106 |
| 0.625% | Yes | 1,148,684 | 3719.75 | 1,141,388 | 1,155,980 | 152 |
| 1.25% | Yes | 1,161,358 | 3636.95 | 1,154,225 | 1,168,492 | 159 |
| 2.5% | Yes | 1,451,769 | 4496.96 | 1,442,949 | 1,460,590 | 104 |
| 5% | Yes | 1,302,171 | 7751.78 | 1,286967 | 1,317,376 | 35 |
| 10% | Yes | 934,000 | 13,238.69 | 908,033 | 959,967 | 12 |
| % EtOH | Vairimorpha Spores Feeding | χ2 | N | Censored | Uncensored | df | p |
|---|---|---|---|---|---|---|---|
| 0% | No | 0.534 | 200 | 177 | 23 | 3 | 0.911 |
| 0.0313% | No | 0.784 | 200 | 175 | 25 | 3 | 0.853 |
| 0.625% | No | 0.539 | 200 | 177 | 23 | 3 | 0.910 |
| 1.25% | No | 0.750 | 200 | 174 | 26 | 3 | 0.861 |
| 2.5% | No | 1.540 | 200 | 125 | 75 | 3 | 0.673 |
| 5% | No | 2.671 | 200 | 80 | 120 | 3 | 0.445 |
| 10% | No | 0.759 | 200 | 39 | 161 | 3 | 0.859 |
| 0% | Yes | 0.273 | 200 | 109 | 91 | 3 | 0.965 |
| 0.0313% | Yes | 0.465 | 200 | 97 | 103 | 3 | 0.926 |
| 0.625% | Yes | 0.135 | 200 | 152 | 48 | 3 | 0.987 |
| 1.25% | Yes | 7.921 | 200 | 146 | 54 | 3 | 0.684 |
| 2.5% | Yes | 0.684 | 200 | 104 | 96 | 3 | 0.877 |
| 5% | Yes | 0.898 | 200 | 35 | 165 | 3 | 0.826 |
| 10% | Yes | 0.888 | 200 | 12 | 188 | 3 | 0.828 |
| Overall test | 1.012 | 2800 | 1608 | 1192 | 3 | 0.798 |
| Compared Groups | The Groups Fed Without Vairimorpha spp. Spores | The Groups Fed with Vairimorpha spp. Spores | ||||||
|---|---|---|---|---|---|---|---|---|
| Z | N | df | p | Z | N | df | p | |
| 0–0.0313% | −0.324 | 400 | 1 | 0.746 | −0.445 | 400 | 1 | 0.656 |
| 0–0.625% | −0.031 | 400 | 1 | 0.975 | 4.225 | 400 | 1 | <0.001 |
| 0–1.25% | −0.505 | 400 | 1 | 0.613 | 3.595 | 400 | 1 | <0.001 |
| 0–2.5% | −6.031 | 400 | 1 | <0.001 | −0.586 | 400 | 1 | 0.558 |
| 0–5% | −10.068 | 400 | 1 | <0.001 | −8.885 | 400 | 1 | <0.001 |
| 0–10% | −13.628 | 400 | 1 | <0.001 | −11.926 | 400 | 1 | <0.001 |
| 0.0313–0.625% | 0.293 | 400 | 1 | 0.769 | 4.700 | 400 | 1 | <0.001 |
| 0.0313–1.25% | −0.185 | 400 | 1 | 0.853 | 4.067 | 400 | 1 | <0.001 |
| 0.0313–2.5% | −2.236 | 400 | 1 | 0.025 | −0.157 | 400 | 1 | 0.875 |
| 0.0313–5% | −9.772 | 400 | 1 | <0.001 | −8.867 | 400 | 1 | <0.001 |
| 0.0313–10% | −13.341 | 400 | 1 | <0.001 | −11.779 | 400 | 1 | <0.001 |
| 0.625–1.25% | −0.476 | 400 | 1 | 0.634 | −0.650 | 400 | 1 | 0.516 |
| 0.625–2.5% | −5.954 | 400 | 1 | <0.001 | −4.742 | 400 | 1 | <0.001 |
| 0.625–5% | −9.971 | 400 | 1 | <0.001 | −11.836 | 400 | 1 | <0.001 |
| 0.625–10% | −13.501 | 400 | 1 | <0.001 | −14.377 | 400 | 1 | <0.001 |
| 1.25–2.5% | −5.501 | 400 | 1 | <0.001 | −4.124 | 400 | 1 | <0.001 |
| 1.25–5% | −9.548 | 400 | 1 | <0.001 | −11.047 | 400 | 1 | <0.001 |
| 1.25–10% | −13.091 | 400 | 1 | <0.001 | −14.021 | 400 | 1 | <0.001 |
| 2.5–5% | −4.505 | 400 | 1 | <0.001 | −8.378 | 400 | 1 | <0.001 |
| 2.5–10% | −8.463 | 400 | 1 | <0.001 | −11.467 | 400 | 1 | <0.001 |
| 5–10% | −4.055 | 400 | 1 | <0.001 | −3.657 | 400 | 1 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuszewska, K. Ethanol Hormesis in Honeybees (Apis mellifera L.) Infected with Vairimorpha (Nosema) spp. Animals 2025, 15, 3316. https://doi.org/10.3390/ani15223316
Kuszewska K. Ethanol Hormesis in Honeybees (Apis mellifera L.) Infected with Vairimorpha (Nosema) spp. Animals. 2025; 15(22):3316. https://doi.org/10.3390/ani15223316
Chicago/Turabian StyleKuszewska, Karolina. 2025. "Ethanol Hormesis in Honeybees (Apis mellifera L.) Infected with Vairimorpha (Nosema) spp." Animals 15, no. 22: 3316. https://doi.org/10.3390/ani15223316
APA StyleKuszewska, K. (2025). Ethanol Hormesis in Honeybees (Apis mellifera L.) Infected with Vairimorpha (Nosema) spp. Animals, 15(22), 3316. https://doi.org/10.3390/ani15223316






