The Formation of a Novel Intergeneric Hybrid Fish Derived from Megalobrama amblycephala (♀) × Culter dabryi (♂)
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Acquisition and Hybridization Preparation of Experimental Fish
2.2. Measurement of Morphological Traits
2.3. Measurement of DNA Content
2.4. Preparation of Chromosome Spreads and Karyotype Analysis
2.5. Examination of 5S rDNA and Sox Genes
2.6. Mitochondrial DNA Sequence Analysis
2.7. Measurement of Growth Performance
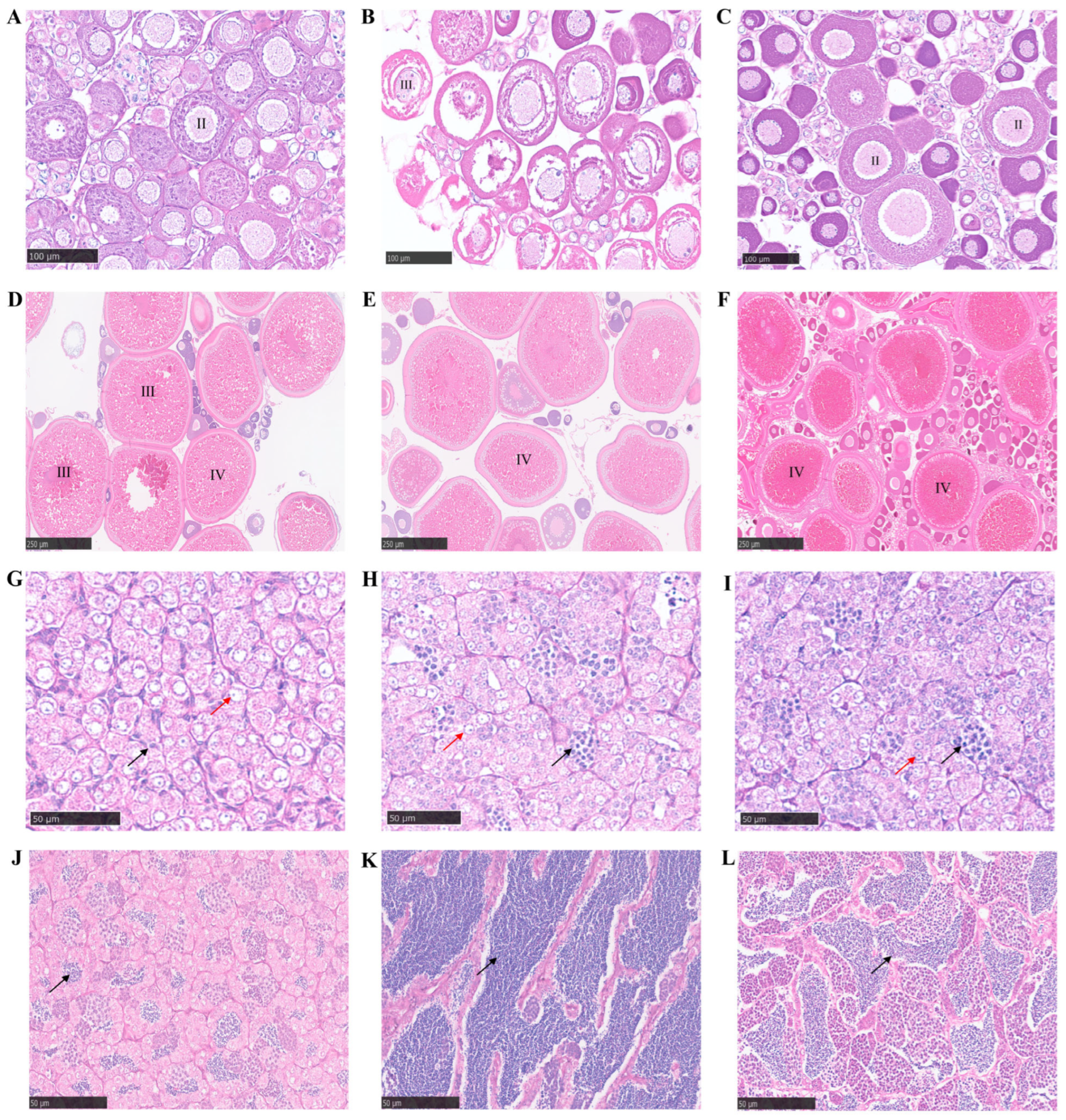
2.8. Gonad Microstructure Analysis
2.9. Preliminary Analysis of Nutritional Components in Muscle of BG and Its Parents
2.10. Heterosis Calculation and Statistical Analysis
3. Results
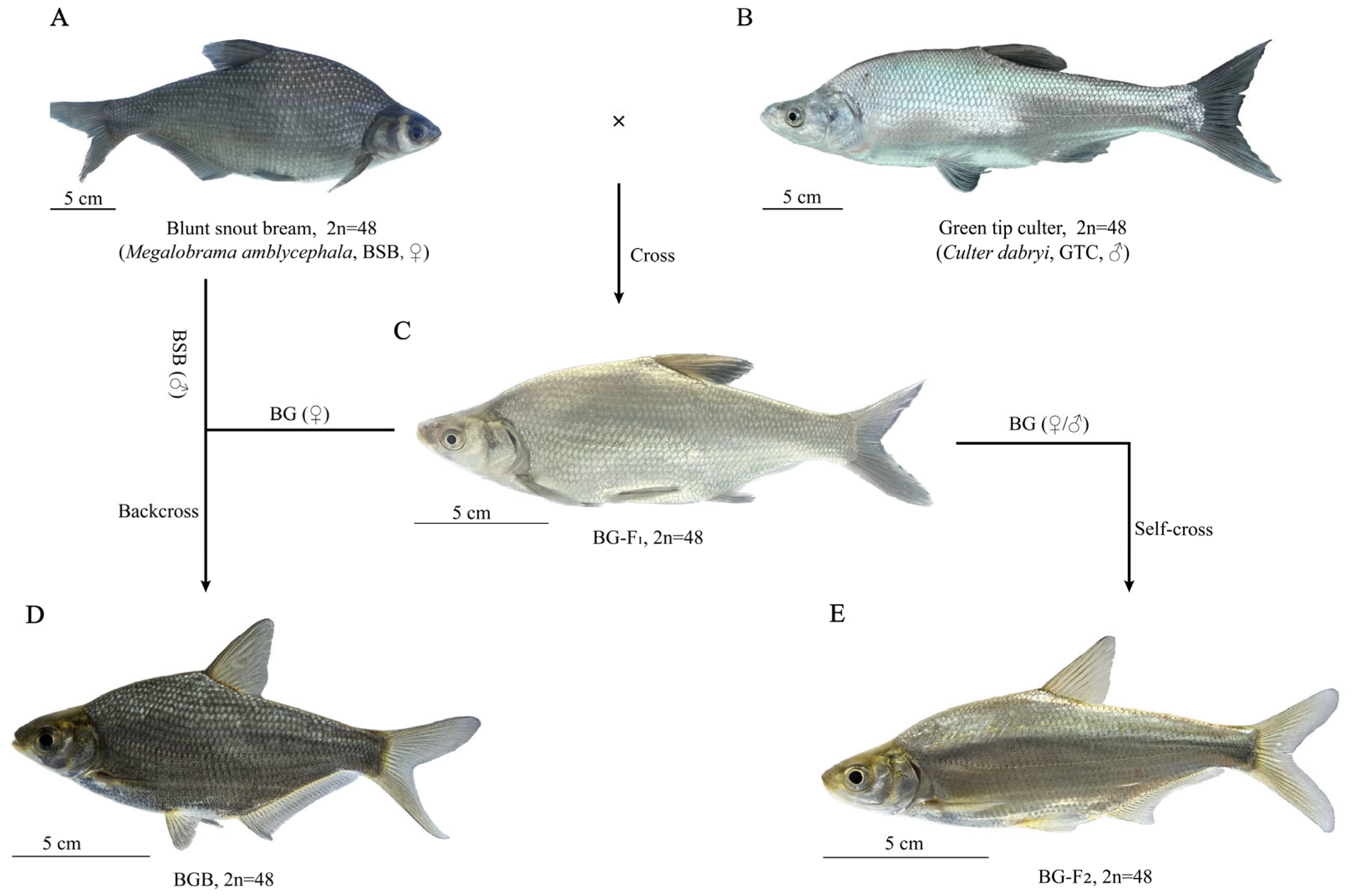
3.1. The Formation and Embryonic Development of BG Hybrid Fish
3.2. Morphological Characteristics
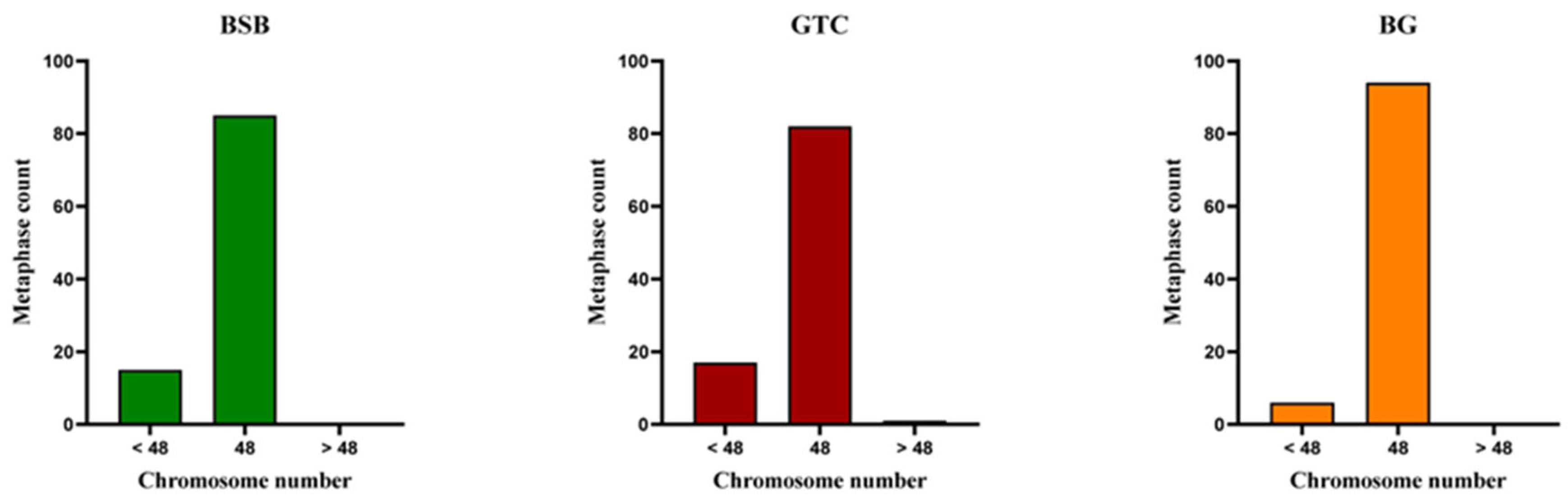
3.3. DNA Content of BSB, GTC, and BG
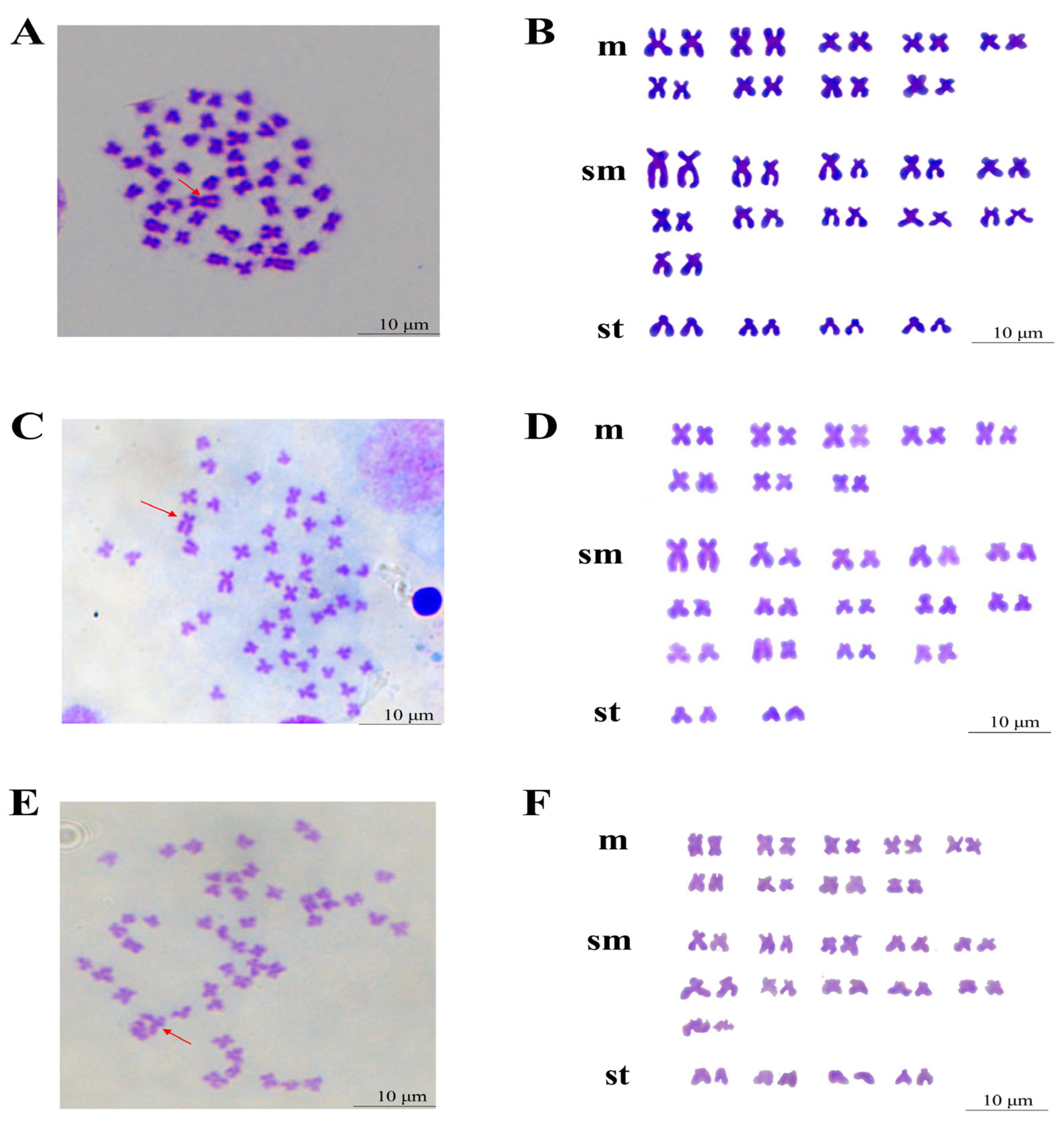
3.4. Chromosome Number and Karyotype Analysis
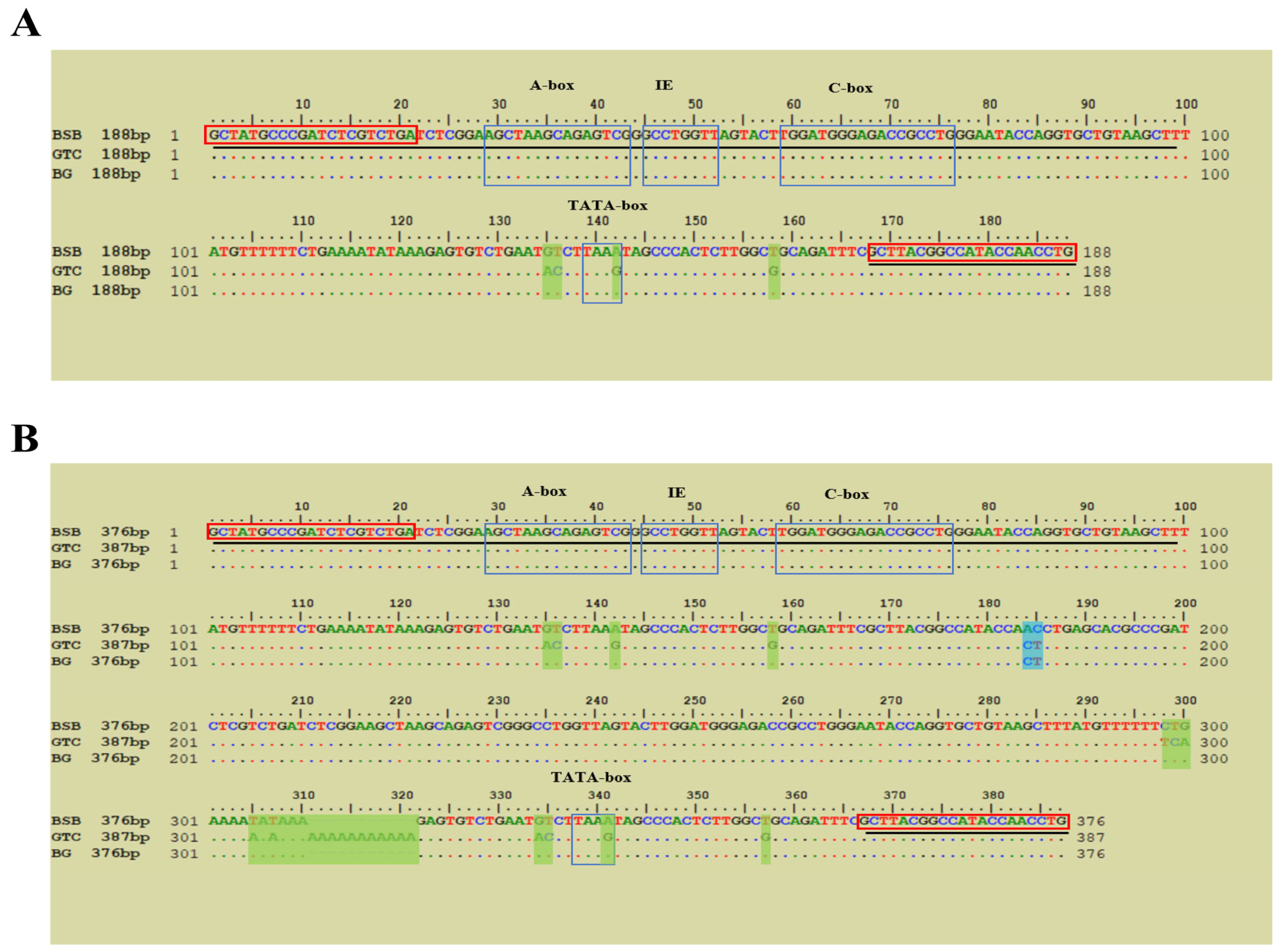
3.5. Molecular Organization of 5S rDNA and Sox Genes
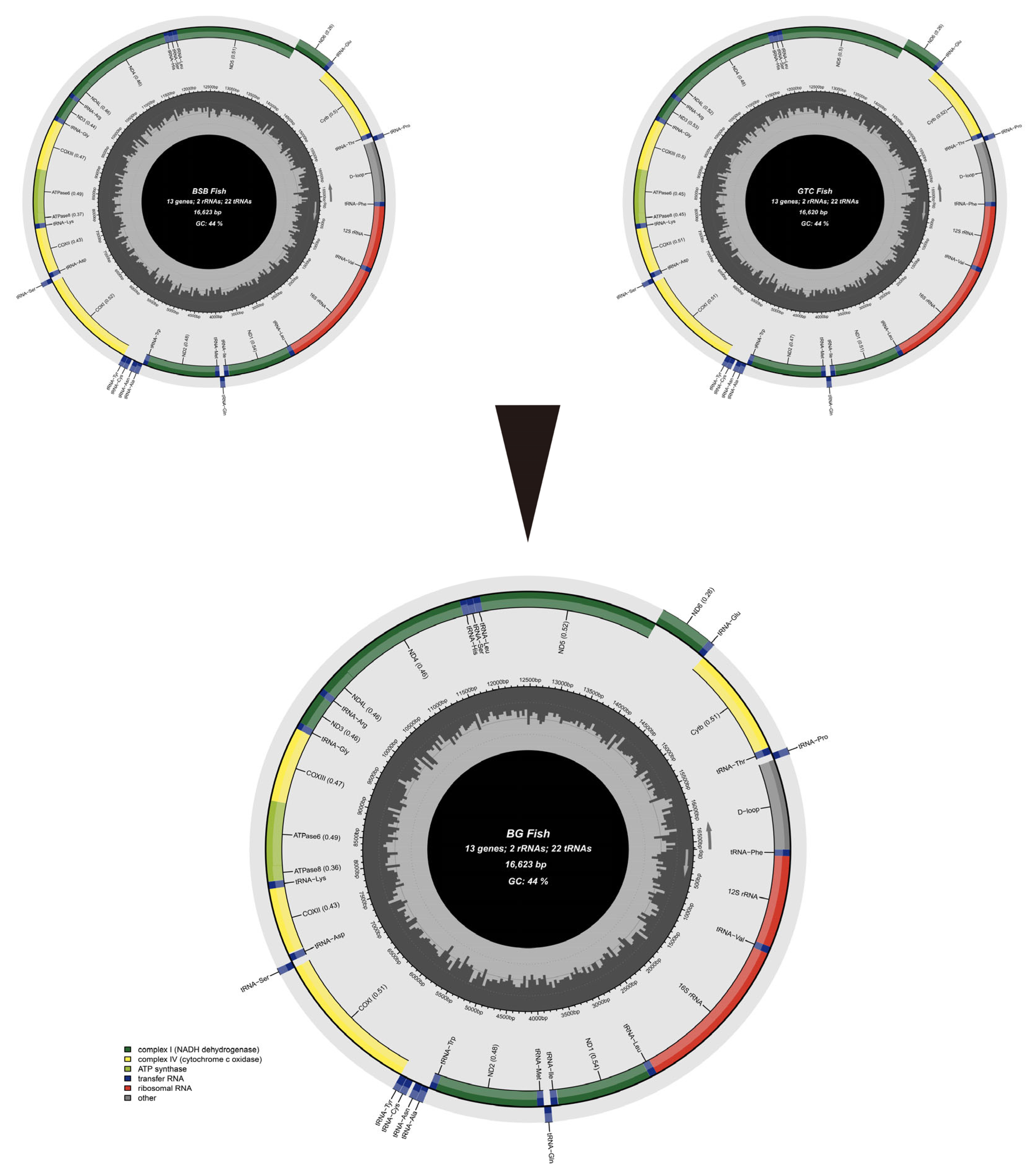
3.6. Mitochondrial Genetic Analysis
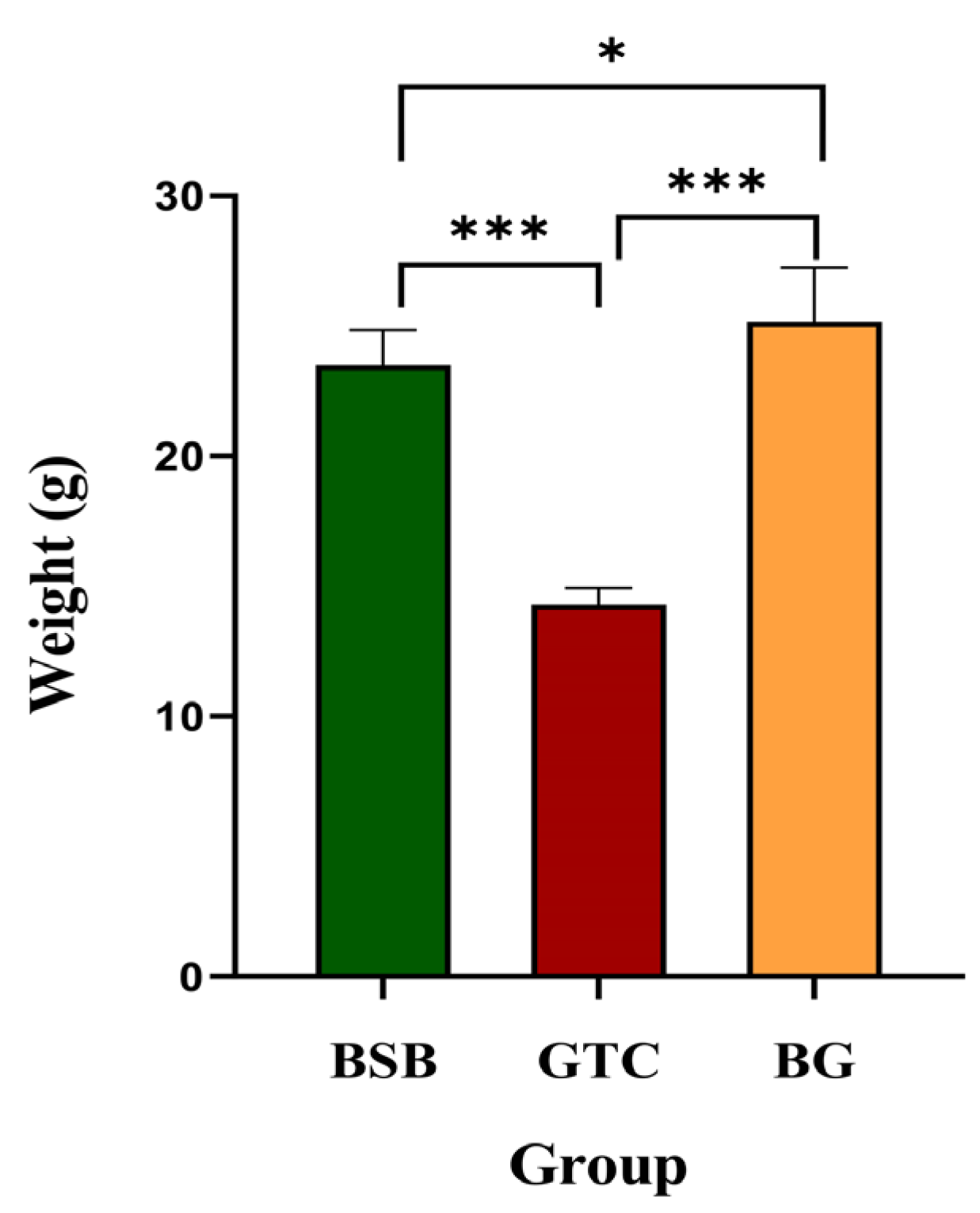
3.7. Growth Performance
3.8. Fertility Analysis
3.9. Preliminary Analysis of Nutritional Components in the Muscle of BG and Its Parents
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Liu, Q.; Wang, S.; Tang, C.; Tao, M.; Zhang, C.; Zhou, Y.; Qin, Q.; Luo, K.; Wu, C.; Hu, F.; et al. The Research Advances in Distant Hybridization and Gynogenesis in Fish. Rev. Aquac. 2024, 17, e12972. [Google Scholar] [CrossRef]
- Guo, H.; Zheng, G.; Wu, C.; Jiang, X.; Zou, S. Comparative Analysis of the Growth Performance and Intermuscular Bone Traits in F1 Hybrids of Black Bream (Megalobrama terminalis) (♀) × topmouth Culter (Culter alburnus) (♂). Aquaculture 2018, 492, 15–23. [Google Scholar] [CrossRef]
- Gao, Z.; Wang, W.; Jiang, E.; Chen, B. Research Advances on Germplasm Resources and Genetic Improvement of Blunt Snout Bream (Megalobrama amblycephala). J. Huazhong Agric. Univ. 2014, 33, 138–144. [Google Scholar] [CrossRef]
- Wang, Y.; Xiong, B.; Ma, X.; Xu, M.; Zhang, L.; Wang, X.; Hu, Q. Fecundity of Culter dabryi in Daoguanhe Reservoir, Wuhan, China. Oceanol. Limnol. Sin. 2007, 38, 180–186. [Google Scholar] [CrossRef]
- Wang, J.; Xiao, J.; Zeng, M.; Xu, K.; Tao, M.; Zehang, C.; Duan, W.; Liu, W.; Luo, K.; Liu, Y.; et al. Genomic Variation in the Hybrids of White Crucian Carp and Red Crucian Carp: Evidence from Ribosomal DNA. Sci. China Life Sci. 2015, 58, 590–601. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Xie, L.; Li, T.; Liu, S.; Xiao, J.; Hu, J.; Wang, J.; Qin, Q.; Liu, Y. The Formation of Diploid and Triploid Hybrids of Female Grass Carp × Male Blunt Snout Bream and Their 5S rDNA Analysis. BMC Genet. 2013, 14, 110. [Google Scholar] [CrossRef] [PubMed]
- Zafar, I.; Iftikhar, R.; Ahmad, S.U.; Rather, M.A. Genome Wide Identification, Phylogeny, and Synteny Analysis of Sox Gene Family in Common Carp (Cyprinus carpio). Biotechnol Rep. 2021, 30, e00607. [Google Scholar] [CrossRef]
- Zhou, J.; Zhang, Y.; Huang, M.; Chen, Y.; Chen, X.; Yao, G. Phylogenetic Relationships among Crotalinae Based on Mitochondrial Cytochrome b Gene Sequence Variations. Acta Zool. Sin. 2001, 47, 361–366. [Google Scholar] [CrossRef]
- Akhter, G.; Ahmed, I.; Ahmad, S.M. Genomic Analysis and Phylogenetic Characterization of Himalayan Snow Trout, Schizothorax Esocinus Based on Mitochondrial Protein-Coding Genes. Mol. Biol. Rep. 2024, 51, 659. [Google Scholar] [CrossRef]
- Modeel, S.; Joshi, B.D.; Yadav, S.; Bharti, M.; Negi, R.K. Mitochondrial DNA Reveals Shallow Population Genetic Structure in Economically Important Cyprinid Fish Labeo Rohita (Hamilton, 1822) from South and Southeast Asia. Mol. Biol. Rep. 2023, 50, 4759–4767. [Google Scholar] [CrossRef]
- Jabeen, F.; Chaudhry, A.S. Chemical Compositions and Fatty Acid Profiles of Three Freshwater Fish Species. Food Chem. 2011, 125, 991–996. [Google Scholar] [CrossRef]
- Zhao, F.; Zhuang, P.; Song, C.; Shi, Z.; Zhang, L. Amino Acid and Fatty Acid Compositions and Nutritional Quality of Muscle in the Pomfret, Pampus Punctatissimus. Food Chem. 2010, 118, 224–227. [Google Scholar] [CrossRef]
- Li, S.; Yang, X.; Fan, S.; Zhou, Z.; Zhou, R.; Wu, C.; Gong, D.; Wen, M.; Wang, Y.; Tao, M.; et al. Comparative Analysis of Muscle Nutrient in Two Types of Hybrid Bream and Native Bream. Reprod. Breed. 2022, 2, 71–77. [Google Scholar] [CrossRef]
- Gong, D.; Tao, M.; Xu, L.; Hu, F.; Wei, Z.; Wang, S.; Wang, Y.; Liu, Q.; Wu, C.; Luo, K.; et al. An Improved Hybrid Bream Derived from a Hybrid Lineage of Megalobrama amblycephala (♀) × Culter alburnus (♂). Sci. China Life Sci. 2022, 65, 1213–1221. [Google Scholar] [CrossRef]
- Wu, C.; Li, J.; Cai, C.; Qin, Q.; Huang, C.; Chen, Z.; Hu, F.; Hu, J.; Huang, H.; Luo, J.; et al. A New Type of Hybrid Golden Pompano “Chenhai No.1” Produced by the Hybridization of (Trachinotus ovatus ♀ × Trachinotus blochii ♂) ♀ × T. ovatus ♂. Reprod. Breed. 2022, 2, 78–82. [Google Scholar] [CrossRef]
- Liu, S.; Qin, Q.; Xiao, J.; Lu, W.; Shen, J.; Li, W.; Liu, J.; Duan, W.; Zhang, C.; Tao, M.; et al. The Formation of the Polyploid Hybrids from Different Subfamily Fish Crossings and Its Evolutionary Significance. Genetics 2007, 176, 1023–1034. [Google Scholar] [CrossRef]
- Xiao, J.; Kang, X.; Xie, L.; Qin, Q.; He, Z.; Hu, F.; Zhang, C.; Zhao, R.; Wang, J.; Luo, K.; et al. The Fertility of the Hybrid Lineage Derived from Female Megalobrama amblycephala × Male Culter alburnus. Anim. Reprod. Sci. 2014, 151, 61–70. [Google Scholar] [CrossRef] [PubMed]
- Levan, A.; Fredga, K.; Sandberg, A.A. Nomenclature for Centromeric Position on Chromosomes. Hereditas 1964, 52, 201–220. [Google Scholar] [CrossRef]
- Chen, L.; Li, W.; Liu, S.; Tao, M.; Long, Y.; Duan, W.; Zhang, C.; Xiao, J.; Qin, Q.; Luo, K.; et al. Novel Genetic Markers Derived from the DNA Fragments of Sox Genes. Mol. Cell. Probes 2009, 23, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Qin, Q.; He, W.; Liu, S.; Wang, J.; Xiao, J.; Liu, Y. Analysis of 5S rDNA Organization and Variation in Polyploid Hybrids from Crosses of Different Fish Subfamilies. J. Exp. Zool. B Mol. Dev. Evol. 2010, 314, 403–411. [Google Scholar] [CrossRef]
- Chen, D.; Chu, W.; Liu, X.; Nong, X.; Li, Y.; Du, S.; Zhang, J. Phylogenetic Studies of Three Sinipercid Fishes (Perciformes: Sinipercidae) Based on Complete Mitochondrial DNA Sequences. Mitochondrial DNA 2012, 23, 70–76. [Google Scholar] [CrossRef]
- Wu, P.; Zeng, Y.; Qin, Q.; Ji, W.; Wu, C.; Zhou, Y.; Zhao, R.; Tao, M.; Zhang, C.; Tang, C.; et al. Formation and Identification of Artificial Gynogenetic Mandarin Fish (Siniperca chuatsi) Induced by Inactivated Sperm of Largemouth Bass (Micropterus salmoides). Aquaculture 2023, 577, 739969. [Google Scholar] [CrossRef]
- Gong, D.; Xu, L.; Liu, Q.; Wang, S.; Wang, Y.; Hu, F.; Wu, C.; Luo, K.; Tang, C.; Zhou, R.; et al. A New Type of Hybrid Bream Derived from a Hybrid Lineage of Megalobrama amblycephala (♀) × Culter alburnus (♂). Aquaculture 2021, 534, 736194. [Google Scholar] [CrossRef]
- Liu, Y. Propagation Physiology of Main Cultivated Fish in China. Beijing Agric. Publ. House 1993, 147, 147–148. [Google Scholar]
- Livesey, G. Energy and Protein Requirements the 1985 Report of the 1981 Joint FAO/WHO/UNU Expert Consultation. Nutr. Bull. 1987, 12, 138–149. [Google Scholar] [CrossRef]
- Wang, K.; Cheng, B.J.; Liu, B.; Chen, X.T.; Hao, Q.R.; Zhao, R.W.; Han, Y. Analysis on Nutritive Composition of Muscle in Wild and Cultured Culter alburnus Populations in Xingkai Lake at Different Ages. J. Fish. Sci. China 2012, 19, 906–912. [Google Scholar] [CrossRef]
- Zhong, H.; Chen, H.; Liu, M.; Sun, Y.; Yu, P.; Zhao, C.; Luo, C.; Zhang, C.; Wu, C.; Wang, X.; et al. Improved Traits of Proximate Compositions, Liver Antioxidant Capacity and Feeding Habits in Diploid Hybrids from Female Micropterus salmoides × Male Lepomis cyanellus. Aquaculture 2024, 587, 740853. [Google Scholar] [CrossRef]
- Gjedrem, T.; Robinson, N.; Rye, M. The Importance of Selective Breeding in Aquaculture to Meet Future Demands for Animal Protein: A Review. Aquaculture 2012, 350–353, 117–129. [Google Scholar] [CrossRef]
- Li, S.; Xie, L.; Xiao, J.; Yuan, L.; Zhou, T.; Luo, K.; Zhang, C.; Zhao, R.; Tao, M.; Liu, S. Diploid Hybrid Fish Derived from the Cross between Female Bleeker’s Yellow Tail and Male Topmouth Culter, Two Cyprinid Fishes Belonging to Different Subfamilies. BMC Genet. 2019, 20, 80. [Google Scholar] [CrossRef]
- Hu, F.; Zhong, H.; Yu, P.; Fan, J.; Wu, C.; Wang, S.; Gong, D.; Sun, Y.; Gao, X.; Wen, M.; et al. Comparative Analysis of Growth Performance, Pharyngeal Teeth and Intestinal Traits in F1 Hybrids of Female Megalobrama amblycephala × Male Culter mongolicus. Aquaculture 2023, 562, 738807. [Google Scholar] [CrossRef]
- Zheng, G.; Zhang, Q.; Li, F.; Chen, J.; Jiang, X.; Zou, S. Genetic Characteristics and Growth Performance of Different Megalobrama amblycephala (♀) × Erythroculter ilishaeformis (♂) Hybrids. J. Fish. Sci. China 2015, 22, 402–409. [Google Scholar] [CrossRef]
- Hu, J.; Liu, S.; Xiao, J.; Zhou, Y.; You, C.; He, W.; Zhao, R.; Song, C.; Liu, Y. Characteristics of Diploid and Triploid Hybrids Derived from Female Megalobrama amblycephala Yih × Male Xenocypris davidi Bleeker. Aquaculture 2012, 364–365, 157–164. [Google Scholar] [CrossRef]
- Zhang, L.; Jia, C.; Xu, W.; Xu, W.; Zheng, G.; Zou, S. Performance and morphological analysis of triploids of Megalobrama amblycephala (♀) × Culter alburnus (♂). Prog. Fish. Sci. 2024, 45, 134–143. [Google Scholar] [CrossRef]
- Pasolini, P.; Costagliola, D.; Rocco, L.; Tinti, F. Molecular Organization of 5S rDNAs in Rajidae (Chondrichthyes): Structural Features and Evolution of Piscine 5S rRNA Genes and Nontranscribed Intergenic Spacers. J. Mol. Evol. 2006, 62, 564–574. [Google Scholar] [CrossRef]
- Jia, Y.; Zheng, J.; Gu, Z.; Chen, L.; Luo, C.; Chi, M.; Liu, S.; Jiang, W.; Cheng, S. The Relationship of Sox9 Expression and Its CpG Island Methylation in Culter alburnus. Acta Hydrobiol. Sin. 2019, 43, 473–478. [Google Scholar] [CrossRef]
- Zheng, J.; Jia, Y.; Liu, S.; Chi, M.; Cheng, S.; Gu, Z. Molecular Characterization and Expression Profiles of Transcription Factor Sox Gene Family in Culter alburnus. Gene Expr. Patterns 2020, 36, 119112. [Google Scholar] [CrossRef]
- Sinclair, A.H.; Berta, P.; Palmer, M.S.; Hawkins, J.R.; Griffiths, B.L.; Smith, M.J.; Foster, J.W.; Frischauf, A.M.; Lovell-Badge, R.; Goodfellow, P.N. A Gene from the Human Sex-Determining Region Encodes a Protein with Homology to a Conserved DNA-Binding Motif. Nature 1990, 346, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Kwok, C.S.-N.; Quah, T.C.; Ariffin, H.; Tay, S.K.-H.; Yeoh, A.E.-J. Mitochondrial D-Loop Polymorphisms and Mitochondrial DNA Content in Childhood Acute Lymphoblastic Leukemia. J. Pediatr. Hematol. Oncol. 2011, 33, e239–e244. [Google Scholar] [CrossRef]
- Mascolo, C.; Ceruso, M.; Sordino, P.; Palma, G.; Anastasio, A.; Pepe, T. Comparison of Mitochondrial DNA Enrichment and Sequencing Methods from Fish Tissue. Food Chem. 2019, 294, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Cao, L.; Chen, P.; Hou, X.; Ma, J.; Yang, N.; Lu, Y.; Huang, H. rDNA and mtDNA Analysis for the Identification of Genetic Characters in the Hybrid Grouper Derived from Hybridization of Cromileptes altivelis (Female) × Epinephelus lanceolatus (Male). BMC Genom. Data 2024, 25, 5–17. [Google Scholar] [CrossRef]
- Ren, L.; Li, W.; Qin, Q.; Dai, H.; Han, F.; Xiao, J.; Gao, X.; Cui, J.; Wu, C.; Yan, X.; et al. The Subgenomes Show Asymmetric Expression of Alleles in Hybrid Lineages of Megalobrama amblycephala × Culter alburnus. Genome Res. 2019, 29, 1805–1815. [Google Scholar] [CrossRef]
- Wu, C.; Chen, Q.; Huang, X.; Hu, F.; Zhu, S.; Luo, L.; Gong, D.; Gong, K.; Zhao, R.; Zhang, C.; et al. Genomic and Epigenetic Alterations in Diploid Gynogenetic Hybrid Fish. Aquaculture 2019, 512, 734383. [Google Scholar] [CrossRef]
- Zhao, H.; Chen, Z.; Xu, H.; Li, H.; Yuan, Z.; Huang, C. Muscular Nutritional Components and Texture Profile of Marine Cultured and Fresh Water Cultured Guam Red Tilapia (Oreochromis spp.). J. South. Agric. 2018, 49, 1396–1402. [Google Scholar] [CrossRef]
- Park, J.-N.; Watanabe, T.; Endoh, K.-I.; Watanabe, K.; Abe, H. Tasteactive Components in a Vietnamese Fish Sauce. Fish. Sci. 2002, 68, 913–920. [Google Scholar] [CrossRef]
- Ding, D.; Chen, X.; Wu, Y.; Chen, H.; Liu, L.; He, Z. Comparative Analysis on Nutritional Quality of Culter alburnus Cultured under Different Aquaculture Modes. China Feed 2021, 1, 89–95. [Google Scholar] [CrossRef]
- Jiang, W.; Wu, P.; Tang, R.; Liu, Y.; Kuang, S.; Jiang, J.; Tang, L.; Tang, W.; Zhang, Y.; Zhou, X.; et al. Nutritive Values, Flavor Amino Acids, Healthcare Fatty Acids and Flesh Quality Improved by Manganese Referring to up-Regulating the Antioxidant Capacity and Signaling Molecules TOR and Nrf2 in the Muscle of Fish. Food Res. Int. 2016, 89, 670–678. [Google Scholar] [CrossRef]
- Ahmed, I.; Jan, K.; Fatma, S.; Dawood, M.A.O. Muscle Proximate Composition of Various Food Fish Species and Their Nutritional Significance: A Review. J. Anim. Physiol. Anim. Nutr. 2022, 106, 690–719. [Google Scholar] [CrossRef]
- Mayneris-Perxachs, J.; Bondia-Pons, I.; Serra-Majem, L.; Castellote, A.I.; López-Sabater, M.C. Long-Chain n-3 Fatty Acids and Classical Cardiovascular Disease Risk Factors among the Catalan Population. Food Chem. 2010, 119, 54–61. [Google Scholar] [CrossRef]


| Fish Type | WL/BL | BL/BH | BL/HL | HL/HH | BH/HH | CPL/CPH |
|---|---|---|---|---|---|---|
| BSB | 1.24 ± 0.01 | 2.39 ± 0.02 ## | 4.42 ± 0.14 # | 1.27 ± 0.04 # | 2.35 ± 0.11 ### | 1.25 ± 0.01 |
| GTC | 1.24 ± 0.03 | 4.13 ± 0.17 ** | 3.74 ± 0.20 * | 1.53 ± 0.08 * | 1.38 ± 0.07 *** | 1.82 ± 0.25 |
| BG | 1.22 ± 0.01 | 2.98 ± 0.03 ***,## | 4.24 ± 0.16 # | 1.34 ± 0.10 | 1.91 ± 0.13 *,## | 1.43 ± 0.05 * |
| Fish Type | Number of lateral Scales | Number of Upper lateral Scales | Number of Lower Lateral Scales | Number of Dorsal Fins a | Number of Abdominal Fins | Number of Anal Fins a |
|---|---|---|---|---|---|---|
| BSB | 50~57 | 12 | 8~9 | III + 7~8 | 8~9 | III + 25~27 |
| GTC | 62~70 | 12~13 | 7~8 | III + 8 | 9 | III + 22~27 |
| BG | 54~61 | 12~13 | 8~9 | III + 8 | 9~10 | III + 25~27 |
| Fish Type | Average DNA Content | Ratio | |
|---|---|---|---|
| Observed | Expected | ||
| BSB | 69.48 | ||
| GTC | 69.12 | ||
| BG | 68.12 | BG/BSB = 0.98 BG/GTC = 0.99 | 1 |
| Fish Type | Crude Protein (g/100 g) | Crude Fat (g/100 g) |
|---|---|---|
| BSB | 17.83 ± 0.35 | 1.27 ± 0.06 |
| GTC | 17.93 ± 0.35 | 1.10 ± 0.10 |
| BG | 18.13 ± 0.85 | 0.87 ± 0.06 **,# |
| Amino Acid Types | BSB | GTC | BG |
|---|---|---|---|
| Asp b (g/100 g) | 1.47 ± 0.06 | 1.54 ± 0.11 | 1.54 ± 0.17 |
| Thr a (g/100 g) | 0.66 ± 0.02 | 0.67 ± 0.03 | 0.68 ± 0.06 |
| Ser (g/100 g) | 0.54 ± 0.03 | 0.57 ± 0.05 | 0.57 ± 0.07 |
| Glu b (g/100 g) | 2.04 ± 0.11 | 2.12 ± 0.18 | 2.15 ± 0.28 |
| Gly b (g/100 g) | 0.79 ± 0.03 | 0.79 ± 0.02 | 0.80 ± 0.12 |
| Ala b (g/100 g) | 0.94 ± 0.02 | 0.98 ± 0.04 | 0.96 ± 0.09 |
| Cys (g/100 g) | 0.11 ± 0.02 | 0.11 ± 0.00 | 0.11 ± 0.01 |
| Val a (g/100 g) | 0.75 ± 0.02 | 0.77 ± 0.03 | 0.77 ± 0.06 |
| Met a (g/100 g) | 0.31 ± 0.02 | 0.33 ± 0.04 | 0.33 ± 0.07 |
| Ile a (g/100 g) | 0.67 ± 0.03 | 0.69 ± 0.03 | 0.68 ± 0.06 |
| Leu a (g/100 g) | 1.25 ± 0.02 | 1.27 ± 0.04 | 1.27 ± 0.10 |
| Tyr (g/100 g) | 0.50 ± 0.03 | 0.50 ± 0.02 | 0.49 ± 0.03 |
| Phe a (g/100 g) | 0.66 ± 0.02 | 0.68 ± 0.05 | 0.67 ± 0.08 |
| Lys a (g/100 g) | 1.51 ± 0.03 | 1.54 ± 0.07 | 1.53 ± 0.14 |
| His (g/100 g) | 0.35 ± 0.00 | 0.38 ± 0.01 | 0.41 ± 0.04 |
| Arg (g/100 g) | 0.90 ± 0.02 | 0.91 ± 0.02 | 0.91 ± 0.08 |
| Pro (g/100 g) | 0.47 ± 0.02 | 0.47 ± 0.01 | 0.48 ± 0.05 |
| ∑DAA | 5.25 ± 0.20 | 5.43 ± 0.33 | 5.45 ± 0.64 |
| ∑EAA | 5.81 ± 0.05 | 5.96 ± 0.26 | 5.94 ± 0.55 |
| ∑TAA | 13.93 ± 0.21 | 14.33 ± 0.67 | 14.37 ± 1.46 |
| ∑DAA/∑TAA | 0.38 ± 0.01 | 0.38 ± 0.01 | 0.38 ± 0.01 |
| ∑EAA/∑TAA | 0.42 ± 0.01 | 0.41 ± 0.01 | 0.41 ± 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Z.; Ouyang, X.; Wu, C.; Fan, S.; Yu, F.; Zhang, L.; Yu, X.; Tang, Z.; Qin, L.; Zhou, Y.; et al. The Formation of a Novel Intergeneric Hybrid Fish Derived from Megalobrama amblycephala (♀) × Culter dabryi (♂). Animals 2025, 15, 3302. https://doi.org/10.3390/ani15223302
Zhou Z, Ouyang X, Wu C, Fan S, Yu F, Zhang L, Yu X, Tang Z, Qin L, Zhou Y, et al. The Formation of a Novel Intergeneric Hybrid Fish Derived from Megalobrama amblycephala (♀) × Culter dabryi (♂). Animals. 2025; 15(22):3302. https://doi.org/10.3390/ani15223302
Chicago/Turabian StyleZhou, Zhifeng, Xinge Ouyang, Chang Wu, Siyu Fan, Faxian Yu, Liran Zhang, Xinxin Yu, Zhong Tang, Lang Qin, Yi Zhou, and et al. 2025. "The Formation of a Novel Intergeneric Hybrid Fish Derived from Megalobrama amblycephala (♀) × Culter dabryi (♂)" Animals 15, no. 22: 3302. https://doi.org/10.3390/ani15223302
APA StyleZhou, Z., Ouyang, X., Wu, C., Fan, S., Yu, F., Zhang, L., Yu, X., Tang, Z., Qin, L., Zhou, Y., Li, S., Wen, M., Wang, Y., Tao, M., & Liu, S. (2025). The Formation of a Novel Intergeneric Hybrid Fish Derived from Megalobrama amblycephala (♀) × Culter dabryi (♂). Animals, 15(22), 3302. https://doi.org/10.3390/ani15223302






