First Observation of Embryonic Development and Paralarvae of Amphioctopus kagoshimensis
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Sampling and Cultivation of Wild A. kagoshimensis
2.2. Cultivation and Observation of A. kagoshimensis Paralarvae
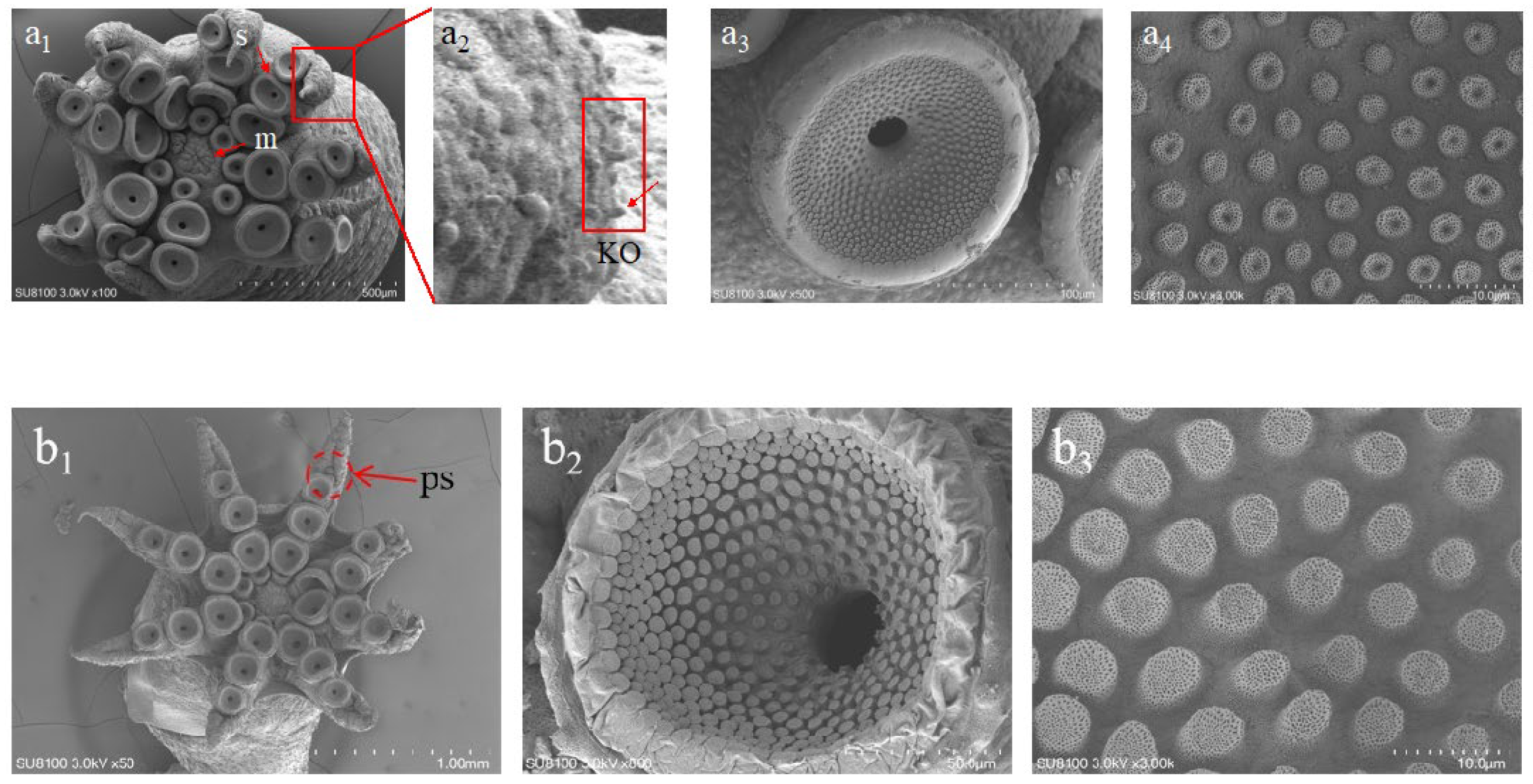
2.3. Sample Preparation for Scanning Electron Microscope (SEM)
2.4. Declaration of Generative AI in Scientific Writing
3. Results
3.1. Morphology, Reproductive Behavior and Early Survival of A. kagoshimensis
3.2. Embryonic Development of A. kagoshimensis
3.2.1. Stage I–III (1–3 Days): Cleavage and Gastrulation
3.2.2. Stage IV–X (Days 4–11): Start of Organogenesis
3.2.3. Stage XI–XV (12–20 Days): Organ Development
3.2.4. Stage XVI–XIX (Days 21–28): Chromatophores and Inversions
3.2.5. Stage XX (29–30 Days): Hatching Stage
3.3. Paralarval Development of A. kagoshimensis
3.3.1. 1-Day Post-Hatching Paralarvae
3.3.2. 3-Day Post-Hatching Paralarvae
3.3.3. 5-Day Post-Hatching Paralarvae
3.3.4. 7-Day Post-Hatching Paralarvae
3.3.5. 10-Day Post-Hatching Paralarvae
3.3.6. 15-Day Post-Hatching Paralarvae
3.3.7. 20-Day Post-Hatching Paralarvae
3.3.8. 30-Day Post-Hatching Paralarvae
3.4. Chromatophores Pattern During Embryonic and Paralarval Development
4. Discussion
4.1. First Successful Artificial Breeding of A. kagoshimensis
4.2. Embryonic Development and Comparative Insights with Other Octopus
4.3. Paralarval Development and Hatchery Applications
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| TL | Total length |
| ML | Mantle length |
| AL | Arm length |
| MW | Mantle width |
| FL | Funnel length |
| FW | Funnel width |
| dph | days post hatching |
References
- Jacquet, J.; Franks, B.; Godfrey-Smith, P.; Sanchez-Suarez, W. The Case against Octopus Farming. Issues Sci. Technol. 2019, 35, 37–44. [Google Scholar]
- Powell, A.L. Octopus Aquaculture: Welfare Practices and Challenges. Can. Vet. J. 2022, 63, 1072. [Google Scholar] [PubMed]
- Xie, S.; Zhou, Y.; Yang, D.; Zhang, B. The reproduction action and embryonic development of Octopus variabilis. J. Dalian Ocean Univ. 2011, 26, 102–107. [Google Scholar]
- Liu, C. Studies on Culture of the Life Cycle of Octopus Minor; Ocean University of China: Qingdao, China, 2013. [Google Scholar]
- Liu, Z.S. Studies on Fundamental Biology and Artificial Reproductive Technique of Octopus Vulgaris; Ocean University of China: Qingdao, China, 2013. [Google Scholar]
- Xu, D.F. Studies on Larvae Development of Octopus Vulgaris and the Effects of Environmental Stress on Larvae; Shanghai Ocean University: Shanghai, China, 2019. [Google Scholar]
- Jiang, D.; Zheng, X.; Qian, Y.; Zhang, Q. Development of Amphioctopus Fangsiao (Mollusca: Cephalopoda) from Eggs to Hatchlings: Indications for the Embryonic Developmental Management. Mar. Life Sci. Technol. 2020, 2, 24–30. [Google Scholar] [CrossRef]
- Jiang, D.; Zheng, X.; Qian, Y.; Zhang, Q. Embryonic Development of Amphioctopus Fangsiao under Elevated Temperatures: Implications for Resource Management and Conservation. Fish. Res. 2020, 225, 105479. [Google Scholar] [CrossRef]
- Villanueva, R.; Norman, M.D. Biology of the Planktonic Stages of Benthic Octopuses. In Oceanography and Marine Biology; CRC Press: Boca Raton, FL, USA, 2008; pp. 111–208. [Google Scholar]
- Uriarte, I.; Iglesias, J.; Domingues, P.; Rosas, C.; Viana, M.T.; Navarro, J.C.; Seixas, P.; Vidal, E.; Ausburger, A.; Pereda, S. Current Status and Bottle Neck of Octopod Aquaculture: The Case of American Species. J. World Aquac. Soc. 2011, 42, 735–752. [Google Scholar] [CrossRef]
- Suzumura, Y.; Matsubara, K.; Morii, S.; Abe, M.; Gleadall, I.G.; Nishikawa, M.; Katayama, A.; Nishitani, G.; Okawara, Y.; Kimura, R.; et al. Shelters for Aquaculture of Octopus Sinensis: Preferences for Gap Width and Horizontal versus Vertical Plates. Fish. Sci. 2022, 88, 285–298. [Google Scholar] [CrossRef]
- Li, M. Study on Reproductive Behaviors and Growth Characteristics of Octopus Sinensis; Shanghai Ocean University: Shanghai, China, 2022. [Google Scholar]
- Yamamoto, M. Egg Size and Fecundity in Relation to Body Weight and Water Temperature in Amphioctopus Fangsiao. Aquac. Sci. 2024, 72, 151–156. [Google Scholar] [CrossRef]
- Ortmann, A. Japanische Cephalopoden. Zool. Jahrbücher Abt. System. 1988, 3, 639–670. [Google Scholar] [CrossRef]
- Norman, M.D.; Hochberg, F.G. The Current State of Octopus Taxonomy. Phuket Mar. Biol. Cent. Res. Bull. 2005, 66, 127–154. [Google Scholar]
- Norman, M.; Finn, J.K.; Hochberg, F.G. Family Octopodidae. In FAO Species Catalogue for Fishery Purposes; FAO: Rome, Italy, 2014. [Google Scholar]
- Norman, M.; Kubodera, T. Taxonomy and Biogeography of an Australian Subtropical Octopus with Japanese Affinities. In Proceedings of the 7th and 8th Symposia on Collection Building and Natural History Studies in Asia and the Pacific Rim; Tomida, Y., Kubodera, T., Akiyama, S., Kitayama, T., Eds.; National Science Museum Monographs: Ibaraki, Japan, 2006; Volume 1, pp. 171–189. [Google Scholar]
- Mangold, K. Octopus Vulgaris. Cephalop. Life Cycles 1983, 1, 335–364. [Google Scholar]
- Naef, A. Cephalopoda: Embryology; Smithsonian Institution and the National Science Foundation: Washington, DC, USA, 1928; Volume 2. [Google Scholar]
- Boletzky, S.V. Embryonic Development of Cephalopods at Low Temperatures. Antarct. Sci. 1994, 6, 139–142. [Google Scholar] [CrossRef]
- Boletzky, S.V. Biology of Early Life Stages in Cephalopod Molluscs. Adv. Mar. Biol. 2003, 44, 144–204. [Google Scholar]
- Boletzky, S.V. Encapsulation of Cephalopod Embryos-a Search for Functional Correlations. Am. Malacol. Bull. 1986, 4, 217–227. [Google Scholar]
- Boletzky, S.V.; Hanlon, R.T. A Review of the Laboratory Maintenance, Rearing and Culture of Cephalopod Molluscs. Mem. Natl. Mus. Vic. Melb. 1983, 44, 147–187. [Google Scholar] [CrossRef]
- Sweeney, M.J.; Roper, C.F.E.; Mangold, K.M.; Clark, M.R.; Boletzky, S.V. “Larval” and Juvenile Cephalopods: A Manual for Their Identification; Smithsonian Institution: Washington, DC, USA, 1992. [Google Scholar]
- Prasopsook, P.; Sukhsangchan, C.; Whanphetch, N. Embryonic Development and External Morphology of Amphioctopus Aegina (Gray, 1849) (Cephalopoda: Octopodidae) in Thailand. ScienceAsia 2022, 48, 393–398. [Google Scholar] [CrossRef]
- Moguel, C.; Mascaró, M.; Avila-Poveda, O.H.; Caamal-Monsreal, C.; Sanchez, A.; Pascual, C.; Rosas, C. Morphological, Physiological and Behavioral Changes during Post-Hatching Development of Octopus Maya (Mollusca: Cephalopoda) with Special Focus on the Digestive System. Aquat. Biol. 2010, 9, 35–48. [Google Scholar] [CrossRef]
- Alejo-Plata, M.d.C.; Alejo, S.H. First Description of Eggs and Paralarvae of Green Octopus Octopus Hubbsorum (Cephalopoda: Octopodidae) under Laboratory Conditions. Am. Malacol. Bull. 2014, 32, 132–139. [Google Scholar] [CrossRef]
- García-Flores, M.; Ceballos-Vázquez, B.P.; Rosales-Velázquez, M.O. Embryonic Development and Fecundity of the Pacific Pygmy Octopus, Paroctopus Digueti. J. Shellfish. Res. 2022, 41, 125–134. [Google Scholar] [CrossRef]
- Ibarra-García, L.E.; Mazón-Suástegui, J.M.; Rosas, C.; Tovar-Ramírez, D.; Bárcenas-Pazos, G.; Civera-Cerecedo, R.; Campa-Córdova, A.I. Morphological and Physiological Changes of Octopus Bimaculoides: From Embryo to Juvenile. Aquaculture 2018, 497, 364–372. [Google Scholar] [CrossRef]
- Ortiz, N.; Ibáñez, C.M.; Farías, A.; Pardo-Gandarillas, M.C.; Uriarte, I. Enteroctopus Megalocyathus, Patagonian Red Octopus. In Octopus Biology and Ecology; Elsevier: Amsterdam, The Netherlands, 2024; pp. 397–417. [Google Scholar]
- Fiorito, G.; Affuso, A.; Basil, J.; Cole, A.; de Girolamo, P.; D’Angelo, L.; Dickel, L.; Gestal, C.; Grasso, F.; Kuba, M.; et al. Guidelines for the Care and Welfare of Cephalopods in Research -A Consensus Based on an Initiative by CephRes, FELASA and the Boyd Group. Lab. Anim. 2015, 49, 1–90. [Google Scholar] [CrossRef]
- Andrews, P.L.R.; Darmaillacq, A.-S.; Dennison, N.; Gleadall, I.G.; Hawkins, P.; Messenger, J.B.; Osorio, D.; Smith, V.J.; Smith, J.A. The Identification and Management of Pain, Suffering and Distress in Cephalopods, Including Anaesthesia, Analgesia and Humane Killing. J. Exp. Mar. Biol. Ecol. 2013, 447, 46–64. [Google Scholar] [CrossRef]
- Wang, W.; Yang, J.; Zhou, Q.; Zheng, X. Reproductive Behavior and Process of Embryonic Development of Octopus Ocellatus. J. Fish. Sci. China 2010, 17, 1157–1165. [Google Scholar]
- Sun, Y.; Yao, C.; Zhu, Y.; Wang, Y.; Zhang, Z. Metabolism Response of Fasting in Octopus Sinensis Paralarvae Revealed by RNA-Seq. Aquaculture 2022, 550, 737859. [Google Scholar] [CrossRef]
- Hanlon, R.T.; Forsythe, J.W. Advances in the Laboratory Culture of Octopuses for Biomedical Research. Lab. Anim. Sci. 1985, 35, 33–40. [Google Scholar]
- Yikang, S. Artificial Hatching of Fertilized Eggs and Study on Growth Performance of Delayed Feeding in Octopus Sinensis; Shanghai Ocean University: Shanghai, China, 2022. [Google Scholar]
- Iglesias, J.; Fuentes, L. Octopus Vulgaris. Paralarval Culture. In Cephalopod Culture; Springer: Berlin/Heidelberg, Germany, 2014; pp. 427–450. [Google Scholar]
- Iglesias, J.; Fuentes, L.; Villanueva, R. Cephalopod Culture; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2014; ISBN 9401786488. [Google Scholar]
- Osborn, S.A. Fecundity and Embryonic Development of Octopus Rubescens Berry from Monterey Bay, California; San Jose State University: San Jose, CA, USA, 1995; ISBN 9798209383680. [Google Scholar]
- Lenz, T.M.; Elias, N.H.; Leite, T.S.; Vidal, E.A.G. First Description of the Eggs and Paralarvae of the Tropical Octopus, Octopus Insularis, under Culture Conditions. Am. Malacol. Bull. 2015, 33, 101–109. [Google Scholar] [CrossRef]
- Caverivière, A.; Domain, F.; Diallo, A. Observations on the Influence of Temperature on the Length of Embryonic Development in Octopus Vulgaris (Senegal). Aquat. Living Resour. 1999, 12, 151–154. [Google Scholar] [CrossRef]
- Ambrose, R.F. Observations on the Embryonic Development and Early Post-Embryonic Behavior of Octopus Bimaculatus (Mollusca: Cephalopoda); California Malacozoological Society: Berkeley, CA, USA, 1981. [Google Scholar]
- Snyder, S. Successful Rearing of Octopus Dofleini from Hatchling to Settlement. In American Association of Zoological Parks and Aquariums 1986 Annual Conference Proceedings; American Association of Zoological Parks and Aquariums: Minneapolis, MN, USA, 1986; pp. 781–783. [Google Scholar]
- Christie, B.L.; Peters, A.; Barord, G.J.; Rehling, M.J. Giant Pacific Octopus (Enteroctopus Dofleini) Care Manual; Association of Zoos and Aquariums: Silver Spring, MD, USA, 2014. [Google Scholar]
- Son, P.W.; Kim, B.-G.; Kim, S.H. Gametogenesis, Mating Behaviour and Spawning of Octopus Ocellatus (Cephalopoda: Octopodidae) in Western Korea. Korean J. Malacol. 2015, 31, 113–121. [Google Scholar] [CrossRef]
- Zhao, J. Studies on Early Growth and Environmental Stress Effect of Octopus Ocellatus; Shanghai Ocean University: Shanghai, China, 2019. [Google Scholar]
- Qian, Y.S.; Jiang, D.H.; Zheng, X.D.; Meng, Y.F.; Zhu, Q.; Zhang, Q.Q. Indoor Cement Pool Breeding and Farming Technology for Octopus Ocellatus Juvenile. Aquac. Feed. 2018, 10, 35–37. [Google Scholar] [CrossRef]
- Hanlon, R.T. Octopus Briareus. Cephalop. Life Cycles 1983, 1, 251–266. [Google Scholar]
- Forsythe, J.W. Octopus Joubini (Mollusca: Cephalopoda): A Detailed Study of Growth through the Full Life Cycle in a Closed Seawater System. J. Zool. 1984, 202, 393–417. [Google Scholar] [CrossRef]
- Forsythe, J.W.; Toll, R.B. Clarification of the Western Atlantic Ocean Pygmy Octopus Complex: The Identity and Life History of Octopus Joubini (Cephalopoda: Octopodinae). Bull. Mar. Sci. 1991, 49, 88–97. [Google Scholar]
- Tranter, D.J.; Augustine, O. Observations on the Life History of the Blue-Ringed Octopus Hapalochlaena Maculosa. Mar. Biol. 1973, 18, 115–128. [Google Scholar] [CrossRef]
- Reynolds, A. Blue-Ringed Octopus Hapalochlaena Maculosa. Scuba Diver 1983, 18, 2022–2024. [Google Scholar]
- Iribarne, O.O. Life History and Distribution of the Small South-Western Atlantic Octopus, Octopus Tehuelchus. J. Zool. 1991, 223, 549–565. [Google Scholar] [CrossRef]
- Forsythe, J.W.; Hanlon, R.T. Effect of Temperature on Laboratory Growth, Reproduction and Life Span of Octopus Bimaculoides. Mar. Biol. 1988, 98, 369–379. [Google Scholar] [CrossRef]
- Van Heukelem, W.F. Octopus Cyanea. In Cephalopod Life Cycles; Boyle, P.R., Ed.; Academic Press: London, UK, 1983; Volume 1, pp. 267–276. [Google Scholar]
- Khen, A.; McCormick, L.R.; Steinke, C.A.; Rouse, G.W.; Zerofski, P.J. First Known Observations of Brooding, Development, and Hatching of Fertilized Eggs for the North Pacific Bigeye Octopus, Octopus Californicus. Ecol. Evol. 2022, 12, e9481. [Google Scholar] [CrossRef]
- Armelloni, E.N.; Lago-Rouco, M.J.; Bartolomé, A.; Felipe, B.C.; Almansa, E.; Perales-Raya, C. Exploring the Embryonic Development of Upper Beak in Octopus Vulgaris Cuvier, 1797: New Findings and Implications for Age Estimation. Fish. Res. 2020, 221, 105375. [Google Scholar] [CrossRef]
- Castro, H.; Olivares, A.; Quintana, A.; Zuñiga, O. Descripción Del Desarrollo Embrionario y Paralarvas de Octopus Mimus Gould 1852 (Mollusca: Cephalopoda) En Cautiverio. Estud. Ocean. 2002, 21, 13–25. [Google Scholar]
- Dan, S.; Iwasaki, H.; Takasugi, A.; Yamazaki, H.; Hamasaki, K. An upwelling system for culturing common octopus paralarvae and its combined effect with supplying natural zooplankton on paralarval survival and growth. Aquaculture 2018, 495, 98–105. [Google Scholar] [CrossRef]
- Dan, S.; Iwasaki, H.; Takasugi, A.; Shibasaki, S.; Yamazaki, H.; Oka, M.; Hamasaki, K. Effects of co-supply ratios of swimming crab Portunus trituberculatus zoeae and Artemia on survival and growth of East Asian common octopus Octopus sinensis paralarvae under an upwelling culture system. Aquac. Res. 2019, 50, 1361–1370. [Google Scholar] [CrossRef]
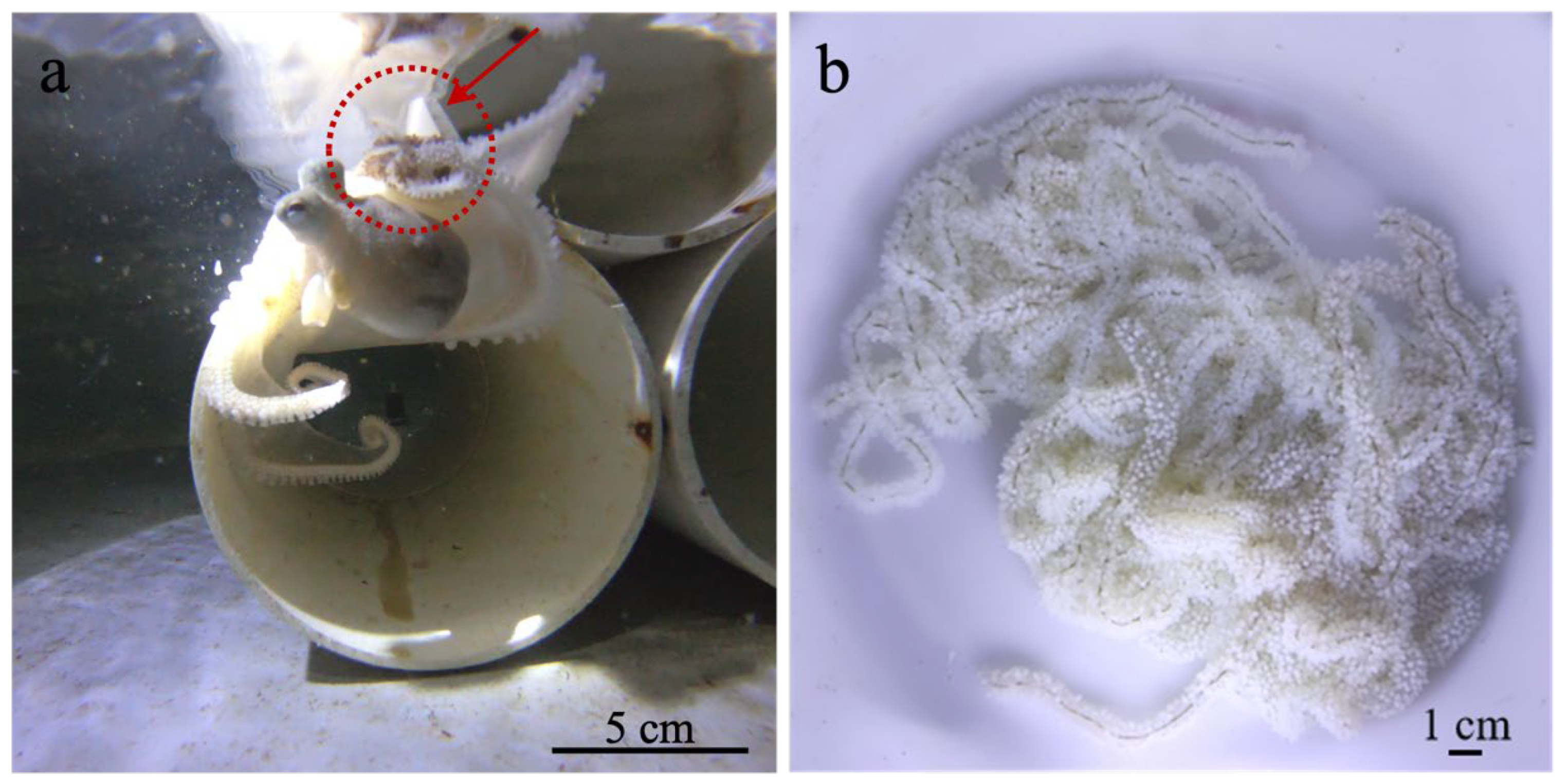

| Stage | Day (Range) | Key Developmental Features |
|---|---|---|
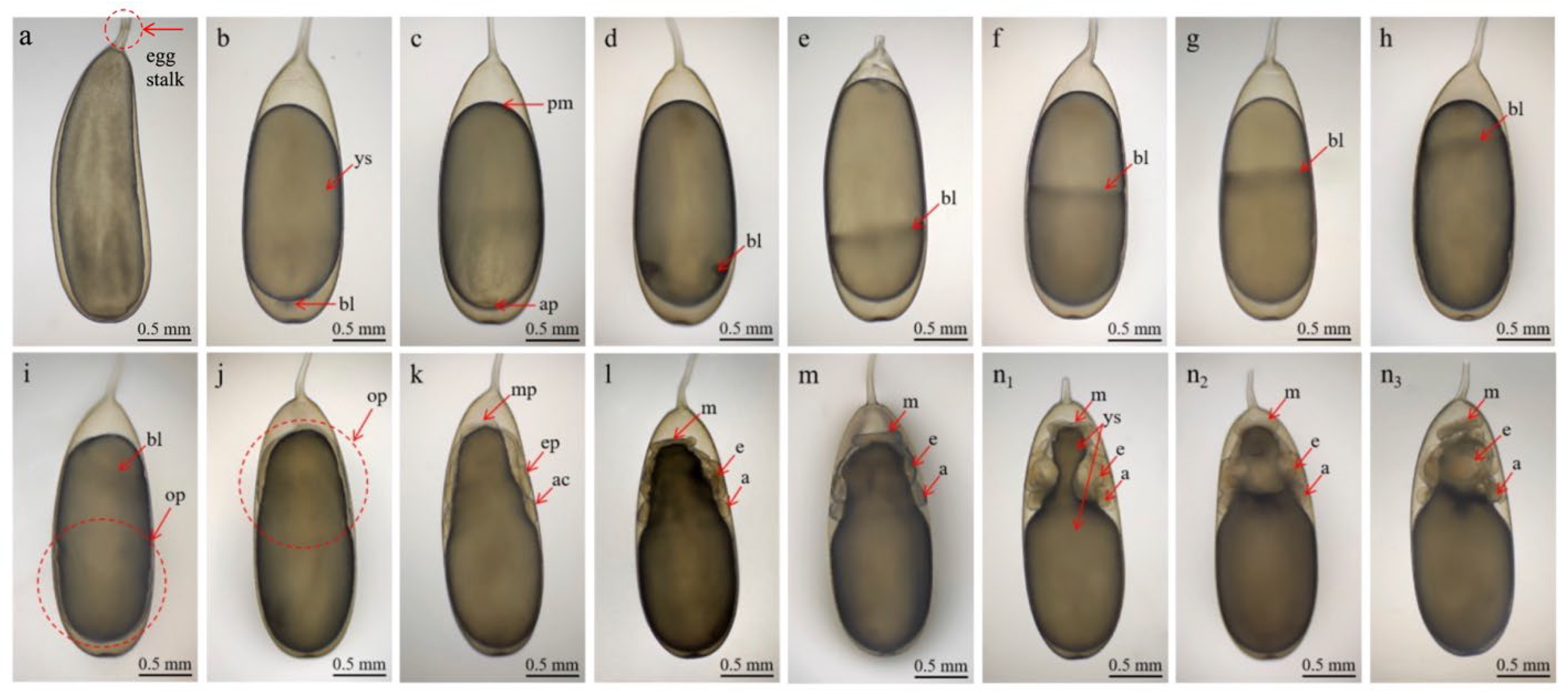
| Stage I | 1 day | Eggs appear white and grain-like, with a slightly narrowed egg stalk. Yolk is transparent and elliptical. The eggs measured 2.55 ± 0.10 mm in length and 1.78 ± 0.05 mm in width (Figure 2b). |
| Stage II | 2 days | Cleavage occurs; first and third divisions are meridional, second and fourth are equatorial (Figure 2c). |
| Stage III | 3 days | Gastrulation begins; mesodermal cells form muscle tissues; yolk epithelium partially envelops yolk (Figure 2d). |
| Stage IV | 4 days | Yolk epithelium extends, covering ~1/3 of yolk; narrow longitudinal groove appears (Figure 2e). |
| Stage V | 5 days | Yolk epithelium covers ~1/2 yolk; organogenesis begins but primordia are not yet distinct (Figure 2f). |
| Stage VI | 6 days | Yolk epithelium covers ~2/3 of yolk; yolk becomes darker. Primordia remain indistinct (Figure 2g). |
| Stage VII | 7 days | Yolk epithelium covers ~4/5 of yolk; the yolk sac is largely depleted, causing a visible rut near the animal pole (Figure 2h). |
| Stage VIII | 8 days | Yolk epithelium reaches nearly the vegetal pole, covering 5/6 of yolk; about half of yolk is consumed (Figure 2i). |
| Stage IX | 9 days | First inversion occurs; yolk sac adheres tightly to the egg membrane; animal pole shifts towards the egg stalk (Figure 2j). |
| Stage X | 10–11 days | Yolk sac consumption accelerates; mantle, eyes, and arms begin to develop (Figure 2k). |
| Stage XI | 12–13 days | Yolk sac shortens; disk-shaped mantle begins to form; eyes and arms become distinguishable; mantle disc: 0.44 mm (L) × 0.07 mm (H) (Figure 2l). |
| Stage XII | 14 days | Mantle, eyes, and arms continue to develop; eight arms form symmetrically around yolk sac; mantle height increased ~0.05 mm (Figure 2m). |
| Stage XIII | 15–16 days | Rapid development in mantle, eyes, and arms. Visceral mass, funnel, statocyst, and brain primordia emerge; eye plates ~20 mm diameter; interocular distance ~0.55 mm; arms ~0.23 mm with round tips (Figure 2(n1–n3)). |
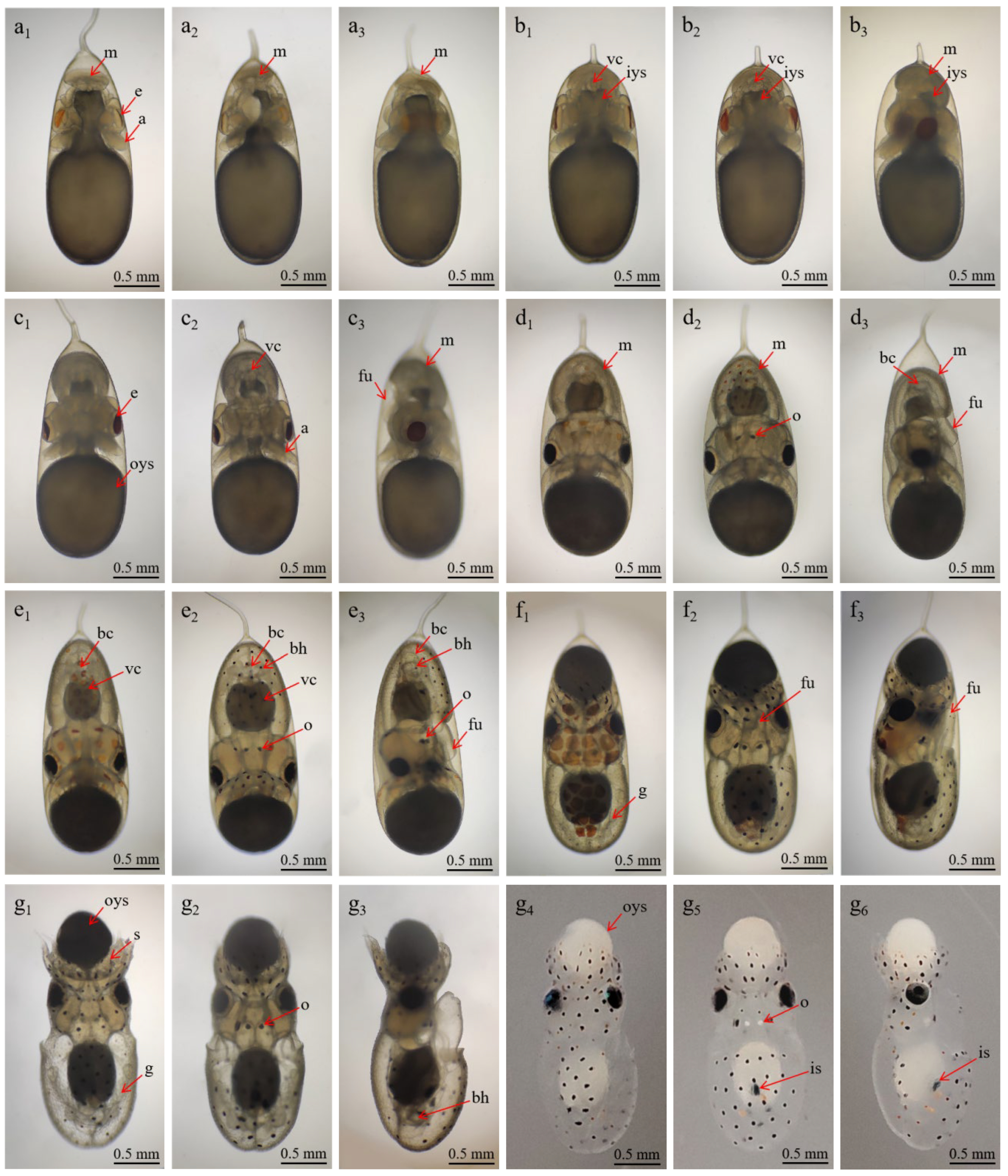
| Stage XIV | 17–18 days | Eye plates turn yellow, dorsal mantle develops rapidly. Heart begins to form; eye plates ~0.25 mm; external eye ~0.65 mm; arms ~0.35 mm (Figure 3(a1–a3)). |
| Stage XV | 19–20 days | Two branchial hearts form and function irregularly. The mantle fully envelops the visceral mass. Branchial hearts ~0.11 × 0.09 mm; mantle dorsal length ~0.70 mm, ventral ~0.35 mm; lens ~0.03 mm; arms ~0.50 mm; funnel primordium ~0.35 × 0.15 mm, opening ~0.08 mm (Figure 3(b1–b3)). |
| Stage XVI | 21–22 days | Yolk sac rapidly consumed. Statocyst “D”-shaped ~0.15 × 0.10 mm; arms ~0.60 mm. Mantle length (dorsal/ventral/height): 0.82/0.40/0.55 mm; yolk sac ~1.35 × 1.15 mm (Figure 3(c1–c3)). |
| Stage XVII | 23–24 days | Juvenile reaches 2.84 ± 0.02 mm in total length. Yellow chromatophores appeared on the head, dorsal, and ventral surfaces; body ~1.30 mm; funnel ~0.45 × 0.25 mm; arms with 3 suckers; yolk ~1.10 mm (Figure 3(d1–d3)). |
| Stage XVIII | 25–26 days | Chromatophores form black/brown spots (head: 10–12; dorsal: 14–17; ventral: 32–38); arms ~0.76 mm; circular muscles ~0.22 mm. Strong heart and gill activity (Figure 3(d1–d3)). |
| Stage XIX | 27–28 days | Second inversion occurs; embryos turn bright orange, yolk sac shrinks. Branchial hearts ~0.28 × 0.15 mm; statocysts ~0.30 × 0.28 mm; statoliths ~0.06 mm (Figure 3(e1–e3)). |
| Stage XX | 29–30 days | Paralarvae hatched, phototactic behavior and ink expulsion observed. TL ~3.53 ± 0.02 mm; yolk sac ~0.35 mm (absorbed in 4–8h); black/yellow chromatophores (Figure 3(g1–g6)). |
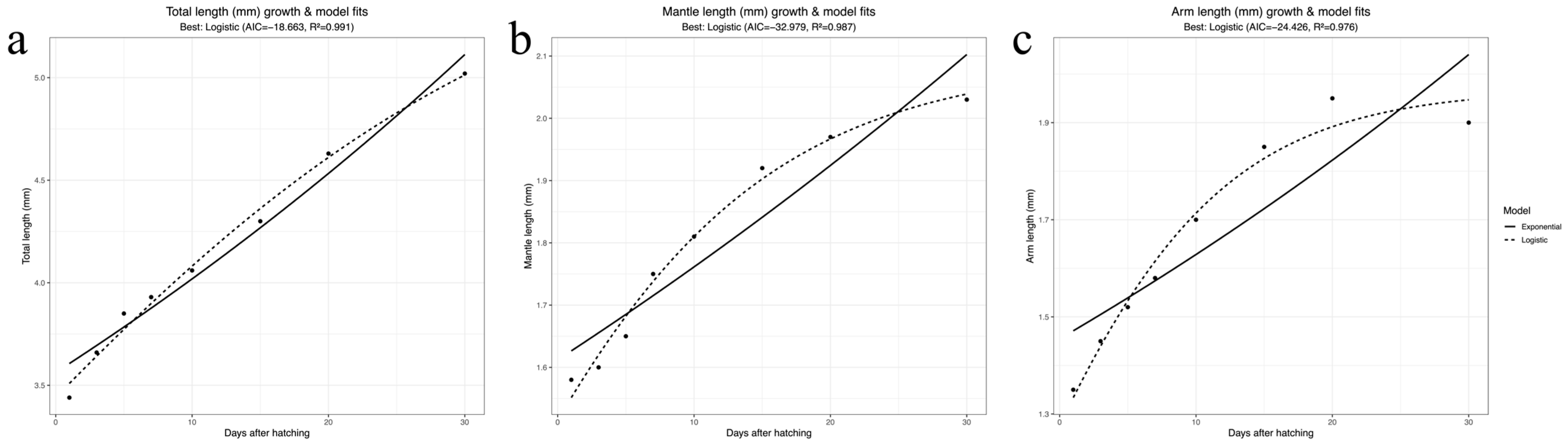
| Days | 1 | 3 | 5 | 7 | 10 | 15 | 20 | 30 |
|---|---|---|---|---|---|---|---|---|
| TL (mm) | 3.44 ± 0.08 | 3.66 ± 0.03 | 3.85 ± 0.02 | 3.93 ± 0.08 | 4.06 ± 0.06 | 4.30 ± 0.07 | 4.63 ± 0.10 | 5.02 ± 0.11 |
| ML (mm) | 1.58 ± 0.03 | 1.6 ± 0.02 | 1.65 ± 003 | 1.75 ± 0.03 | 1.81 ± 0.07 | 1.92 ± 0.10 | 1.97 ± 0.11 | 2.03 ± 0.08 |
| MW (mm) | 1.4 ± 0.06 | 1.45 ± 0.04 | 1.6 ± 0.02 | 1.63 ± 0.05 | 1.67 ± 0.07 | 1.78 ± 0.07 | 1.82 ± 0.08 | 1.9 ± 0.10 |
| AL (mm) | 1.35 ± 0.04 | 1.45 ± 0.03 | 1.52 ± 0.07 | 1.58 ± 0.05 | 1.7 ± 0.06 | 1.85 ± 0.06 | 1.95 ± 0.08 | 1.90 ± 0.013 |
| FL (mm) | 0.8 ± 0.04 | - | - | 0.8 ± 0.03 | - | - | - | 1.15 ± 0.05 |
| FW (mm) | 0.65 ± 0.04 | - | - | 0.65 ± 0.05 | - | - | - | 0.95 ± 0.08 |
| Developmental Stage | Head | Dorsal Mantle | Ventral Mantle | Arms (per Arm) | Funnel |
|---|---|---|---|---|---|
| Stage XVIII (embryo) | 11 ± 1 | 15.5 ± 1.5 | 35 ± 1.5 | 5 | - |
| Stage XIX (embryo) | 11 ± 1 | 16 ± 1 | 36 ± 2 | 7 | 10 ± 1 (two rows) |
| Stage XX (hatching) | 11 ± 1 | 16 ± 1 | 36 ± 2 | 7 | 10 ± 1 |
| 5 dph paralarvae | 11 ± 1 | 16 ± 1 | 36 ± 2 | 9 | 10 ± 1 |
| 15 dph paralarvae | 11 ± 1 | 16 ± 1 | 36 ± 2 | 13 ± 1 | 10 ± 1 |
| 30 dph paralarvae | 11 ± 1 | 16 ± 1 | 36 ± 2 | 23 ± 2 (9 large + 14 small) | 10 ± 1 |
| Species | Adult Size ML (cm) | Egg Size (mm) | Birth Size ML (mm) | Development Days/ Temperature (°C) | Fecundity (No. Eggs) | Source |
|---|---|---|---|---|---|---|
| Merobenthic octopus (Indirect development) | ||||||
| Amphioctopus kagoshimensis | 8 | 2.6 | 1.58 | 30/22.0–24.5 °C | 4000–5000 | Present study |
| Octopus rubescens | 8–10 | 3–4 | 1.7–2 | 52/17.7 °C 91/14.8 °C | 4000–45,000 1000–19,000 | [24,39] |
| Octopus insularis | 12 | 2.13- 2.29 | 1.68 | 30–38/26 °C | 85,000 | [40] |
| Octopus vulgaris | 25 | 1.5–2 | 2.18 2.4 2–3 | 29–49/22–23 °C 87/17 °C 15–28/27 °C | 100,000–600,000 100,000–500,000 | [18,24,41] |
| Octopus bimaculatus | 20 | 2.5–4 | 2.6 | 31/16 °C, 50/19 °C | 20,000 | [42] |
| Octopus dofleini | 36 | 6–8 | 3–3.5 | 548/5 °C | 30,000–180,000 | [24] |
| Octopus hubbsorum | 11 | 1.6 | 1.22 | 45/24–26.5 °C | 105,000–144,000 | [27] |
| Octopus defilippi | 9 | 1.5–2.1 | 1.3–1.5 | 10,000+ | [24] | |
| Amphioctopus aegina | 5.83 | 2.6 | 1.9 | 18–22/28 °C | 5607–13,640 | [25] |
| Octopus sinensis | 13.3–17.9 | 2.3–2.7 2.4 ± 0.2 | 1.87–2.16 | 21–24/22.4–23.5 °C 25–35/20.4–23.6 °C | 30,000–180,000 90,000–130,000 | [5,12] |
| Enteroctopus dofleini | 40–60 | 6–11 | 6–8 | 155–223/9–13 °C | 20,000–180,000 | [43,44] |
| Enteroctopus megalocyathus | >30 | 7.5–12 | 7–9.55 | 131–136/14–18 °C 256–280/8 °C | 3000–8600 | [30] |
| Holobenthic octopus (Direct development) | ||||||
| Amphioctopus fangsiao | 10.1 | 4.12 | 30/18–25 °C | 294–660 | [45,46,47] | |
| Paroctopus digueti | 4–5 | 7–10 7–8 | 5.5 4.5–6 | 38/27 °C 42/25 °C | 50–300 50–150 | [28,35] |
| Octopus joubini | 4.5 3 | 6–7 6–10 2.5 | 5.8 5.5 2.5 | 35–40/25 °C 35–42/25 °C 32–42/22–24 °C | 50–200 25–300 136–2400 | [48,49,50] |
| Octopus fitchi | 4.5 | 4–10 | 4 | 30–45/25 °C | 150–300 | [24] |
| Hapalochlaena maculosa | 5.7 | 6–7 6–9 | 4 | 40–50/20 °C 60/20.8–22.5 °C | 150 100–200 | [51,52] |
| Octopus tehuelchus | 4.95 | 9–12 | 6.64 | 120–150/4–19 °C | 80+ | [53] |
| Octopus minor | 8 | 21–22 | 8.5–11.5 | 72–89/21–25 °C | 50–200 | [38] |
| Octopus bimaculoides | 8.5 | 10–17 | 6–7 6.5 | 55/24 °C, 85/18 °C 46–50/23 °C | 250–750 | [35,54] |
| Octopus briareus | 12 | 12–13 10–14 | 7 | 60–70/25 °C | 300–700 150–950 | [24,48] |
| Octopus maya | 12 | 11–17 | 7 4–9 | 45/25 °C 50–65/wild | 300–500 500–5 000 | [26,55] |
| Octopus californicus | 14 12–14 | 14–17 17.7–24.8 | 9.9–12.0 | 74–77/8–10 °C | 50–100 200 | [24,56] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Yu, J.; Chen, S.; Zhang, T.; Chang, Q.; Bian, L. First Observation of Embryonic Development and Paralarvae of Amphioctopus kagoshimensis. Animals 2025, 15, 3249. https://doi.org/10.3390/ani15223249
Zhu J, Yu J, Chen S, Zhang T, Chang Q, Bian L. First Observation of Embryonic Development and Paralarvae of Amphioctopus kagoshimensis. Animals. 2025; 15(22):3249. https://doi.org/10.3390/ani15223249
Chicago/Turabian StyleZhu, Jinchao, Juanwen Yu, Siqing Chen, Tianshi Zhang, Qing Chang, and Li Bian. 2025. "First Observation of Embryonic Development and Paralarvae of Amphioctopus kagoshimensis" Animals 15, no. 22: 3249. https://doi.org/10.3390/ani15223249
APA StyleZhu, J., Yu, J., Chen, S., Zhang, T., Chang, Q., & Bian, L. (2025). First Observation of Embryonic Development and Paralarvae of Amphioctopus kagoshimensis. Animals, 15(22), 3249. https://doi.org/10.3390/ani15223249






