The Effects of Cold Tolerance on the Distribution of Two Extreme Altitude Lizard Species in the Qinghai–Tibetan Plateau
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Acquisition
2.2. Predicting the CTmin and Cold Stress Frequency for Phrynocephalus
2.2.1. Measuring the CTmin in Phrynocephalus theobaldi
2.2.2. Cold Stress Frequency During the Overwintering Period
2.3. Traditional Environmental Variables
2.4. Species Distribution Modelling
3. Results
3.1. CTmin in Phrynocephalus theobaldi
3.2. Model Performance and Evaluation
3.3. Main Environmental Variables Among Different SDMs
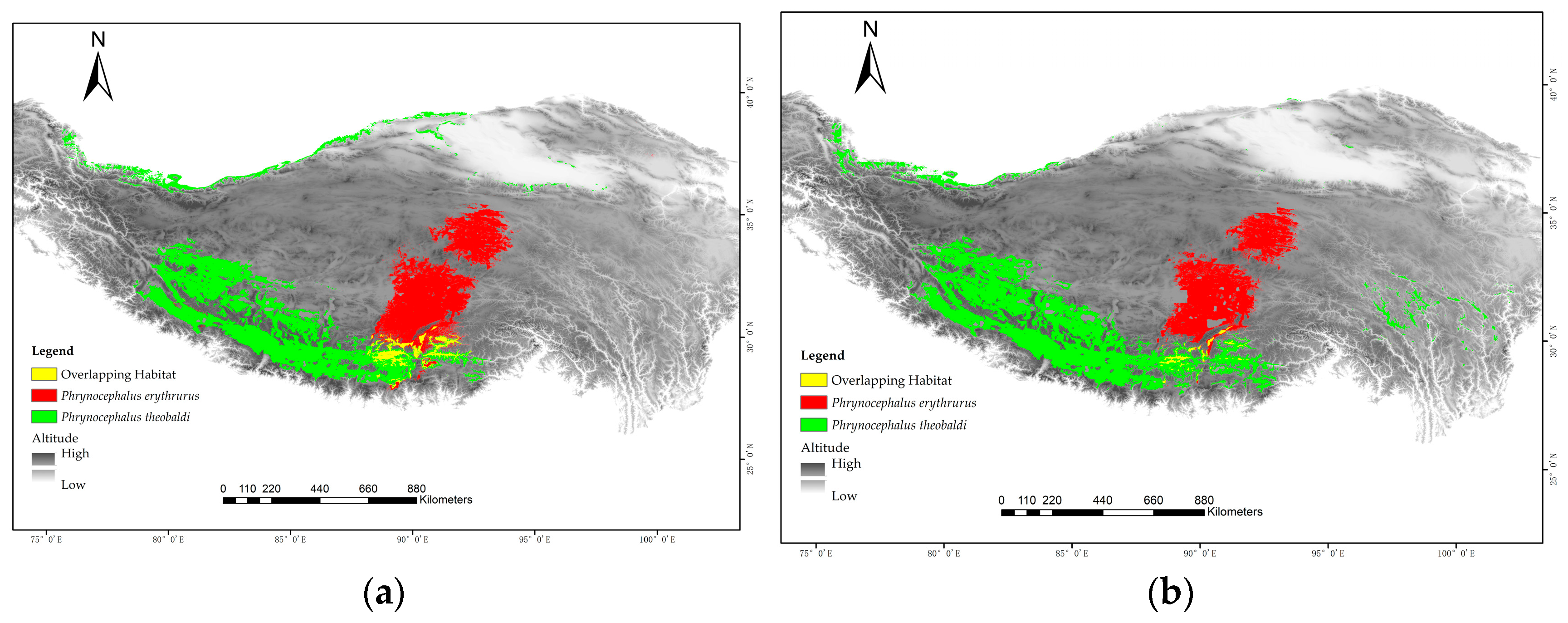
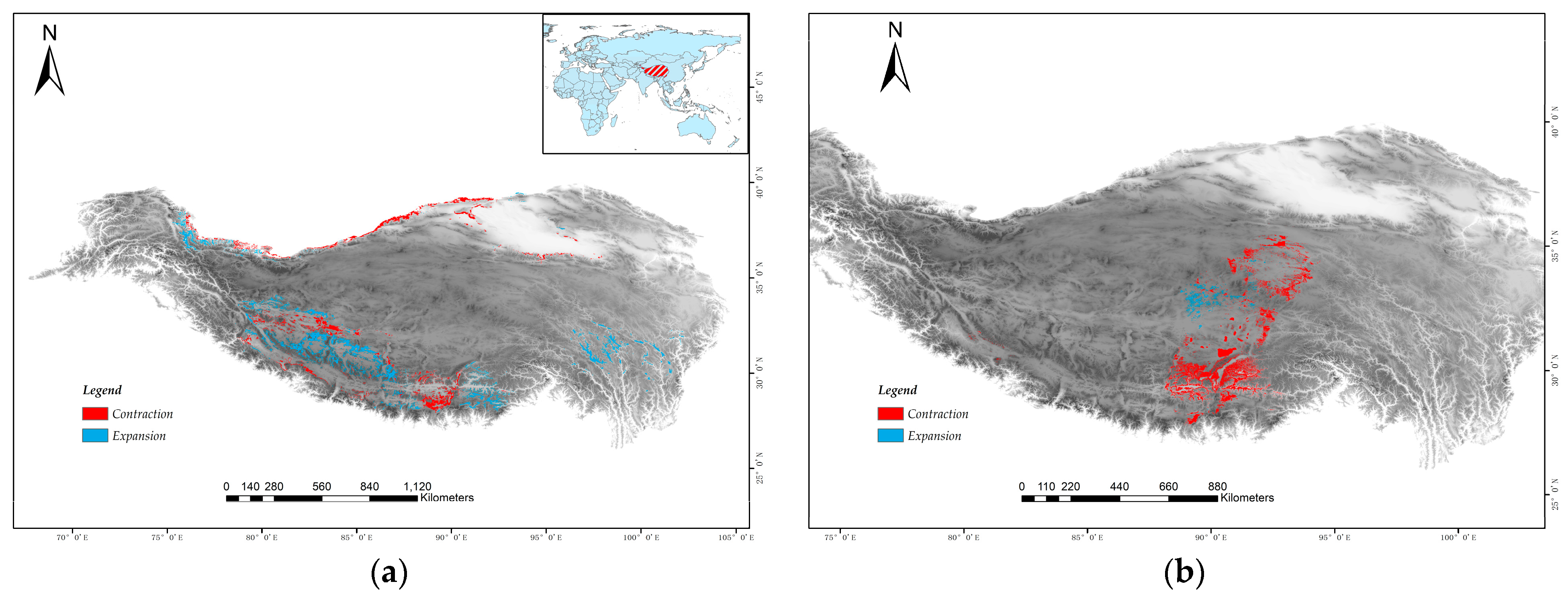
3.4. Effects of Incorporating CTmin on the Prediction of Suitable Habitat Areas
4. Discussion
4.1. Comparison of CTmin Between Phrynocephalus theobaldi and Other Lizards in Qinghai–Tibetan Plateau
4.2. Model Limitations and Behavioral Buffering
4.3. Effects of Traditional Environmental Factors
4.4. Effects of Incorporating Temperature Tolerance Data on the SDMs
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Travis, J. Climate change and habitat destruction: A deadly anthropogenic cocktail. Proc. R. Soc. B 2003, 270, 467–473. [Google Scholar] [CrossRef]
- Elith, J.; Graham, C.H. Do they? How do they? WHY do they differ? On finding reasons for differing performances of species distribution models. Ecography 2009, 32, 66–77. [Google Scholar] [CrossRef]
- Rodríguez, L.; García, J.J.; Carreño, F.; Martínez, B. Integration of physiological knowledge into hybrid species distribution modelling to improve forecast of distributional shifts of tropical corals. Divers. Distrib. 2019, 25, 715–728. [Google Scholar] [CrossRef]
- Gamliel, I.; Buba, Y.; Guy-Haim, T.; Garval, T.; Willette, D.; Rilov, G.; Belmaker, J. Incorporating physiology into species distribution models moderates the projected impact of warming on selected Mediterranean marine species. Ecography 2020, 43, 1090–1106. [Google Scholar] [CrossRef]
- Sunday, J.M.; Bates, A.E.; Kearney, M.R.; Colwell, R.K.; Dulvy, N.K.; Longino, J.T.; Huey, R.B. Thermal-safety margins and the necessity of thermoregulatory behavior across latitude and elevation. Proc. Natl. Acad. Sci. USA 2014, 111, 5610–5615. [Google Scholar] [CrossRef]
- Gangloff, E.J.; Telemeco, R.S. High temperature, oxygen, and performance: Insights from reptiles and amphibians. Integr. Comp. Biol. 2018, 58, 9–24. [Google Scholar] [CrossRef] [PubMed]
- Niu, Z.; Li, M.; Pu, P.; Wang, H.; Zhang, T.; Tang, X.; Chen, Q. Effects of temperature on the locomotor performance and contraction properties of skeletal muscle from two Phrynocephalus lizards at high and low altitude. J. Comp. Physiol. B 2021, 191, 907–916. [Google Scholar] [CrossRef]
- Irwin, J.T.; Lee, R.E., Jr. Cold winter microenvironments conserve energy and improve overwintering survival and potential fecundity of the goldenrod gall fly, Eurosta solidaginis. Oikos 2003, 100, 71–78. [Google Scholar] [CrossRef]
- Hahn, D.A.; Denlinger, D.L. Energetics of insect diapause. Annu. Rev. Entomol. 2011, 56, 103–121. [Google Scholar] [CrossRef]
- Du, W.G.; Shine, R. The behavioural and physiological strategies of bird and reptile embryos in response to unpredictable variation in nest temperature. Biol. Rev. 2015, 90, 19–30. [Google Scholar] [CrossRef] [PubMed]
- Noble, D.W.; Stenhouse, V.; Schwanz, L.E. Developmental temperatures and phenotypic plasticity in reptiles: A systematic review and meta-analysis. Biol. Rev. 2018, 93, 72–97. [Google Scholar] [CrossRef] [PubMed]
- Logan, M.L.; Huynh, R.K.; Precious, R.A.; Calsbeek, R.G. The impact of climate change measured at relevant spatial scales: New hope for tropical lizards. Glob. Change Biol. 2013, 19, 3093–3102. [Google Scholar] [CrossRef] [PubMed]
- Kubisch, E.L.; Fernández, J.B.; Ibargüengoytía, N.R. Vulnerability to climate warming of Liolaemus pictus (Squamata, Liolaemidae), a lizard from the cold temperate climate in Patagonia, Argentina. J. Comp. Physiol. B 2016, 186, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Valdecantos, S.; Martínez, V.; Lobo, F.; Cruz, F.B. Thermal biology of Liolaemus lizards from the high Andes: Being efficient despite adversity. J. Therm. Biol. 2013, 38, 126–134. [Google Scholar] [CrossRef]
- Freeman, B.G.; Tobias, J.A.; Schluter, D. Behavior influences range limits and patterns of coexistence across an elevational gradient in tropical birds. Ecography 2019, 42, 1832–1840. [Google Scholar] [CrossRef]
- Sinervo, B.; Mendez-De-La-Cruz, F.; Miles, D.B.; Heulin, B.; Bastiaans, E.; Villagrán-Santa Cruz, M.; Lara-Resendiz, R.; Martínez-Méndez, N.; Calderón-Espinosa, M.L.; Meza-Lázaro, R.N.; et al. Erosion of lizard diversity by climate change and altered thermal niches. Science 2010, 328, 894–899. [Google Scholar] [CrossRef]
- Bombi, P.; Calò, F.; Salvi, D. Living Beyond the Edge: Impacts of Climate Change on Rock Lizards at the Niche Margin. Anim. Conserv. 2025, 28, 699–710. [Google Scholar] [CrossRef]
- Ma, L.; Buckley, L.B.; Huey, R.B.; Du, W. A global test of the cold-climate hypothesis for the evolution of viviparity of squamate reptiles. Glob. Ecol. Biogeogr. 2018, 27, 679–689. [Google Scholar] [CrossRef]
- Moore, D.; Stow, A.; Kearney, M.R. Under the weather?—The direct effects of climate warming on a threatened desert lizard are mediated by their activity phase and burrow system. J. Anim. Ecol. 2018, 87, 660–671. [Google Scholar] [CrossRef]
- Yan, C.; Song, M.; Jiang, D.; Ren, J.; Lv, Y.; Chang, J.; Huang, S.; Zaher, H.; Li, J. Genomic evidence reveals intraspecific divergence of the hot-spring snake (Thermophis baileyi), an endangered reptile endemic to the Qinghai-Tibet plateau. Mol. Ecol. 2023, 32, 1335–1350. [Google Scholar] [CrossRef]
- IUCN. Phrynocephalus theobaldi. Available online: https://www.iucnredlist.org/species/170415/123299193 (accessed on 10 October 2025).
- IUCN. Phrynocephalus erythrurus. Available online: https://www.iucnredlist.org/species/107660333/107660347 (accessed on 10 October 2025).
- Jin, Y.; Liu, N. Phylogeography of Phrynocephalus erythrurus from the Qiangtang Plateau of the Tibetan Plateau. Mol. Phylogenet. Evol. 2010, 54, 933–940. [Google Scholar] [CrossRef]
- Warren, D.; Dinnage, R. ENMTools: Analysis of Niche Evolution Using Niche and Distribution Models. R Package Version 1.1.5. Available online: https://CRAN.R-project.org/package=ENMTools (accessed on 10 October 2025).
- R Core Team, R. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 10 October 2025).
- Dayananda, B.; Murray, B.R.; Webb, J.K. Hotter nests produce hatchling lizards with lower thermal tolerance. J. Exp. Biol. 2017, 220, 2159–2165. [Google Scholar] [CrossRef]
- Zhao, E.; Zhao, K.; Zhou, K. Fauna Sinica, Reptilia, Volume 2, Squamata, Lacertilia; Science Press: Beijing, China, 1999. [Google Scholar]
- Wang, Z.; Ma, L.; Shao, M.; Ji, X. Are viviparous lizards more vulnerable to climate warming because they have evolved reduced body temperature and heat tolerance? Oecologia 2017, 185, 573–582. [Google Scholar] [CrossRef]
- Li, X.; Wang, Y.; Li, M.; Men, S.; Pu, P.; Tang, X.; Chen, Q. Comparison of cold hardiness of two toad-headed lizards from different altitudes. Sichuan J. Zool. 2017, 36, 300–305. [Google Scholar]
- Sun, B.; Ma, L.; Wang, Y.; Mi, C.; Buckley, L.B.; Levy, O.; Lu, H.; Du, W. Latitudinal embryonic thermal tolerance and plasticity shape the vulnerability of oviparous species to climate change. Ecol. Monogr. 2021, 91, e01468. [Google Scholar] [CrossRef]
- Thuiller, W.; Georges, D.; Gueguen, M.; Engler, R.; Breiner, F.; Lafourcade, B.; Patin, R.; Blancheteau, H. biomod2: Ensemble Platform for Species Distribution Modeling. R Package Version 4.2-6-2. Available online: https://CRAN.R-project.org/package=biomod2 (accessed on 10 October 2025).
- Barbet-Massin, M.; Jiguet, F.; Albert, C.H.; Thuiller, W. Selecting pseudo-absences for species distribution models: How, where and how many? Methods Ecol. Evol. 2012, 3, 327–338. [Google Scholar] [CrossRef]
- Fielding, A.H.; Bell, J.F. A review of methods for the assessment of prediction errors in conservation presence/absence models. Environ. Conserv. 1997, 24, 38–49. [Google Scholar] [CrossRef]
- Allouche, O.; Tsoar, A.; Kadmon, R. Assessing the accuracy of species distribution models: Prevalence, kappa and the true skill statistic (TSS). J. Appl. Ecol. 2006, 43, 1223–1232. [Google Scholar] [CrossRef]
- Hutchison, V.H. Factors influencing thermal tolerances of individual organisms. In Thermal Ecology, Vol. II; Esch, G.W., McFarlane, R.M., Eds.; National Technical Information Service; Springfield: Augusta, GA, USA, 1976; pp. 10–26. [Google Scholar]
- Shu, L.; Zhang, Q.; Qu, Y.; Ji, X. Thermal tolerance, selected body temperature and thermal dependence of food assimilation and locomotor performance in the Qinghai toad headed lizard, Phrynocephalus vlangalii. Acta Ecol. Sin. 2010, 30, 2036–2042. [Google Scholar]
- Luo, L.; Qu, Y.; Ji, X. Thermal dependence of food assimilation and sprint speed in a lacertid lizard (Eremias argus) from northern China. Acta Zool. Sin. 2006, 52, 256–262. [Google Scholar]
- Zhang, Y.; Ji, X. The thermal dependence of food assimilation and locomotor performance in southern grass lizards, Takydromus sexlineatus (Lacertidae). J. Therm. Biol. 2004, 29, 45–53. [Google Scholar] [CrossRef]
- Ji, X.; Du, W.; Sun, P. Body temperature, thermal tolerance and influence of temperature on sprint speed and food assimilation in adult grass lizards, Takydromus septentrionalis. J. Therm. Biol. 1996, 21, 155–161. [Google Scholar] [CrossRef]
- Jia, X.; Lee, H.F.; Cui, M.; Liu, C.; Zeng, L.; Yue, R.P.; Zhao, Y.; Lu, H. Habitat variability and ethnic diversity in Northern Tibetan Plateau. Sci. Rep. 2017, 7, 918. [Google Scholar] [CrossRef]
- Díaz de la Vega-Pérez, A.H.; Barrios-Montiel, R.; Jiménez-Arcos, V.H.; Bautista, A.; Bastiaans, E. High-mountain altitudinal gradient influences thermal ecology of the Mesquite Lizard (Sceloporus grammicus). Can. J. Zool. 2019, 97, 659–668. [Google Scholar] [CrossRef]
- Li, S.; Hao, X.; Wang, Y.; Sun, B.; Bi, J.; Zhang, Y.; Janzen, F.J.; Du, W. Female lizards choose warm, moist nests that improve embryonic survivorship and offspring fitness. Funct. Ecol. 2018, 32, 416–423. [Google Scholar] [CrossRef]
- Doody, J.S.; Guarino, E.; Georges, A.; Corey, B.; Murray, G.; Ewert, M. Nest site choice compensates for climate effects on sex ratios in a lizard with environmental sex determination. Evol. Ecol. 2006, 20, 307–330. [Google Scholar] [CrossRef]
- Üveges, B.; Mahr, K.; Szederkényi, M.; Bókony, V.; Hoi, H.; Hettyey, A. Experimental evidence for beneficial effects of projected climate change on hibernating amphibians. Sci. Rep. 2016, 6, 26754. [Google Scholar] [CrossRef] [PubMed]
- Maussion, F.; Scherer, D.; Mölg, T.; Collier, E.; Curio, J.; Finkelnburg, R. Precipitation seasonality and variability over the Tibetan Plateau as resolved by the High Asia Reanalysis. J. Clim. 2014, 27, 1910–1927. [Google Scholar] [CrossRef]
- Chen, K.; Ma, L.; Jiang, W.; Wang, L.; Wei, L.; Zhang, H.; Yang, R. Anthropogenic Disturbance and Climate Change Impacts on the Suitable Habitat of Sphenomorphus incognitus in China. Ecol. Evol. 2025, 15, e70848. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Q.; Cui, X.; Peng, J.; Zhou, S.; Wang, F.; Zhong, L.; Wang, X.; Zheng, H.; Yang, C.; et al. Prediction of Potential Suitable Habitats for Elaphodus cephalophus in China Under Climate Change Scenarios. Ecol. Evol. 2025, 15, e72194. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, L.; Wu, Q.; Hu, C.; Qu, Y.; Ji, X. Geological and climatic influences on population differentiation of the Phrynocephalus vlangalii species complex (Sauria: Agamidae) in the northern Qinghai-Tibet Plateau. Mol. Phylogenet. Evol. 2022, 169, 107394. [Google Scholar] [CrossRef]
- Xu, R.; Song, Q.; Chen, D.; Guo, X. Lineage Diversification and Population Dynamics of the Qinghai Toad-Headed Agama (Phrynocephalus vlangalii) on the Qinghai-Tibet Plateau, with Particular Attention to the Northern Slope of the Kunlun-Arjin Mountains. Animals 2025, 15, 400. [Google Scholar] [CrossRef]
- Moore, I.D.; Grayson, R.B.; Ladson, A.R. Digital terrain modelling: A review of hydrological, geomorphological, and biological applications. Hydrol. Process. 1991, 5, 3–30. [Google Scholar] [CrossRef]
- Stanton, J.C.; Pearson, R.G.; Horning, N.; Ersts, P.; Reşit Akçakaya, H. Combining static and dynamic variables in species distribution models under climate change. Methods Ecol. Evol. 2012, 3, 349–357. [Google Scholar] [CrossRef]
- Byrne, M.; Gall, M.; Wolfe, K.; Agüera, A. From pole to pole: The potential for the Arctic seastar Asterias amurensis to invade a warming Southern Ocean. Glob. Change Biol. 2016, 22, 3874–3887. [Google Scholar] [CrossRef] [PubMed]
- Coulin, C.; de la Vega, G.J.; Chifflet, L.; Calcaterra, L.A.; Schilman, P.E. Linking thermo-tolerances of the highly invasive ant, Wasmannia auropunctata, to its current and potential distribution. Biol. Invasions 2019, 21, 3491–3504. [Google Scholar] [CrossRef]
- Laeseke, P.; Martínez, B.; Mansilla, A.; Bischof, K. Future range dynamics of the red alga Capreolia implexa in native and invaded regions: Contrasting predictions from species distribution models versus physiological knowledge. Biol. Invasions 2020, 22, 1339–1352. [Google Scholar] [CrossRef]
- Gong, S.; Gao, Y.; Duan, H.; Ge, Y.; Wei, Y. Incorporating physiological data into species distribution models to predict the potential distribution range of the red-eared slider in China. Ecol. Indic. 2023, 154, 110749. [Google Scholar] [CrossRef]
- Gong, S.; Yang, J.; Ge, Y.; Gaillard, D. Extent and Mechanisms of the Increasing Geographic Distribution of the Alien Red-Eared Slider (Trachemys scripta elegans) in China. Chin. J. Wildl. 2018, 39, 373–378. [Google Scholar]
- Zhao, M.; Cao, G.; Cao, S.; Liu, F.; Yuan, J.; Zhang, Z.; Diao, E.; Fu, J. Spatial-temporal variation characteristics of land surface temperature in Qinghai Province from 1980 to 2017. Arid. Zone. Res. 2021, 38, 178–187. [Google Scholar]
- Cong, J.; Tao, Z.; Xu, Y.; Li, X. From thermal tolerance to distribution resilience: Physiology-informed species distribution models moderated range shifts for an intertidal crab species under climate change. Glob. Ecol. Conserv. 2025, 62, e03809. [Google Scholar] [CrossRef]
- Jiang, Z.; Ma, L.; Mi, C.; Du, W. Effects of hypoxia on the thermal physiology of a high-elevation lizard: Implications for upslope-shifting species. Biol. Lett. 2021, 17, 20200873. [Google Scholar] [CrossRef] [PubMed]
- Tao, X.; Xie, F.; Zhu, L.; Wu, Q. Correlation of Morphology and Metabolism of Reproductive Traits in the Genus Phrynocephalus Around the Qinghai-Tibetan Plateau. Ecol. Evol. 2025, 15, e72029. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Z.; Du, W.; Zhang, C.; Yu, W.; Zhao, X.; Liu, Z.; Zeng, Z. Altitudinal variation in thermal vulnerability of Qinghai-Tibetan Plateau lizards under climate warming. Curr. Zool. 2025, 71, 99–108. [Google Scholar] [CrossRef] [PubMed]

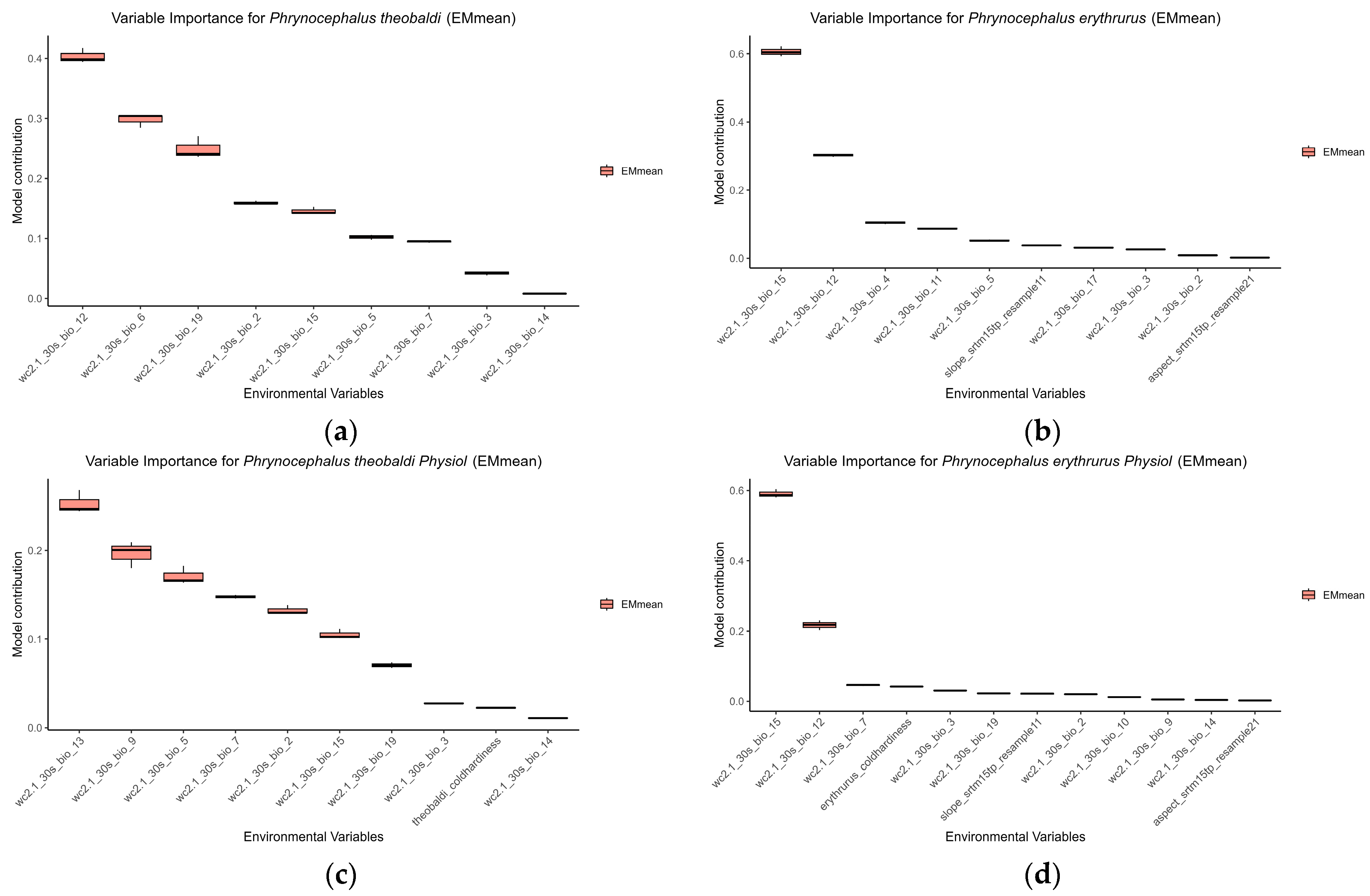
| Types | Variables | Description | Phrynocephalus theobald | Phrynocephalus erythrurus | ||
|---|---|---|---|---|---|---|
| Without CTmin Data | Incorporating CTmin Data | Without CTmin Data | Incorporating CTmin Data | |||
| Climatic variables | Bio1 | Annual Mean Temperature | ||||
| Bio2 | Mean Diurnal Range (Mean of monthly (max temp–min temp)) | ✓ | ✓ | ✓ | ✓ | |
| Bio3 | Isothermality (Bio2/Bio7) (×100) | ✓ | ✓ | ✓ | ✓ | |
| Bio4 | Temperature Seasonality (standard deviation × 100) | ✓ | ||||
| Bio5 | Max Temperature of Warmest Month | ✓ | ✓ | ✓ | ||
| Bio6 | Min Temperature of Coldest Month | ✓ | ||||
| Bio7 | Temperature Annual Range (Bio5–Bio6) | ✓ | ✓ | ✓ | ||
| Bio8 | Mean Temperature of Wettest Quarter | |||||
| Bio9 | Mean Temperature of Driest Quarter | ✓ | ✓ | |||
| Bio10 | Mean Temperature of Warmest Quarter | ✓ | ||||
| Bio11 | Mean Temperature of Coldest Quarter | ✓ | ||||
| Bio12 | Annual Precipitation | ✓ | ✓ | ✓ | ||
| Bio13 | Precipitation of Wettest Month | ✓ | ||||
| Bio14 | Precipitation of Driest Month | ✓ | ✓ | ✓ | ||
| Bio15 | Precipitation Seasonality (Coefficient of Variation) | ✓ | ✓ | ✓ | ✓ | |
| Bio16 | Precipitation of Wettest Quarter | |||||
| Bio17 | Precipitation of Driest Quarter | ✓ | ||||
| Bio18 | Precipitation of Warmest Quarter | |||||
| Bio19 | Precipitation of Coldest Quarter | ✓ | ✓ | ✓ | ||
| Geographical factors | Altitude | Digital elevation model | ||||
| Slope | Derived from DEM | ✓ | ✓ | |||
| Aspect | Derived from DEM | ✓ | ✓ | |||
| Physiological factors | Cold stress frequency | total number of cold stress days during the overwintering period | ✓ | ✓ | ||
| Population | N | Snout-Vent Length/mm | Critical Thermal Minimum/°C | Range |
|---|---|---|---|---|
| Gar | 12 | 43.53 ± 1.58 | 0.9 ± 0.2 | 0.5–1.2 |
| Zhongba | 12 | 49.10 ± 3.62 | 0.8 ± 0.1 | 0.6–1.0 |
| Species | Phrynocephalus theobaldi | Phrynocephalus erythrurus | |||||
|---|---|---|---|---|---|---|---|
| Model | KAPPA | AUC | TSS | KAPPA | AUC | TSS | |
| Traditional SDMs | CTA | 0.530 ± 0.155 | 0.795 ± 0.086 | 0.557 ± 0.159 | 0.688 ± 0.147 | 0.904 ± 0.068 | 0.799 ± 0.144 |
| GAM | 0.692 ± 0.125 | 0.924 ± 0.047 | 0.715 ± 0.114 | 0.566 ± 0.189 | 0.895 ± 0.073 | 0.650 ± 0.198 | |
| GBM | 0.669 ± 0.117 | 0.921 ± 0.043 | 0.681 ± 0.123 | 0.633 ± 0.198 | 0.954 ± 0.048 | 0.629 ± 0.217 | |
| GLM | 0.683 ± 0.130 | 0.893 ± 0.066 | 0.695 ± 0.130 | 0.533 ± 0.193 | 0.805 ± 0.109 | 0.600 ± 0.216 | |
| MARS | 0.704 ± 0.129 | 0.932 ± 0.054 | 0.708 ± 0.122 | 0.623 ± 0.196 | 0.938 ± 0.054 | 0.691 ± 0.199 | |
| MAXENT | 0.533 ± 0.161 | 0.765 ± 0.084 | 0.530 ± 0.167 | 0.587 ± 0.177 | 0.836 ± 0.097 | 0.672 ± 0.193 | |
| RF | 0.697 ± 0.122 | 0.936 ± 0.039 | 0.687 ± 0.130 | 0.615 ± 0.244 | 0.956 ± 0.040 | 0.575 ± 0.260 | |
| XGBOOST | 0.605 ± 0.165 | 0.877 ± 0.069 | 0.600 ± 0.169 | 0.629 ± 0.205 | 0.923 ± 0.085 | 0.622 ± 0.218 | |
| EM * | 0.673 | 0.978 | 0.877 | 0.706 | 0.992 | 0.944 | |
| SDMs With CTmin data | CTA | 0.662 ± 0.159 | 0.870 ± 0.084 | 0.697 ± 0.152 | 0.707 ± 0.135 | 0.902 ± 0.061 | 0.803 ± 0.121 |
| GAM | 0.705 ± 0.114 | 0.934 ± 0.038 | 0.720 ± 0.117 | 0.617 ± 0.195 | 0.916 ± 0.078 | 0.674 ± 0.217 | |
| GBM | 0.763 ± 0.113 | 0.948 ± 0.041 | 0.769 ± 0.115 | 0.707 ± 0.168 | 0.964 ± 0.034 | 0.722 ± 0.182 | |
| GLM | 0.681 ± 0.139 | 0.885 ± 0.073 | 0.694 ± 0.139 | 0.532 ± 0.202 | 0.814 ± 0.109 | 0.616 ± 0.225 | |
| MARS | 0.737 ± 0.122 | 0.932 ± 0.049 | 0.749 ± 0.121 | 0.654 ± 0.181 | 0.942 ± 0.046 | 0.727 ± 0.183 | |
| MAXENT | 0.603 ± 0.140 | 0.801 ± 0.074 | 0.602 ± 0.148 | 0.512 ± 0.188 | 0.798 ± 0.102 | 0.595 ± 0.203 | |
| RF | 0.757 ± 0.102 | 0.954 ± 0.027 | 0.757 ± 0.107 | 0.694 ± 0.200 | 0.969 ± 0.032 | 0.657 ± 0.220 | |
| XGBOOST | 0.656 ± 0.144 | 0.904 ± 0.056 | 0.652 ± 0.145 | 0.686 ± 0.181 | 0.925 ± 0.072 | 0.685 ± 0.199 | |
| EM * | 0.705 | 0.982 | 0.904 | 0.674 | 0.989 | 0.965 | |
| Species | Phrynocephalus theobaldi | Phrynocephalus erythrurus | ||
|---|---|---|---|---|
| Model | Traditional SDMs | SDMs with CTmin Data | Traditional SDMs | SDMs with CTmin Data |
| CTA | 18 | 54 | 72 | 67 |
| GAM | 59 | 56 | 37 | 34 |
| GBM | 44 | 68 | 38 | 53 |
| GLM | 49 | 50 | 26 | 30 |
| MARS | 48 | 66 | 40 | 45 |
| MAXENT | 15 | 21 | 36 | 23 |
| RF | 40 | 69 | 34 | 43 |
| XGBOOST | 27 | 36 | 40 | 47 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tao, X.; Li, Y.; Li, J. The Effects of Cold Tolerance on the Distribution of Two Extreme Altitude Lizard Species in the Qinghai–Tibetan Plateau. Animals 2025, 15, 3297. https://doi.org/10.3390/ani15223297
Tao X, Li Y, Li J. The Effects of Cold Tolerance on the Distribution of Two Extreme Altitude Lizard Species in the Qinghai–Tibetan Plateau. Animals. 2025; 15(22):3297. https://doi.org/10.3390/ani15223297
Chicago/Turabian StyleTao, Xiaqiu, Yiyi Li, and Jiasheng Li. 2025. "The Effects of Cold Tolerance on the Distribution of Two Extreme Altitude Lizard Species in the Qinghai–Tibetan Plateau" Animals 15, no. 22: 3297. https://doi.org/10.3390/ani15223297
APA StyleTao, X., Li, Y., & Li, J. (2025). The Effects of Cold Tolerance on the Distribution of Two Extreme Altitude Lizard Species in the Qinghai–Tibetan Plateau. Animals, 15(22), 3297. https://doi.org/10.3390/ani15223297








