Sperm Cell Membranes of Bulls and Bucks Associated with Sperm Fertility and Freezability
Simple Summary
Abstract
1. Introduction
2. Sperm Cell Membranes and Fertility in Mammals
2.1. Lipid Composition and Membrane Dynamics
2.2. Membrane Proteins
3. Sperm Cell Membrane and Freezability of Mammalian Sperm
3.1. Cryoprotectant Mechanisms
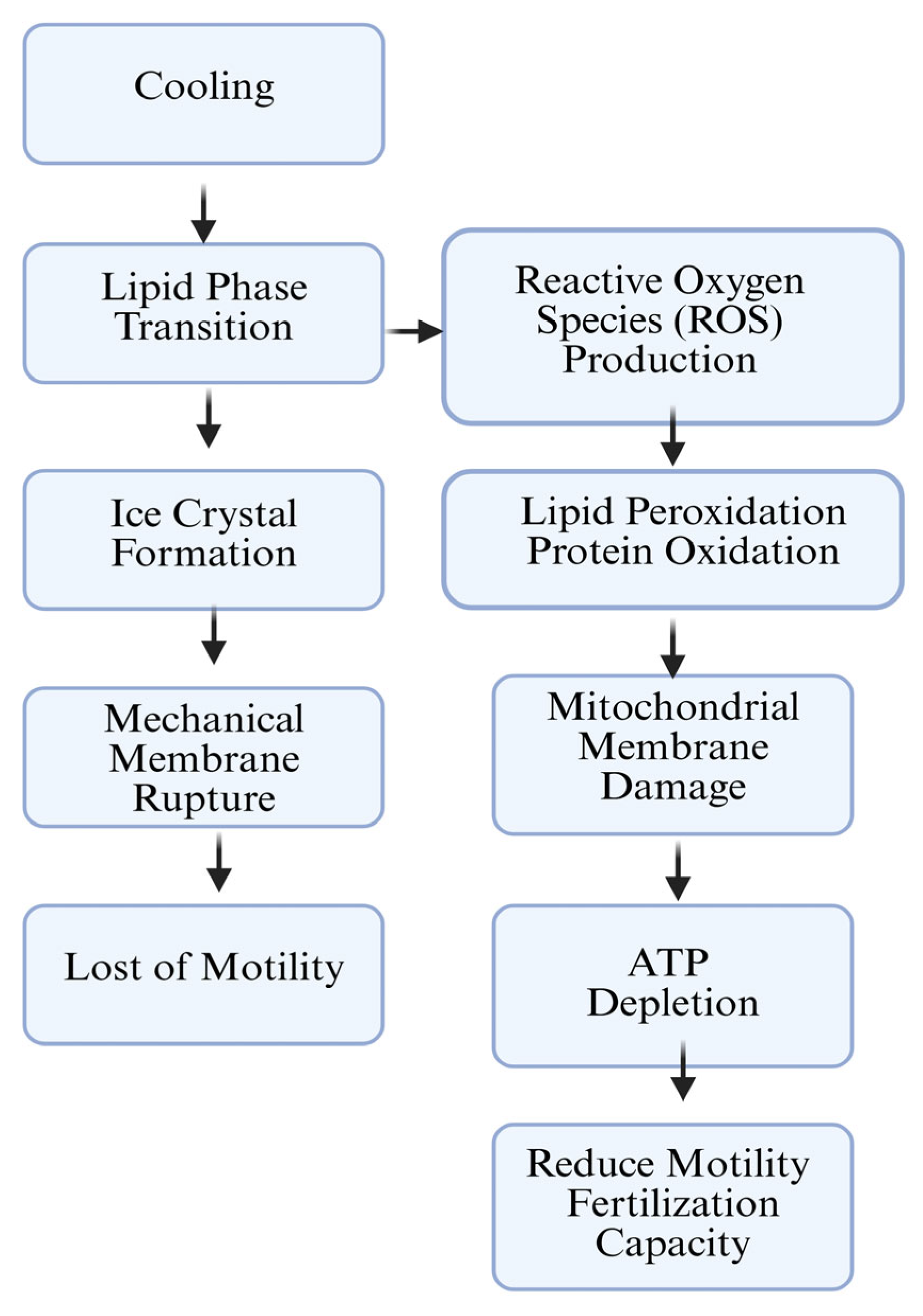
3.2. Freeze–Thaw Damage and ROS Effect
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hotea, I.; Dragomirescu, M.; Berbecea, A.; Radulov, I. The Role of Nutrition in Enhancing Sustainability in Sheep Production. In Sheep Farming—Sustainability from Traditional to Precision Production; IntechOpen: London, UK, 2023. [Google Scholar] [CrossRef]
- Herrero, M.; Grace, D.; Njuki, J.; Johnson, N.; Enahoro, D.; Silvestri, S.; Rufino, M.C. The roles of livestock in developing countries. Animal 2013, 7, 3–18. [Google Scholar] [CrossRef]
- Tanga, B.M.; Qamar, A.Y.; Raza, S.; Bang, S.; Fang, X.; Yoon, K.; Cho, J. Semen evaluation: Methodological advancements in sperm quality-specific fertility assessment—A review. Anim. Biosci. 2021, 34, 1253–1270. [Google Scholar] [CrossRef]
- Bodu, M.; Hitit, M.; Memili, E. Harnessing the value of fertility biomarkers in bull sperm for buck sperm. Anim. Reprod. Sci. 2025, 272, 107643. [Google Scholar] [CrossRef]
- Waberski, D.; Suarez, S.S.; Henning, H. Assessment of sperm motility in livestock: Perspectives based on sperm swimming conditions in vivo. Anim. Reprod. Sci. 2022, 246, 106849. [Google Scholar] [CrossRef]
- Carvalho, F.E.; Ferraz, J.B.S.; Pedrosa, V.B.; Matos, E.C.; Eler, J.P.; Silva, M.R.; Guimarães, J.D.; Bussiman, F.O.; Silva, B.C.A.; Cançado, F.A.; et al. Genetic parameters for various semen production and quality traits and indicators of male and female reproductive performance in Nellore cattle. BMC Genom. 2023, 24, 150. [Google Scholar] [CrossRef]
- Khan, M.Z.; Chen, W.; Naz, S.; Liu, X.; Liang, H.; Chen, Y.; Kou, X.; Liu, Y.; Ashraf, I.; Han, Y.; et al. Determinant genetic markers of semen quality in livestock. Front. Endocrinol. 2024, 15, 1456305. [Google Scholar] [CrossRef]
- Kalwani, D. Management strategies for improving production and reproduction performance of sheep and goat. Int. J. Vet. Sci. Anim. Husb. 2023, 8, 155–160. [Google Scholar] [CrossRef]
- Salisbury, G.; VanDemark, N. Physiology of Reproduction and Artificial İnsemination of Cattle; W. H. Freeman & Company: San Francisco, CA, USA; London, UK, 1961; pp. xii + 639. [Google Scholar]
- Zammit, S. Physical Activity and Semen Quality Parameters in Adults. Bachelor’s Thesis, University of Malta, Msida, Malta, 2022. [Google Scholar]
- Barth, A.D.; Brito, L.F.C.; Kastelic, J.P. The effect of nutrition on sexual development of bulls. Theriogenology 2008, 70, 485–494. [Google Scholar] [CrossRef]
- Dayoub, M.; Shnaigat, S.; Tarawneh, R.A.; Al-Yacoub, A.N.; Al-Barakeh, F.; Al-Najjar, K. Enhancing Animal Production through Smart Agriculture: Possibilities, Hurdles, Resolutions, and Advantages. Ruminants 2024, 4, 22–46. [Google Scholar] [CrossRef]
- Dyce, K.M.; Sack, W.O.; Wensing, C.J.G. Textbook of Veterinary Anatomy-E-Book; Elsevier Health Sciences: St. Louis, MO, USA, 2009; p. 63043. [Google Scholar]
- Fawcett, D.W. The Structure of the Mammalian Spermatozoon. In International Review of Cytology; Bourne, G.H., Danielli, J.F., Eds.; Academic Press: New York, NY, USA, 1958; Volume 7, pp. 195–234. [Google Scholar] [CrossRef]
- Leung, E.T.Y.; Lee, B.K.M.; Lee, C.-L.; Tian, X.; Lam, K.K.W.; Li, R.H.W.; Ng, E.H.Y.; Yeung, W.S.B.; Ou, J.-P.; Chiu, P.C.N. The role of spermatozoa-zona pellucida interaction in selecting fertilization-competent spermatozoa in humans. Front. Endocrinol. 2023, 14, 1135973. [Google Scholar] [CrossRef]
- Skowronek, M.F.; Pietroroia, S.; de Cola, G.; Ramos, M.; Silvera, D.; Casanova, G.; Lecumberry, F.; Cassina, A.; Sapiro, R. Mitochondrial morphology in fertile and infertile men: Image processing and morphometric analysis of the sperm midpiece. Front. Cell Dev. Biol. 2025, 13, 1609081. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.; Braga, P.C.; Rebelo, I.; Oliveira, P.F.; Alves, M.G. Mitochondria Quality Control and Male Fertility. Biology 2023, 12, 827. [Google Scholar] [CrossRef]
- Amaral, A.; Lourenço, B.; Marques, M.; Ramalho-Santos, J. Mitochondria functionality and sperm quality. Reproduction 2013, 146, R163–R174. [Google Scholar] [CrossRef]
- Ruiz-Pesini, E.; Wallace, D.C. Evidence for adaptive selection acting on the tRNA and rRNA genes of human mitochondrial DNA. Hum. Mutat. 2006, 27, 1072–1081. [Google Scholar] [CrossRef]
- Litscher, E.S.; Wassarman, P.M. Chapter Seven—The mammalian egg’s zona pellucida, fertilization, and fertility. In Current Topics in Developmental Biology; Wassarman, P.M., Litscher, E.S., Eds.; Academic Press: Cambridge, MA, USA, 2025; Volume 162, pp. 207–258. [Google Scholar] [CrossRef]
- Hopper, R.M. Bovine Reproduction; John Wiley & Sons: Hoboken, NJ, USA, 2021. [Google Scholar]
- Lindemann, C.B.; Lesich, K.A. Flagellar and ciliary beating: The proven and the possible. J. Cell Sci. 2010, 123, 519–528. [Google Scholar] [CrossRef]
- Beer-Ljubić, B.; Aladrović, J.; Marenjak, T.S.; Laškaj, R.; Majić-Balić, I.; Milinković-Tur, S. Cholesterol concentration in seminal plasma as a predictive tool for quality semen evaluation. Theriogenology 2009, 72, 1132–1140. [Google Scholar] [CrossRef]
- Yániz, J.L.; Capistrós, S.; Vicente-Fiel, S.; Hidalgo, C.O.; Santolaria, P. A comparative study of the morphometry of sperm head components in cattle, sheep, and pigs with a computer-assisted fluorescence method. Asian J. Androl. 2016, 18, 840–843. [Google Scholar] [CrossRef]
- Gulaya, N.M.; Margitich, V.M.; Govseeva, N.M.; Klimashevsky, V.M.; Gorpynchenko, I.I.; Boyko, M.I. Phospholipid composition of human sperm and seminal plasma in relation to sperm fertility. Arch. Androl. 2001, 46, 169–175. [Google Scholar] [CrossRef]
- Bodu, M.; Hitit, M.; Woldesenbet, S.; Uğur, M.R.; Erdoğan, Z.; Greenwood, O.C.; Murray, R.D.; Cervantes, A.P.; Memili, E. Lipidomic Landscapes of Cryopreserved Sperm from Alpine and Spanish–Creole Bucks. Animals 2025, 15, 1897. [Google Scholar] [CrossRef]
- Gahlay, G.K.; Rajput, N. The enigmatic sperm proteins in mammalian fertilization: An overview. Biol. Reprod. 2020, 103, 1171–1185. [Google Scholar] [CrossRef]
- Medeiros, C.M.O.; Forell, F.; Oliveira, A.T.D.; Rodrigues, J.L. Current status of sperm cryopreservation: Why isn’t it better? Theriogenology 2002, 57, 327–344. [Google Scholar] [CrossRef]
- Hernández-Falcó, M.; Sáez-Espinosa, P.; López-Botella, A.; Aizpurua, J.; Gómez-Torres, M.J. The Role of Sperm Proteins IZUMO1 and TMEM95 in Mammalian Fertilization: A Systematic Review. Int. J. Mol. Sci. 2022, 23, 3929. [Google Scholar] [CrossRef] [PubMed]
- Satouh, Y.; Inoue, N.; Ikawa, M.; Okabe, M. Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1. J. Cell Sci. 2012, 125, 4985–4990. [Google Scholar] [CrossRef]
- Holt, W. Fundamental aspects of sperm cryobiology: The importance of species and individual differences. Theriogenology 2000, 53, 47–58. [Google Scholar] [CrossRef] [PubMed]
- Sugkraroek, P.; Kates, M.; Leader, A.; Tanphaichitr, N. Levels of cholesterol and phospholipids in freshly ejaculated sperm and Percoll-gradient-pelletted sperm from fertile and unexplained infertile men. Fertil. Steril. 1991, 55, 820–827. [Google Scholar] [CrossRef]
- Khodadadi, E.; Moradi, M. Cholesterol concentration effect on bilayer membranes and its role in designing efficient liposomal drug delivery systems. Biophys. J. 2023, 122, 365a. [Google Scholar] [CrossRef]
- Krause, M.R.; Regen, S.L. The Structural Role of Cholesterol in Cell Membranes: From Condensed Bilayers to Lipid Rafts. Acc. Chem. Res. 2014, 47, 3512–3521. [Google Scholar] [CrossRef]
- Nsairat, H.; Ibrahim, A.A.; Jaber, A.M.; Abdelghany, S.; Atwan, R.; Shalan, N.; Abdelnabi, H.; Odeh, F.; El-Tanani, M.; Alshaer, W. Liposome bilayer stability: Emphasis on cholesterol and its alternatives. J. Liposome Res. 2024, 34, 178–202. [Google Scholar] [CrossRef]
- Ji, R.; Chiozzi, R.Z.; van den Toorn, H.; Leung, M.; Zeev-Ben-Mordehai, T.; Burke, N.D.; Bromfield, E.G.; Reiding, K.R.; Heck, A.J.R. Spatial Organization of the Sperm Cell Glycoproteome. Mol. Cell. Proteom. 2025, 24, 100893. [Google Scholar] [CrossRef]
- Bravo, A.; Sánchez, R.; Zambrano, F.; Uribe, P. Exogenous Oxidative Stress in Human Spermatozoa Induces Opening of the Mitochondrial Permeability Transition Pore: Effect on Mitochondrial Function, Sperm Motility and Induction of Cell Death. Antioxidants 2024, 13, 739. [Google Scholar] [CrossRef]
- Rotimi, D.E.; Iyobhebhe, M.; Oluwayemi, E.T.; Olajide, O.P.; Akinsanola, B.A.; Evbuomwan, I.O.; Asaleye, R.M.; Ojo, O.A. Energy metabolism and spermatogenesis. Heliyon 2024, 10, e38591. [Google Scholar] [CrossRef]
- Sugita, H.; Takarabe, S.; Kageyama, A.; Kawata, Y.; Ito, J. Molecular Mechanism of Oocyte Activation in Mammals: Past, Present, and Future Directions. Biomolecules 2024, 14, 359. [Google Scholar] [CrossRef]
- Akgün, N.; Cimşit Kemahlı, M.N.; Pradas, J.B. The effect of dietary habits on oocyte/sperm quality. J. Turk. Ger. Gynecol. Assoc. 2023, 24, 125–137. [Google Scholar] [CrossRef]
- Yuan, Y.; Yu, M.; Chen, L.; Ren, X.; Qu, Y.; Shari, A.; Li, G. Comparative analysis of different metabolites in semen of Guanzhong dairy goats with different motility rates. Theriogenology 2023, 210, 53–61. [Google Scholar] [CrossRef]
- Singson, A.; Zannoni, S.; Kadandale, P. Molecules that function in the steps of fertilization. Cytokine Growth Factor. Rev. 2001, 12, 299–304. [Google Scholar] [CrossRef]
- Wei, Y.; Wang, J.; Qu, R.; Zhang, W.; Tan, Y.; Sha, Y.; Li, L.; Yin, T. Genetic mechanisms of fertilization failure and early embryonic arrest: A comprehensive review. Hum. Reprod. Update 2023, 30, 48–80. [Google Scholar] [CrossRef]
- Merc, V.; Frolikova, M.; Komrskova, K. Role of Integrins in Sperm Activation and Fertilization. Int. J. Mol. Sci. 2021, 22, 11809. [Google Scholar] [CrossRef]
- Bodu, M.; Hitit, M.; Donmez, H.; Kaya, A.; Ugur, M.R.; Memili, E. Exploration of Small Non-Coding RNAs as Molecular Markers of Ram Sperm Fertility. Int. J. Mol. Sci. 2025, 26, 2690. [Google Scholar] [CrossRef]
- Argov-Argaman, N.; Mahgrefthe, K.; Zeron, Y.; Roth, Z. Variation in lipid profiles within semen compartments—The bovine model of aging. Theriogenology 2013, 80, 712–721. [Google Scholar] [CrossRef]
- Shan, S.; Xu, F.; Hirschfeld, M.; Brenig, B. Sperm Lipid Markers of Male Fertility in Mammals. Int. J. Mol. Sci. 2021, 22, 8767. [Google Scholar] [CrossRef]
- Bodu, M.; Hitit, M.; Greenwood, O.C.; Murray, R.D.; Memili, E. Extender development for optimal cryopreservation of buck sperm to increase reproductive efficiency of goats. Front. Vet. Sci. 2025, 12, 1554771. [Google Scholar] [CrossRef]
- Gautier, C.; Aurich, C. “Fine feathers make fine birds”—The mammalian sperm plasma membrane lipid composition and effects on assisted reproduction. Anim. Reprod. Sci. 2022, 246, 106884. [Google Scholar] [CrossRef]
- Bustani, G.S.; Baiee, F.H. Semen extenders: An evaluative overview of preservative mechanisms of semen and semen extenders. Vet. World 2021, 14, 1220–1233. [Google Scholar] [CrossRef]
- Ofosu, J.; Nartey, M.A.; Mo, X.; Ye, J.; Zhang, Y.; Zeng, C.; Zhang, M.; Fang, Y.; Zhou, G. Ram sperm cryopreservation disrupts metabolism of unsaturated fatty acids. Theriogenology 2023, 204, 8–17. [Google Scholar] [CrossRef]
- Parks, J.E.; Lynch, D.V. Lipid composition and thermotropic phase behavior of boar, bull, stallion, and rooster sperm membranes. Cryobiology 1992, 29, 255–266. [Google Scholar] [CrossRef]
- Rajoriya, J.S.; Prasad, J.K.; Ramteke, S.S.; Perumal, P.; Ghosh, S.K.; Singh, M.; Pande, M.; Srivastava, N. Enriching membrane cholesterol improves stability and cryosurvival of buffalo spermatozoa. Anim. Reprod. Sci. 2016, 164, 72–81. [Google Scholar] [CrossRef]
- Waterhouse, K.E.; Hofmo, P.O.; Tverdal, A.; Miller, R.R. Within and between breed differences in freezing tolerance and plasma membrane fatty acid composition of boar sperm. Reproduction 2006, 131, 887–894. [Google Scholar] [CrossRef]
- Ozimic, S.; Ban-Frangez, H.; Stimpfel, M. Sperm Cryopreservation Today: Approaches, Efficiency, and Pitfalls. Curr. Issues Mol. Biol. 2023, 45, 4716–4734. [Google Scholar] [CrossRef]
- Mostafa, A.A.; El-Belely, M.S.; Ismail, S.T.; El-Sheshtawy, R.I.; Shahba, M.I. Effect of cholesterol-loaded cyclodextrin enriched extenders on the quality of prefrozen and frozen buffalo semen. Asian Pac. J. Reprod. 2022, 11, 146–152. [Google Scholar] [CrossRef]
- Delgado-Bermúdez, A. Insights into crucial molecules and protein channels involved in pig sperm cryopreservation. Anim. Reprod. Sci. 2024, 269, 107547. [Google Scholar] [CrossRef]
- De Leeuw, F.E.; Chen, H.-C.; Colenbrander, B.; Verkleij, A.J. Cold-induced ultrastructural changes in bull and boar sperm plasma membranes. Cryobiology 1990, 27, 171–183. [Google Scholar] [CrossRef]
- Karabinus, D.S.; Evenson, D.P.; Kaproth, M.T. Effects of Egg Yolk-Citrate and Milk Extenders on Chromatin Structure and Viability of Cryopreserved Bull Sperm. J. Dairy. Sci. 1991, 74, 3836–3848. [Google Scholar] [CrossRef]
- Malo, C.; Crichton, E.G.; Skidmore, J.A. Optimization of the cryopreservation of dromedary camel semen: Cryoprotectants and their concentration and equilibration times. Cryobiology 2017, 74, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Pezo, F.; Contreras, M.J.; Zambrano, F.; Uribe, P.; Risopatron, J.; Andrade, A.F.C.d.; Yeste, M.; Sánchez, R. Thawing of cryopreserved sperm from domestic animals: Impact of temperature, time, and addition of molecules to thawing/insemination medium. Anim. Reprod. Sci. 2024, 268, 107572. [Google Scholar] [CrossRef]
- Khalil, W.A.; El-Deghaidy, R.M.; Sakr, A.M.; Swelum, A.A.; Abdelnour, S.A.; El-Harairy, M.A. Impacts of adding sucrose or trehalose to extenders with different glycerol concentrations on freezablility and fertility of buffalo bull semen. Vet. Res. Commun. 2024, 49, 22. [Google Scholar] [CrossRef]
- Esmeryan, K.D.; Fedchenko, Y.I.; Chaushev, T.A. Probing the impact of commercial cryoprotectants and freezing technique on the motility of human spermatozoa cryopreserved onto extremely water-repellent soot-coated surfaces. Cryobiology 2025, 118, 105195. [Google Scholar] [CrossRef]
- Whaley, D.; Damyar, K.; Witek, R.P.; Mendoza, A.; Alexander, M.; Lakey, J.R. Cryopreservation: An Overview of Principles and Cell-Specific Considerations. Cell Transplant. 2021, 30, 0963689721999617. [Google Scholar] [CrossRef]
- Shah, S.A.H.; Andrabi, S.M.H.; Ahmed, H.; Qureshi, I.Z. Cryoprotection synergism between glycerol and dimethyl sulfoxide improves the mitochondrial transmembrane potential, plasmalemma, acrosomal and DNA integrities, and in vivo fertility of water buffalo (Bubalus bubalis) spermatozoa. Cytotechnology 2016, 68, 2335–2344. [Google Scholar] [CrossRef][Green Version]
- Am-in, N.; Kirkwood, R.N.; Techakumphu, M.; Tantasuparuk, W. Lipid profiles of sperm and seminal plasma from boars having normal or low sperm motility. Theriogenology 2011, 75, 897–903. [Google Scholar] [CrossRef]
- Yánez-Ortiz, I.; Catalán, J.; Rodríguez-Gil, J.E.; Miró, J.; Yeste, M. Advances in sperm cryopreservation in farm animals: Cattle, horse, pig and sheep. Anim. Reprod. Sci. 2022, 246, 106904. [Google Scholar] [CrossRef]
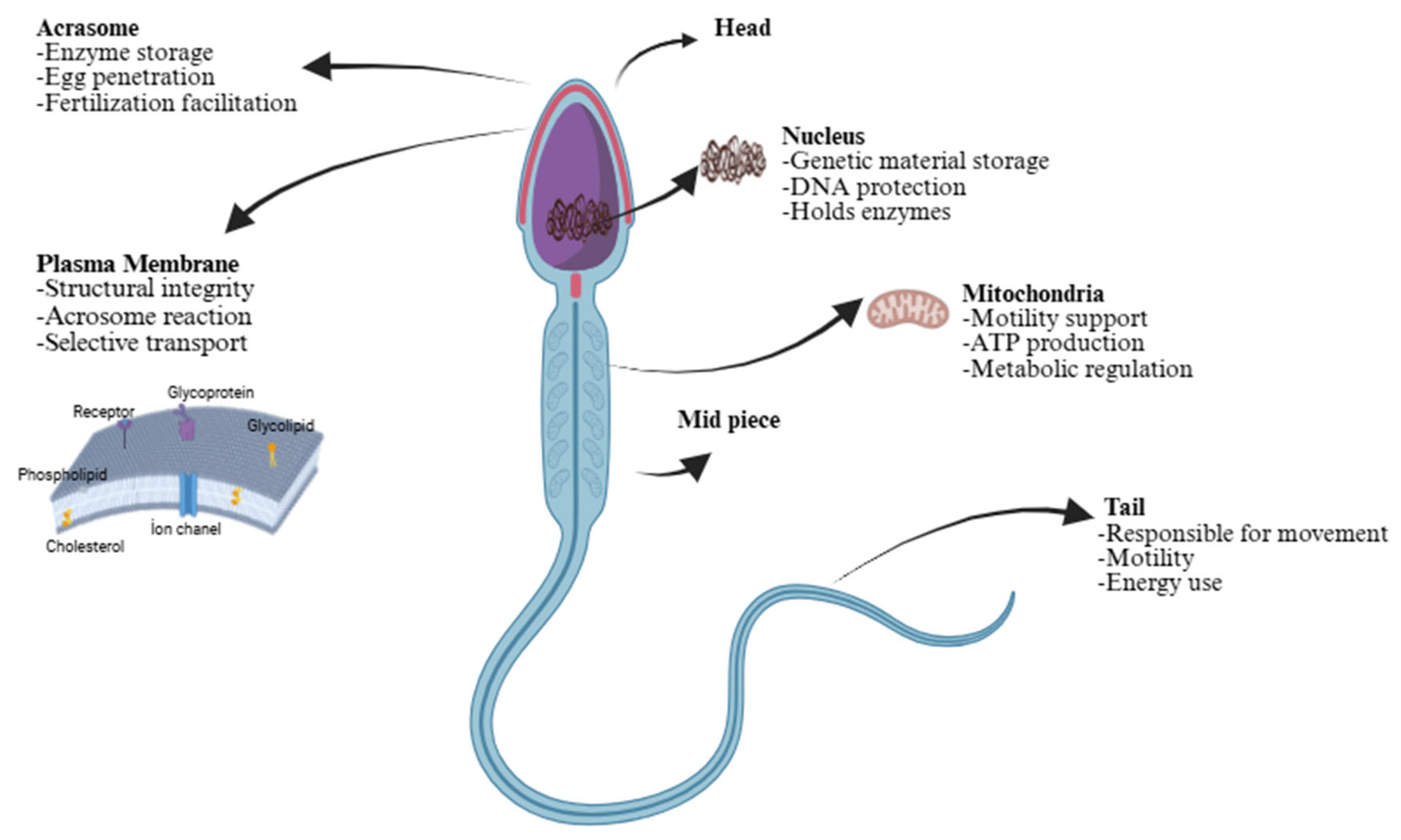
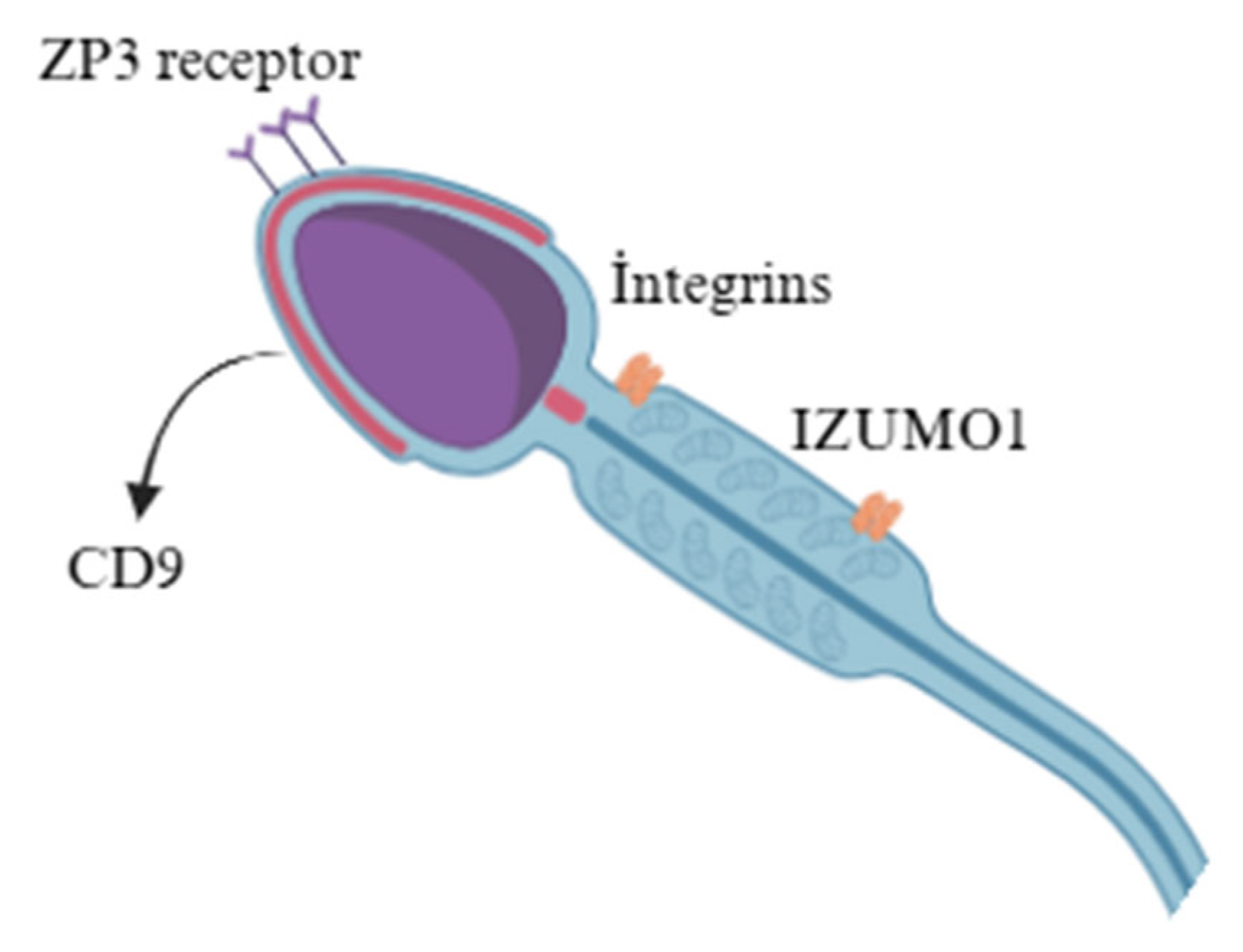
| Component | Bull Sperm Membrane | Buck Sperm Membrane |
|---|---|---|
| Fatty Acids | Predominantly saturated and monounsaturated fatty acids; typically, 60–70% of total lipids. PUFAs represent ~20–25%. | Enriched in PUFAs such as arachidonic acid and docosahexaenoic acid; overall unsaturation index higher than in bulls, contributing to greater membrane fluidity. |
| Proteins | Major proteins include adhesion molecules (integrins, CD9), enzymes (hyaluronidase), and ZP3 receptor proteins. | Similar protein repertoire but relatively higher levels of sperm adhesins, suggesting species-specific modulation of sperm–egg interaction. |
| Cholesterol | Cholesterol–phospholipid ratio ≈ 0.45–0.50; contributes to membrane rigidity and stability. | Cholesterol–phospholipid ratio ≈ 0.30–0.35 (lower than bull), promoting a more fluid membrane and facilitating the acrosome reaction. |
| Glycolipids | Dominated by GM3 gangliosides, associated with membrane stabilization and sperm–egg recognition. | Enriched in GD3 gangliosides, potentially enhancing signal transduction during fertilization. |
| Glycoproteins | Contains ZP3-binding glycoproteins and sperm adhesins, key for zona pellucida binding. | Similar glycoproteins but higher sperm adhesin abundance, possibly improving sperm–egg interaction efficiency. |
| Membrane Phase Transition (Tm) | Estimated Tm ≈ 22–25 °C (reflecting higher saturation and cholesterol). | Estimated Tm ≈ 15–18 °C, indicating greater unsaturation and fluidity; quantitative confirmation needed. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Simsek, S.; Hitit, M.; Bodu, M.; Memili, E. Sperm Cell Membranes of Bulls and Bucks Associated with Sperm Fertility and Freezability. Animals 2025, 15, 3248. https://doi.org/10.3390/ani15223248
Simsek S, Hitit M, Bodu M, Memili E. Sperm Cell Membranes of Bulls and Bucks Associated with Sperm Fertility and Freezability. Animals. 2025; 15(22):3248. https://doi.org/10.3390/ani15223248
Chicago/Turabian StyleSimsek, Seher, Mustafa Hitit, Mustafa Bodu, and Erdogan Memili. 2025. "Sperm Cell Membranes of Bulls and Bucks Associated with Sperm Fertility and Freezability" Animals 15, no. 22: 3248. https://doi.org/10.3390/ani15223248
APA StyleSimsek, S., Hitit, M., Bodu, M., & Memili, E. (2025). Sperm Cell Membranes of Bulls and Bucks Associated with Sperm Fertility and Freezability. Animals, 15(22), 3248. https://doi.org/10.3390/ani15223248






