Effect of Cryodiluent and Time of Glycerol Addition on Cryopreservation and In Vitro Fertilization of Domestic Cat Epididymal Spermatozoa
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Reagents
2.2. Collection of Epididymal Sperm Samples
2.3. Evaluation of Sperm Traits
2.4. Sperm Cryopreservation
2.5. Sperm Thawing
2.6. Sperm Preparation for IVF: Swim-Up and Pentoxifylline Addition
2.7. Oocyte Recovery and In Vitro Maturation (IVM)
2.8. IVF of In Vitro Matured Oocytes with Frozen–Thawed Spermatozoa
2.9. Statistical Analysis
3. Results
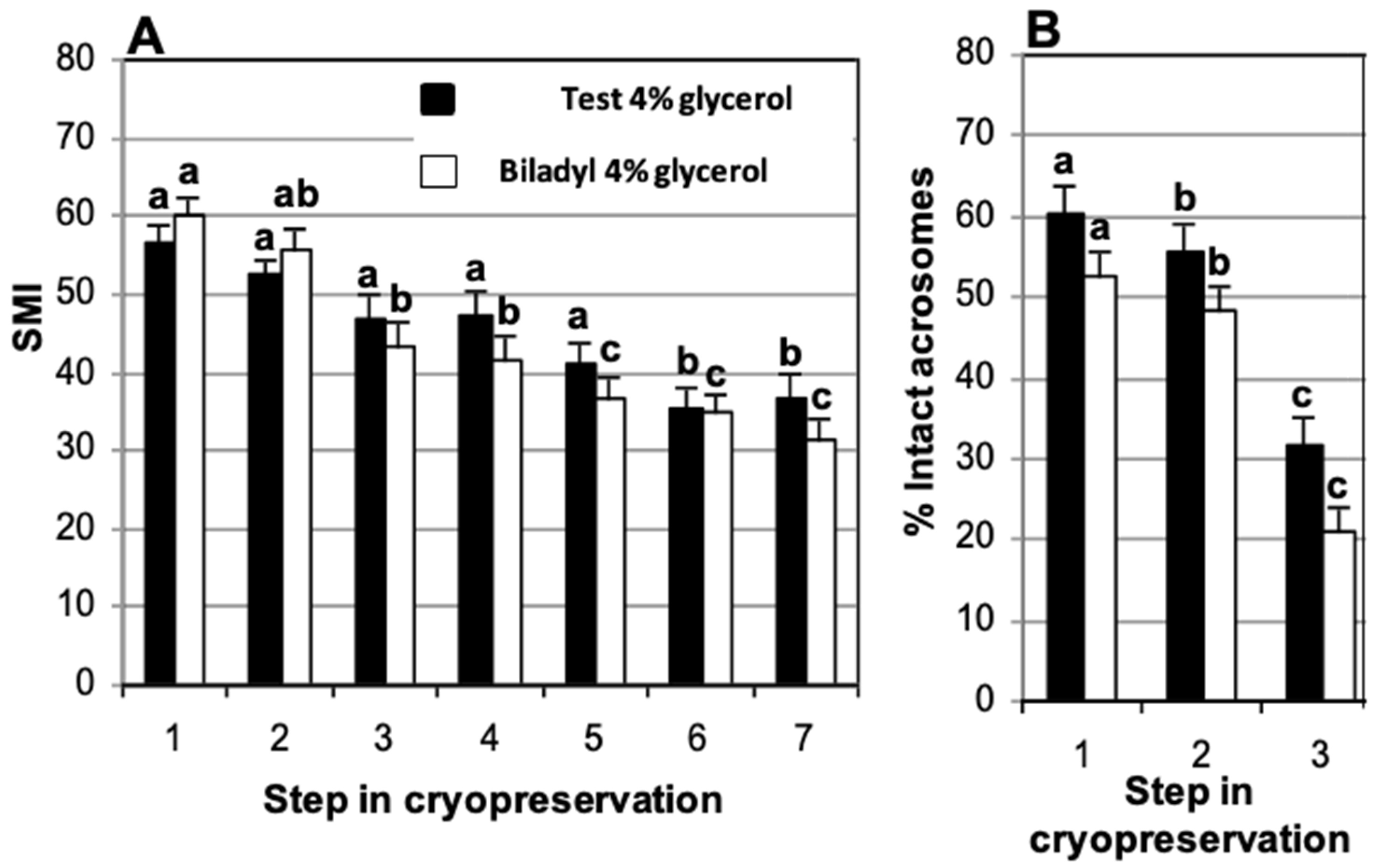
3.1. Effect of Cryodiluent: TEST vs. Biladyl
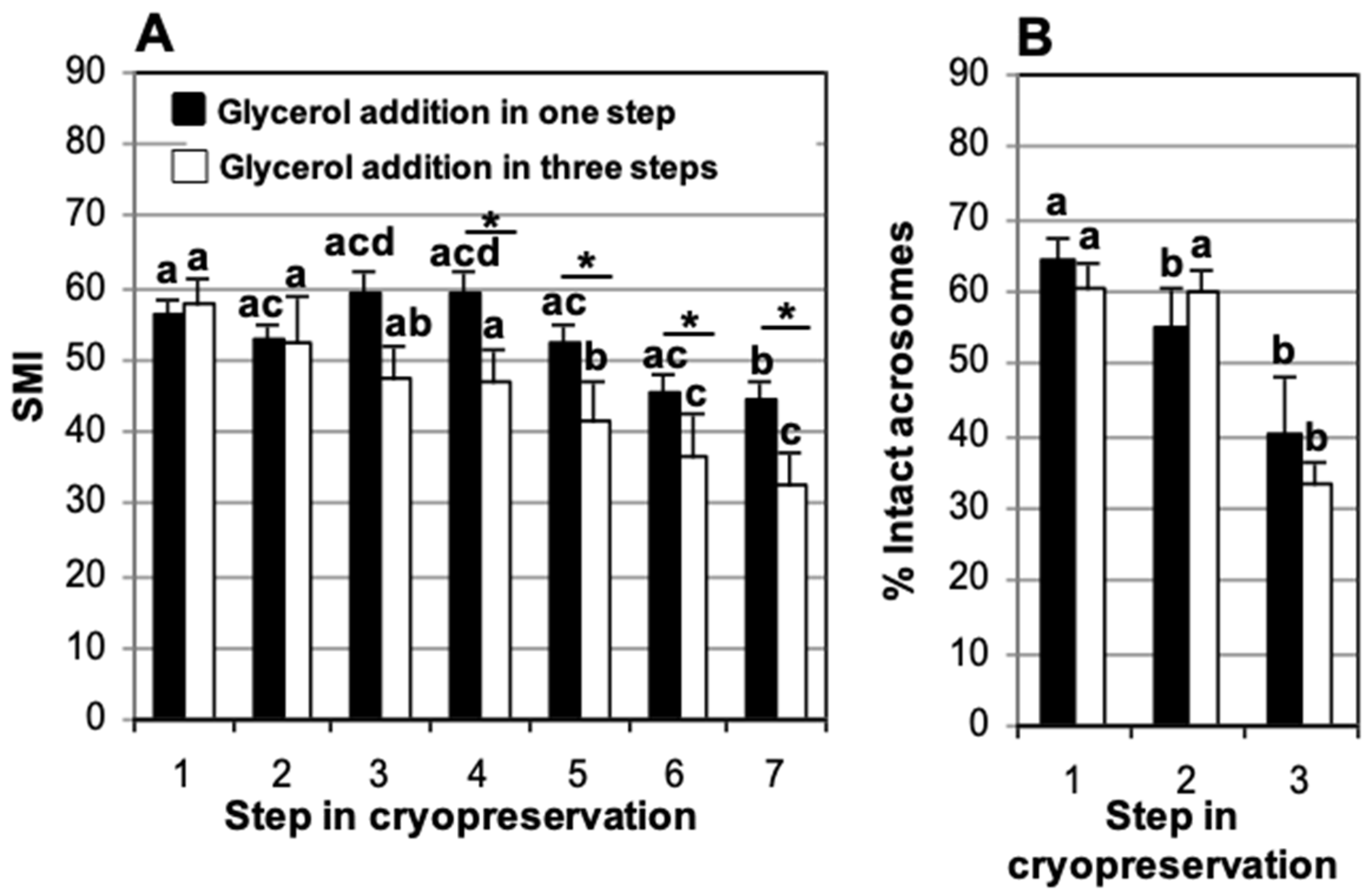
3.2. Effect of Glycerol Addition System in TEST Cryodiluent: One vs. Three Steps
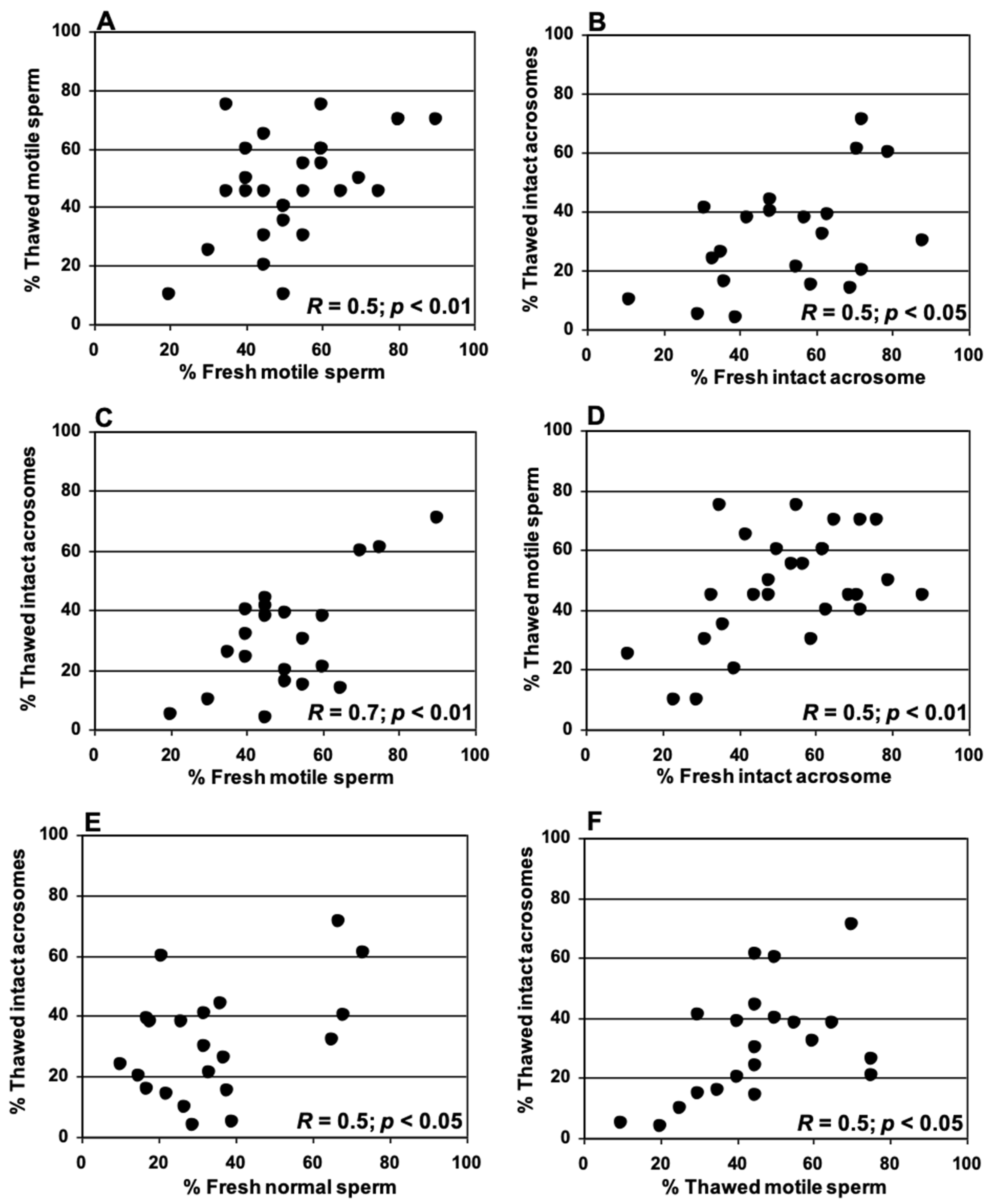
3.3. Relationships Between Sperm Parameters Before and After Cryopreservation
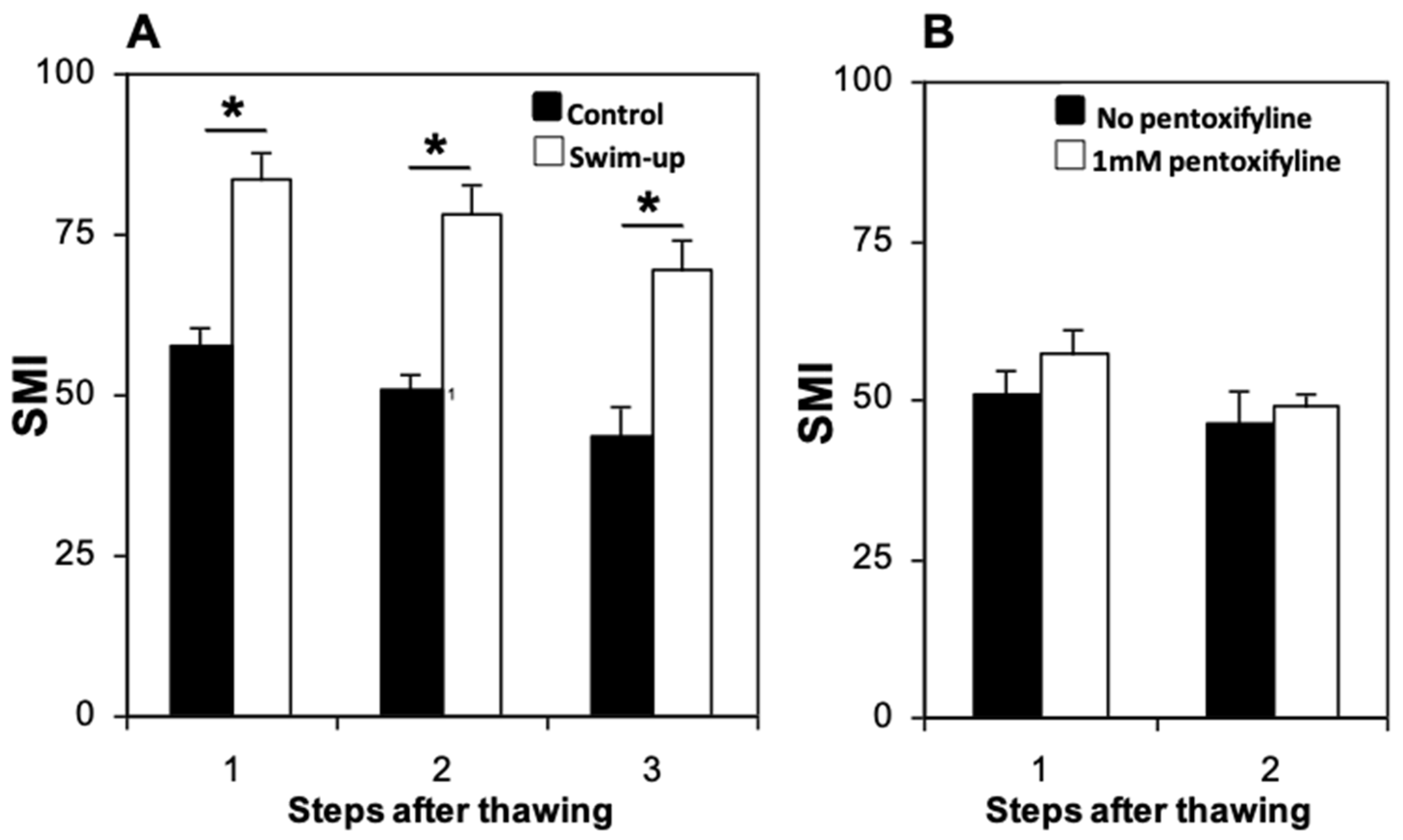
3.4. Sperm Preparation for IVF
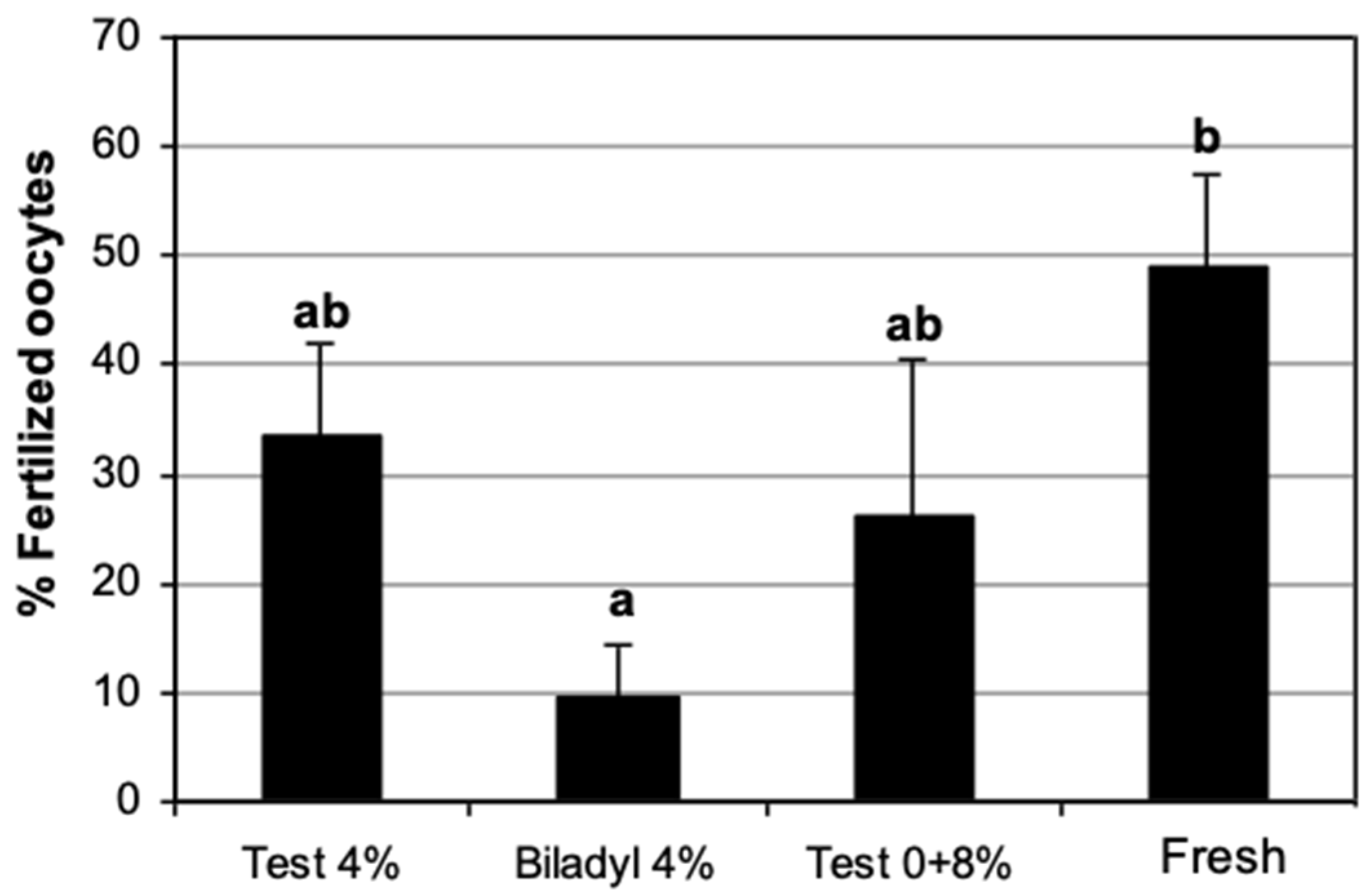
3.5. IVF of In Vitro Matured Oocytes with Frozen–Thawed Domestic Cat Epididymal Spermatozoa
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wildt, D.E.; Rall, W.F.; Crister, J.K.; Monfort, S.L.; Seal, U.S. Genome Resource Banks Living collections for biodiversity conservation. Bioscience 1997, 47, 689–698. [Google Scholar] [CrossRef]
- Roldan, E.R.S.; Garde, J.J. Biotecnología de la reproducción y conservación de especies en peligro de extinción. In Los Retos Medioambientales del siglo XXI. La Conservación de la Biodiversidad en España; Gomendio, M., Ed.; CSIC-FBBVA: Madrid, Spain, 2004; pp. 283–303. [Google Scholar]
- Pukazhenthi, B.; Comizzoli, P.; Travis, A.J.; Wildt, D.E. Applications of emerging technologies to the study and conservation of threatened and endangered species. Reprod. Fert. Dev. 2006, 18, 77–90. [Google Scholar] [CrossRef] [PubMed]
- Swanson, W.F.; Stoops, M.A.; Magarey, G.M.; Herrick, J.R. Sperm cryopreservation in endangered felids: Developing linkage of in situ-ex situ populations. In Spermatology; Roldan, E.R.S., Gomendio, M., Eds.; Nottingham University Press: Nottingham, UK, 2007; pp. 417–432. [Google Scholar]
- Klaus, C.; Eder, S.; Franz, C.; Müller, K. Successful cryopreservation of domestic cat (Felis catus) epididymal sperm after slow equilibration to 15 or 10°C. Reprod. Domest. Anim. 2016, 51, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Eder, S.; Bailey, L.D.; Müller, K. Equilibration in freezing extender alters in vitro sperm-oviduct binding in the domestic cat (Felis catus). Theriogenology 2020, 149, 79–87. [Google Scholar] [CrossRef]
- Wildt, D.E.; Schiewe, M.C.; Schmidt, P.M.; Goodrowe, K.L.; Howard, J.G.; Phillips, L.G.; O’Brien, S.J.; Bush, M. Developing animals models system for embryo technologies in rare and endangered wildlife. Theriogenology 1986, 25, 33–51. [Google Scholar] [CrossRef]
- Swanson, W.F. Research in non-domestic species: Experiences in Reproductive physiology research for conservation of endangered felids. ILAR J. 2003, 4, 307–316. [Google Scholar] [CrossRef]
- Pope, C.E. Embryo technology in conservation efforts for endangered felids. Theriogenology 2000, 53, 163–174. [Google Scholar] [CrossRef]
- Luvoni, G.C.; Kalchschmidt, E.; Leoni, S.; Ruggiero, C. Conservation of feline semen. Part I: Cooling and freezing protocols. J. Feline Med. Surg. 2003, 5, 203–208. [Google Scholar] [CrossRef]
- Goodrowe, K.L.; Hay, M. Characteristics and zona binding ability of fresh and cooled domestic cat epididymal spermatozoa. Theriogenology 1993, 40, 967–975. [Google Scholar] [CrossRef]
- Lengwinat, T.; Blottner, S. In vitro fertilization of follicular oocytes of domestic cat using fresh and cryopreserved spididymal spermatozoa. Anim. Reprod. Sci. 1994, 35, 291–301. [Google Scholar] [CrossRef]
- Kashiwazaki, N.; Yamaguchi, R.; Uesugi, R.; Hishiyama, N.; Kim, M.; Nakatsukasa, E.; Kojima, Y.; Okuda, Y.; Hisamatsu, S.; Inomata, T.; et al. Sperm motility, plasma membrane integrity, and binding capacity to homologous zona pellucida of cryopreserved epididymal spermatozoa in the domestic cat. J. Reprod. Dev. 2005, 51, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Zambelli, D.; Merlo, B.; Iacono, E.; Prati, F.; Belluzzi, S. Fertilizing ability of electro-ejaculated cryopreserved semen in the domestic cat. Reprod. Domest. Anim. 2006, 41, 137–141. [Google Scholar] [CrossRef] [PubMed]
- Zambelli, D.; Prati, F.; Cunto, M.; Iacono, E.; Merlo, B. Quality and in vitro fertilizing ability of cryopreserved cat spermatozoa obtained by urethral catheterization after medetomidine administration. Theriogenology 2008, 69, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Bogliolo, L.; Leoni, G.; Ledda, S.; Naitana, S.; Zedda, M.; Carluccio, A.; Pau, S. Intracytoplasmic sperm injection of in vitro matured oocytes of domestic cats with frozen-thawed epididymal spermatozoa. Theriogenology 2001, 56, 955–967. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Fujiki, K.; Alam, M.E.; Tsukamoto, M.; Azuma, R.; Kanegi, R.; Anzai, M.; Inaba, T.; Sugiura, K.; Hatoya, S. Development of feline embryos produced by piezo-actuated intracytoplasmic sperm injection of elongated spermatids. J. Reprod. Dev. 2019, 65, 245–250. [Google Scholar] [CrossRef]
- Tsujimoto, Y.; Kaneko, T.; Yoshida, T.; Kimura, K.; Inaba, T.; Sugiura, K.; Hatolya, S. Development of feline embryos produced using freeze-dried sperm. Theriogenology 2020, 147, 71–76. [Google Scholar] [CrossRef]
- Tsutsui, T. Artificial insemination in domestic cats (Felis catus). Theriogenology 2006, 66, 122–125. [Google Scholar] [CrossRef]
- Villaverde, A.I.; Melo, C.M.; Martin, I.; Ferreira, T.H.; Papa, F.O.; Taconeli, C.A.; Lopes, M.D. Comparison of efficiency between two artificial insemination methods using frozen-thawed semen in domestic cat (Felis catus): Artificial insemination in domestic cats. Anim. Reprod. Sci. 2009, 114, 434–442. [Google Scholar] [CrossRef]
- Karimi, I.; Mohammad, L.J.; Suvitha, A.; Haidari, Z.; Schiöth, H.B. Comprehensive overview of the toxicities of small-molecule cryoprotectants for carnivorous spermatozoa foundation for computational cryobiotechnology. Front. Toxicol. 2025, 7, 1477822. [Google Scholar] [CrossRef]
- Gao, D.Y.; Liu, J.; McGann, L.E.; Watson, P.F.; Kleinhans, F.W.; Mazur, P.; Critser, E.S.; Critser, J.K. Prevention of osmotic injury to human spermatozoa during addition and removal of glycerol. Hum. Reprod. 1995, 10, 1109–1122. [Google Scholar] [CrossRef]
- Pukazhenthi, B.; Spindler, R.; Wildt, D.E.; Bush, L.M.; Howard, J.G. Osmotic properties of spermatozoa from felids producing different proportions of pleimorphisms: Influence of adding and removing cryoprotectant. Cryobiology 2002, 44, 288–300. [Google Scholar] [CrossRef] [PubMed]
- Glover, T.E.; Watson, P.F. The effect of buffer osmolality on the survival of cat (Felis catus) spermatozoa at 5°C. Theriogenology 1985, 24, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Hay, M.A.; Goodrowe, K.L. Comparative cryopreservation and capacitation of spermatozoa from epididymides and vasa deferentia of the domestic cat. J. Reprod. Fert. Suppl. 1993, 47, 297–305. [Google Scholar]
- Gualtieri, R.; Kalthur, G.; Barbato, V.; Di Nardo, M.; Adiga, S.K.; Talevi, R. Mitochondrial Dysfunction and Oxidative Stress Caused by Cryopreservation in Reproductive Cells. Antioxidants 2021, 10, 337. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.L.H.; Mermillod, P.; Grasseau, I.; Brillard, J.-P.; Gérard, N.; Reynaud, K.; Chen, L.-R.; Blesbois, E.; Carvalho, A.V. Is glycerol a good cryoprotectant for sperm cells? New exploration of its toxicity using avian model. Anim. Reprod. Sci. 2023, 258, 107330. [Google Scholar] [CrossRef]
- Hemmati, S. Expanding the cryoprotectant toolbox in biomedicine by multifunctional antifreeze peptides. Biotechnol. Adv. 2025, 81, 108545. [Google Scholar] [CrossRef]
- Nelson, K.L.; Crichton, E.G.; Doty, L.; Volenec, D.E.; Morato, R.G.; Pope, C.E.; Dresser, B.L.; Brown, C.S.; Armstrong, D.L.; Loskutoff, N.M. Heterologous and homologous fertilizing capacity of cryopreserved felid sperm: A model for endangered species. Theriogenology 1999, 51, 290. [Google Scholar] [CrossRef]
- Thuwanut, P.; Chatdarong, K.; Johannisson, A.; Bergqvist, A.S.; Soderquist, L.; Axner, E. Cryopreservation of epididymal cat spermatozoa: Effects of in vitro antioxidative enzyme supplementation and lipid peroxidation induction. Theriogenology 2010, 73, 1076–1087. [Google Scholar] [CrossRef]
- Villaverde, A.I.; Fioratti, E.G.; Penitenti, M.; Ikoma, M.R.V.; Tsunemi, M.H.; Papa, F.O.; Lopes, M.D. Cryoprotective effect of different glycerol concentrations on domestic cat spermatozoa. Theriogenology 2013, 80, 730–737. [Google Scholar] [CrossRef]
- Vick, M.M.; Bateman, H.L.; Lambo, C.A.; Swanson, W.F. Improved cryopreservation of domestic cat sperm in a chemically defined medium. Theriogenology 2012, 78, 2120–2128. [Google Scholar] [CrossRef]
- Buranaamnuay, K. Determination of appropriate cryopreservation protocols for epididymal cat spermatozoa. Reprod. Dom. Anim. 2015, 50, 378–385. [Google Scholar] [CrossRef] [PubMed]
- Hassan, H.A.; Banchi, P.; Domain, G.; Vanderheyden, L.; Prochowska, S.; Nizanski, W.; Van Soom, A. Mito-Tempo improves acrosome integrity of frozen-thawed epididymal spermatozoa in tomcats. Front. Vet. Sci. 2023, 10, 1170347. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.L.; Bush, L.M.; Wildt, D.E.; Weiss, R.B. Scimitar horned oryx (Oryx dammah) spermatozoa are functionally competent in a heterologous bovine in vitro fertilization system after cryopreservation system after cryopreservation on dry ice, in a dry shipper, or over liquid nitrogen. Biol. Reprod. 1990, 60, 493–498. [Google Scholar] [CrossRef]
- Pope, C.E. Aspects of in vivo oocyte production, blastocyst development, and embryo transfer in the cat. Theriogenology 2014, 81, 126–137. [Google Scholar] [CrossRef]
- Howard, J.G. Semen collection and analysis in carnivores. In Zoo and Wild Animal Medicine III; Fowler, M.E., Ed.; WB Saunders WB: Philadelphia, PA, USA, 1993; pp. 390–399. [Google Scholar]
- Luvoni, G.C. Gamete cryopreservation in the domestic cat. Theriogenology 2006, 66, 101–111. [Google Scholar] [CrossRef]
- Stachecki, J.J.; Ginsburg, K.A.; Armant, D.R. Stimulation of cryopreserved epididymal spermatozoa of the domestic cat using the motility stimulants caffeine, pentoxifylline, and 2’-deoxyadenosine. J. Androl. 1994, 15, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Gala, B.; Badge, A.; Bawaskar, P.; Gajbe, U.; Singh, B.R.; Kohale, M. The potential of theophylline and pentoxifylline in sperm optimization and its intracytoplasmic sperm injection outcomes. Cureus 2023, 15, e48192. [Google Scholar] [CrossRef]
- Terriou, P.; Hans, E.; Giorgetti, C.; Spach, J.L.; Salzmann, J.; Urrutia, V.; Roulier, R. Pentoxifylline initiates motility in spontaneously immotile epididymal and testicular spermatozoa and allows normal fertilization, pregnancy, and birth after intracytoplasmic sperm injection. J. Assist. Reprod. Genet. 2000, 17, 194–199. [Google Scholar] [CrossRef]
- Ponce, A.A.; Fiol, M.; Ruiz, R.D.; Vincenti, L.M.; Santilla, M.E.; Stutz, G.; Lacuara, J.L. Influence of pentoxifylline on sperm membrane functional integrity. Arch. Androl. 2009, 43, 77–84. [Google Scholar] [CrossRef]
- Gañán, N.; Gomendio, M.; Roldan, E.R.S. Effect of storage of domestic cat (Felis catus) epididymides at 5 °C on sperm quality and cryopreservation. Theriogenology 2009, 72, 1268–1277. [Google Scholar] [CrossRef]
- Gañán, N.; Sanchez-Rodriguez, A.; Roldan, E.R.S. Factors affecting cryopreservation of domestic cat (Felis catus) epididymal spermatozoa. Animals 2025, 15, 949. [Google Scholar] [CrossRef] [PubMed]
- Howard, J.G. Feline semen analysis and artificial insemination. In Kirk’s Current Veterinary Therapy. XI Small Animal Practice; Kirk, R.W., Bonagura, J.D., Eds.; WB Saunders: Philadelphia, PA, USA, 1992; pp. 929–934. [Google Scholar]
- Pukazhenthi, B.; Santymire, R.; Crosier, A.; Howard, J.G.; Wildt, D.E. Challenges in cryopreserving endangered mammal spermaozoa: Morphology and the value of acrosomal integrity as markers of cryo-survival. In Spermatology; Roldan, E.R.S., Gomendio, M., Eds.; Nottingham University Press: Nottingham, UK, 2007; pp. 433–446. [Google Scholar]
- Gómez, M.C.; Pope, E.; Harris, R.; Mikota, S.; Dresser, B.L. Development of in vitro matured, in vitro fertilized domestic cat embryos following cryopreservation, culture and transfer. Theriogenology 2003, 60, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Gañán, N.; González, R.; Sestelo, A.; Garde, J.J.; Sánchez, I.; Aguilar, J.M.; Gomendio, M.; Roldan, E.R.S. Male reproductive traits, semen cryopreservation and heterologous in vitro fertilization in the bobcat (Lynx rufus). Theriogenology 2009, 72, 341–352. [Google Scholar] [CrossRef]
- Pope, C.E.; Gomez, M.C.; Dresser, B.L. In vitro embryo production and embryo transfer in domestic and non-domestic cats. Theriogenology 2006, 66, 1518–1524. [Google Scholar] [CrossRef] [PubMed]
- Díaz-Hernández, E.; Bóveda, P.; Picazo, C.M.; Laborda-Gomariz, J.A.; Serralle, M.; Ramón, M.; Gallego, R.; Montoro, V.; García-Álvarez, O.; Fernández-Santos, R.; et al. Influence of liquid extender, preservation temperature and time on the sheep sperm quality. Reprod. Dom. Anim. 2024, 59 (Suppl. S3), e14653. [Google Scholar] [CrossRef]
- Jimenez-Rabadán, P.; Ramón, M.; García-Álvarez, O.; Maroto-Morales, A.; del Olmo, E.; Pérez-Guzmán, M.D.; Bisbal, A.; Fernández-Santos, M.R.; Garde, J.J.; Soler, A.J. Effect of semen collection method (artificial vagina vs. electroejaculation), extender and centrifugation on post-thaw sperm quality of Blanca-Celtibérica buck ejaculates. Anim. Reprod. Sci. 2012, 132, 88–95. [Google Scholar] [CrossRef]
- Muiño, R.; Fernández, M.; Peña, A.I. Post-thaw survival and longevity of bull spermatozoa frozen with an egg yolk-based or two egg yolk-free extenders after an equilibration period of 18 h. Reprod. Domest. Anim. 2007, 42, 305–311. [Google Scholar] [CrossRef]
- Terrell, K.A.; Wildt, D.E.; Anthony, N.M.; Bavister, B.D.; Leibo, S.P.; Penfold, L.M.; Marker, L.L.; Crosier, A.E. Glycolytic enzyme activity is essential for domestic cat (Felis catus) and cheetah (Acinonyx jubatus) sperm and viability in a sugar-free medium. Biol. Repr. 2011, 84, 1198–1206. [Google Scholar] [CrossRef][Green Version]
- Minter, L.J.; Pinto, C.R.F.; Davis, M.; Kozink, D.M. Influence of extender and packaging on post-thaw survival of epididymal cat spermatozoa. Theriogenology 2007, 68, 501–502. [Google Scholar] [CrossRef]
- Byers, A.P.; Hunter, A.G.; Scal, U.S.; Binezik, G.A.; Graham, E.F.; Reind, N.J.; Tilson, R.L. In-vitro induction of capacitation of fresh and frozen spermatozoa of the Siberian tiger (Panthera tigris). J. Reprod. Fert. 1989, 86, 599–607. [Google Scholar] [CrossRef]
- Donoghue, A.M.; Johnston, L.A.; Seal, U.S.; Armstrong, D.L.; Simmons, L.G.; Gross, T.; Tilson, R.L.; Wildt, D.E. Ability of thawed tiger (Panthera tigris) spermatozoa to fertilize conspecific eggs and bind and penetrate domestic cat eggs in vitro. J. Reprod. Fert. 1992, 96, 555–564. [Google Scholar] [CrossRef] [PubMed]
- Manee-In, S.; Parmornsupornvichit, S.; Kraiprayoon, S.; Tharasanit, T.; Chanapiwat, P.; Kaeoket, K. L-carnitine supplemented extender improves cryopreserved-thawed cat epididymal sperm motility. Asian-Australas. J. Anim. Sci. 2014, 27, 791–796. [Google Scholar] [CrossRef]
- Chatdarong, K.; Thuwanut, P.; Manee-in, S.; Lohachit, C.; Axner, E. Effects of thawing temperature and post-thaw dilution on the quality of cat spermatozoa. Reprod. Domestic Anim. 2010, 45, 221–227. [Google Scholar] [CrossRef]
- Thiangtum, K.; Swanson, W.; Howard, J.G.; Tunwattana, W.; Tongthainam, D.; Wichasilpa, W.; Patumrattanathan, P.; Pinyopoommintr, T. Assessment of basic seminal characteristics, sperm cryopreservation and heterologous in vitro fertilisation in the fishing cat (Prionailurus viverrinus). Reprod. Fertil. Dev. 2006, 18, 373–382. [Google Scholar] [CrossRef]
- Tebet, J.M.; Martins, M.I.M.; Chirinea, V.H.; Souza, F.F.; Campagnol, D.; Lopes, M.D. Cryopreservation effects on domestic cat epididymal versus electroejaculated spermatozoa. Theriogenology 2006, 66, 1629–1632. [Google Scholar] [CrossRef]
- Hermansson, U.; Axnér, E. Epididymal and ejaculated cat spermatozoa are resistant to cold shock but egg yolk promotes sperm longevity during cold storage at 4ºC. Theriogenology 2007, 67, 1239–1248. [Google Scholar] [CrossRef]
- Comizzoli, P.; Amelkina, O.; Chavez, D.R.; Rowlison, T.R.; Lee, P.-C. Current knowledge in the biology of gametes and embryos from Carnivora. Theriogenology 2023, 196, 254–263. [Google Scholar] [CrossRef]
- Fukuda, M.; Morales, P.; Overstreet, J.W. Acrosomal function of human spermatozoa with normal and abnormal head morphology. Gamete Res. 1989, 24, 59–65. [Google Scholar] [CrossRef]
- Crosier, A.E.; Pukazhenthi, B.S.; Henghali, J.N.; Howard, J.G.; Dickman, A.J.; Marker, L.; Wildt, D.E. Cryopreservation of spermatozoa from wild-born Namibian cheetah (Acinonyx jubatus) and influence of glycerol on cryosurvival. Cryobiology 2006, 52, 169–181. [Google Scholar] [CrossRef]
- Axnér, E.; Hermansson, U.; Linde-Forsberg, C. The effect of Equex STM paste and sperm morphology on post-thaw survival of cat epididymal spermatozoa. Anim. Reprod. Sci. 2004, 84, 179–191. [Google Scholar] [CrossRef]
- Watson, P.F. The causes of reduced fertility with cryopreserved semen. Anim. Reprod. Sci. 2000, 60–61, 481–492. [Google Scholar] [CrossRef] [PubMed]
- Swanson, W.F.; Maggs, D.J.; Clarke, H.E.; Newell, A.E.; Bond, J.B.; Bateman, H.L.; Kennedy-Stoskopf, S. Assessment of viral presence in semen and reproductive function of frozen-thawed spermatozoa from Pallas’ cats (Otocolobus manul) infected with feline herpervirus. J. Zoo. Wildl. Med. 2006, 37, 336–346. [Google Scholar] [CrossRef] [PubMed]
- Mahaldashtian, M.; Khalili, M.A.; Nottola, S.A.; Woodward, B.; Macchiarelli, G.; Miglietta, S. Does in vitro application of pentoxifylline have beneficial effects in assisted male reproduction? Andrologia 2020, 53, e13722. [Google Scholar] [CrossRef]
- Stachecki, J.J.; Dresser, B.L.; Pope, C.E.; Armant, D.R. Stimulation of ejaculated domestic cat sperm motility with caffeine, pentoxifylline, and 2’-deoxyadenosine. Arch. Androl. 1995, 34, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Roth, T.L.; Howard, J.G.; Donoghue, A.M.; Swanson, W.F.; Wildt, D.E. Function and culture requirements of snow leopard (Panthera uncia) spermatozoa in vivo. J. Reprod. Fertil. 1994, 101, 563–569. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Amann, R.P. Can the fertility potential of a seminal sample be predicted accurately? J. Androl. 1989, 10, 89–98. [Google Scholar] [CrossRef]
- Amann, R.P.; Hammerstedt, R.H. Detection of differences in fertility. J. Androl. 2002, 23, 317–325. [Google Scholar] [CrossRef]
| Component | TEST | Biladyl |
|---|---|---|
| Tes | 4.83% | - |
| Tris | 1.15% | 2.42% |
| Glucose | 0.4% | - |
| Fructose | - | 1.00% |
| Citric Acid | - | 1.38% |
| Egg yolk | 20% | 20% |
| Glycerol (*) | 4% | 4% |
| Penicillin (IU/mL) | 200 | 200 |
| Streptomycin (µg/mL) | 200 | 200 |
| pH | 7.2 | 7.0 |
| Osmolarity (mOsm/L) | 360 | 340 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gañán, N.; González, R.; Sanchez-Rodriguez, A.; Roldan, E.R.S. Effect of Cryodiluent and Time of Glycerol Addition on Cryopreservation and In Vitro Fertilization of Domestic Cat Epididymal Spermatozoa. Animals 2025, 15, 1680. https://doi.org/10.3390/ani15121680
Gañán N, González R, Sanchez-Rodriguez A, Roldan ERS. Effect of Cryodiluent and Time of Glycerol Addition on Cryopreservation and In Vitro Fertilization of Domestic Cat Epididymal Spermatozoa. Animals. 2025; 15(12):1680. https://doi.org/10.3390/ani15121680
Chicago/Turabian StyleGañán, Natalia, Raquel González, Ana Sanchez-Rodriguez, and Eduardo R. S. Roldan. 2025. "Effect of Cryodiluent and Time of Glycerol Addition on Cryopreservation and In Vitro Fertilization of Domestic Cat Epididymal Spermatozoa" Animals 15, no. 12: 1680. https://doi.org/10.3390/ani15121680
APA StyleGañán, N., González, R., Sanchez-Rodriguez, A., & Roldan, E. R. S. (2025). Effect of Cryodiluent and Time of Glycerol Addition on Cryopreservation and In Vitro Fertilization of Domestic Cat Epididymal Spermatozoa. Animals, 15(12), 1680. https://doi.org/10.3390/ani15121680






