MiRNAs in Poultry Health and Production: Progress and Challenges
Simple Summary
Abstract
1. Introduction
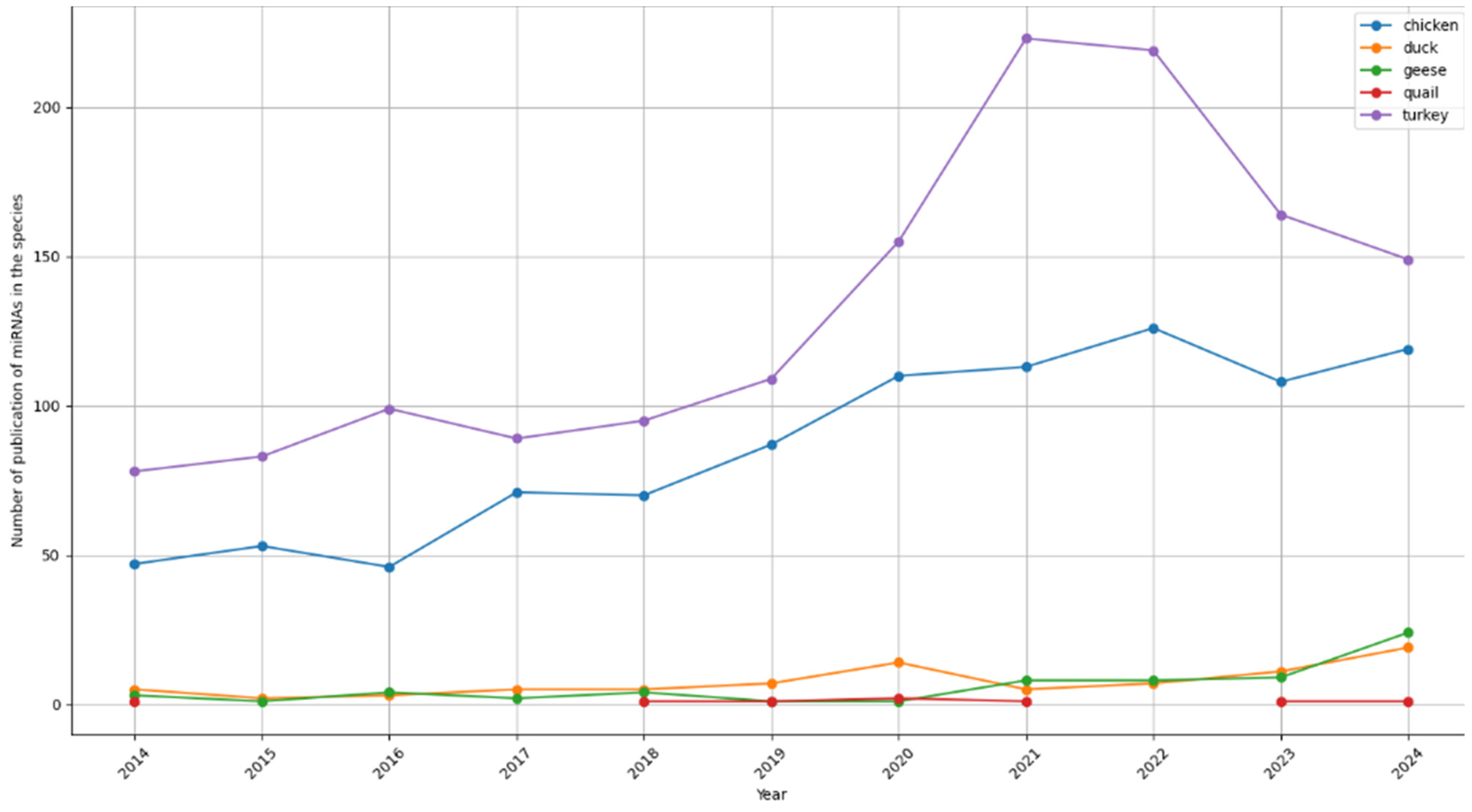
2. Poultry Genomes and MicroRNAs Research
3. Potential Functions of microRNAs in Poultry
3.1. MicroRNAs in Chicken
3.1.1. MicroRNAs Related to Production Traits in Chicken
MicroRNAs Involved in Muscle Growth and Meat Quality in Chickens
MicroRNAs in Egg Production-Related Traits
MicroRNA in Chicken Feed Efficiency
3.1.2. MicroRNAs in Chicken Immune Functions and Diseases
Regulation of Bacterial Infections
Regulation of Viral Infections
3.1.3. Role of MicroRNAs in Exterior Traits in Chickens
Feather Color
Comb Type
Skin Pigmentation
Body Size
3.1.4. Role of MicroRNAs in Physiological Traits in Chickens
Metabolism
MicroRNAs and Stress Response
3.2. MicroRNAs in Other Poultry Species
3.2.1. MicroRNAs in Turkey
3.2.2. MicroRNAs in Duck
3.2.3. MicroRNAs in Geese
3.2.4. MicroRNAs in Quails
4. Prospects and Challenges
4.1. Prospects
4.2. Challenges
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Henchion, M.; Hayes, M.; Mullen, A.M.; Fenelon, M.; Tiwari, B. Future Protein Supply and Demand: Strategies and Factors Influencing a Sustainable Equilibrium. Foods 2017, 6, 53. [Google Scholar] [CrossRef]
- Mottet, A.; Tempio, G. Global Poultry Production: Current State and Future Outlook and Challenges. Worlds Poult. Sci. J. 2017, 73, 245–256. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA Function in Animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Ha, M.; Kim, V.N. Regulation of microRNA Biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- O’Connell, R.M.; Rao, D.S.; Baltimore, D. microRNA Regulation of Inflammatory Responses. Annu. Rev. Immunol. 2012, 30, 295–312. [Google Scholar] [CrossRef]
- Shang, R.; Lee, S.; Senavirathne, G.; Lai, E.C. microRNAs in action: Biogenesis, function and regulation. Nat Rev Genet. 2023, 24, 816–833. [Google Scholar] [CrossRef]
- Sohel, M.M.H. Circulating microRNAs as Biomarkers in Cancer Diagnosis. Life Sci. 2020, 248, 117473. [Google Scholar] [CrossRef]
- Kim, V.N.; Nam, J.W. Genomics of microRNA. Trends Genet. 2006, 22, 165–173. [Google Scholar] [CrossRef]
- Wang, J.; Yang, L.Z.; Zhang, J.S.; Gong, J.X.; Wang, Y.H.; Zhang, C.L.; Chen, H.; Fang, X.T. Effects of microRNAs on Skeletal Muscle Development. Gene 2018, 668, 107–113. [Google Scholar] [CrossRef]
- Wang, X.; Gu, Z.; Jiang, H. MicroRNAs in Farm Animals. Animal 2013, 7, 1567–1575. [Google Scholar] [CrossRef]
- Wang, Y. Research Progress on MicroRNAs Involved in the Regulation of Chicken Diseases. J. Poult. Sci. 2020, 57, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.; Zheng, S.J. The Roles of MicroRNAs (miRNAs) in Avian Response to Viral Infection and Pathogenesis of Avian Immunosuppressive Diseases. Int. J. Mol. Sci. 2019, 20, 5454. [Google Scholar] [CrossRef] [PubMed]
- Hillier, L.W.; Miller, W.; Birney, E.; Warren, W.; Hardison, R.C.; Ponting, C.P.; Bork, P.; Burt, D.W.; Groenen, M.A.M.; Delany, M.E.; et al. Sequence and Comparative Analysis of the Chicken Genome Provide Unique Perspectives on Vertebrate Evolution. Nature 2004, 432, 695–716. [Google Scholar] [CrossRef] [PubMed]
- Duan, X.; Wang, L.; Sun, G.; Yan, W.; Yang, Y. Understanding the Cross-Talk between Host and Virus in Poultry from the Perspectives of microRNA. Poult. Sci. 2020, 99, 1838–1846. [Google Scholar] [CrossRef]
- Shi, H.; He, Y.; Li, X.; Du, Y.; Zhao, J.; Ge, C. Regulation of Non-Coding RNA in the Growth and Development of Skeletal Muscle in Domestic Chickens. Genes 2022, 13, 1033. [Google Scholar] [CrossRef]
- Gilyazova, I.; Korytina, G.; Kochetova, O.; Savelieva, O.; Mikhaylova, E.; Vershinina, Z.; Chumakova, A.; Markelov, V.; Abdeeva, G.; Karunas, A.; et al. Advances in Genomics and Postgenomics in Poultry Science: Current Achievements and Future Directions. Int. J. Mol. Sci. 2025, 26, 8285. [Google Scholar] [CrossRef]
- Scheuermann, G.N.; Bilgili, S.F.; Hess, J.B.; Mulvaney, D.R. Breast Muscle Development in Commercial Broiler Chickens. Poult. Sci. 2003, 82, 1648–1658. [Google Scholar] [CrossRef]
- Sweetman, D.; Goljanek, K.; Rathjen, T.; Oustanina, S.; Braun, T.; Dalmay, T.; Münsterberg, A. Specific Requirements of MRFs for the Expression of Muscle Specific microRNAs, miR-1, miR-206 and miR-133. Dev. Biol. 2008, 321, 491–499. [Google Scholar] [CrossRef]
- Petracci, M.; Cavani, C. Muscle growth and poultry meat quality issues. Nutrients 2012, 4, 1–12. [Google Scholar] [CrossRef]
- Luo, W.; Nie, Q.; Zhang, X. MicroRNAs Involved in Skeletal Muscle Differentiation. J. Genet. Genomics 2013, 40, 107–116. [Google Scholar] [CrossRef]
- Collins, A.A.; Zou, K.; Zhang, L.; Su, Y. MechanisM and Functions of Identified miRnas in poultRy Skeletal Muscle development—A Review. Ann. Anim. Sci. 2019, 19, 887–904. [Google Scholar] [CrossRef]
- Güller, I.; Russell, A.P. MicroRNAs in Skeletal Muscle: Their Role and Regulation in Development, Disease and Function. J. Physiol. 2010, 588, 4075–4087. [Google Scholar] [CrossRef]
- Li, P.; Wei, X.; Guan, Y.; Chen, Q.; Zhao, T.; Sun, C.; Wei, L. MicroRNA-1 Regulates Chondrocyte Phenotype by Repressing Histone Deacetylase 4 during Growth Plate Development. FASEB J. 2014, 28, 3930. [Google Scholar] [CrossRef]
- Wu, P.; Zhou, K.; Zhang, J.; Ling, X.; Zhang, X.; Zhang, L.; Li, P.; Wei, Q.; Zhang, T.; Wang, X. Identification of Crucial circRNAs in Skeletal Muscle during Chicken Embryonic Development. BMC Genom. 2022, 23, 330. [Google Scholar] [CrossRef]
- Chen, J.-F.; Tao, Y.; Li, J.; Deng, Z.; Yan, Z.; Xiao, X.; Wang, D.-Z. microRNA-1 and microRNA-206 Regulate Skeletal Muscle Satellite Cell Proliferation and Differentiation by Repressing Pax7. J. Cell Biol. 2010, 190, 867–879. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Huang, W.; Chen, B.; Jebessa Bekele, E.; Chen, X.; Cai, B.; Nie, Q. Gga-Mir-133a-3p Regulates Myoblasts Proliferation and Differentiation by Targeting PRRX1. Front. Genet. 2018, 9, 577. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chen, Y.; Jin, W.; Fu, S.; Li, D.; Zhang, Y.; Sun, G.; Jiang, R.; Han, R.; Li, Z. Analyses of MicroRNA and mRNA Expression Profiles Reveal the Crucial Interaction Networks and Pathways for Regulation of Chicken Breast Muscle Development. Front. Genet. 2019, 10, 197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; He, M.; Wu, P.; Zhang, X.; Zhou, K.; Li, T.; Zhang, T.; Xie, K.; Dai, G.; Wang, J. MicroRNA-27b-3p Targets the Myostatin Gene to Regulate Myoblast Proliferation and Is Involved in Myoblast Differentiation. Cells 2021, 10, 423. [Google Scholar] [CrossRef]
- Sun, G.; Li, F.; Ma, X.; Sun, J.; Jiang, R.; Tian, Y.; Han, R.; Li, G.; Wang, Y.; Li, Z. Gga-miRNA-18b-3p Inhibits Intramuscular Adipocytes Differentiation in Chicken by Targeting the ACOT13 Gene. Cells 2019, 8, 556. [Google Scholar] [CrossRef]
- Zhang, M.; Li, D.-H.; Li, F.; Sun, J.-W.; Jiang, R.-R.; Li, Z.-J.; Han, R.-L.; Li, G.-X.; Liu, X.-J.; Kang, X.-T. Integrated Analysis of MiRNA and Genes Associated with Meat Quality Reveals That Gga-MiR-140-5p Affects Intramuscular Fat Deposition in Chickens. Cell. Physiol. Biochem. 2018, 46, 2421–2433. [Google Scholar] [CrossRef]
- Liu, Y.-F.; Zhang, M.; Shan, Y.-J.; Pang, L.-C.; Ji, G.-G.; Ju, X.-J.; Tu, Y.-J.; Shi, S.-Y.; Bai, H.; Zou, J.-M. Transcriptome Sequencing Analysis of the Role of miR-499-5p and SOX6 in Chicken Skeletal Myofiber Specification. Front. Genet. 2022, 13, 1008649. [Google Scholar] [CrossRef]
- Li, X.; Wang, J.; Jia, Z.; Cui, Q.; Zhang, C.; Wang, W.; Chen, P.; Ma, K.; Zhou, C. MiR-499 Regulates Cell Proliferation and Apoptosis during Late-Stage Cardiac Differentiation via Sox6 and Cyclin D1. PLoS ONE 2013, 8, e74504. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, M.; Shan, Y.; Ji, G.; Ju, X.; Tu, Y.; Sheng, Z.; Xie, J.; Zou, J.; Shu, J. miRNA–mRNA Network Regulation in the Skeletal Muscle Fiber Phenotype of Chickens Revealed by Integrated Analysis of miRNAome and Transcriptome. Sci. Rep. 2020, 10, 10619. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, H.; Liao, Y.; Zhou, P.; Xu, Y.; Zhao, Y.; Xie, S.; Zhao, S.; Li, X. miR-208b modulating skeletal muscle development and energy homoeostasis through targeting distinct targets. RNA Biol. 2020, 17, 743–754. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Bi, H.; Xie, S.; Cui, W. MiR-208b Regulates the Conversion of Skeletal Muscle Fiber Types by Inhibiting Mettl8 Expression. Front. Genet. 2022, 13, 820464. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Sun, T.; Jiang, S.; Yang, Z.; Xiao, C.; Deng, J.; Zhou, B.; Yang, X. Comprehensive Analysis of Non-Coding RNAs in the Ovaries of High and Low Egg Production Hens. Anim. Reprod. Sci. 2025, 276, 107836. [Google Scholar] [CrossRef]
- Wang, W.; Wu, K.; Jia, M.; Sun, S.; Kang, L.; Zhang, Q.; Tang, H. Dynamic Changes in the Global microRNAome and Transcriptome Identify Key Nodes Associated with Ovarian Development in Chickens. Front. Genet. 2018, 9, 491. [Google Scholar] [CrossRef]
- Wu, N.; Zhu, Q.; Chen, B.; Gao, J.; Xu, Z.; Li, D. High-Throughput Sequencing of Pituitary and Hypothalamic microRNA Transcriptome Associated with High Rate of Egg Production. BMC Genom. 2017, 18, 1–13. [Google Scholar] [CrossRef]
- Wu, N.; Gaur, U.; Zhu, Q.; Chen, B.; Xu, Z.; Zhao, X.; Yang, M.; Li, D. Expressed Micro RNA Associated with High Rate of Egg Production in Chicken Ovarian Follicles. Anim. Genet. 2017, 48, 205–216. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Q.; Ning, C.; Yang, M.; Zhu, Q.; Li, D.; Wang, T.; Li, F. miRNA Profiling of Chicken Follicles during Follicular Development. Sci. Rep. 2024, 14, 2212. [Google Scholar] [CrossRef]
- Isa, A.M.; Sun, Y.; Li, Y.; Wang, Y.; Ni, A.; Yuan, J.; Ma, H.; Shi, L.; Tesfay, H.H.; Fan, J.; et al. MicroRNAs with Non-Additive Expression in the Ovary of Hybrid Hens Target Genes Enriched in Key Reproductive Pathways That May Influence Heterosis for Egg Laying Traits. Front. Genet. 2022, 13, 974619. [Google Scholar] [CrossRef]
- Wu, H.; Fan, F.; Liang, C.; Zhou, Y.; Qiao, X.; Sun, Y.; Jiang, Y.; Kang, L. Variants of Pri-miR-26a-5p Polymorphisms Are Associated with Values for Chicken Egg Production Variables and Affects Abundance of Mature miRNA. Anim. Reprod. Sci. 2019, 201, 93–101. [Google Scholar] [CrossRef]
- Li, J.; Hou, L.; Sun, Y.; Xing, J.; Jiang, Y.; Kang, L. Single Nucleotide Polymorphism Rs737028527 (G > A) Affect miR-1b-3p Biogenesis and Effects on Chicken Egg-Laying Traits. Anim. Reprod. Sci. 2020, 218, 106476. [Google Scholar] [CrossRef]
- Yuan, J.; Chen, S.; Shi, F.; Wu, G.; Liu, A.; Yang, N.; Sun, C. Genome-Wide Association Study Reveals Putative Role of Gga-miR-15a in Controlling Feed Conversion Ratio in Layer Chickens. BMC Genom. 2017, 18, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.; Sun, L.; Ma, J.; Wang, J.; Qu, H.; Shu, D. Association of Single Nucleotide Polymorphisms in the Micro RNA miR-1596 Locus with Residual Feed Intake in Chickens. Anim. Genet. 2015, 46, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Marchesi, J.A.P.; Ono, R.K.; Cantão, M.E.; Ibelli, A.M.G.; Peixoto, J.d.O.; Moreira, G.C.M.; Godoy, T.F.; Coutinho, L.L.; Munari, D.P.; Ledur, M.C. Exploring the Genetic Architecture of Feed Efficiency Traits in Chickens. Sci. Rep. 2021, 11, 4622. [Google Scholar] [CrossRef] [PubMed]
- Chodkowska, K.A.; Barszcz, M.; Tuśnio, A. MicroRNA Expression and Oxidative Stress Markers in Pectoral Muscle of Broiler Chickens Fed Diets Supplemented with Phytobiotics Composition. Sci. Rep. 2024, 14, 4413. [Google Scholar] [CrossRef]
- Tian, W.-H.; Wang, Z.; Yue, Y.-X.; Li, H.; Li, Z.-J.; Han, R.-L.; Tian, Y.-D.; Kang, X.-T.; Liu, X.-J. miR-34a-5p Increases Hepatic Triglycerides and Total Cholesterol Levels by Regulating ACSL1 Protein Expression in Laying Hens. Int. J. Mol. Sci. 2019, 20, 4420. [Google Scholar] [CrossRef]
- Mashima, R. Physiological Roles of miR-155. Immunology 2015, 145, 323–333. [Google Scholar] [CrossRef]
- Wen, J.; Wu, Y.; Tian, Y.; Han, J.; Wang, Q.; Liu, Y.; Man, C. Circulating miR-155, a Potential Regulator of Immune Responses to Different Vaccines in Chicken. Res. Vet. Sci. 2022, 152, 670–677. [Google Scholar] [CrossRef]
- Bondada, M.S.; Yao, Y.; Nair, V. Multifunctional miR-155 Pathway in Avian Oncogenic Virus-Induced Neoplastic Diseases. Non-Coding RNA 2019, 5, 24. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Wen, J.; Zhang, W.; Zhang, R.; Xu, X.; Jiang, Y.; Wang, X.; Man, C. CircMYO1B/miR-155 Pathway Is a Common Mechanism of Stress-Induced Immunosuppression Affecting Immune Response to Three Vaccines in Chicken. Int. Immunopharmacol. 2024, 130, 111719. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.; Teng, M.; Li, H.-Z.; Ma, S.-M.; Lu, Q.-X.; Hao, H.-F.; Zhao, D.; Zhou, E.-M.; Zhang, G.-P.; Luo, J. Marek’s Disease Virus Type 1 Encoded Analog of miR-155 Promotes Proliferation of Chicken Embryo Fibroblast and DF-1 Cells by Targeting hnRNPAB. Vet. Microbiol. 2017, 207, 210–218. [Google Scholar] [CrossRef] [PubMed]
- Do, D.N.; Dudemaine, P.-L.; Mathur, M.; Suravajhala, P.; Zhao, X.; Ibeagha-Awemu, E.M. miRNA Regulatory Functions in Farm Animal Diseases, and Biomarker Potentials for Effective Therapies. Int. J. Mol. Sci. 2021, 22, 3080. [Google Scholar] [CrossRef]
- Hicks, J.A.; Liu, H.-C. Current State of Marek’s Disease Virus microRNA Research. Avian Dis. 2013, 57, 332–339. [Google Scholar] [CrossRef]
- Zhao, Y.; Zou, M.; Sun, Y.; Zhang, K.; Peng, X. Gga-miR-21 Modulates Mycoplasma gallisepticum (HS Strain)-Induced Inflammation via Targeting MAP3K1 and Activating MAPKs and NF-κB Pathways. Vet. Microbiol. 2019, 237, 108407. [Google Scholar] [CrossRef]
- Rayner, K.J.; Suárez, Y.; Dávalos, A.; Parathath, S.; Fitzgerald, M.L.; Tamehiro, N.; Fisher, E.A.; Moore, K.J.; Fernández-Hernando, C. MiR-33 Contributes to the Regulation of Cholesterol Homeostasis. Science 2010, 328, 1570–1573. [Google Scholar] [CrossRef]
- Lai, L.; Azzam, K.M.; Lin, W.-C.; Rai, P.; Lowe, J.M.; Gabor, K.A.; Madenspacher, J.H.; Aloor, J.J.; Parks, J.S.; Näär, A.M.; et al. MicroRNA-33 Regulates the Innate Immune Response via ATP Binding Cassette Transporter-Mediated Remodeling of Membrane Microdomains. J. Biol. Chem. 2016, 291, 19651–19660. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, Y.; Zou, M.; Wang, T.; Wang, L.; Peng, X. Lnc90386 Sponges miR-33-5p to Mediate Mycoplasma gallisepticum-Induced Inflammation and Apoptosis in Chickens via the JNK Pathway. Front. Immunol. 2022, 13, 887602. [Google Scholar] [CrossRef]
- Zhao, Y.; Hou, Y.; Zhang, K.; Yuan, B.; Peng, X. Identification of Differentially Expressed miRNAs through High-Throughput Sequencing in the Chicken Lung in Response to Mycoplasma Gallisepticum HS. Comp. Biochem. Physiol. Part D Genom. Proteom. 2017, 22, 146–156. [Google Scholar] [CrossRef]
- Zhao, Y.; Wang, Z.; Hou, Y.; Zhang, K.; Peng, X. Gga-miR-99a Targets SMARCA5 to Regulate Mycoplasma gallisepticum (HS Strain) Infection by Depressing Cell Proliferation in Chicken. Gene 2017, 627, 239–247. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Bi, D.; Hou, Y.; Zhao, Y.; Sun, J.; Peng, X. Gga-miR-101-3p Plays a Key Role in Mycoplasma gallisepticum (HS Strain) Infection of Chicken. Int. J. Mol. Sci. 2015, 16, 28669–28682. [Google Scholar] [CrossRef]
- Hu, Q.; Zhao, Y.; Wang, Z.; Hou, Y.; Bi, D.; Sun, J.; Peng, X. Chicken Gga-miR-19a Targets ZMYND11 and Plays an Important Role in Host Defense against Mycoplasma gallisepticum (HS Strain) Infection. Front. Cell. Infect. Microbiol. 2016, 6, 102. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Brahmakshatriya, V.; Zhu, H.; Lupiani, B.; Reddy, S.M.; Yoon, B.-J.; Gunaratne, P.H.; Kim, J.H.; Chen, R.; Wang, J.; et al. Identification of Differentially Expressed miRNAs in Chicken Lung and Trachea with Avian Influenza Virus Infection by a Deep Sequencing Approach. BMC Genom. 2009, 10, 512. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Brahmakshatriya, V.; Lupiani, B.; Reddy, S.M.; Soibam, B.; Benham, A.L.; Gunaratne, P.; Liu, H.; Trakooljul, N.; Ing, N.; et al. Integrated Analysis of microRNA Expression and mRNA Transcriptome in Lungs of Avian Influenza Virus Infected Broilers. BMC Genom. 2012, 13, 278. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Gao, Q.S.; Zhou, L.; Chen, Z.H.; Lu, S.; Huang, H.J.; Zhan, C.Y.; Xiang, M. MicroRNAs in Avian Influenza Virus H9N2-Infected and Non-Infected Chicken Embryo Fibroblasts. Genet. Mol. Res. GMR 2015, 14, 9081–9091. [Google Scholar] [CrossRef]
- O’Dowd, K.; Emam, M.; El Khili, M.R.; Emad, A.; Ibeagha-Awemu, E.M.; Gagnon, C.A.; Barjesteh, N. Distinct miRNA Profile of Cellular and Extracellular Vesicles Released from Chicken Tracheal Cells Following Avian Influenza Virus Infection. Vaccines 2020, 8, 438. [Google Scholar] [CrossRef]
- Vu, T.H.; Heo, J.; Kang, S.; Kim, C.; Lillehoj, H.S.; Hong, Y.H. Chicken miR-26a-5p Modulates MDA5 during Highly Pathogenic Avian Influenza Virus Infection. Dev. Comp. Immunol. 2023, 149, 104921. [Google Scholar] [CrossRef]
- Ma, X.; Wang, Q.; Xu, X.; Zhang, W.; Zhang, R.; Jiang, Y.; Wang, X.; Man, C. miR-214-PTEN Pathway Is a Potential Mechanism for Stress-Induced Immunosuppression Affecting Chicken Immune Response to Avian Influenza Virus Vaccine. Virology 2024, 595, 110094. [Google Scholar] [CrossRef]
- Wang, J.; Xing, Y.; Chen, L.; Han, S.; Wang, Y.; Zhao, Z.; Li, G.; Li, W.; He, H. Mechanisms of miR-18a-5p Target NEDD9-Mediated Suppression of H5N1 Influenza Virus in Mammalian and Avian Hosts. Vet. Sci. 2025, 12, 240. [Google Scholar] [CrossRef]
- Ivanyi, J.; Morris, R. Immunodeficiency in the Chicken. IV. An Immunological Study of Infectious Bursal Disease. Clin. Exp. Immunol. 1976, 23, 154–165. [Google Scholar]
- Mahgoub, H.A. An Overview of Infectious Bursal Disease. Arch. Virol. 2012, 157, 2047–2057. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Wang, S.; Liu, Y.; Qi, X.; Gao, Y. Advances on Adaptive Immune Responses Affected by Infectious Bursal Disease Virus in Chicken. Front. Immunol. 2024, 14, 1330576. [Google Scholar] [CrossRef] [PubMed]
- Berg, T.P.V.D. Acute Infectious Bursal Disease in Poultry: A Review. Avian Pathol. 2000, 29, 175–194. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.; Wang, Y.; Sun, H.; Zhang, X.; Xia, X. Inhibition of infectious bursal disease virus replication in chicken embryos by miRNAs delivered by recombinant avian adeno-associated viral vector. Wei Sheng Wu Xue Bao 2011, 51, 256–261. [Google Scholar]
- Ouyang, W.; Wang, Y.; Du, X.; Liu, H.; Zhang, H. Gga-miR-9* Inhibits IFN Production in Antiviral Innate Immunity by Targeting Interferon Regulatory Factor 2 to Promote IBDV Replication. Vet. Microbiol. 2015, 178, 41–49. [Google Scholar] [CrossRef]
- Ouyang, W.; Wang, Y.; Meng, K.; Pan, Q.; Wang, X.; Xia, X.; Zhu, Y.; Bi, Z.; Zhang, H.; Luo, K. Gga-miR-2127 Downregulates the Translation of Chicken P53 and Attenuates Chp53-Mediated Innate Immune Response against IBDV Infection. Vet. Microbiol. 2017, 198, 34–42. [Google Scholar] [CrossRef]
- Fu, M.; Wang, B.; Chen, X.; He, Z.; Wang, Y.; Li, X.; Cao, H.; Zheng, S.J. MicroRNA Gga-miR-130b Suppresses Infectious Bursal Disease Virus Replication via Targeting of the Viral Genome and Cellular Suppressors of Cytokine Signaling 5. J. Virol. 2018, 92, e01646-17. [Google Scholar] [CrossRef]
- Chen, Z.; Leng, M.; Liang, Z.; Zhu, P.; Chen, S.; Xie, Q.; Chen, F.; Lin, W. Gga-miR-20b-5p Inhibits Infectious Bursal Disease Virus Replication via Targeting Netrin 4. Vet. Microbiol. 2023, 279, 109676. [Google Scholar] [CrossRef]
- Huang, X.; Li, Y.; Li, J.; Jiang, Y.; Cui, W.; Zhou, H.; Tang, L. The Long Noncoding RNA Loc107053557 Acts as a Gga-miR-3530-5p Sponge to Suppress the Replication of vvIBDV through Regulating STAT1 Expression. Virulence 2024, 15, 2333237. [Google Scholar] [CrossRef]
- Jiang, Y.; Tian, Y.; Han, J.; Wang, X.; Zhang, R.; Xu, X.; Ma, X.; Zhang, W.; Man, C. CircITSN2-miR-17-5p/20a-5p/20b-5p-PD-L1 Regulatory Network Is a Potential Molecular Mechanism of PD-L1 Gene Involving in Immune Response to IBDV. Avian Pathol. J. WVPA 2025, 54, 512–520. [Google Scholar] [CrossRef] [PubMed]
- Payne, L.N.; Nair, V. The Long View: 40 Years of Avian Leukosis Research. Avian Pathol. J. WVPA 2012, 41, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Gao, Y.; Ji, X.; Qi, X.; Qin, L.; Gao, H.; Wang, Y.; Wang, X. Differential Expression of microRNAs in Avian Leukosis Virus Subgroup J-Induced Tumors. Vet. Microbiol. 2013, 162, 232–238. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhou, D.; Zhou, J.; Du, X.; Zhang, X.; Liu, X.; Ding, L.; Cheng, Z. miR-155 Facilitates the Synergistic Replication between Avian Leukosis Virus Subgroup J and Reticuloendotheliosis Virus by Targeting a Dual Pathway. J. Virol. 2023, 97, e0093723. [Google Scholar] [CrossRef]
- Li, H.; Shang, H.; Shu, D.; Zhang, H.; Ji, J.; Sun, B.; Li, H.; Xie, Q. Gga-miR-375 Plays a Key Role in Tumorigenesis Post Subgroup J Avian Leukosis Virus Infection. PLoS ONE 2014, 9, e90878. [Google Scholar] [CrossRef]
- Liu, D.; Dai, M.; Zhang, X.; Cao, W.; Liao, M. Subgroup J Avian Leukosis Virus Infection of Chicken Dendritic Cells Induces Apoptosis via the Aberrant Expression of microRNAs. Sci. Rep. 2016, 6, 20188. [Google Scholar] [CrossRef]
- Li, H.; Ji, J.; Xie, Q.; Shang, H.; Zhang, H.; Xin, X.; Chen, F.; Sun, B.; Xue, C.; Ma, J.; et al. Aberrant Expression of Liver microRNA in Chickens Infected with Subgroup J Avian Leukosis Virus. Virus Res. 2012, 169, 268–271. [Google Scholar] [CrossRef]
- Dai, Z.; Ji, J.; Yan, Y.; Lin, W.; Li, H.; Chen, F.; Liu, Y.; Chen, W.; Bi, Y.; Xie, Q. Role of Gga-miR-221 and Gga-miR-222 during Tumour Formation in Chickens Infected by Subgroup J Avian Leukosis Virus. Viruses 2015, 7, 6538–6551. [Google Scholar] [CrossRef]
- Li, Z.; Chen, B.; Feng, M.; Ouyang, H.; Zheng, M.; Ye, Q.; Nite, Q.; Zhang, X. MicroRNA-23b Promotes Avian Leukosis Virus Subgroup J (ALV-J) Replication by Targeting IRF1. Sci. Rep. 2015, 5, 10294. [Google Scholar] [CrossRef]
- Li, Z.; Luo, Q.; Xu, H.; Zheng, M.; Abdalla, B.A.; Feng, M.; Cai, B.; Zhang, X.; Nie, Q.; Zhang, X. MiR-34b-5p Suppresses Melanoma Differentiation-Associated Gene 5 (MDA5) Signaling Pathway to Promote Avian Leukosis Virus Subgroup J (ALV-J)-Infected Cells Proliferaction and ALV-J Replication. Front. Cell. Infect. Microbiol. 2017, 7, 17. [Google Scholar] [CrossRef][Green Version]
- Ji, J.; Shang, H.; Zhang, H.; Li, H.; Ma, J.; Bi, Y.; Xie, Q. Temporal Changes of microRNA Gga-Let-7b and Gga-Let-7i Expression in Chickens Challenged with Subgroup J Avian Leukosis Virus. Vet. Res. Commun. 2017, 41, 219–226. [Google Scholar] [CrossRef]
- Zhou, D.; Xue, J.; He, S.; Du, X.; Zhou, J.; Li, C.; Huang, L.; Nair, V.; Yao, Y.; Cheng, Z. Reticuloendotheliosis Virus and Avian Leukosis Virus Subgroup J Synergistically Increase the Accumulation of Exosomal miRNAs. Retrovirology 2018, 15, 45. [Google Scholar] [CrossRef]
- Burnside, J.; Morgan, R.W. Genomics and Marek’s Disease Virus. Cytogenet. Genome Res. 2007, 117, 376–387. [Google Scholar] [CrossRef]
- Teng, M.; Zhu, Z.J.; Yao, Y.; Nair, V.; Zhang, G.P.; Luo, J. Critical roles of non-coding RNAs in lifecycle and biology of Marek’s disease herpesvirus. Sci. China Life Sci. 2023, 66, 251–268. [Google Scholar] [CrossRef]
- Heidari, M.; Zhang, L.; Zhang, H. MicroRNA Profiling in the Bursae of Marek’s Disease Virus-Infected Resistant and Susceptible Chicken Lines. Genomics 2020, 112, 2564–2571. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhang, S.; Wang, G.; Feng, S.; Han, K.; Han, L.; Han, L. Role of microRNA and Long Non-Coding RNA in Marek’s Disease Tumorigenesis in Chicken. Res. Vet. Sci. 2021, 135, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Tian, F.; Luo, J.; Zhang, H.; Chang, S.; Song, J. MiRNA Expression Signatures Induced by Marek’s Disease Virus Infection in Chickens. Genomics 2012, 99, 152–159. [Google Scholar] [CrossRef] [PubMed]
- Teng, M.; Luo, J.; Zhang, Y.; Reddy, V.R.A.P.; Samuel, P.; Yao, Y.; Nair, V. Viral miRNA Delivered by Exosomes from Marek’s Disease Virus-Transformed Lymphoma Cell Line Exerts Regulatory Function in Internalized Primary Chicken Embryo Fibroblast Cells. Tumour Virus Res. 2024, 18, 200286. [Google Scholar] [CrossRef] [PubMed]
- Lian, L.; Li, X.; Zhao, C.; Han, B.; Qu, L.; Song, J.; Liu, C.; Yang, N. Chicken Gga-miR-181a Targets MYBL1 and Shows an Inhibitory Effect on Proliferation of Marek’s Disease Virus-Transformed Lymphoid Cell Line. Poult. Sci. 2015, 94, 2616–2621. [Google Scholar] [CrossRef]
- Lian, L.; Qu, L.; Chen, Y.; Lamont, S.J.; Yang, N. A Systematic Analysis of miRNA Transcriptome in Marek’s Disease Virus-Induced Lymphoma Reveals Novel and Differentially Expressed miRNAs. PLoS ONE 2012, 7, e51003. [Google Scholar] [CrossRef]
- Hicks, J.A.; Liu, H.-C. Impact of HVT Vaccination on Splenic miRNA Expression in Marek’s Disease Virus Infections. Genes 2019, 10, 115. [Google Scholar] [CrossRef]
- Stik, G.; Dambrine, G.; Pfeffer, S.; Rasschaert, D. The Oncogenic microRNA OncomiR-21 Overexpressed during Marek’s Disease Lymphomagenesis Is Transactivated by the Viral Oncoprotein Meq. J. Virol. 2013, 87, 80–93. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Lian, L.; Zhang, D.; Qu, L.; Yang, N. Gga-miR-26a Targets NEK6 and Suppresses Marek’s Disease Lymphoma Cell Proliferation. Poult. Sci. 2014, 93, 1097–1105. [Google Scholar] [CrossRef] [PubMed]
- Han, B.; Lian, L.; Li, X.; Zhao, C.; Qu, L.; Liu, C.; Song, J.; Yang, N. Chicken Gga-miR-103-3p Targets CCNE1 and TFDP2 and Inhibits MDCC-MSB1 Cell Migration. G3 2016, 6, 1277–1285. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Li, X.; Han, B.; You, Z.; Qu, L.; Liu, C.; Song, J.; Lian, L.; Yang, N. Gga-miR-219b Targeting BCL11B Suppresses Proliferation, Migration and Invasion of Marek’s Disease Tumor Cell MSB1. Sci. Rep. 2017, 7, 4247. [Google Scholar] [CrossRef]
- Heidari, M.; Zhang, H.; Sunkara, L. MDV-Induced Differential microRNA Expression in the Primary Lymphoid Organ of Thymus. Microb. Pathog. 2022, 170, 105688. [Google Scholar] [CrossRef]
- Fang, G.; Jia, X.; Li, H.; Tan, S.; Nie, Q.; Yu, H.; Yang, Y. Characterization of microRNA and mRNA Expression Profiles in Skin Tissue between Early-Feathering and Late-Feathering Chickens. BMC Genom. 2018, 19, 399. [Google Scholar] [CrossRef]
- Bao, W.; Greenwold, M.J.; Sawyer, R.H. Expressed miRNAs Target Feather Related mRNAs Involved in Cell Signaling, Cell Adhesion and Structure during Chicken Epidermal Development. Gene 2016, 591, 393–402. [Google Scholar] [CrossRef]
- Tu, Y.; Liu, Y.; Zhang, M.; Shan, Y.; Ji, G.; Ju, X.; Zou, J.; Shu, J. Identifying Signatures of Selection Related to Comb Development. J. Poult. Sci. 2021, 58, 5–11. [Google Scholar] [CrossRef]
- Ng, C.S.; Li, W.H. Genetic and Molecular Basis of Feather Diversity in Birds. Genome Biol. Evol. 2018, 10, 2572–2586. [Google Scholar] [CrossRef]
- Price-Waldman, R.; Stoddard, M.C. Avian coloration genetics: Recent advances and emerging questions. J. Heredity 2021, 112, 395–416. [Google Scholar] [CrossRef]
- Itoh, T.; Fukatani, K.; Nakashima, A.; Suzuki, K. MicroRNA-141-3p and microRNA-200a-3p Regulate α-Melanocyte Stimulating Hormone-Stimulated Melanogenesis by Directly Targeting Microphthalmia-Associated Transcription Factor. Sci. Rep. 2020, 10, 2149. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.; Luo, M.; Wang, L.; Yin, H.; Zhu, W.; Fu, J. MicroRNA-206 Regulation of Skin Pigmentation in Koi Carp (Cyprinus carpio L.). Front. Genet. 2020, 11, 47. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, H.; He, X.; Li, G.; Xu, H.; Jia, X.; Nie, Q.; Zhang, X. Deep Sequencing Analysis of miRNA Expression in Breast Muscle of Fast-Growing and Slow-Growing Broilers. Int. J. Mol. Sci. 2015, 16, 16242–16262. [Google Scholar] [CrossRef] [PubMed]
- Hicks, J.A.; Liu, H.-C. Centennial Review: Metabolic microRNA—Shifting Gears in the Regulation of Metabolic Pathways in Poultry. Poult. Sci. 2020, 100, 100856. [Google Scholar] [CrossRef]
- Hicks, J.A.; Porter, T.E.; Liu, H.-C. Identification of microRNAs Controlling Hepatic mRNA Levels for Metabolic Genes during the Metabolic Transition from Embryonic to Posthatch Development in the Chicken. BMC Genom. 2017, 18, 687. [Google Scholar] [CrossRef]
- Zhu, L.; Liao, R.; Huang, J.; Yan, H.; Xiao, C.; Yang, Y.; Wang, H.; Yang, C. The miR-216/miR-217 Cluster Regulates Lipid Metabolism in Laying Hens With Fatty Liver Syndrome via PPAR/SREBP Signaling Pathway. Front. Vet. Sci. 2022, 9, 913841. [Google Scholar] [CrossRef]
- Yue, Y.; Liu, Z.; Zhang, K.; Jia, Q.; Wang, D.; Wang, Z.; Guo, Y.; Li, D.; Jiang, R.; Li, Z.; et al. Functional and miRNA Regulatory Characteristics of INSIG Genes Highlight the Key Role of Lipid Synthesis in the Liver of Chicken (Gallus gallus). Poult. Sci. 2023, 102, 102380. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Xie, H.-L.; Yang, Y.-W.; Wen, J.; Liu, R.-R.; Zhao, G.-P.; Tan, X.-D.; Liu, Z.; Zheng, Y.; Zhang, J.-B. miR-375 Upregulates Lipid Metabolism and Inhibits Cell Proliferation Involved in Chicken Fatty Liver Formation and Inheritance via Targeting Recombination Signal Binding Protein for Immunoglobulin Kappa J Region (RBPJ). Poult. Sci. 2023, 102, 102218. [Google Scholar] [CrossRef]
- Safdar, M.; Özaslan, M. MicroRNAs as Potential Biomarkers for Heat Stress in Livestock. Zeugma Biol. Sci. 2023, 4, 6–12. [Google Scholar] [CrossRef]
- Ahanda, M.-L.E.; Zerjal, T.; Dhorne-Pollet, S.; Rau, A.; Cooksey, A.; Giuffra, E. Impact of the Genetic Background on the Composition of the Chicken Plasma MiRNome in Response to a Stress. PLoS ONE 2014, 9, e114598. [Google Scholar] [CrossRef] [PubMed]
- Dalloul, R.A.; Long, J.A.; Zimin, A.V.; Aslam, L.; Beal, K.; Ann Blomberg, L.; Bouffard, P.; Burt, D.W.; Crasta, O.; Crooijmans, R.P. Multi-Platform next-Generation Sequencing of the Domestic Turkey (Meleagris gallopavo): Genome Assembly and Analysis. PLoS Biol. 2010, 8, e1000475. [Google Scholar] [CrossRef] [PubMed]
- Lecchi, C.; Marques, A.; Redegalli, M.; Meani, S.; Vinco, L.; Bronzo, V.; Ceciliani, F. Circulating Extracellular miR-22, miR-155, and miR-365 as Candidate Biomarkers to Assess Transport-Related Stress in Turkeys. Animal 2016, 10, 1213–1217. [Google Scholar] [CrossRef] [PubMed]
- Velleman, S.; Harding, R. Regulation of Turkey Myogenic Satellite Cell Migration by MicroRNAs miR-128 and miR-24. Poult. Sci. 2017, 96, 1910–1917. [Google Scholar] [CrossRef]
- Wu, N.; Gu, T.; Lu, L.; Cao, Z.; Song, Q.; Wang, Z.; Zhang, Y.; Chang, G.; Xu, Q.; Chen, G. Roles of miRNA-1 and miRNA-133 in the Proliferation and Differentiation of Myoblasts in Duck Skeletal Muscle. J. Cell. Physiol. 2019, 234, 3490–3499. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, F.; Liang, Z.; Wu, Y.; Pi, J.; Wang, L.; Du, J.; Shen, J.; Pan, A.; Pu, Y. Analysis of miRNAs and Their Target Genes Associated with Mucosal Damage Caused by Transport Stress in the Mallard Duck Intestine. PLoS ONE 2020, 15, e0237699. [Google Scholar] [CrossRef]
- Luo, X.; Huang, L.; Guo, Y.; Yang, Y.; Gong, P.; Ye, S.; Wang, L.; Feng, Y. Identification of Potential Candidate miRNAs Related to Semen Quality in Seminal Plasma Extracellular Vesicles and Sperms of Male Duck (Anas Platyrhynchos). Poult. Sci. 2024, 103, 103928. [Google Scholar] [CrossRef]
- He, J.; Wang, W.; Lu, L.; Tian, Y.; Niu, D.; Ren, J.; Dong, L.; Sun, S.; Zhao, Y.; Chen, L. Analysis of miRNAs and Their Target Genes Associated with Lipid Metabolism in Duck Liver. Sci. Rep. 2016, 6, 27418. [Google Scholar] [CrossRef]
- Wang, Z.; Yang, C.; Dong, B.; Chen, A.; Song, Q.; Bai, H.; Jiang, Y.; Chang, G.; Chen, G. Whole Transcriptome Sequencing Reveals miRNAs and ceRNA Networks in Duck Abdominal Fat Deposition. Animals 2025, 15, 506. [Google Scholar] [CrossRef]
- Dellar, E.R.; Hill, C.; Melling, G.E.; Carter, D.R.; Baena-Lopez, L.A. Unpacking extracellular vesicles: RNA cargo loading and function. J. Exp. Biol. 2022, 1, e40. [Google Scholar]
- Lu, L.; Chen, Y.; Wang, Z.; Li, X.; Chen, W.; Tao, Z.; Shen, J.; Tian, Y.; Wang, D.; Li, G. The Goose Genome Sequence Leads to Insights into the Evolution of Waterfowl and Susceptibility to Fatty Liver. Genome Biol. 2015, 16, 89. [Google Scholar] [CrossRef]
- Yu, J.; He, K.; Ren, T.; Lou, Y.; Zhao, A. High-Throughput Sequencing Reveals Differential Expression of miRNAs in Prehierarchal Follicles of Laying and Brooding Geese. Physiol. Genom. 2016, 48, 455–463. [Google Scholar] [CrossRef]
- Guo, P.; Chen, J.; Luo, L.; Zhang, X.; Li, X.; Huang, Y.; Wu, Z.; Tian, Y. Identification of Differentially Expressed Genes and MicroRNAs in the Gray and White Feather Follicles of Shitou Geese. Animals 2024, 14, 1508. [Google Scholar] [CrossRef] [PubMed]
- He, K.; Ren, T.; Zhu, S.; Liang, S.; Zhao, A. Transiently Expressed Pattern during Myogenesis and Candidate miRNAs of Tmem8C in Goose. J. Genet. 2017, 96, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Zhang, H.; Li, J.; Tian, Y.; Xu, J.; Chen, L.; Wei, J.; Zhao, N.; Yang, X.; Zhang, W. Identification of Differentially Expressed miRNAs in the Fatty Liver of Landes Goose (Anser anser). Sci. Rep. 2017, 7, 16296. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Chen, J.; Dong, X.; Zhang, X.; Luo, W. Transcriptome Data Revealed the circRNA–miRNA–mRNA Regulatory Network during the Proliferation and Differentiation of Myoblasts in Shitou Goose. Animals 2024, 14, 576. [Google Scholar] [CrossRef]
- Li, R.; Wang, Y.; Xie, F.; Tong, X.; Li, X.; Ren, M.; Hu, Q.; Li, S. Construction and Analysis of miRNA–mRNA Interaction Network in Ovarian Tissue of Wanxi White Geese Across Different Breeding Stages. Animals 2024, 14, 3258. [Google Scholar] [CrossRef]
- Kocamis, H.; Hossain, M.M.; Cinar, M.; Salilew-Wondim, D.; Mohammadi-Sangcheshmeh, A.; Tesfaye, D.; Hölker, M.; Schellander, K. Expression of microRNA and microRNA Processing Machinery Genes during Early Quail (Coturnix japonica) Embryo Development. Poult. Sci. 2013, 92, 787–797. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, L.; Liu, Y.; Liu, Y.; Yang, J. Comprehensive Analysis of mRNA and miRNA Expression Profiles in Follicular Development in Quail. Br. Poult. Sci. 2025, 66, 439–452. [Google Scholar] [CrossRef]
- Chojnacka-Puchta, L.; Sawicka, D. CRISPR/Cas9 Gene Editing in a Chicken Model: Current Approaches and Applications. J. Appl. Genet. 2020, 61, 221–229. [Google Scholar] [CrossRef]
- Correia de Sousa, M.; Gjorgjieva, M.; Dolicka, D.; Sobolewski, C.; Foti, M. Deciphering miRNAs’ Action through miRNA Editing. Int. J. Mol. Sci. 2019, 20, 6249. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, K.; Matsuzaki, M.; Ezaki, R.; Horiuchi, H. Genome Editing in Chickens. Gene Genome Ed. 2022, 3, 100015. [Google Scholar] [CrossRef]
- Idoko-Akoh, A.; Goldhill, D.H.; Sheppard, C.M.; Bialy, D.; Quantrill, J.L.; Sukhova, K.; Brown, J.C.; Richardson, S.; Campbell, C.; Taylor, L. Creating Resistance to Avian Influenza Infection through Genome Editing of the ANP32 Gene Family. Nat. Commun. 2023, 14, 6136. [Google Scholar] [CrossRef] [PubMed]
- Parveen, A.; Mustafa, S.H.; Yadav, P.; Kumar, A. Applications of Machine Learning in miRNA Discovery and Target Prediction. Curr. Genom. 2019, 20, 537–544. [Google Scholar] [CrossRef]
- Amin, N.; McGrath, A.; Chen, Y.-P.P. Evaluation of Deep Learning in Non-Coding RNA Classification. Nat. Mach. Intell. 2019, 1, 246–256. [Google Scholar] [CrossRef]
- Taleb, H.M.; Mahrose, K.; Abdel-Halim, A.A.; Kasem, H.; Ramadan, G.S.; Fouad, A.M.; Khafaga, A.F.; Khalifa, N.E.; Kamal, M.; Salem, H.M. Using Artificial Intelligence to Improve Poultry Productivity–a Review. Ann. Anim. Sci. 2025, 25, 23–33. [Google Scholar] [CrossRef]
- Milosevic, B.; Ciric, S.; Lalic, N.; Milanovic, V.; Savic, Z.; Omerovic, I.; Doskovic, V.; Djordjevic, S.; Andjusic, L. Machine Learning Application in Growth and Health Prediction of Broiler Chickens. Worlds Poult. Sci. J. 2019, 75, 401–410. [Google Scholar] [CrossRef]
- Benefo, E.O.; Karanth, S.; Pradhan, A.K. A Machine Learning Approach to Identifying Salmonella Stress Response Genes in Isolates from Poultry Processing. Food Res. Int. 2024, 175, 113635. [Google Scholar] [CrossRef]
- Tian, H.; Cheng, L.; Liang, Y.; Lei, H.; Qin, M.; Li, X.; Ren, Y. MicroRNA Therapeutic Delivery Strategies: A Review. J. Drug Deliv. Sci. Technol. 2024, 93, 105430. [Google Scholar] [CrossRef]
- Dasgupta, I.; Chatterjee, A. Recent Advances in miRNA Delivery Systems. Methods Protoc. 2021, 4, 10. [Google Scholar] [CrossRef]
- Sartini, I.; Giorgi, M. Veterinary Pharmacology: A World Almost Unexplored with Huge Potential. Vet. Anim. Sci. 2022, 16, 100251. [Google Scholar] [CrossRef]
- Naeem, S.; Zhang, J.; Zhang, Y.; Wang, Y. Nucleic Acid Therapeutics: Past, Present, and Future. Mol. Ther. Nucleic Acids 2025, 36, 102440. [Google Scholar] [CrossRef]


| Name | Scientific Name | Genome Size (Gb) | Year of Complete Genomics | Number of Genes | Number of miRNAs | Version of Genome Assembly in NCBI |
|---|---|---|---|---|---|---|
| Chicken | Gallus gallus | 1.1 | 2004 | 18,023 | 474 | GCF_016699485.2 |
| Turkey | Meleagris gallopavo | 1.1 | 2010 | 17,974 | 353 | GCF_000146605.3 |
| Duck | Anas platyrhynchos | 1.3 | 2021 | 17,676 | ~300 | GCF_047663525.1 |
| Goose | Anser anser | 1.3 | 2014 | ~19,000 | ~250 | GCA_964211835.1 |
| Quail | Coturnix japonica | 1.24 | 2013 | ~17,000 | ~200 | GCF_001577835.2 |
| Guinea Fowl | Numida meleagris | 1 | 2021 | 21,846 | ~200 | GCF_002078875.1 |
| Ostrich | Struthio camelus | 1.5 | 2015 | 23,381 | Data limited | GCA_040807025.1 |
| Emu | Dromaius novaehollandiae | 1.5 | 2019 | 24,513 | Data limited | GCF_036370855.1 |
| Pheasant | Phasianus colchicus | 1 | Genome incomplete | 19,413 | Data limited | GCF_004143745.1 |
| Peacock | Pavo cristatus | 1 | Genome incomplete | 15,970–17,490 | 540 | GCA_045791835.1 |
| Trait | miRNAs | Target Genes |
|---|---|---|
| Muscle growth | miR-1 | HDAC4 |
| miR-206 | Pax7 | |
| miR-133 | SRF | |
| miR-27a | PPARγ | |
| miR-499 | SOX6 | |
| miR-208 | MSTN | |
| miR-29 | IGF-1 | |
| miR-486 | PTEN | |
| Egg production and quality | miR-202-5p | BMPR2 |
| miR-26a | PTEN | |
| miR-181a | ZP3 | |
| miR-143 | ERK5 | |
| miR-21 | PDCD4 | |
| miR-155 | SOCS1 | |
| miR-146a | IRAK1 | |
| Feed efficiency | miR-15a | FOXO1, PDPK1, |
| PRKAR2A | ||
| miR-142-5p | FOXO3 |
| Disease | Pathogens | Tissues | MiRNAs |
|---|---|---|---|
| Marek’s disease | Gallid herpesvirus 2 | Spleen and liver | miR-221, miR-140, miR-199, miR-181a, miR-146b, miR-146c, and miR-26a |
| Spleen | miR-15, miR-456 and let-7i | ||
| Spleen | miR-21 | ||
| Spleen and liver | miR-21 | ||
| Spleen | miR-26a | ||
| Spleen and liver | miR-103 | ||
| Spleen and liver | miR-219b | ||
| Avian leukosis virus | Avian leukosis virus | Liver | (miR-221, miR-222, miR-1456, miR-1704, miR-1777, miR-1790, and miR-2127), let-7b, let-7i, miR-125b, miR-375, and miR-458 |
| Liver | miR-375 | ||
| Liver | ga-miR-221, miR-193a, miR-193b, and miR-125b | ||
| Liver | miR-221, miR-222, | ||
| Liver | miR-23 | ||
| Liver | miR-34b-5p | ||
| Liver | let-7b and let-7i | ||
| Bursal disease | Bursal disease virus | miR-9 | |
| miR-2127 | |||
| miR-130b | |||
| Avian influenza | Avian influenza viruses | Lung and trachea | as miR-146, miR-15 or miR-21 |
| Lung | -miR-34a, 122–1, 122–2, 146a, 155, 206, 1719, 1594, 1599, and 451, | ||
| Embryo fibroblasts | miR-146c, miR-181a, miR-181b, miR-30b, miR-30c, miR-30e, and miR-455, miR-1599 and miR-1416 | ||
| Chronic respiratory disease (CRD) | Mycoplasma gallisepticum | miR-8 family, miR-499 family, miR-17 family | |
| miR-99a | |||
| miR-101-3p | |||
| Chicken embryonic lungs and DF-1 cells | miR-19a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, T.V.; Tat, T.H.; Do, D.N. MiRNAs in Poultry Health and Production: Progress and Challenges. Animals 2025, 15, 3230. https://doi.org/10.3390/ani15223230
Nguyen TV, Tat TH, Do DN. MiRNAs in Poultry Health and Production: Progress and Challenges. Animals. 2025; 15(22):3230. https://doi.org/10.3390/ani15223230
Chicago/Turabian StyleNguyen, Thanh Van, Tan Hy Tat, and Duy Ngoc Do. 2025. "MiRNAs in Poultry Health and Production: Progress and Challenges" Animals 15, no. 22: 3230. https://doi.org/10.3390/ani15223230
APA StyleNguyen, T. V., Tat, T. H., & Do, D. N. (2025). MiRNAs in Poultry Health and Production: Progress and Challenges. Animals, 15(22), 3230. https://doi.org/10.3390/ani15223230





