Simple Summary
Finches are a large and diverse group of birds found worldwide, yet little is known about the parasites of the family Syringophilidae that live inside their quill feathers. These parasites form close and often long-lasting relationships with their bird hosts. In this study, we investigated which species of syringophilid mites inhabit finches and how specific these mites are to particular bird species. We identified 20 different mite species on 51 finch species, including 4 species newly described for science. Our research indicates that most syringophilids are not strictly limited to a single bird species, but rather occupy birds that are closely related. Interestingly, we did not find any mites that switched between phylogenetically not closely related finch groups. These findings suggest that syringophilid mites and finches have evolved together over long periods of time. This kind of research helps us understand how parasites adapt to their hosts and how these hidden relationships shape biodiversity. It also shows the importance of studying old museum specimens, which can still reveal new scientific discoveries even after many decades.
Abstract
Quill mites belonging to the family Syringophilidae are highly specialised external parasites that live inside feather quills. However, parasitologists have limited knowledge about their diversity and patterns of host specificity, particularly regarding how restricted syringophilids are in their choice of avian hosts across different bird lineages. This research presents the first complete overview of mites of the subfamily Syringophiline found on True Finches (Fringillidae). Based on a combination of all published records and extensive examinations of museum specimens of fringillids housed at the Bavarian State Collection of Zoology in Munich, Germany, we report 20 mite species from 5 genera parasitising 51 finch species across all 3 subfamilies: Fringillinae, Euphoninae, and Carduelinae. Four species are described here as new to science: Aulobia ruppelae sp. n., Aulonastus ritzerfeldi sp. n., Torotrogla enucleator sp. n., and T. janhafti sp. n. Herein, we also recognised that most syringophilid mites associated with the True Finches, parasitise hosts belonging to the same or phylogenetically closely related genera, suggesting strong phylogenetic conservatism. The variation in host range and speciation rate across mite genera suggests that quill mite diversification does not always proceed synchronously with host evolution. These discoveries offer an important understanding of how host–parasite relationships evolve over time.
1. Introduction
Mites associated with birds comprise representatives of numerous families and display a remarkable diversity of ecological strategies, ranging from commensalism, in which the mite benefits without causing measurable harm to its host, to parasitism, which can lead to direct damage through feeding on host tissues or resources [1]. Over 2500 mite species from about 40 families are closely associated with birds, occupying virtually every conceivable niche on the host body, including the skin, respiratory passages, and feathers [2]. Among the most intriguing groups are the quill mites of the family Syringophilidae (Prostigmata: Cheyletoidea). As their name suggests, these mites inhabit the interior of feather quills (calamus), a microhabitat that also harbours members of several, often distantly related, mite families, such as the astigmatan families Ascouracaridae, Syringobiidae, and Kiwilichiidae belonging to the superfamily Pterolichoidea [3,4,5,6]; Apionacaridae, Dermoglyphidae, Gaudoglyphidae, and Laminosioptidae belonging to the superfamily Analgoidea [7,8,9,10,11,12]; and members of the tribes Cheletosomatini and Metacheyletiini of the prostigmatan family Cheyletidae, which are close relatives of the family Syringophilidae [13,14,15,16].
Unlike other quill mites, syringophilids are unique in feeding on the fluid tissues of their avian hosts, piercing the calamus wall with elongated, needle-like chelicerae to access host nutrients, lymph, or blood [17]. This specialised feeding mode, combined with pronounced host and habitat specificity, has driven their diversification into one of the most taxonomically rich groups of quill-inhabiting mites, with approximately 400 described species in 63 genera and 2 subfamilies, recorded from hosts representing 27 avian orders [18,19]. However, historical sampling of this mite fauna has often been fragmentary, with new species described opportunistically and without a broader taxonomic or ecological framework. Only recently have systematic surveys begun to target entire avian genera [20,21], families [22,23] or orders [24,25], revealing substantial hidden diversity and clarifying host–parasite relationships. As part of the continuing effort to characterise syringophilid diversity in passerines, the most species-rich avian order, this study concentrates on their occurrence on the representatives of the family Fringillidae (Passeriformes). This group of birds represents a widespread lineage of songbirds, making it an excellent model for exploring patterns of host specificity, diversity, and distribution of syringophilid mites.
The True Finches (Fringillidae) represent one of the most morphologically and ecologically diverse families among oscine passerines. Despite wide variation in plumage and bill shape, they share several core traits, including a predominantly granivorous and frugivorous diet that extends even to feeding nestlings. Their broad ecological tolerance allows them to inhabit environments ranging from arid scrublands to alpine zones and tropical rainforests [26]. The Fringillidae family is nested within the superfamily Passeroidea, one of the most extensive and evolutionarily successful radiations among oscine passerines. Although early molecular data suggested a sister-group relationship with Passeridae [27], most subsequent analyses support a closer affinity between Fringillidae and the New World nine-primaried oscines (Passerellidae, Thraupidae, Icteridae, Cardinalidae, and Parulidae) [28,29,30,31,32,33]. Within Fringillidae, three subfamilies are currently recognised: Fringillinae represents the most basal lineage; Euphoniinae, formerly classified within Thraupidae; and Carduelinae, the most species-rich group [26,33,34].
The wide distribution and complex evolutionary history of Fringillidae, most diverse in the Old World, particularly in Eurasia and Africa, make its members especially valuable for studying host–parasite associations. Among their diverse ectoparasites, quill mites represent a group whose diversity and host relationships remain incompletely understood. To address this gap, we present a comprehensive assessment of Syringophilinae, one of the two subfamilies of syringophilid mites associated with the True Finches, focusing on species richness, patterns of host specificity, and implications for host–parasite coevolution. By examining a broad taxonomic spectrum of Fringillidae, we provide new data that advance an integrated understanding of quill mite diversity and the evolutionary dynamics of finch–mite interactions. In addition to ecological and evolutionary analyses, we also provide an identification key to the syringophilid species parasitising Fringillidae, updated diagnoses of previously known species, and descriptions of four new species. These systematic contributions form the basis for a more accurate taxonomic and evolutionary framework of these mites and their hosts.
2. Materials and Methods
The newly acquired material of quill mites was obtained from dry-preserved bird specimens deposited at the Bavarian State Collection of Zoology (SNSB-ZSM) in Munich, Germany. From each finch specimen, a standardised set of feathers was selected for examination, i.e., approximately ten contour feathers from the cloaca region, along with two upper-tail coverts, two under-tail coverts, and one secondary wing covert. Selected feathers were screened for the presence of syringophilid mites. The infested quills were placed in Nesbitt’s solution and kept at room temperature for three days to allow softening of mite tissues [35,36]. Subsequently, each quill was longitudinally dissected with fine-tipped forceps to extract the mites. The recovered specimens were rinsed in 70% ethanol and mounted on permanent microscope slides in Hoyer’s medium [37] for morphological analysis. Slide-mounted mites were examined using a ZEISS Axioscope light microscope (Carl Zeiss AG, Oberkochen, Germany) fitted with differential interference contrast (DIC) optics and a camera lucida.
In the description, the terminology for idiosomal setation adheres to Grandjean’s system [38], as modified for Prostigmata by Kethley [39]. Leg chaetotaxy follows Grandjean’s nomenclature [40], while general morphological terminology is based on Kethley [41] and Skoracki [42]. All measurements are expressed in micrometres, with ranges for paratypes provided in brackets following the holotype data.
Collected mite specimens have been deposited in two institutional collections: the Department of Animal Morphology at Adam Mickiewicz University in Poznań (AMU), Poland, and the Bavarian State Collection for Zoology (SNSB-ZSM), Germany.
Avian taxonomy and nomenclature adhere to the classifications of Winkler et al. [26] and the Clements et al. [43].
The geographical distributions of host species were determined using BirdLife International data [44,45]. The delineation of zoogeographic regions relies on the frameworks proposed by Holt et al. [46] and Ficetola et al. [47]
Host specificity for particular mite species follows Caira et al. [48] and Skoracki et al. [49]. In this context, we delineate four distinct categories: monoxenous parasites which are limited to a singular host species; oligoxenous parasites inhabiting multiple host species, yet all are contained within the same genus; mesostenoxenous parasites are linked with host species from various genera, while still being classified within the same subfamily; and metastenoxenous parasites utilise hosts from diverse subfamilies, but remain confined to the family Fringillidae.
The bar chart was generated in the R (ver. 4.0.) statistical environment [50].
3. Results
3.1. Syringophilinae Species Richness Associated with the True Finches
3.1.1. Aulobia cardueli Skoracki, Hendricks & Spicer, 2010
Diagnosis. Female. Total body length 815–855. Infracapitulum densely punctate. Each medial branch of peritremes with 3–4 chambers, each lateral branch with 18–20 chambers. Stylophore 180 long. Propodonotal shield punctate near bases of setae ve and si. Hysteronotal shield not fused to pygidial shield, apunctate. Pygidial shield, large and punctate posteriorly. Coxal fields I–IV punctate. Fan-like tarsal setae III–IV p′ and p″ with 7–8 tines. Lengths of setae: vi 30–40, ve 40–60, si 50–75, se 205–225, c1 215–245, c2 210–235, d1 160–190, d2 160–185, e2 140–170, f1 60–85, f2 190–210, h1 60–90, h2 295–325, ps1 and ps2 25–30, g1 and g2 55–60, ag1 70–75, ag2 75–80, ag3 100–115, 3b 35–40, 3c 70–80.
Hosts and Distribution
Mesostenoxenous parasite recorded from representatives of the subfamily Carduelinae in the Holarctic region: Redpoll Acanthis flammea (Linnaeus) in Poland, Slovakia and Germany [42,51]; European Goldfinch Carduelis carduelis (Linnaeus) in Kazakhstan and Poland [42,52]; Citril Finch Carduelis citrinella (Pallas) in Poland [42]; Twite Linaria flavirostris (Linnaeus) in Poland and Germany [42,51]; Lesser Goldfinch Spinus psaltria (Say) in the United States [53], and Eurasian Siskin Spinus spinus (Linnaeus) in Russia and Kazakhstan [42].
3.1.2. Aulobia leucostictus Skoracki, 2011
Diagnosis. Female. Total body length 700–745. Infracapitulum sparsely punctate. Each medial branch of peritremes with 3–4 chambers, each lateral branch with 11–13 chambers. Stylophore 200–215 long. Propodonotal shield punctate near bases of setae ve and si. Hysteronotal shield small and weakly sclerotised, not fused to pygidial shield, apunctate. Pygidial shield apunctate. Coxal fields I–IV punctate. Fan-like tarsal setae III–IV p′ and p″ with 9–11 tines. Lengths of setae: vi 45–55, ve 60–70, si 75–95, se 280–305, c1 280 315, c2 280–310, d1 210–270, d2 220–240, e2 285–205, f1 65–85, f2 200–285, h1 60–80, h2 355–395, ps1 and ps2 25–35, g1 and g2 65–75, ag1 100–115, ag2 80–95, ag3 125–135, 3b 25, 3c 70.
Host and Distribution
Monoxenous parasite associated with a member of the subfamily Carduelinae: Asian Rosy-Finch Leucosticte arctoa (Pallas) in Japan (current paper).
New Material Examined
Ex Leucosticte arctoa (host reg. no. SNSB-ZSM 1339, male); Japan: Hokkaido, Sapporo, May 1896, coll. A. Owston—four females and one male deposited in the AMU (reg. no. AMU-MS 24-1025-095).
Remarks
In a previous publication [42], the host Leucosticte arctoa was reported as originating from Europe. However, this is almost certainly a labelling error, as none of the currently recognised subspecies of L. arctoa are known to occur in the European continent. The nominate subspecies L. a. arctoa inhabits south-central Russian Siberia, northeastern Kazakhstan, and northwestern Mongolia; L. a. cognata occurs from the eastern Sayan region to central Baikal and northern Mongolia; and L. a. sushkini is restricted to the Khangai Mountains in west-central Mongolia [54]. Therefore, we conclude that the type host identification is valid, but the geographic label indicating “Europe” as the collection locality is incorrect. We thus exclude Europe from the distributional range of both Aulobia leucostictus and its host association.
3.1.3. Aulobia ruppelae sp. n. (Figure 1 and Figure 2)
Description. Female, holotype. Total body length 870 (780–910 in 10 paratypes). Gnathostoma. Infracapitulum apunctate. Hypostomal apex without protuberances but with two pairs of unequal in size, finger-like lips. Stylophore rounded posteriorly, 240 (210–235) long, exposed portion of stylophore apunctate, 200 (180–195) long. Length of movable cheliceral digit 170 (160–170). Medial branch of peritremes with 3 chambers, each lateral branch with 10–11 delicately striated chambers. Idiosoma. Propodonotal shield well-sclerotised, 160 (155–160) long, 120 (120–125) wide on level of setae si, rectangular in shape, punctate between bases of setae ve and si, bearing all propodonotal setae except c2. Setae se and c1 situated at same transverse level. Setae vi, ve, and si short and subequal in length. Hysteronotal shield absent. Pygidial shield with rounded anterior margin, well-sclerotised, apunctate, 100 (100–105) long and 80 (75–80) wide. Setae f2 about 3 times longer than f1. Setae f1 and h1 subequal in length. Both pairs of pseudanal setae ps1 and ps2 equal in length. All coxal fields well-sclerotised and sparsely punctate. Setae ag1 slightly (1.1–1.3 times) longer than ag2. Genital plate present, bearing bases of genital setae g1 and g2, bases of setae ag2 and ag3 situated out of this plate. Both pairs of genital setae equal in length. Lengths of setae: vi 40 (35–45), ve 45 (45–55), si 45 (45–70), se (260–310), c1 300 (300–330), c2 285 (270–285), d1 variable in length 130 (100–230), d2 variable in length (100–210), e2 variable in length 145 (130–215), f1 70 (70–90), f2 230 (230–250), h1 75 (70–90), h2 (320–370), ps1 and ps2 20 (20–30), g1 and g2 60 (50–60), ag1 100 (105–110), ag2 75 (80–90), ag3 150 (140–150), 3b 35 (35–40), 3c 80 (80–95), 4b 40 (30–40), 4c 85 (85–90), l’RIII 55 (45–55), l’RIV 40 (30–40).
Male. Total body length 580–600 in three paratypes. Gnathostoma. Infracapitulum apunctate. Hypostomal apex without protuberances. Stylophore slightly constricted posteriorly, 190–195 long, exposed portion of stylophore apunctate, 160–165 long. Length of movable cheliceral digit 150. Medial branch of peritremes with 3–4 chambers, each lateral branch with 9–11. Idiosoma. Propodonotal shield 120–125 long, 90–95 wide on level of setae si, weakly sclerotised, punctate laterally, bearing bases of setae vi, ve, si, and c1, bases of setae se on or near this shield, bases c2 out of this shield. Setae se situated anterior to level of setae c1. Setae vi, ve, and si short and subequal in length. Hysteronotal shield apunctate, weakly sclerotised, bearing bases of setae d1 and e2. Hysteronotal setae d1, d2, and e2 short. Pygidial shield small, apunctate and weakly sclerotised, anterior margin indiscernible, bearing bases of setae f2 and h2 on lateral margins. Aggenital series with three pairs of setae. Lengths of setae: vi 30–40, ve 30–50, si 20–35, se 165–175, c1 155–165, c2 145–160, d1 10–15, d2 20, e2 10–15, f2 20, h2 175, ag1 40–55, ag2 30, ag3 50, 3b 25–30, 3c 50–60, 4b 25–30, 4c 50–60, l’RIII 30–40, l’RIV 25–30.
Type Material
Female holotype and paratypes: 10 females and 3 males from Pallas’s Rosefinch Carpodacus roseus (Pallas) (Carduelinae) (host reg. no. SNSB-ZSM 03.1693, male); Russia: Amur Region, 19 March 1903, coll. R. Tancre.
Type Material Deposition
Holotype and paratypes are deposited in the SNSB-ZSM (reg. no. ZSMA20250027), except three female paratypes and one male paratype deposited in the AMU (reg. no AMU-MS 24-1025-081).
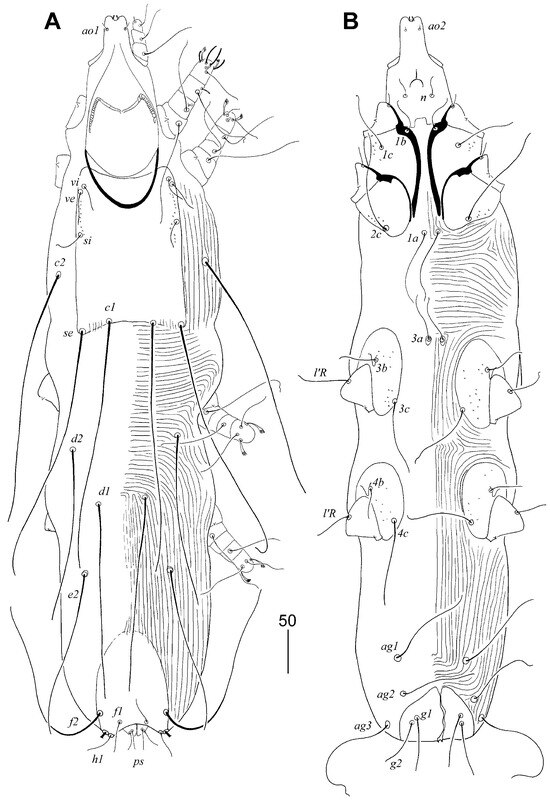
Figure 1.
Aulobia ruppelae sp. n., female. (A) Dorsal view; (B) ventral view.
Additional Material
Ex. Beautiful Rosefinch Carpodacus pulcherrimus (Moore) (Carduelinae) (host reg. no. SNSB-ZSM 62.2767, male); Nepal: Khumjung, Khumbu, 20 July 1962, coll. unknown—four females and one male deposited in the AMU (reg. no. AMU-MS 24-1025-072).
Differential Diagnosis
Aulobia ruppelae sp. n. is morphologically similar to Aulobia cardueli Skoracki, Hendricks and Spicer, 2010. In females of both species, the hypostomal apex bears two pairs of unequal lips, and the propodonotal setae vi, ve, and si are short and subequal in length. This new species can be easily distinguished from A. cardueli by the following features: in females of A. ruppelae sp. n., the infracapitulum is apunctate, each lateral branch of the peritremes has 10–11 chambers, and the hysteronotal shield is absent. In contrast, in females of A. cardueli, the infracapitulum is densely punctate, each lateral branch of the peritremes has 18–20 chambers, and the hysteronotal shield is present.
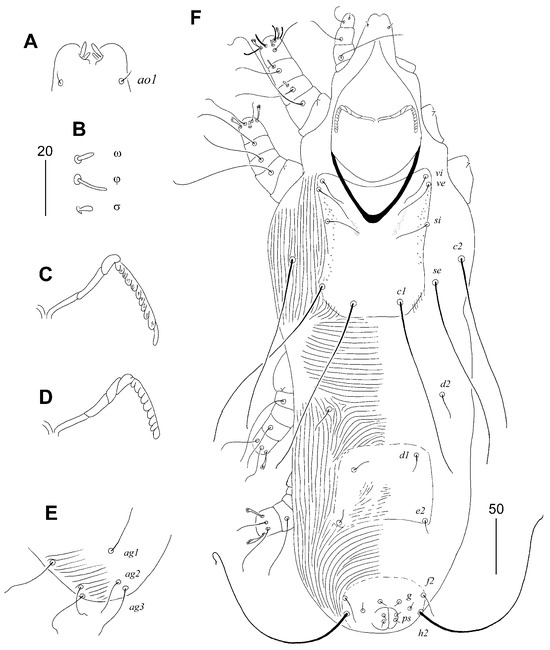
Figure 2.
Aulobia ruppelae sp. n., female (A–C): (A) hypostomal apex; (B) solenidia of legs I; (C) peritreme. Male (D–F): (D) peritreme; (E) opisthosoma in ventral view; (F) body in dorsal view. Scale bars: (A–D) = 20 µm, (E,F) = 50 µm.
Etymology
This species is named in honour of Barbara Ruppel, a scientific illustrator with a deep interest in nature. She has long been a friend to us, and it is a pleasure that she is now finally receiving a species dedicated to her.
3.1.4. Aulonastus fringillus Skoracki, 2011
Diagnosis. Females. Total body length 450–480. Infracapitulum sparsely punctate. Each medial branch of peritremes with 2 chambers, each lateral branch with 4–5 chambers. Stylophore 120–125 long. Propodonotal shield punctate. Setae c1 1.2–1.3 times longer than se. Length ratio of setae d2:c1 1:1.9–2. Hysteronotal shield fused to pygidial shield, apunctate. Setae f2 twice as long as f1. Setae h2 6.6–7.6 times longer than f2. Aggenital setae ag1 and ag2 subequal in length. All coxal fields apunctate. Fan-like tarsal setae III–IV p′ and p″ of legs III and IV with six tines. Lengths of setae: se 120–125, c1 140–165, c2 90–100, d2 80–90, f1 20, f2 40–50, h1 15–20, h2 305–330, ag1 90–95, ag2 80–90, ag3 125–135.
Host and Distribution
Monoxenous parasite associated with Common Chaffinch Fringilla coelebs Linnaeus (Fringillinae) in Poland [42].
Remarks
The mite material collected from Linaria cannabina was previously misidentified as Aulonastus fringillus [51]. However, re-examination of the specimens from this host clearly indicates that the material belongs to Aulonastus loxius. Consequently, Linaria cannabina should be excluded from the host spectrum of Aulonastus fringillus and instead included in the host spectrum of Aulonastus loxius (see below).
3.1.5. Aulonastus loxius Skoracki, 2011
Diagnosis. Females. Total body length 400–445. Infracapitulum apunctate. Each medial branch of peritremes with 2 chambers, each lateral branch with 3–4 chambers. Stylophore 120–130 long. Propodonotal shield punctate. Setae c1 1.4 times longer than se. Length ratio of setae d2:c1 1:1.3. Hysteronotal shield fused to pygidial shield, apunctate. Setae f2 about four times longer than f1. Setae h2 2.7–3.2 times longer than f2. Setae ag1 1.2 times longer than ag2. Coxal fields IV sparsely punctate. Fan-like tarsal setae III–IV p′ and p″ of III–IV legs with 6–7 tines. Lengths of setae: se 145–170, c1 170–205, c2 120–155, d2 130–145, f1 25, f2 80–105, h1 20–25, h2 255–325, ag1 70–80, ag2 60–70, ag3 100–120.
Hosts and Distribution
Mesostenoxenous parasite associated with birds of the subfamily Carduelinae: Red Crossbill Loxia curvirostra Linnaeus in Poland [42], and Eurasian Linnet Linaria cannabina (Linnaeus) (Carduelinae) in Germany ([51], current paper).
New Material Examined
Ex Linaria cannabina; Germany: Darmstadt, Griesheim, March 1972, coll. H. Friemann—2 females deposited in the AMU (reg. no. AMU-MS 25-0516-001).
3.1.6. Aulonastus neotropicalis Sikora, Unsoeld, Melzer, Friedrich, Hromada and Skoracki, 2025
Diagnosis. Females. Total body length 460–475. Infracapitulum apunctate. Each medial branch of peritremes with two chambers; each lateral branch with five chambers. Stylophore 130–140 long. Propodonotal shield apunctate. Setae c1 1.2 times longer than se. Length ratio of setae d2:c1 1:1.2. Hysteronotal shield fused to pygidial shield, apunctate. Setae f2 3–3.5 times longer than f1. Setae h2 4–4.4 times longer than f2. Aggenital setae ag1 1.3–1.7 longer than ag2. All coxal fields apunctate. Fan-like setae p′ and p″ of legs III and IV with 5–6 tines. Lengths of setae: se 160–180, c1 190–210, c2 150–165, d2 130–165, f1 20–25, f2 70–80, h1 20–25, h2 270–320, ag1 65–85, ag2 40–50, ag3 105–110.
Hosts and Distribution
Mesostenoxenous parasite associated with birds of the subfamily Euphoninae: Blue-naped Chlorophonia Chlorophonia cyanea (Thunberg) in Venezuela and Bolivia; Lesser Antillean Euphonia Chlorophonia flavifrons (Sparrman) in Guadeloupe (Lesser Antillean Creole); Golden-rumped Euphonia Chlorophonia cyanocephala (Vieillot), Scrub Euphonia Euphonia affinis (Lesson), Velvet-fronted Euphonia Euphonia concinna Sclater, all three in Colombia; Yellow-crowned Euphonia Euphonia luteicapilla (Cabanis) in Panama; Tawny-capped Euphonia Euphonia anneae Cassin in Costa Rica; Orange-bellied Euphonia Euphonia xanthogaster Sundevall in Peru; White-lored Euphonia Euphonia chrysopasta Sclater and Salvin in Venezuela, and Golden-sided Euphonia Euphonia cayennensis (Gmelin) in French Guiana [55].
3.1.7. Aulonastus ritzerfeldi sp. n. (Figure 3)
Description. Female, holotype. Total body length 510 (480–540 in six paratypes). Gnathostoma. Infracapitulum apunctate. Hypostomal apex smooth, without protuberances. Stylophore rounded posteriorly, 140 (140–145) long, exposed portion of stylophore apunctate, 105 (100–105) long. Movable cheliceral digit 95 (95) long. Medial branch of peritremes with two chambers, each lateral branch with four delicately striated chambers. Idiosoma. Propodonotal shield weakly sclerotised, 115 (115–120) long, 60 (60–65) wide on level of setae si, rectangular in shape, apunctate, bearing bases of setae ve, si and c1, bases of setae se situated on or near this shield, bases of setae c2 out of this shield. Setae se and c1 situated at same transverse level, or setae se slightly anterior to level of setae c1. Setae ve and si short and subequal in length. Setae c1 1.4–1.5 times longer than se. Hysteronotal shield apunctate, fused to pygidial shield, anterior margin reach level of setal bases d1, hysteronotal shield 170 long and 40 wide on level of setae e2. Setae d2 distinctly (9–13 times) longer than d1 and e2 but about 2 times shorter than c1. Setae f2 3 times longer than f1. Setae f1 and h1 subequal in length. Both pairs of genital setae equal in length. All coxal fields weakly sclerotised and sparsely punctate. Aggenital setae ag1 and ag2 subequal in length. Genital plate absent. Legs. Fan-like tarsal setae p′ and p″ of legs III and IV with 7–8 tines. Lengths of setae: ve 20 (15–20), si 20 (15–20), se 125 (120–140), c1 190 (170–190), c2 115 (115–125), d1 10 (10), d2 80 (70–80), e2 15 (10–15), f1 (20–25), f2 60 (60–70), h1 20 (20–25), h2 (305–340), ps1 15 (15), g1 and g2 25 (25), ag1 95 (85–95), ag2 100 (95–105), ag3 130 (135–150), 3b 25 (20–30), 3c 60 (55–60), 4b 25 (25), 4c (60–65), l′RIII 30 (30–35), l′RIV (25–30).
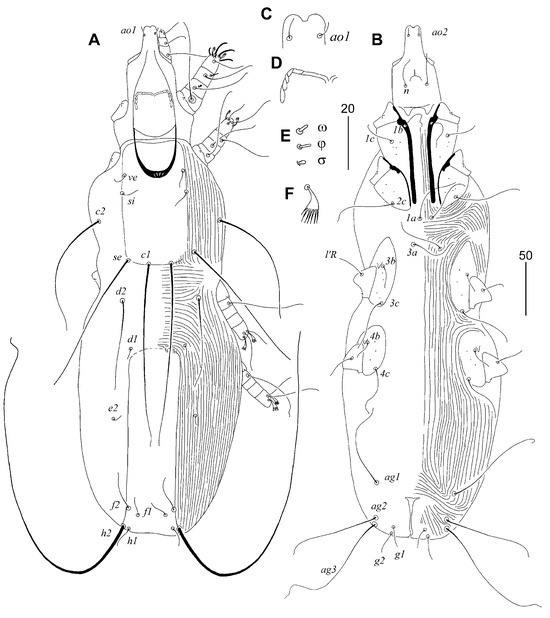
Figure 3.
Aulonastus ritzerfeldi sp. n., female: (A) dorsal view; (B) ventral view; (C) hypostomal apex; (D) peritreme; (E) solenidia of leg I; (F) fan-like tarsal seta III. Scale bars: (A,B) = 50 µm, (C–F) = 20 µm.
Male. Not found.
Type Material
Female holotype and six female paratypes from Asian Rosy-Finch Leucosticte arctoa (Pallas) (Carduelinae) (host reg. no. ZSM 28.1041, male); Japan: Kobe, 16 April 1915, coll. J. Gengler.
Type Material Deposition
Holotype and most paratypes are deposited in the SNSB-ZSM (reg. no. ZSMA20250028), except two female paratypes in the AMU (reg. no. MS 24-1025-094).
Etymology
This species is named in honour of our colleague and good friend Marc Ritzerfeld from the SNSB, Bavarian State Collection for Zoology, for his great expertise and tireless diligence in recording and maintaining natural history collection data.
Differential Diagnosis
Aulonastus ritzerfeldi sp. n. is morphologically similar to A. fringillus Skoracki, 2011. In females of both species, each medial and lateral branch of the peritremes has a similar number of chambers (two and four, respectively); the movable cheliceral digit is 90 µm long; setae se and c1 are situated at the same transverse level; setae c1 are approximately twice as long as d2; aggenital setae ag1 and ag2 are subequal in length; the genital plate is absent; and both pairs of genital setae are subequal in length. This new species differs from A. fringillus in the following features: in females of A. ritzerfeldi sp. n., the infracapitulum and the propodonotal shield are apunctate; the length of the stylophore is 140–145 µm; the propodonotal shield is rectangular in shape; the anterior margin of the hysteronotal shield reach the level of the setal bases d1; setae f2 are approximately three times longer than f1; all coxal fields are sparsely punctate; fan-like setae p′ and p″ of legs III and IV have 7–8 tines, and the lengths of setae are c1 and f2 are 170–190 µm and 60–70 µm, respectively. In contrast, in females of A. fringillus, the infracapitulum and the propodonotal shield are punctate; the stylophore is 120–125 µm long; the propodonotal shield has a concave anterior margin; the anterior margin of the hysteronotal shield not reach the level of the setal bases d1; setae f2 are about twice as long as f1; all coxal fields are apunctate; fan-like setae p′ and p″ of legs III and IV have six tines, and the lengths of setae c1 and f2 are: 140–165 µm and 40–50 µm, respectively.
3.1.8. Syringophiloidus carpodaci Bochkov and Apanaskevich, 2001
Diagnosis. Females. Total body length 690–745. Infracapitulum densely punctate. Each medial branch of peritremes with 7–8 chambers, each lateral branch with 8–9 chambers. Stylophore 155–160 long. Propodonotal shield punctate in anterior part. Length ratio of setae vi:ve:si 1:1.6–1.8:3. Propodonotal setae thin with discernible ornament. Hysteronotal shield apunctate, not fused to pygidial shield. Pygidial shield punctate in posterior part. Setae d2 1.2–1.3 times longer than e2. Length ratio of setae ag1:ag2:ag3 1:1:1.2. Setae ps1 and ps2 subequal in length. Genital plate weakly sclerotised. Coxal fields I–IV punctate. Fan-like setae p′ and p″ of legs III and IV with 7–8 tines. Lengths of setae: vi 40–45, ve 70–75, si 125–130, se 230, c1 220–230, c2 225, d1 140–180, d2 180–215, e2 140–175, f1 and h1 40, f2 235–275, h2 300–320, ps1 and ps2 25, g1 and g2 40–45, ag1 170–185, ag2 180–215, ag3 225–230, tc′III–IV 35, tc″III–IV 65–70, 3b 25, 3c 100–110, l′RIII 35.
Hosts and Distribution
Oligoxenous parasite associated with birds of the genus Carpodacus (Carduelinae): Common Rosefinch Carpodacus erythrinus (Pallas) in Poland, Kazakhstan, Russia, and Nepal ([42,56], current paper), and Scarlet Finch Carpodacus sipahi (Hodgson) in Nepal [current paper].
New Material Examined
Ex Carpodacus erythrinus (host reg. no. ZSM 62.1712, male); Nepal: Khumjung, Khumbu, 7 August 1962, coll. unknown—eight females and four males deposited in the AMU (reg. no. AMU-MS 24-1025-065).
Ex Carpodacus sipahi (host reg. no. ZSM A.1246, male); Nepal: no other data—10 females and 4 males deposited in the AMU (reg. no. AMU-MS 24-1025-074).
3.1.9. Syringophiloidus coccothraustes Skoracki, 2011
Diagnosis. Females. Total body length 680–720. Infracapitulum sparsely punctate. Each medial branch of peritremes with 2–3 chambers, each lateral branch with 8–9 chambers. Stylophore 140–145 long. Propodonotal shield sparsely punctate on whole surface. Length ratio of setae vi:ve:si 1:1:2–3. Setae vi and ve thin and smooth, other propodonotal setae with delicate ornament. Hysteronotal shield not fused to pygidial shield, sparsely punctate. Setae d2 and e2 subequal in length. Pygidial shield densely punctate posteriorly. Length ratio ag1:ag2:ag3 1:1:1–1.2. Setae ps2 1.7 times longer than ps1. Genital plate present. Coxal fields I–IV punctate. Fan-like setae p′ and p″ of legs III and IV with six tines. Lengths of setae: vi 25–35, ve 25–35, si 55–90, se 195–200, c1 190–205, c2 160–170, d1 145–160, d2 140–170, e2 140–150, f1 30, f2 200–230, h1 30, h2 305–330, ps1 12–15, ps2 20–25, g1 35–40, g2 35, ag1 130–160, ag2 140–155, ag3 145–175.
Host and Distribution
Monoxenous parasite associated with Hawfinch Coccothraustes coccothraustes (Linnaeus) (Carduelinae) in Poland [42].
3.1.10. Syringophiloidus klimovi Skoracki and Bochkov, 2010
Diagnosis. Females. Total body length 680–705. Infracapitulum punctate. Each medial branch of peritremes with 2 chambers, each lateral branch with 6–7 chambers. Stylophore apunctate, 145 long. Propodonotal shield punctate anteriorly. Setae vi, ve, and si thin and smooth. Setae se and c1 enlarged in basal part. Setae vi and ve subequal in length, both 1.8–2.2 times shorter than si. Hysteronotal shield absent, punctate area near bases of setae d2 present. Small pygidial shield punctate and restricted to bases of setae f1, f2, h1 and h2. Setae h2 1.5 times longer than f2. Setae ag1 and ag3 slightly (1.2–1.4 times) longer than ag2. Setae ps1 and ps2 subequal in length. Coxal fields I–IV punctate. Fan-like setae p′ and p″ of legs III and IV with 7–9 tines. Lengths of setae: vi 30–40, ve 30–40, si 70–75, se 140–150, c1 155–170, c2 130–150, d1 105, d2 50–55, e2 110, f1 30, f2 170–180, h1 30–35, h2 255, ps1 and ps2 15–20, g1 and g2 30, ag1 115–125, ag2 100, ag3 130–140.
Hosts and Distribution
Mesostenoxenous parasite associated with birds of the subfamily Carduelinae: European Greenfinch Chloris chloris (Linnaeus) in Kazakhstan and England [42,52] and Desert Finch Rhodospiza obsoleta (Lichtenstein) in Germany [51].
3.1.11. Syringophiloidus serini Bochkov, Fain & Skoracki, 2004
Diagnosis. Females. Each medial branch of peritremes with 8 chambers, each lateral branch with 10–11 chambers. Propodonotal shield punctate. Setae vi, ve, and si enlarged basally and serrate. Hysterosomal plate fused to pygidial plate, punctate. Fan-like setae p′ and p″ of legs III and IV with 5–6 tines. Lengths of setae: vi 30–35, ve 40–55, si 115–130, se 210–240, c2 180–200, c1 215–220, d1 120–145, f1 17–22, h1 29–35, d2 150–165, e2 130–140, f2 165–180, h2 330–380, ag1 110–135, ag2 110–150, ag3 165–200, g1 and g2 ca. 25, ps1 and ps2 ca. 15.
Hosts and Distribution
Mesostenoxenous parasite associated with birds of the subfamily Carduelinae: Yellow-rumped Seedeater Crithagra atrogularis (Smith), Thick-billed Seedeater Crithagra burtoni (Gray), African Citril Crithagra citrinelloides (Rüppell), all from Tanzania [current paper], Yellow-fronted Canary Crithagra mozambica (Müller) in Central Africa [57], Twite Linaria flavirostris (Linnaeus) in Kyrgyzstan, and Yellow-crowned Canary Serinus flavivertex (Blanford) in Tanzania [current paper].
New Material Examined
Ex Crithagra atrogularis (host reg. no. ZSM 61.40); Tanzania: Manyara Region, Magugu, 21 July 1960, coll. v. Nagy—2 females deposited in the AMU (reg. no. AMU-MS 24-1025-113).
Ex Crithagra burtoni (host reg. no. ZSM 64.732, female); Tanzania: near Lake Tanganyika, 31 January 1951, coll. Th. Andersen—11 females and 2 males deposited in the AMU (reg. no. AMU-MS 24-1025-122).
Ex Crithagra citrinelloides (host reg. no. ZSM 60.1253, female); Tanzania: Arusha Region, Meru District, Usa River, 1200 m a.s.l., 19 April 1960, coll. v. Nagy—10 females deposited in the AMU (reg. no. AMU-MS 24-1025-110).
Ex Linaria flavirostris (host reg. no. ZSM 10.1744); Kyrgyzstan: Naryn Province, Naryn, 13 January 1910, coll. Merzbacher—five females and two males deposited in the AMU (reg. no. AMU-MS 24-1025-124).
Ex Serinus flavivertex (host reg. no. ZSM uncatalogued, male); Tanzania: Njombe District, 21 September 1950, coll. Th. Andersen—nine females and one male deposited in the AMU (reg. no. AMU-MS 24-1025-153).
3.1.12. Syringophiloidus stawarczyki Skoracki, 2004
Diagnosis. Females. Total body length 605–695. Infracapitulum punctate. Each medial branch of peritremes with 2–3 chambers, each lateral branch with 11–12 chambers. Stylophore 170–195 long. Propodosomal shield punctate. Setae vi and ve thin, slightly serrate and subequal in length. Setae si variable in length but always longer than vi and ve. Hysteronotal shield not fused to pygidial shield, punctate, anterior margin reach above level of setal bases d2. Pygidial shield punctate. Setae ps2 longer than ps1. Setae ag1 and ag2 subequal in length. All coxal field punctate. Fan-like setae p′ and p″ of legs III–IV with 7–8 tines. Lengths of setae: vi 15, ve 15–20, si variable 30–60, se 165–170, c1 195, d1 145–160, d2 115–125, e2 145–150, f1 25, f2 220, h1 25, h2 305, ps1 10, ps2 15–20, ag1 130–135, ag2 125–135, ag3 165.
Hosts and Distribution
Mesostenoxenous parasite associated with birds of the subfamily Euphoninae: Blue-naped Chlorophonia Chlorophonia cyanea (Thunberg) in Brazil and Colombia [55]; Golden-rumped Euphonia Chlorophonia cyanocephala (Vieillot) in Brazil, Paraguay and Colombia [55,58]; Purple-throated Euphonia Euphonia chlorotica (Linnaeus) in Paraguay; White-lored Euphonia Euphonia chrysopasta (Sclater & Salvin) in Venezuela and Bolivia; Velvet-fronted Euphonia Euphonia concinna (Sclater) in Colombia; Thick-billed Euphonia Euphonia laniirostris (d’Orbigny & Lafresnaye) in Venezuela, and Violaceous Euphonia Euphonia violacea (Linnaeus) in Trinidad and Tobago [55].
3.1.13. Syringophilopsis euphonicus Sikora, Unsoeld, Melzer, Friedrich, Hromada and Skoracki, 2025
Diagnosis. Females. Total body length 950–1040. Infracapitulum apunctate. Hypostomal apex with one pair of small and sharp-ended protuberances. Stylophore 230–250 long. Each medial branch of peritremes with 2–3 chambers, each lateral branch with 12 chambers. Propodonotal shield sparsely punctate near bases of setae ve and si. Bases of setae se and c1 situated at same transverse level. Bases of setae c2 situated posterior to level of setal bases si. Length ratio of setae vi:ve:si 1:1.5–2:3.5–4. Hysteronotal shields absent. Pygidial shield apunctate. Setae ag2 3.8–4 times longer than g1 and g2. Setae ag1 and ag2 long and subequal in length. Coxal fields III–IV densely punctate. Apodemes I and II fused in anterior part of apodemes II. Lengths of setae: vi 90–95, ve 140–180, si 315–355, se 335–390, c1 320–380, c2 325–350, d1 320–410, d2 320–360, e2 300–400, f1 > 250, f2 400, h1 290–345, h2 415–470, g1 and g2 35–50, ag1 195–295, ag2 190–235, ag3 230–300.
Hosts and Distribution
Oligoxenous parasite associated with birds of the subfamily Euphoninae: White-vented Euphonia Euphonia minuta (Cabanis) in Colombia; Orange-bellied Euphonia Euphonia xanthogaster (Sundevall) in Peru, and Trinidad Euphonia Euphonia trinitatis (Strickland) in Venezuela [55].
3.1.14. Syringophilopsis fringillae (Fritsch, 1958)
Diagnosis. Females. Total body length 1200–1310. Infracapitulum apunctate. Hypostomal apex with one pair of short protuberances. Each medial branch of peritremes with 3–5 chambers, each lateral branch with 13–14 chambers. Stylophore 255 long. Propodonotal shield punctate near bases of setae ve and si. Length ratio of setae vi:ve:si 1:1.5:1.5–2. Setae se and c1 situated at same transverse level. Hysteronotal shields apunctate. Small pygidial shield sparsely punctate. Setae h1 1.8–2 times shorter than f1, both shorter than f2 and h2. Genital setae shorter than aggenital setae ag1 and ag3. Length ratio of setae g1:ag2 1:2.8–3. Setae ag1 1.2 times longer than ag2. Coxal fields I–IV punctate. Fan-like setae p′ and p″ of legs III and IV with 15–17 tines. Apodemes I and II fused in anterior part of apodemes II. Lengths of setae: vi 205–215, ve 310–315, si 325–395, se 405–410, c1 425, c2 390–395, d1 425, d2 395, e2 455, f1 185–190, f2 470–505, h1 340–350, h2 470–505, ps1 and ps2 55, g1 and g2 90–100, ag1 370–395, ag2 285–325, ag3 395.
Hosts and Distribution
Oligoxenous parasite associated with birds of the subfamily Fringillinae: Common Chaffinch Fringilla coelebs Linnaeus in England, Germany, Poland, Slovakia, Russia and Kazakhstan [42,52,58,59,60,61,62], and Brambling Fringilla montifringilla Linnaeus in Germany [51].
3.1.15. Syringophilopsis kirgizorum Bochkov, Mironov and Kravtsova, 2000
Diagnosis. Females. Total body length 900–1180. Infracapitulum apunctate. Hypostomal apex with one pair of short and sharp-ended protuberances. Each medial branch of peritremes with 3–4 chambers, each lateral branch with 9–11 chambers. Stylophore 240–245 long. Propodonotal shield punctate near bases of setae ve and si. Length ratio of setae vi:ve:si 1:2:3.5–4. Setae se and c1 situated at same transverse level. Hysteronotal shields absent. Small pygidial shield punctate. Genital setae shorter than aggenital setae ag1 and ag3. Length ratio of setae g1:ag2 1:2.5–3. Setae ag1 1.3 times longer than ag2. Coxal fields I–IV punctate. Fan-like setae p′ and p″ of legs III and IV with 11–13 tines. Apodemes I and II fused in middle part of apodemes II. Lengths of setae: vi 60, ve 90–130, si 195–220, se 240–295, c1 305–325, c2 255–295, d1 215–280, d2 215–275, e2 255–280, f1 80, f2 375, h1 80, ps1 and ps2 45, g1 and g2 60–70, ag1 200–220, ag2 160–180, ag3 300–370.
Hosts and Distribution
Mesostenoxenous parasite associated with birds of the subfamily Carduelinae: European Goldfinch Carduelis carduelis (Linnaeus) in Poland and Russia [42,58]; European Greenfinch Chloris chloris (Linnaeus) in Germany, Poland, England, Jordan, and Kyrgyzstan [42,51,58,63,64,65]; Eurasian Linnet Linaria cannabina (Linnaeus) in Jordan [65]; Oriole Finch Linurgus olivaceus (Fraser) [66], and Desert Finch Rhodospiza obsoleta (Lichtenstein) in Kyrgyzstan [63].
3.1.16. Torotrogla cardueli Bochkov and Mironov, 1999
Diagnosis. Females. Total body length 800–875. Hypostomal apex with one pair of medium-sized, blunt-ended and bill-like protuberances. Each medial branch of peritremes with 3–4 chambers, each lateral branch with 6–7 chambers. Propodonotal shield densely punctate on whole surface. Length ratio of setae vi:ve:si 1:1.3:3. Setae c2 situated anterior to level of setae se. Hysteronotal shields punctate, bases of setae d1 near these shields. Pygidial shield punctate. Setae f1 and h1 subequal in length. Coxal fields I–IV sparsely punctate. Fan-like setae p′ and p″ of legs III and IV with 10–11 tines. Lengths of setae: vi 55–75, ve 75, si 165–185, se 175, c1 215, c2 175, d1 150–170, d2 140, e2 145–170, h1 70–75, h2 440–490, f1 75–85, f2 375.
Hosts and Distribution
Mesostenoxenous parasite associated with birds of the subfamily Carduelinae: European Goldfinch Carduelis carduelis Linnaeus in Poland; Common Linnet Linaria cannabina (Linnaeus) in Poland and Slovakia; Red Crossbill Loxia curvirostra Linnaeus in Poland; White-winged Crossbill Loxia leucoptera Gmelin in Poland; Parrot Crossbill Loxia pytyopsittacus Borkhausen in Finland; Atlantic Canary Serinus canaria (Linnaeus) in Europe [42]; Fire-fronted Serin Serinus pusillus (Pallas) in Kyrgyzstan [current paper], and Eurasian Siskin Spinus spinus (Linnaeus) in Poland, Slovakia, and Russia [42,67].
New Material Examined
Ex Serinus pusillus (host reg. no. ZSM 17.3034, male); Kyrgyzstan: Naryn Province, Naryn, 9 April 1910, coll. Akulin—1 female and 10 males deposited in the AMU (reg. no. AMU-MS 24-1025-152).
3.1.17. Torotrogla coccothraustes Bochkov, Flannery and Spicer, 2009
Diagnosis. Females. Total body length 1360–1400. Hypostomal apex with one pair of slim and elongated protuberances. Each lateral branch of peritremes with six chambers, each medial branch with three chambers. Propodonotal shield concave on anterior and posterior margins, apunctate. Length ratio of setae vi:ve:si 1:1.4:1.9. Setae c2 situated anterior to level of setae se. Hysteronotal shields punctate, bases of setae d1 situated on these shields. Pygidial shield punctate. Setae f1 and h1 subequal in length. Coxal fields I–IV sparsely punctate. Fan-like setae p′ and p″ of legs III and IV with 6–9 tines. Lengths of setae: vi 135–140, ve 150–165, si 245–270, se 285–300, c1 260–280, c2 300–330, d1 280–320, d2 280–320, e2 280–320, h1 120–140, h2 380–420, f1 110–125, f2 400–420, ps1 and ps2 40, g1 and g2 50.
Host and Distribution
Monoxenous parasite associated with Evening Grosbeak Coccothraustes vespertinus (Cooper) in the United States [68].
3.1.18. Torotrogla gaudi Bochkov and Mironov, 1998
Diagnosis. Females. Total body length 800–875. Hypostomal apex with one pair of blunt-ended protuberances. Each medial branch of peritremes with 3–4 chambers, each lateral branch with 8–10 chambers. Propodonotal shield sparsely punctate near lateral margins. Length ratio of setae vi:ve:si 1:1.6–2:3.6–4. Setae c2 situated anterior to level of setae se. Hysteronotal shields punctate, bases of setae d1 near this shields. Pygidial shield apunctate. Coxal fields I–IV punctate. Fan-like setae p′ and p″ of legs III and IV with 10–12 tines. Lengths of setae: vi 65, ve 105–135, si 220–260, se 225–245, c1 320, c2 215–270, d1 205–275, d2 175, e2 230–250, f1 105–165, f2 610, h1 210–295, h2 500–645, ps1 and ps2 35–45, g1 60, g2 40–45.
Hosts and Distribution
Mesostenoxenous parasite associated with birds of the subfamily Fringillinae: Common Chaffinch Fringilla coelebs Linnaeus in Poland and Russia [42,60], and Brambling Fringilla montifringilla Linnaeus in Poland and Slovakia [42,69].
Remark
The species Torotrogla gaudi was also previously recorded from the Eurasian Bullfinch Pyrrhula pyrrhula (Linnaeus) in Poland [69]. However, re-examination of the available material (3 females ex P. pyrrhula from Poland, West Pomeranian Voivodeship, near Gryfino, June 2001, coll. G. Kiljan, deposited in the AMU (reg. no. AMU MS 25-0706-001)) revealed that the mites collected from this host cannot be assigned to T. gaudi, as they exhibit markedly longer setae vi, ve, and h1, and probably represent a new, undescribed species. Nevertheless, the poor preservation of the available specimens precludes their unambiguous identification. Therefore, we propose excluding the Eurasian Bullfinch from the host spectrum of T. gaudi until new, well-preserved specimens from this host can be obtained.
3.1.19. Torotrogla enucleator sp. n. (Figure 4)
Description. Female, holotype. Total body length 1140 (1080–1150 in four paratypes). Gnathosoma. Hypostomal apex with one pair of medium-sized and blunt-ended protuberances. Each medial branch of peritremes with 4–5 chambers, each lateral branch with 9–10 chambers. Stylophore constricted posteriorly, 370 (300–390) long, exposed portion of stylophore apunctate, 300 (250–330) long. Idiosoma. Propodonotal shield rectangular in shape, cleft on anterior margin, punctate laterally, width at level of setae si 160 (150–160). Bases of setae se situated anterior to level of setal bases c1, c2 anterior to se. Length ratio of setae vi:ve:si 1:1.5–1.7:2.4–2.6. Pair of hysteronotal shields situated in close proximity to each other, punctate, bearing bases of setae d1 on anterior margin. Pygidial shield apunctate, with rounded anterior margin, 180 long. Setae h1 distinctly longer than f1. Setae g1 slightly (1.2–1.3 times) longer than g2. Setae ps1 and ps2 subequal in length. Coxal fields I–IV punctate. Setae 3c 1.8 times longer than 3b. Legs. Fan-like setae p′ and p″ of legs III and IV with 6–9 tines. Lengths of setae: vi 105 (105–110), ve 165 (165–180), si 260 (255–270), se 260 (260–270), c1 (260–305), c2 (280–300), d1 215 (190–220), d2 190 (190–210), e2 200 (190–220), f1 (80–90), f2 430 (400–450), h1 (445–495), h2 470 (460–530), ps1 60 (50–60), ps2 55 (45–55), g1 80 (80–90), g2 60 (60–65), l’RI 50 (50), l’RII 55 (55–60), l’RIII (75–90), l’RIV 75 (70–75), 3b 95 (85–95), 3c 170 (170–185), all aggenital setae (ag) longer than 300.
Male. Not found.
Type Material
Female holotype and four female paratypes from Pine Grosbeak Pinicola enucleator (Linnaeus) (host reg. no. ZSM uncatalogued, female); Japan: Kuril Islands, Iturup, 28 August 1900, coll. Haberer.
Type Material Deposition
Holotype and two female paratypes are deposited in the SNSB-ZSM (reg. no. ZSMA20250029), except two female and one male paratypes in the AMU (reg. no. AMU MS 24-1025-082).
Additional Material
Ex Plain Mountain-Finch Leucosticte nemoricola (Hodgson) (host reg. no. ZSM 17.4752); Kyrgyzstan: Tian Shan, vicinity of Naryn, 2 February 1910, coll. Neschiwjow—1 female deposited in the AMU (reg. no. AMU MS 24-1025-093).
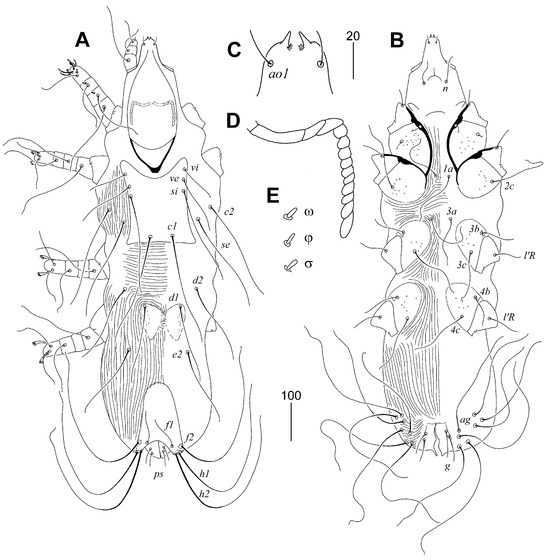
Figure 4.
Torotrogla enucleator sp. n., female: (A) dorsal view; (B) ventral view; (C) hypostomal apex; (D) peritreme; (E) solenidia of leg I. Scale bars: (A,B) = 50 µm, (C–E) = 20 µm.
Differential Diagnosis
Torotrogla enucleator sp. n. is morphologically similar to T. gaudi Bochkov and Mironov, 1998. In females of both species, the hypostomal apex bears a pair of medium-sized and blunt-ended protuberances; each medial branch of the peritremes has 3–4 chambers, and each lateral branch has 8–10 chambers; the propodonotal shield is rectangular in shape and sparsely punctate near the lateral margins; setae h1 are distinctly longer than f1; the pygidial shield is apunctate, and coxal fields I–IV are punctate. This new species differs from T. gaudi in the following features: in females of T. enucleator, setae h1 are 4–5 times longer than f1; the lengths of setae vi and ve are 105–110 µm and 165–180 µm, respectively, and fan-like setae p′ and p″ of legs III and IV have 6–9 tines. In contrast, in females of T. gaudi, setae f1 are half the length of h1; the lengths of setae vi and ve are 65 µm and 105–135 µm, respectively, and fan-like setae p′ and p″ of legs III and IV have 10–12 tines.
Etymology
This species name is taken from the specific name of the type host species—Pinicola enucleator.
3.1.20. Torotrogla janhafti sp. n. (Figure 5 and Figure 6)
Description. Female, holotype. Total body length 925 (830–950 in six paratypes). Gnathosoma. Hypostomal apex with one pair of medium-sized and blunt-ended protuberances. Each medial branch of peritremes with 4 chambers, each lateral branch with 7–10 chambers. Stylophore constricted posteriorly, 345 (300–345) long, exposed portion of stylophore apunctate, 285 (250–280) long. Idiosoma. Propodonotal shield rectangular in shape, apunctate, width at level of setae si 140 (135–145). Bases of setae se situated anterior to level of setal bases c1, bases c2 anterior to se. Length ratio of setae vi:ve:si 1:1.5–1.6:3–4. Hysteronotal shields punctate, bearing bases of setae d1 on anterior margin. Pygidial shield apunctate, with rounded anterior margin, 120 long. Setae f1 and h1 short and subequal in length. Genital and pseudanal setae subequal in length. Coxal fields I–IV apunctate. Setae 3c about twice as long as 3b. Legs. Fan-like setae p′ and p″ of legs III and IV with 7–8 tines. Lengths of setae: vi 75 (55–75), ve 100 (90–110), si 230 (205–230), se 240 (215–240), c1 295 (235–275), c2 (215–250), d1 210 (210–220), d2 190 (190–225), e2 210 (205–230), f1 80 (80–100), f2 495 (490–505), h1 (120–170), h2 530 (515–530), ps1 and ps2 40 (30–40), g1 40 (40–45), g2 35 (30–35), l’RIII 65 (60–65), l’RIV 65 (60–65), 3b 55 (55–65), 3c 100 (100–125), all setae ag longer than 300.
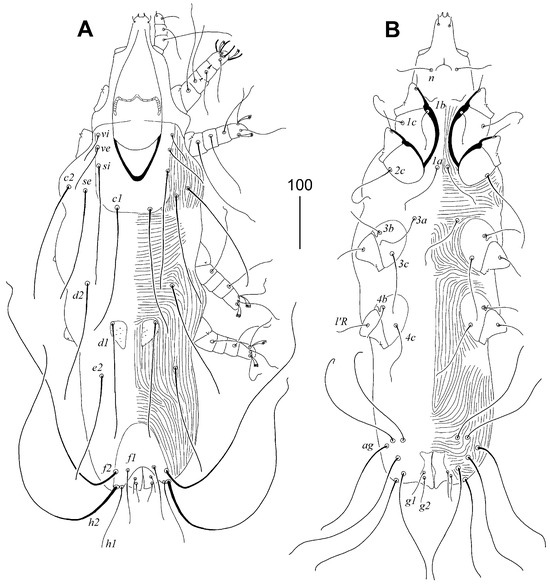
Figure 5.
Torotrogla janhafti sp. n., female: (A) dorsal view; (B) ventral view.
Male. Total body length 690–710. Gnathosoma. Hypostomal apex with one pair of small and blunt-ended protuberances. Each medial branch of peritremes with 3–4 chambers, each lateral branch with 8–10 chambers. Stylophore constricted posteriorly, 230–240 long, exposed portion of stylophore apunctate, 190–200 long. Propodonotal shield with all margins concave, laterally punctate, width at level of setae si 125–130. Bases of setae se situated anterior to level of setal bases c1, c2 situated anterior to se. Length ratio of setae vi:ve:si 1:2.8:2.5. Hysteronotal shield fused to pygidial shield, about 255 long, bearing bases of setae d1, e2, f2 and h2, anterior margin not reaching bases of setae d2. Setae d2 1.3–1.6 times longer than d1. Coxal fields I–IV apunctate. Legs. Fan-like setae p′ and p″ of legs III and IV with six tines. Lengths of setae: vi 55, ve 70–85, si 130–140, se 125, c1 155, c2 125–150, d1 25, d2 20–40, e2 20, f2 55–60, h2 200–270, l’RIII–IV 40.
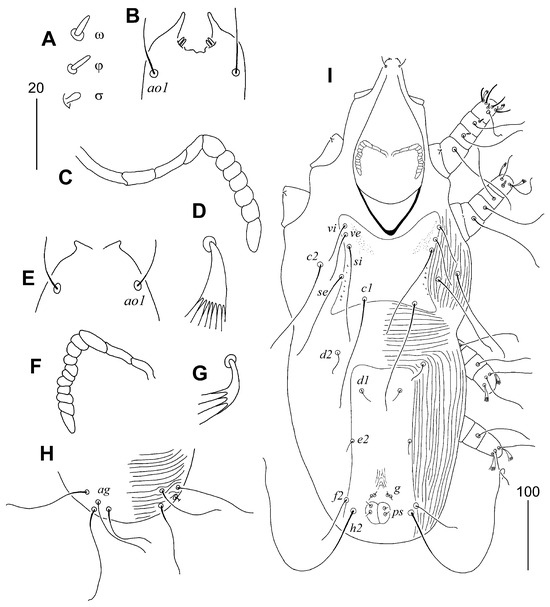
Figure 6.
Torotrogla janhafti sp. n., female (A–D): (A) solenidia of leg I; (B) hypostomal apex; (C) peritreme; (D) fan-like seta p′III. Male (E–I): (E) hypostomal apex; (F) peritreme; (G) fan-like seta p′III; (H) opisthosoma in ventral view; (I) body in dorsal view. Scale bars: (A–G) = 20 µm, (H,I) = 50 µm.
Type Material
Female holotype and paratypes: six females and two males from Red-mantled Rosefinch Carpodacus rhodochlamys (Brandt) (host reg. no. ZSM 09.4519, male); Kyrgyzstan: Naryn, 21 March 1908, coll. Merzbacher.
Type Material Deposition
Holotype and most paratypes are deposited in the SNSB-ZSM (reg. no. ZSMA20250030), except two female and one male paratypes in the AMU (reg. no. AMU MS 24-1025-075).
Additional Material
Ex. Siberian Long-tailed Rosefinch Carpodacus sibiricus (Pallas) (host reg. no. ZSM 33.110, male); Japan: Hokkaido, Sapporo, 8 November 1906, coll. R. Fenk—eight females deposited in the ZSM (ZSMA20250031), eight females and one male in the AMU (reg. no. AMU MS 24-1025-079).
Ex Himalayan White-browed Rosefinch Carpodacus thura Bonaparte and Schlegel (host reg. no. ZSM 62.1733, male); Nepal: Khumbu Region, Khumjung, 14 June 1962, coll. unknown—two females and three males deposited in the AMU (reg. no. AMU MS 24-1025-080).
Ex Sinai Rosefinch Carpodacus synoicus (Temminck) (host reg. no. ZSM 1910/2, female); Egypt: Sinai, Wadi Debbet, 3 April 1909, coll. Schloesser—two females and two males deposited in the AMU (reg. no. MS 24-1025-076).
Differential Diagnosis
Torotrogla janhafti sp. n. is morphologically most similar to T. cardueli Bochkov and Mironov, 1999, in having a hypostomal apex with a pair of medium-sized blunt-ended protuberances, medial peritremal branches with 3–4 chambers, lateral branches with 6–7 chambers, setae c2 situated anterior to se, punctate hysteronotal shields, and setae f1 and h1 subequal in length. The new species differs from T. cardueli in the following features: in females of T. janhafti, the propodonotal shield and coxal fields I–IV are apunctate, the fan-like setae p′ and p″ of legs III and IV have 7–8 tines, the length ratio of setae vi:ve is 1:1.5–1.6, and the lengths of setae si, se, d2, and f2 are 205–230 µm, 215–240 µm, 190–225 µm, and 490–505 µm, respectively; in males, setae vi and ve measure 55 µm and 70–85 µm, respectively, the coxal fields I–IV are apunctate, and the fan-like setae p′ and p″ of legs III and IV have six tines. In contrast, in females of T. cardueli, the propodonotal shield and coxal fields I–IV are densely punctate, the fan-like setae p′ and p″ of legs III and IV have 10–11 tines, the length ratio of setae vi:ve is 1:1.3, and the lengths of setae si, se, d2, and f2 are 165–185 µm, 175 µm, 140 µm, and 375 µm, respectively; in males, setae vi and ve measure 20 µm and 45 µm, respectively, the coxal fields I–IV are punctate, and the fan-like setae p′ and p″ of legs III and IV have 8–9 tines.
Etymology
The species is named in honour of the German nature and wildlife filmmaker Jan Michael Haft for his great work in producing particularly extraordinary and impressive nature documentaries.
3.2. Summarised Diversity of Syringophilinae Mites Associated with Fringillidae Birds
To date, representatives of the subfamily Syringophilinae have been recorded from numerous finch genera across all major zoogeographic regions inhabited by this bird family. The following table summarises the current knowledge on their species richness, host associations, and distribution, integrating both previously published records and new data from the present study (Table 1).

Table 1.
Quill mites of the family Syringophilidae associated with birds of the family Fringillidae. AFRO—Afrotropic, PALA—Palaearctic, NEAR—Nearctic, SIJA—Sino-Japanese, NEOT—Neotropic, PANA—Panamanian, SAAR—Saharo-Arabian; *—type host species, c.p.—current paper. The ditto mark (″) indicates repetition of the species name.
3.3. Key to Syringophilinae Species Associated with Birds of the Family Fringillidae
| 1. | Propodonotal region with 5 pairs of setae (vi absent)—Aulonastus | 2 |
| Propodonotal region with 6 pairs of setae (vi present) | 5 | |
| 2. | Setae f2 twice as long as f1 | Aulonastus fringillus |
| Setae f2 3–4 times longer than f1 | 3 | |
| 3. | Propodonotal shield punctate | Aulonastus loxius |
| Propodonotal shield apunctate | 4 | |
| 4. | Setae c1 twice longer than d2 | Aulonastus ritzerfeldi sp. n. |
| Setae c1 1.2 times longer than d2 | Aulonastus neotropicalis | |
| 5. | Leg setae dGII absent—Syringophiloidus | 6 |
| Leg setae dGII present | 10 | |
| 6. | Medial branch of peritremes with 8 chambers | 7 |
| Medial branch of peritremes with 2–3 chambers | 8 | |
| 7. | All propodonotal setae thick and enlarged basally. Hysteronotal shield punctate | Syringophiloidus serini |
| All propodonotal setae thin and hair-like. Hysteronotal shield apunctate | Syringophiloidus carpodaci | |
| 8. | Setae d1 and e2 twice as long as d2 | Syringophiloidus klimovi |
| Setae d1, d2, and e2 subequal in length | 9 | |
| 9. | Lateral branch of peritreme with 8–9 chambers. Infracapitulum sparsely punctate. Length of stylophore 140–145 μm | Syringophiloidus coccothraustes |
| Lateral branch of peritreme with 11–12 chambers. Infracapitulum densely punctate. Length of stylophore 170–195 μm | Syringophiloidus stawarczyki | |
| 10. | Aggenital setal series with more than 4 pairs—Torotrogla | 11 |
| Aggenital setal series with 3 pairs | 15 | |
| 11. | Total body length 1360–1400 μm | Torotrogla coccothraustes |
| Total body length less than 1100 μm | 12 | |
| 12. | Setae h1 distinctly longer than f1 | 13 |
| Setae f1 and h1 subequal in length | 14 | |
| 13. | Setae h1 210–295 μm long | Torotrogla gaudi |
| Setae h1 445–495 μm long | Torotrogla enucleator sp. n. | |
| 14. | Propodonotal shield and coxal fields I–IV apunctate | Torotrogla janhafti sp. n. |
| Propodonotal shield and coxal fields I–IV densely punctate | Torotrogla cardueli | |
| 15. | Apodemes I parallel and not fused to apodemes II—Aulobia | 16 |
| Apodemes I divergent and fused to apodemes II—Syringophilopsis | 18 | |
| 16. | Each lateral branch of peritremes with 18–20 chambers | Aulobia cardueli |
| Each lateral branch of peritremes with 10–13 chambers | 17 | |
| 17. | Hysteronotal shield absent | Aulobia ruppelae sp. n. |
| Hysteronotal shield present | Aulobia leucostictus | |
| 18. | Hysteronotal shield absent | 19 |
| Hysteronotal shield present | Syringophilopsis fringillae | |
| 19. | Setae si 315–355 μm long. Pygidial shield apunctate | Syringophilopsis euphonicus |
| Setae si 195–220 μm long. Pygidial shield punctate | Syringophilopsis kirgizorum |
4. Discussion
The family Fringillidae comprises 236 species grouped into 49 genera [26], of which 17 species and 7 genera are considered extinct [43]. Considering only extant taxa, the family currently comprises 219 species belonging to 42 genera in 3 subfamilies: Carduelinae (176 species in 39 genera), Euphoninae (35 species in 2 genera), and Fringillinae (8 species in 1 genus). The global diversity of quill mites of the subfamily Syringophilinae, associated with the Fringillidae, currently comprises 20 species belonging to 5 genera. They have so far been recorded from 51 bird species, representing 23% of all extant Fringillidae species. These infested hosts belong to 18 genera, accounting for 43% of all fringillid genera, and span all 3 subfamilies (100%) (Figure 7). While the subfamilies Fringillinae and Euphoninae have been fully surveyed at the genus level, only 39% (15 out of 39 genera) of Carduelinae genera have been examined for quill mites. The following 24 genera in Carduelinae remain unexplored, many of which are monotypic or contain only a few species: Agraphospiza (1 species), Bucanetes (2), Callacanthis (1), Chlorodrepanis (3), Chrysocorythus (2), Drepanis (1), Eophona (2), Haemorhous (3), Hemignathus (1), Himatione (1), Loxioides (1), Loxops (3), Magumma (1), Mycerobas (4), Oreomystis (1), Palmeria (1), Paroreomyza (2), Procarduelis (1), Psittirostra (1), Pseudonestor (1), Pyrrhoplectes (1), Rhodopechys (1), Rhynchostruthus (3), and Telespiza (2). The number of unexamined genera within Carduelinae suggests that many new species of syringophilines may still await discovery and description. However, given that the major phylogenetic lineages of Fringillidae have already been studied, we do not expect the presence of additional quill mite genera beyond the five reported in this work.
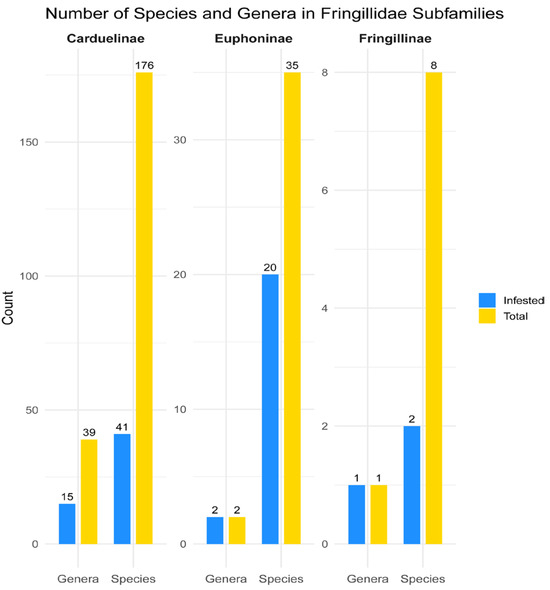
Figure 7.
Representation of quill mite infestation across species and genera of Fringillidae subfamilies; the blue bars represent the number of host species/genera infested with quill mites, and the yellow bars indicate the total number of extant species within each subfamily.
The present-day structure of the Fringillidae family is the result of decades of anatomical research [70,71,72,73] and recent advances in molecular phylogenetics [27,28,29,30,31,32,33,34]. Traditionally, the True Finches have been divided into three subfamilies: Fringillinae, which represents the most basal lineage; Euphoninae, the sister group to Carduelinae; and Carduelinae, the most derived and speciose clade [33,34] (Figure 8). The split between the Fringillidae and their sister family, the Emberizidae, is estimated to have occurred around 16.8 million years ago (mya) [34,74]. Likely, syringophilid mites were already associated with the common ancestor of these two lineages, and the Fringillidae inherited this parasitic association after the divergence. The diversification of quill mites in Fringillidae likely occurred later, in parallel with the radiation of their avian hosts. Among the three subfamilies, Carduelinae exhibits the highest diversity of associated syringophiline mites, hosting all five recorded genera: Aulobia, Aulonastus, Syringophiloidus, Syringophilopsis, and Torotrogla. In contrast, Euphoninae and Fringillinae each harbour only three genera of quill mites. This pattern may reflect both historical loss and limited opportunity for host–parasite co-diversification within the less speciose lineages. We hypothesise that the ancestral syringophiline community associated with the early Fringillidae comprised all five genera listed above. Following the divergence of Fringillinae around 14.6 mya, only Aulonastus, Syringophilopsis, and Torotrogla were retained within that clade. Similarly, the later split between Euphoninae and Carduelinae, estimated at 13.8 mya [34,74], led to the persistence of Aulonastus and Syringophilopsis in Euphoninae, but with the apparent loss of Torotrogla and the retention of Syringophiloidus [55]. Only Carduelinae, likely due to its extensive radiation, retained representatives of all five genera. A plausible explanation for the reduced diversity of quill mite genera observed in Fringillinae and Euphoninae lies in the limited taxonomic diversity of these host lineages. Euphoninae currently comprises just 35 species in 2 genera, while Fringillinae includes only 8 species in a single genus. In contrast, Carduelinae is markedly more diverse, with 176 species across 39 genera, offering greater opportunity for parasite speciation and persistence. This clear link between host taxonomic diversity and parasite genus richness suggests that the evolutionary and taxonomic structure of host lineages plays a pivotal role in shaping the composition and complexity of their parasite assemblages. This observation is consistent with Eichler’s rule, which posits a positive correlation between host and parasite diversity in coevolutionary systems.
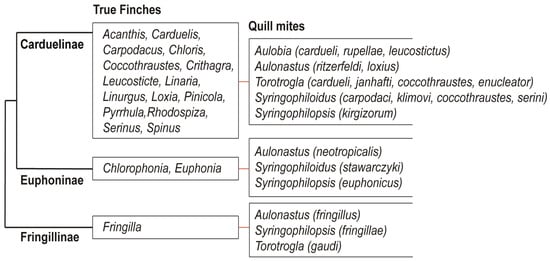
Figure 8.
Phylogeny of the family Fringillidae on the subfamily level (after Zuccon et al. [33]) with the records of quill mites.
For a long time, quill mites of the family Syringophilidae were considered to be predominantly monoxenous parasites or, at most, oligoxenous (parasitising hosts belonging to a single genus) [42,49]. However, increasingly frequent studies on the distribution of quill mites across entire bird families or orders indicate that these mites exhibit greater host diversity, suggesting a more substantial presence of oligoxenous or mesostenoxenous species, which infest hosts belonging to closely related genera [20,21,22,23,24,25]. In our study (see Table 1), we observed that the number of monoxenous species amounts to five: Aulonastus fringillus, A. ritzerfeldi sp. n., Aulobia leucostictus, Syringophiloidus coccothraustes, and Torotrogla coccothraustes—representing 25% of the total fauna. We identified six oligoxenous species—Syringophiloidus carpodaci associated with birds of the genus Carpodacus; Syringophilopsis euphonicus parasitising birds of the genus Euphonia; Syringophilopsis fringillae and Torotrogla gaudi from Fringilla; and T. janhafti and Aulobia ruppellae sp. n., both from Carpodacus—which make up 30% of all species. The largest proportion consists of mesostenoxenous species (45%, nine species), including Aulobia cardueli (associated with Acanthis, Carduelis, Linaria, Spinus), Aulonastus loxius (Loxia, Linaria), A. neotropicalis (Chlorophonia, Euphonia), Syringophiloidus klimovi (Chloris, Rhodospiza), S. serini (Crithagra, Linaria, Serinus), S. stawarczyki (Chlorophonia, Euphonia), Syringophilopsis kirgizorum (Carduelis, Chloris, Linaria, Linurgus, Rhodospiza), Torotrogla cardueli (Carduelis, Linaria, Loxia, Serinus, Spinus), and T. enucleator (Pinicola, Leucosticte). Importantly, no parasite species in our dataset was found to inhabit hosts from different subfamilies of Fringillidae, suggesting a strong phylogenetic constraint in host exploitation. The observed ratio of monoxenous (25%) to non-monoxenous (75%) species may reflect a general pattern in syringophilid mites associated with taxonomically diverse avian groups. This distribution supports the hypothesis of close co-phylogenetic relationships between parasites and their hosts. The presence of specific mite species on phylogenetically related hosts suggests a long-term evolutionary association within particular avian lineages.
The exploration of host–parasite cospeciation has intrigued scientists for over a century [75]. Given the deep evolutionary ties between parasites and their hosts, early researchers often considered parasites as extensions of their host phenotypes, anticipating that parasite phylogenies would closely mirror those of their hosts, an assumption encapsulated in Fahrenholz’s Rule [76]. However, it has become increasingly clear that the evolutionary histories of hosts and parasites are far more complex, with a range of events capable of obscuring or disrupting strict co-phylogenetic patterns [77,78,79]. Based on the results of our study, several key observations can be made. First, quill mites associated with the family Fringillidae do not exhibit a random host distribution, a characteristic expected of obligate parasites. Instead, individual mite species are typically restricted to either a single host species or a group of closely related hosts, fitting the definitions of oligoxenous or mesostenoxenous parasites, respectively (Figure 9). The presence of the same quill mite species on representatives of hosts belonging to phylogenetically closely related genera may result from delayed parasite speciation relative to host speciation, a phenomenon referred to as retardation, which appears to be common among quill mites. An exceptionally fascinating aspect of the evolutionary trajectory of syringophilids is the observation that the rate of speciation exhibits a remarkable degree of variability among the different genera within this group. For instance, when examining the Carduelini clade, one can observe that the species known as Syringophilopsis kirgizorum has a widespread distribution and is found inhabiting multiple species of hosts, in stark contrast to the genus Syringophiloidus, which is characterised by the presence of two species, S. serini and S. klimovi, that are both located within the same lineage of hosts. Such patterns of distribution and speciation suggest that the process of diversification among quill mites does not necessarily unfold in a manner that is synchronous with the evolutionary progress of their avian hosts. Instead, these apparent discrepancies may serve to illustrate the existence of intricate coevolutionary dynamics, wherein the speciation of parasites is influenced by a range of factors that extend beyond the mere phylogenetic relationships of their hosts.
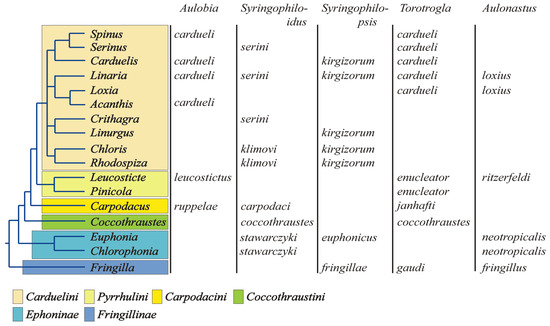
Figure 9.
Phylogeny of the family Fringillidae (after Zuccon et al. [33], modified) with the records of quill mite genera and species.
Among other coevolutionary processes, we found no evidence of host switching; that is, no quill mite species in our dataset parasitising hosts outside the Fringillidae or coming from non-fringillid hosts. We also did not observe any cases of duplication or synhospitality cases (i.e., parasite speciation within a single host lineage [80]). However, we did identify likely sorting events, where parasite lineages have disappeared from certain host lineages, during the early stages of the diversification of the quill mite fauna within the Fringillidae (see Chapter 4.2). All these findings highlight the potential of quill mites as a model system for exploring host–parasite coevolutionary processes, even though much remains to be uncovered.
5. Conclusions
This research provides the most thorough evaluation to date of the syringophilinae biodiversity and host specificity within the avian family Fringillidae. We identified 20 distinct mite species, including 4 new to science, across 5 genera, parasitising 51 finch species belonging to all 3 subfamilies: Carduelinae, Euphoninae, and Fringillinae. The greatest diversity of quill mites was observed in the subfamily Carduelinae, which demonstrates the highest taxonomic richness concerning host species and genera. This association strongly suggests that the taxonomic diversity of hosts has a significant influence on the structure and complexity of parasite assemblages. Moreover, our results reveal that the majority of syringophiline mites associated with finches do not exhibit narrow host specificity. While five species (25%) are monoxenous, the majority are oligoxenous or mesostenoxenous, confined to hosts from phylogenetically related genera. In several instances, the presence of identical mite species among closely related hosts may indicate a delayed divergence of parasites relative to their hosts. Furthermore, significant differences in diversification rates among mite genera were noted; for example, Syringophilopsis displays extensive host ranges, while others, such as Syringophiloidus, seem to be more constrained. Such patterns of distribution and speciation suggest that the process of diversification among quill mites does not necessarily unfold in a manner that is synchronous with the speciation of their hosts.
Despite these advancements, substantial gaps in our understanding persist. A considerable percentage of Carduelinae genera, especially those that are monotypic or have few species, remains unsurveyed for quill mites. These unexamined lineages are likely to contain numerous undescribed species, and their inclusion in forthcoming studies will be crucial for refining estimates of parasite diversity and host specificity. Equally important is the expansion of research to encompass related avian families. Specifically, the Emberizidae, the sister group to the Fringillidae, remains notably understudied regarding quill mite associations, with only four host species analysed to date. This lack of data currently hinders meaningful comparisons of genus-level parasite assemblages between the two families or evaluations of shared evolutionary histories.
In summary, the Fringillidae–Syringophilinae system presents a compelling model for examining host–parasite co-phylogenetic patterns, host specificity, and parasite diversification over an extensive evolutionary timescale. However, achieving a more comprehensive understanding of these dynamics will necessitate enhanced sampling across underrepresented host taxa and a greater integration of morphological, ecological, and molecular data in future investigations.
Author Contributions
Conceptualisation, M.S., B.S. and M.U.; methodology, M.S. and M.U.; validation, M.S., B.S. and M.U.; formal analysis, M.S., B.S. and M.U.; investigation, M.S., B.S. and M.U.; material collection, M.S. and M.U.; data curation, M.S.; writing—original draft preparation, M.S., B.S., M.U., R.R.M. and S.F.; writing—review and editing, M.S., B.S., M.U., R.R.M. and S.F.; visualisation, M.S.; supervision, M.S. and B.S.; project administration, M.S., B.S., R.R.M. and S.F.; funding acquisition, M.S., B.S. and R.R.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Society Freunde der Zoologischen Staatssammlung München e. V. and by the SNSB collection panel (project 808: “New species from old collections”). Additional support was provided by the Excellence Initiative Program “Mobility” of the UAM Research University—UAM ID-UB: 185/07/POB1/0011 (to M.S.), 185/07/POB1/0010 (to B.S.), and 146/01/POB1/003 (to R.R.M.).
Institutional Review Board Statement
The ethics was not applied. The research was conducted solely and exclusively on dry bird skins deposited in the ornithological collection of the museum.
Informed Consent Statement
Not applicable.
Data Availability Statement
All necessary data are available in the text.
Acknowledgments
The corresponding authors (B.S. and M.S.) extend their heartfelt gratitude to the administration of the SNSB—Bavarian State Collections of Natural History and to all members of the Society Freunde der Zoologischen Staatssammlung München e. V. for their invaluable support during our research tenure at the Bavarian State Collection of Zoology, Munich, Germany. We are deeply indebted to Burcin Yurdakul (ZSM) for her kind assistance throughout this study. We also wish to express our sincere appreciation to the anonymous reviewers for their constructive comments and critical evaluation of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Proctor, H.C. Feather mites (Acari: Astigmata): Ecology, behavior, and evolution. Annu. Rev. Entomol. 2003, 48, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Proctor, H.; Owens, I.I. Mites and birds: Diversity, parasitism and coevolution. Trends Ecol. Evol. 2000, 15, 358–364. [Google Scholar] [CrossRef]
- Dabert, J.; Ehrnsberger, R. Vassilevascus gen. nov., a new genus of the family Ascouracaridae Gaud et Atyeo, 1976 (Astigmata; Pterolichoidea). Osnabrücker Naturw. Mitt. 1995, 20/21, 95–100. [Google Scholar]
- Hernandes, F.A.; O’Connor, B.M. Cystoidosoma hermaphroditus sp. n., the first representative of the quill mite family Ascouracaridae (Acari: Astigmata: Pterolichoidea) from an owl (Aves: Strigiformes). Folia Parasitol. 2015, 62, 037. [Google Scholar] [CrossRef] [PubMed]
- Dabert, J. The feather mite family Syringobiidae Trouessart, 1896 (Acari, Astigmata, Pterolichoidea). I. Systematics of the family and description of new taxa. Acta Parasitol. 2003, 48 (Suppl. S1), 1–184. [Google Scholar]
- Dabert, J. Kiwilichidae fam. nov., eine neue Federmilbenfamilie (Astigmata, Pterolichoidea). Entomol. Mitt. Zool. Mus. Hamb. 1994, 11, 101–110. [Google Scholar]
- Gaud, J.; Atyeo, W.T. Ovacaridae, une famille nouvelle de Sarcoptiformes plumicoles. Acarologia 1975, 17, 169–176. [Google Scholar]
- Gaud, J.; Atyeo, W.T. A new name for Ovacarus and Ovacaridae (Acarina: Analgoidea). Acarologia 1977, 18, 568–569. [Google Scholar]
- Mironov, S.V. The first find of the feather mite from Apionacaridae family (Astigmata: Analgoidea) on the passerines (Passeriformes). Parazitologiia 2001, 35, 284–290. (In Russian) [Google Scholar]
- Lukoschus, F.S.; Lombert, H.A.P.M. Five new species of quill wall mites from European birds (Astigmata: Laminosioptidae: Fainocoptinae). Int. J. Acarol. 1980, 6, 63–78. [Google Scholar] [CrossRef]
- Skoracki, M.; Kavetska, K.; Ozminski, M.; Zawierucha, K. Calamicoptes anatidus sp. nov., a new quill wall mite (Acari: Laminosioptidae) from the Greater Scaup Aythya marila (L.) (Aves: Anseriformes). Acta Parasitol. 2014, 59, 426–432. [Google Scholar] [CrossRef]
- Skoracki, M.; Kosicki, J.Z.; Hromada, M. Unusual parasite from an enigmatic host—A new group of mites infesting feather quills of the hoatzin. Eur. Zool. J. 2021, 88, 9–17. [Google Scholar] [CrossRef]
- Bochkov, A.V.; Fain, A. Phylogeny and system of the Cheyletidae (Acari: Prostigmata) with special reference to their host-parasite associations. Bull. Inst. R. Sci. Nat. Belg. 2001, 71, 5–36. [Google Scholar]
- Bochkov, A.V.; O’Connor, B.M. New cheyletid mites (Acari, Cheyletidae) associated with birds. Acta Parasitol. 2003, 48, 265–279. [Google Scholar]
- Fain, A.; Bochkov, A.V. A new species of the genus Metacheyletia Fain, 1972 (Acari: Cheyletidae) parasitising Serinus mozambicus (Passeriformes: Fringillidae) in Central Africa. Int. J. Acarol. 2003, 29, 119–121. [Google Scholar] [CrossRef]
- Atyeo, W.T.; Kethley, J.B.; Pérez, T.M. Paedomorphosis in Metacheyletia (Acari: Cheyletidae), with the description of a new species. J. Med. Entomol. 1984, 21, 125–131. [Google Scholar] [CrossRef]
- Kethley, J.B. Population regulation in quill mites (Acari: Syringophilidae). Ecology 1971, 52, 1113–1118. [Google Scholar] [CrossRef]
- Glowska, E.; Chrzanowski, M.; Kaszewska, K. Checklist of the quill mites (Acariformes: Syringophilidae) of the World. Zootaxa 2015, 3968, 1–81. [Google Scholar] [CrossRef]
- Zmudzinski, M.; Skoracki, M.; Sikora, B. An Updated Checklist of Quill Mites of the Family Syringophilidae (Acariformes: Prostigmata). 2023. Available online: https://figshare.com/articles/dataset/An_updated_checklist_of_quill_mites_of_the_family_Syringophilidae_Acariformes_Prostigmata_/16529574 (accessed on 15 September 2025).
- Skoracki, M.; Hromada, M.; Prevuznakova, P.; Wamiti, W. Mites of the family Syringophilidae (Acariformes: Cheyletoidea) parasitising waxbills of the genus Estrilda (Passeriformes: Estrildidae). Syst. Appl. Acarol. 2019, 24, 1799–1808. [Google Scholar] [CrossRef]
- Szymański, P.; Niśkiewicz, M.; Budka, M.; Zampa, L.; Osiejuk, T.S.; Skoracki, M. Quill mites of the family Syringophilidae (Acariformes: Prostigmata) parasitising doves of the genus Turtur (Columbiformes: Columbidae). Syst. Appl. Acarol. 2023, 28, 1466–1475. [Google Scholar] [CrossRef]
- Sikora, B.; Unsoeld, M.; Melzer, R.R.; Friedrich, S.; Hromada, M. Revealing the Complexity of Host-Parasite Relationships between Syringophilid Mites and Sunbirds in Their Global Range. Animals 2025, 15, 110. [Google Scholar] [CrossRef]
- Sikora, B.; Kosicki, J.Z.; Patan, M.; Marcisova, I.; Hromada, M.; Skoracki, M. Diversity and Interactions between Picobiine Mites and Starlings. Animals 2024, 14, 2517. [Google Scholar] [CrossRef]
- Marciniak-Musial, N.; Skoracki, M.; Kosicki, J.Z.; Unsöld, M.; Sikora, B. Host-Parasite Relationships of Quill Mites (Syringophilidae) and Parrots (Psittaciformes). Diversity 2023, 15, 1. [Google Scholar] [CrossRef]
- Kaszewska-Gilas, K.; Kosicki, J.Z.; Hromada, M.; Skoracki, M. Global Studies of the Host-Parasite Relationships between Ectoparasitic Mites of the Family Syringophilidae and Birds of the Order Columbiformes. Animals 2021, 11, 3392. [Google Scholar] [CrossRef] [PubMed]
- Winkler, D.W.; Billerman, S.M.; Lovette, I.J. Finches, Euphonias, and Allies (Fringillidae), version 1.0. In Birds of the World; Billerman, S.M., Keeney, B.K., Rodewald, P.G., Schulenberg, T.S., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Ericson, P.G.; Johansson, U.S. Phylogeny of Passerida (Aves: Passeriformes) based on nuclear and mitochondrial sequence data. Mol. Phylogenet. Evol. 2003, 29, 126–138. [Google Scholar] [CrossRef] [PubMed]
- Yuri, T.; Mindell, D.P. Molecular phylogenetic analysis of Fringillidae, “New World nine-primaried oscines” (Aves: Passeriformes). Mol. Phylogenet. Evol. 2002, 23, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Johansson, U.S.; Fjeldså, J.; Bowie, R.C. Phylogenetic relationships within Passerida (Aves: Passeriformes): A review and a new molecular phylogeny based on three nuclear intron markers. Mol. Phylogenet. Evol. 2008, 48, 858–876. [Google Scholar] [CrossRef]
- Treplin, S.; Siegert, R.; Bleidorn, C.; Thompson, H.S.; Fotso, R.; Tiedemann, R. Molecular phylogeny of songbirds (Aves: Passeriformes) and the relative utility of common nuclear marker loci. Cladistics 2008, 24, 328–349. [Google Scholar] [CrossRef]
- Barker, F.K.; Cibois, A.; Schikler, P.; Feinstein, J.; Cracraft, J. Phylogeny and diversification of the largest avian radiation. Proc. Natl. Acad. Sci. USA 2004, 101, 11040–11045. [Google Scholar] [CrossRef]
- Barker, F.K.; Burns, K.J.; Klicka, J.; Lanyon, S.M.; Lovette, I.J. Going to extremes: Contrasting rates of diversification in a recent radiation of New World passerine birds. Syst. Biol. 2013, 62, 298–320. [Google Scholar] [CrossRef]
- Zuccon, D.; Prys-Jones, R.; Rasmussen, P.C.; Ericson, P.G.P. The phylogenetic relationships and generic limits of finches (Fringillidae). Mol. Phylogenet. Evol. 2012, 62, 581–596. [Google Scholar] [CrossRef]
- Imfeld, T.S.; Barker, F.K.; Brumfield, R.T. Mitochondrial genomes and thousands of ultraconserved elements resolve the taxonomy and historical biogeography of the Euphonia and Chlorophonia finches (Passeriformes: Fringillidae). Auk 2020, 137, ukaa016. [Google Scholar] [CrossRef]
- Martin, J.E.H. The Insects and Arachnids of Canada. Part 1. Collecting, Preparing and Preserving Insects, Mites, and Spiders; Publication 1643; Canada Department of Agriculture, Biosystematics Research Institute: Ottawa, ON, Canada, 1978; pp. 1–182. [Google Scholar]
- Neuhaus, B.; Schmid, T.; Riedel, J. Collection management and study of microscope slides: Storage, profiling, deterioration, restoration procedures, and general recommendations. Zootaxa 2017, 4322, 1–173. [Google Scholar] [CrossRef]
- Walter, D.E.; Krantz, G.W. Collecting, rearing, and preparing specimens. In A Manual of Acarology, 3rd ed.; Krantz, G.W., Walter, D.E., Eds.; Texas Tech University Press: Lubbock, TX, USA, 2009; pp. 83–96. [Google Scholar]
- Grandjean, F. Les segments post-larvaires de l’hystérosoma chez les Oribates (Acariens). Bull. Soc. Zool. Fr. 1939, 64, 273–284. [Google Scholar]
- Kethley, J.B. Acarina: Prostigmata (Actinedida). In Soil Biology Guide; Dindal, D.L., Ed.; John Wiley & Sons: New York, NY, USA, 1990; pp. 667–756. [Google Scholar]
- Grandjean, F. Observations sur les Acariens de la famille des Stigmaeidae. Arch. Sci. Phys. Nat. 1944, 26, 103–131. [Google Scholar]
- Kethley, J.B. A revision of the family Syringophilidae (Prostigmata: Acarina). Contrib. Am. Entomol. Inst. 1970, 5, 1–76. [Google Scholar]
- Skoracki, M. Quill mites (Acari: Syringophilidae) of the Palaearctic region. Zootaxa 2011, 2840, 1–414. [Google Scholar] [CrossRef]
- Clements, J.F.; Rasmussen, P.C.; Schulenberg, T.S.; Iliff, M.J.; Fredericks, T.A.; Gerbracht, J.A.; Lepage, D.; Spencer, A.; Billerman, S.M.; Sullivan, B.L.; et al. The eBird/Clements Checklist of Birds of the World. 2024. Available online: https://www.birds.cornell.edu/clementschecklist/download/ (accessed on 20 September 2025).
- BirdLife International. Species factsheets. 2024. Available online: https://www.birdlife.org (accessed on 13 August 2025).
- BirdLife International. Bird Species Distribution Maps of the World. Version 2024.1. Available online: http://datazone.birdlife.org/species/requestdis (accessed on 13 August 2025).
- Holt, B.G.; Lessard, J.P.; Borregaard, M.K.; Fritz, S.A.; Araújo, M.B.; Dimitrov, D.; Fabre, P.H.; Graham, C.H.; Graves, G.R.; Jønsson, K.A.; et al. An update of Wallace’s zoogeographic regions of the World. Science 2013, 339, 74–78. [Google Scholar] [CrossRef]
- Ficetola, G.; Mazel, F.; Thuiller, W. Global determinants of zoogeographical boundaries. Nat. Ecol. Evol. 2017, 1, 89. [Google Scholar] [CrossRef]
- Caira, J.N.; Jensen, K.; Holsinger, K.I. On a new index of host specificity. In Taxonomie, Écologie et Évolution des Metazoaires Parasites; Combes, C., Jourdane, J., Eds.; Presses Universitaires de Perpignan: Perpignan, France, 2003; Volume 1, pp. 161–201. [Google Scholar]
- Skoracki, M.; Sikora, B.; Spicer, G.S. A review of the subfamily Picobiinae Johnston and Kethley, 1973 (Acariformes: Prostigmata: Syringophilidae). Zootaxa 2016, 4113, 1–95. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2023; Available online: https://www.R-project.org/ (accessed on 15 August 2025).
- Zmudziński, M.; Unsoeld, M. Quill mites (Acariformes: Syringophilidae) parasitising birds in Germany: New host records and descriptions of two new species from Limosa lapponica (L.) (Aves: Scolopacidae). Syst. Appl. Acarol. 2019, 24, 362–376. [Google Scholar] [CrossRef]
- Skoracki, M.; Bochkov, A.V. Syringophilid mites (Acari: Syringophilidae) of Kazakhstan. Zootaxa 2010, 2546, 52–68. [Google Scholar] [CrossRef]
- Skoracki, M.; Hendricks, S.; Spicer, G. Systematics of the ectoparasitic quill mites of the genus Aulobia Kethley, 1970 (Acari: Syringophilidae) with the description of a new species. Zootaxa 2010, 2399, 31–41. [Google Scholar] [CrossRef]
- Clement, P.; Arkhipov, V.Y. Asian Rosy-Finch (Leucosticte arctoa), version 1.0. In Birds of the World; del Hoyo, J., Elliott, A., Sargatal, J., Christie, D.A., de Juana, E., Eds.; Cornell Lab of Ornithology: Ithaca, NY, USA, 2020. [Google Scholar]
- Sikora, B.; Unsoeld, M.; Melzer, R.R.; Friedrich, S.; Hromada, M.; Skoracki, M. Quill Mites of the Family Syringophilidae (Acariformes: Cheyletoidea) Parasitising Birds of the Subfamily Euphoninae (Passeriformes: Fringillidae). Animals 2025, 15, 764. [Google Scholar] [CrossRef]
- Bochkov, A.V.; Apanaskevich, D. Two new species of the family Syringophilidae (Acari: Cheyletoidea) from passeriform birds collected in the Altai. Folia Parasitol. 2001, 48, 321–325. [Google Scholar] [CrossRef]
- Bochkov, A.V.; Fain, A.; Skoracki, M. New quill mites of the family Syringophilidae (Acari: Cheyletoidea). Syst. Parasitol. 2004, 57, 135–150. [Google Scholar] [CrossRef] [PubMed]
- Skoracki, M. A review of quill mites of the genus Syringophiloidus Kethley, 1970 (Acari: Prostigmata: Syringophilidae) parasitising quills of passeriform birds, with descriptions of four new species. Genus 2004, 15, 281–300. [Google Scholar]
- Fritsch, W. Die Milbengattung Syringophilus Heller, 1880 (Subordo Trombidiformes, Fam. Myobiidae Megnin, 1877). Zool. Jahrb. Syst. 1958, 86, 227–234. [Google Scholar]
- Bochkov, A.V.; Mironov, S.V. Quill mites of the family Syringophilidae Lavoipierre, 1953 (Acariformes: Prostigmata) parasitic on birds (Aves) of the fauna of the former USSR. Acarina 1998, 6, 3–16. [Google Scholar]
- Nattress, B.; Skoracki, M. A new species and further records of quill mites (Acari: Cheyletoidea: Syringophilidae) parasitic on birds (Aves) in England. Zootaxa 2009, 2133, 49–54. [Google Scholar] [CrossRef]
- Klimovičová, M.; Hromada, M. New hosts and localities of quill mites (Acari: Syringophilidae) parasitising birds in Slovakia. Acta Univ. Prešov., Folia Oecol. 2014, 11, 31–42. [Google Scholar]
- Bochkov, A.V.; Mironov, S.V.; Kravtsova, N.T. Two new syringophilid mites (Acari: Syringophilidae) from the Greenfinch Carduelis chloris (Passeriformes: Fringillidae) from Kirghizia. Genus 2000, 11, 351–358. [Google Scholar]
- Nattress, B.; Skoracki, M. Quill mites of the family Syringophilidae Lavoipierre, 1953 (Acari: Cheyletoidea) parasitic on birds in England. Genus 2007, 18, 139–145. [Google Scholar]
- Glowska, E.; Skoracki, M.; Khourly, F. A new species and new records of syringophilid mites (Acari: Prostigmata: Syringophilidae) from birds of Jordan. Zootaxa 2007, 1635, 63–68. [Google Scholar] [CrossRef]
- Skoracki, M.; Antczak, M.; Riegert, J.; Fainova, D.; Mikes, V. New species and new records of quill mites (Acari: Syringophilidae) inhabiting African passerines (Aves: Passeriformes). Acta Zool. Acad. Sci. Hung. 2009, 55, 123–137. [Google Scholar]
- Bochkov, A.V.; Mironov, S.V. New quill mite species of the family Syringophilidae (Acari: Cheyletoidea) from European part of Russia. Acarina 1999, 7, 35–45. [Google Scholar]
- Bochkov, A.V.; Flannery, M.E.; Spicer, G.S. Mites of the genus Torotrogla (Prostigmata: Syringophilidae) from North American passerines. J. Med. Entomol. 2009, 46, 183–197. [Google Scholar] [CrossRef]
- Skoracki, M. New data on systematics of the quill mites of the genus Torotrogla Kethley, 1970 (Acari: Syringophilidae). Belg. J. Entomol. 2004, 6, 303–314. [Google Scholar]
- Beecher, W.J. A phylogeny of the Oscines. Auk 1953, 70, 270–333. [Google Scholar] [CrossRef]
- Ziswiler, V. Neue Aspekte zur Systematik körnerfressender Singvögel. Verhand. Schweizerisch. Naturforsch. Gesellsch. 1964, 144, 133–134. [Google Scholar]
- Ziswiler, V. Zur Kenntnis des Samenöffnens und der Struktur des hörneren Gaumens bei körnerfressenden Oscines. J. Orn. 1965, 106, 1–48. [Google Scholar] [CrossRef]
- Zusi, R.L. The interorbital septum in cardueline finches. Bull. Brit. Orn. Club 1978, 98, 5–10. [Google Scholar]
- Oliveros, C.H.; Field, D.J.; Ksepka, D.T.; Barker, F.K.; Aleixo, A.; Andersen, M.J.; Alström, P.; Benz, B.W.; Braun, E.L.; Braun, M.J.; et al. Earth history and the passerine superradiation. Proc. Natl. Acad. Sci. USA 2019, 116, 7916–7925. [Google Scholar] [CrossRef] [PubMed]
- Klassen, G.J. Coevolution: A history of the macroevolutionary approach to studying host parasite associations. J. Parasitol. 1992, 78, 573–587. [Google Scholar] [CrossRef] [PubMed]
- Fahrenholz, H. Ectoparasiten und abstammungslehre. Zool. Anz. 1913, 41, 371–374. [Google Scholar]
- Page, R.D.M. (Ed.) Tangled Trees: Phylogeny, Cospeciation, and Coevolution; The University of Chicago Press: Chicago, IL, USA, 2003. [Google Scholar]
- Poulin, R. Evolutionary Ecology of Parasites, 2nd ed.; Princeton University Press: Priceton, NJ, USA, 2007. [Google Scholar]
- Page, R.D.M.; Charleston, M.A. Trees within trees—Phylogeny and historical associations. Trends Ecol. Evol. 1998, 13, 356–359. [Google Scholar] [CrossRef]
- Bochkov, A.V.; Mironov, S.V. The phenomenon of phylogenetic synhospitality in acariform mites (Acari: Acariformes)—The permanent parasites of vertebrates. Parazitologiia 2008, 42, 81–100. (In Russian) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).