Effect of Meal and Whole Larvae of Black Soldier Fly (Hermetia illucens) on the Performance, Blood Lipid Profile, Slaughter Characteristics, Sensory Properties and Fatty Acid Composition of Pheasant (Phasianus colchicus L.) Muscles
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Birds and Housing
2.2. Diet
2.3. Analytical Procedures
2.4. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Braasch, T.; Pes, T.; Michel, S.; Jacken, H. The subspecies of the common pheasant Phasianus colchicus in the wild and captivity. Int. J. Gall. Conserv. 2011, 2, 6–13. [Google Scholar]
- Beeger, S.; Wójcik, M.; Flis, M.; Marecki, M.; Pyrkosz, R.; Dziedzic, R. Anatomo-morphological features of free-living and farmed pheasant. Med. Wet. 2017, 73, 370–374. [Google Scholar] [CrossRef]
- Glutz, V.; Blotzheim, U.N.; Bauer, K.; Bezzel, E. Handbuch der Vögel Mitteleuropas; Akademisches Verlagsgesselschaft: Frankfurt am Main, Germany, 1973; pp. 322–372. [Google Scholar]
- Grahn, M. Mortality in the pheasant Phasianus colchicus during the breeding season. Behav. Ecol. Sociobiol. 1993, 32, 95–101. [Google Scholar] [CrossRef]
- Geaumont, B.A.; Sedivec, K.K.; Schauer, C.S. Ring-necked Pheasant use of post—Conservation reserve program lands. Ranel. Ecol. Manag. 2017, 70, 569–575. [Google Scholar] [CrossRef]
- Dordevic, M.; Pekec, S.; Popovic, Z.; Dordevic, N. Production losses and mortality of pheasants depending on cultivation technology in voliers, nutrition and hunting conditions. In Proceedings of the International Symposium on Animal Science, Belgrade, Serbia, 22–23 November 2018; pp. 83–89. [Google Scholar]
- Madden, J.R.; Hall, A.; Whiteside, M.A. Why do many pheasants released in the UK die, and how can we best reduce their natural mortality? Eur. J. Wildl. Res. 2018, 64, 40. [Google Scholar] [CrossRef]
- Flis, M.; Czyżowski, P.; Beeger, S.; Grela, E.R. Effect of dietary supplementation with insect meal on anatomical and morphological traits of pheasants (Phasianus colchicus). Animals Sci. Genet. 2024, 24, 65–75. [Google Scholar] [CrossRef]
- Czyżowski, P. Functioning of pheasant (Phasianus colchicus L.) population in western Lublin region. Pt. 1. Effects of introduction. Ann. Univ. Mariae Curie-Sklodowska. Sect. EE Zoot. 2003, 57, 31–38. [Google Scholar]
- Dordevic, M.; Pekec, S.; Popovic, Z.; Dordevic, N. Influence of dietary protein levels on production results and mortality in pheasants reared under controlled conditions. Acta Vet. 2010, 60, 79–88. [Google Scholar] [CrossRef]
- Flis, M.; Józefiak, D.; Bielak, A.; Kasperek, K.; Kierończyk, B.; Grela, E.R. Insects as a natural component of pheasant diets: Effects of full-fat Hermetia illucens meal on egg production and quality, hatchability, and selected physicochemical egg indices. J. Anim. Feed Sci. 2024, 33, 217–225. [Google Scholar] [CrossRef]
- Gugała, D.; Flis, M. Breeding aviary pheasants—Passion or source of income? Wiad Zoot 2018, 4, 211–216. (In Polish) [Google Scholar]
- Tucak, Z.; Škrivanko, M.; Posavčević, Š.; Periškić, M.; Bošković, I.; Jumić, V. The influence of keeping pheasants in captivity vs. nature on the biological value of meat and its use in human nutrition. Coll. Antropol. 2008, 32, 959–962. [Google Scholar]
- Mieczkowska, A.; Kokoszyński, D.; Wasilewski, R.; Bernacki, Z. Skład tuszki i jakość mięsa bażantów zwyczajnych (Phasianus colchicus colchicus) w zależności od płci ptaków. Żyw. Nauka Technol. Jak. 2015, 3, 95–106. [Google Scholar] [CrossRef]
- Gašparovič, M.; Hrnčár, C.; Gálik, B. The effect of feed additives in pheasants fattening: A review. J. Cent. Eur. Agric. 2017, 18, 749–761. [Google Scholar] [CrossRef]
- Adamski, M.; Kuźniacka, J. The effect of age and sex on slaughter traits of pheasants (Phasianus colchicus L.). Anim. Sci. Pap. Rep. 2006, 24, 11–18. [Google Scholar]
- Brudnicki, A.; Kułakowska, A.; Pietruszyńska, D.; Łożycka-Kapłon, M.; Wach, J. Differences in the amino acid composition of the breast muscle of wild and farmed pheasant. Czech J. Food Sci. 2012, 30, 309–313. [Google Scholar] [CrossRef]
- Franco, D.; Lorenzo, J.M. Meat quality nutritional composition of pheasants (Phasianus colchicus) reared in an extensive system. Br. Poult. Sci. 2013, 54, 594–602. [Google Scholar] [CrossRef]
- Kokoszyński, D.; Bernacki, Z.; Korytkowska, H.; Wilkanowska, A. Effect of different feeding regimens for game pheasants on carcass composition, fatty acid profile and mineral content of meat. Eur. Poul. Sci. 2014, 78, 1–10. [Google Scholar] [CrossRef]
- Quaresma, M.A.G.; Pimentel, F.B.; Ribeiro, A.P.; Ferreira, J.D.; Alves, S.P.; Rocha, I.; Bessa, R.J.B.; Oliveira, M.B.P.P. Lipid and protein quality of common pheasant (Phasianus colchicus) reared in semi-extensive conditions. J. Food Comp. Anal. 2016, 46, 88–95. [Google Scholar] [CrossRef]
- Flis, M.; Grela, E.R.; Gugała, A.; Kołodziejski, A. Carcass composition and fatty acid profile of pectoral muscle of male and female pheasants (Phasianus colchicus). Żyw. Nauka Technol. Jak. 2019, 26, 111–124. (In Polish) [Google Scholar] [CrossRef]
- Kokoszyński, D.; Żochowska-Kujawska, J.; Kotowicz, M.; Piątek, H.; Włodarczyk, K.; Arpášova, H.; Biesiada-Drzazga, B.; Wegner, M.; Saleh, M.; Imański, M. The effects of slaughter age and sex on carcass traits, meat quality, and leg bone characteristics of farmed common pheasant (Phasianus colchicus L.). Animals 2024, 14, 1050. [Google Scholar] [CrossRef] [PubMed]
- Šperanda, M.; Florijančić, T.; Bošković, I.; Bogut, I.; Gutzmirtl, H.; Grgurić, D.; Senčić, Đ.; Antunović, Z. The effects of organic selenium and mannan oligosaccharides on the productivity and health of pheasant chicken (Phasianus colchicus). Acta Veter. (Beograd) 2008, 58, 63–73. [Google Scholar] [CrossRef]
- Biesiada-Drzazga, B.; Socha, S.; Janocha, A.; Banaszkiewicz, T.; Koncerewicz, A. Assessment of slaughter value and quality of meat in common game pheasant (Phasianus colchicus). Żyw. Nauk. Technol. Jak. 2011, 1, 79–86. [Google Scholar]
- Haščík, P.; Trembecká, L.; Bobko, M.; Čuboň, J.; Bučko, O.; Tkáčová, J. Evaluation of meat quality after application of different feed additives in diet of broiler chickens. Slovak J. Food Sci./Potravin. 2015, 9, 174–182. [Google Scholar] [CrossRef]
- Abo Ghanima, M.M.; Elsadek, M.F.; Taha, A.E.; Abd El-Hack, M.E.; Alagawany, M.; Ahmed, B.M.; Elshafie, M.M.; El-Sabrout, K. Effect of housing system and rosemary and cinnamon essential oils on layers’ performance, egg quality, haematological traits, blood chemistry, immunity, and antioxidant. Animals 2020, 10, 245. [Google Scholar] [CrossRef]
- El-Sabrout, K.; Dantas, M.R.T.; Souza-Junior, J.B.F. Herbal and bee products as nutraceuticals for improving poultry health and production. World Poul. Sci. J. 2023, 79, 223–242. [Google Scholar] [CrossRef]
- Hanzal, V.; Divišová, M.; Murawska, D.; Janiszewski, P. The effect of dietary bio-alginate supplementation of the growth rate and body weight of common pheasant (Phasianus colchicus) chicks. Pol. J. Nat. Sci. 2016, 31, 363–371. [Google Scholar]
- Grela, E.R.; Knaga, S.; Mieczan, A.; Zięba, G. Effects of dietary alfalfa protein concentrate supplementation on performance, egg quality, and fatty acid composition of raw, freeze-dried, and hard-boiled eggs from Polbar laying hens. Poult. Sci. 2020, 99, 2256–2265. [Google Scholar] [CrossRef]
- Gugała, D.; Flis, M.; Grela, E.R. The effect of zinc, iron, calcium, and copper from organic sources in pheasant diet on the performance, hatching, minerals, and fatty acid composition of eggs. Poul. Sci. 2019, 98, 4640–4647. [Google Scholar] [CrossRef]
- Józefiak, D.; Józefiak, A.; Kierończyk, B.; Rawski, M.; Świątkiewicz, S.; Długosz, J.; Engberg, R.M. Insects—A natural nutrient source for poultry—A review. Ann. Animal Sci. 2016, 16, 297–313. [Google Scholar] [CrossRef]
- Chatzidimitriou, E.; Davis, H.; Maurer, V.; Leiber, F.; Leifert, C.; Stergiadis, S.; Butler, G. Egg fatty acid profiles and potential health risk from defatted insect meal in laying hens’ diets. J. Insects Food Feed. 2022, 8, 1085–1095. [Google Scholar] [CrossRef]
- Zamri, M.Z.; Ramiah, S.K.; Jamein, E.S.; Zulkifli, I.; Lokman, I.H.; Amirul, F.M.; Fadzlin, S.; Mohd Zamri, S.; Jayanegara, A.; Hassim, H.A. Potential use of black soldier fly, Hermetia illucens larvae in chicken feed as a protein replacer: A review. J. Anim. Feed Sci. 2023, 32, 341–353. [Google Scholar] [CrossRef]
- Rytlewski, G.; Flis, M.; Jaworski, H.; Piórkowski, J.; Grela, E.R. Effectiveness of black soldier fly (Hermetia illucens) as meal or whole larvae in feeding pheasants on production outcomes, chemical composition, and fatty acid profile of eggs. Poult. Sci. 2025, 104, 10. [Google Scholar] [CrossRef]
- Marono, S.; Loponte, R.; Lombardi, P.; Vassalotti, G.; Pero, M.E.; Russo, F.; Gasco, L.; Parisi, G.; Piccolo, G.; Nizza, S.; et al. Productive performance and blood profiles of laying hens fed Hermetia illucens larvae meal as total replacement of soybean meal from 24 to 45 weeks of age. Poult. Sci. 2017, 96, 1783–1790. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, X.; Yao, Y.; Qu, X.; Chen, J.; Xie, K.; Wang, X.; Yi, Y.; Xiao, B.; He, C. Effects of different levels of Hermetia illucens larvae meal on performance, egg quality, yolk fatty acid composition and oxidative status of laying hens. Ital. J. Anim. Sci. 2021, 20, 256–266. [Google Scholar] [CrossRef]
- Secci, G.; Bovera, F.; Parisi, G.; Moniello, G. Quality of eggs and albumen technological properties as affected by Hermetia illucens larvae meal in hens’ diet and hen age. Animals 2020, 10, 81. [Google Scholar] [CrossRef]
- Yan, Y.; Zhang, J.; Chen, X.; Wang, Z. Effects of Black soldier fly larvae (Hermetia illucens) meal on the production performance and caecal microbiota of hens. Vet. Sci. 2023, 10, 364. [Google Scholar] [CrossRef]
- NRC (National Research Council). Nutrient Requirements of Ring-Necked Pheasants, Japanese Quail, and Bobwhite Quail, 9th ed.; Table 6-2. Nutrient Requirements of Poultry; National Academic Press: Washington, DC, USA; NRC: Ottawa, ON, Canada, 1994. [Google Scholar]
- Kierończyk, B.; Sypniewski, J.; Mikołajczak, Z.; Rawski, M.; Pruszyńska-Oszmałek, E.; Sassek, M.; Kołodziejski, P.; Józefiak, D. Replacement of soybean oil with cold-extracted fat from Hermetia illucens in young turkey diets: Effects on performance, nutrient digestibility, selected organ measurements, meat and liver tissue traits, intestinal microbiota modulation, and physiological and immunological status. Anim. Feed Sci. Technol. 2022, 286, 115210. [Google Scholar] [CrossRef]
- Mikołajczak, Z.; Rawski, M.; Mazurkiewicz, J.; Kierończyk, B.; Kołodziejski, P.; Pruszyńska-Oszmałek, E.; Józefiak, D. The first insight into black soldier fly meal in brown trout nutrition as an environmentally sustainable fish meal replacement. Animal 2022, 16, 100516. [Google Scholar] [CrossRef]
- Council Regulation (EC) No 1099/2009 of 24 September 2009 on the Protection of Animals at the Time of Killing. OJ L 2009, 303.1. Available online: https://eur-lex.europa.eu/eli/reg/2009/1099/oj/eng (accessed on 2 November 2025).
- AOAC. Official Methods of Analysis of AOAC International, 20th ed.; Association of Official Analytical Chemists International: Gaithersburg, MD, USA, 2016. [Google Scholar]
- Friedewald, W.T.; Levy, R.I.; Fredrickson, D.S. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin. Chem. 1972, 18, 499–502. [Google Scholar] [CrossRef]
- Botsoglou, N.; Fletouris, D.; Psomas, I.; Mantis, A. Rapid gas chromatographic method for simultaneous determination of cholesterol and α-tocopherol in eggs. J. AOAC Int. 1998, 81, 1177–1183. [Google Scholar] [CrossRef] [PubMed]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Baryłko-Pikielna, N. Outline of Food Sensory Analysis; PWN: Warszawa, Poland, 1975; pp. 241–295. [Google Scholar]
- Krełowska-Kulas, M. The Study of Quality Food Products; PWE: Warszawa, Poland, 1993; pp. 399–427. [Google Scholar]
- Fisher, C.; McNab, J.M. Techniques for Determining the Metabolizable Energy (ME) Content of Poultry Feeds. In Recent Advances in Animal Nutrition; Haresign, W., Cole, J.A.D., Eds.; Butterworths: London, UK, 1987; pp. 3–18. [Google Scholar]
- Gadzama, I.U. Black soldier fly larvae as animal feed. Bulg. J. Anim. Husb. 2025, 62, 48–64. [Google Scholar] [CrossRef]
- Schiavone, A.; Castillo, A. Incorporating whole insect larvae into poultry diet: State of the art and future perspectives. Ital. J. Anim. Sci. 2024, 23, 1–14. [Google Scholar] [CrossRef]
- Krystianiak, S.; Torgowski, J. The effect of two feeding systems on rearing results of pheasants (Phasianus colchicus L.) of Polish and French varieties. Anim. Prod. Rev. Appl. Sci. Rep. 1998, 36, 201–209. [Google Scholar]
- Straková, E.; Suchý, P.; Karásková, K.; Jámbor, M.; Navrátil, P. Comparison of nutritional values of pheasant and broiler chicken meats. Acta Vet. Brno 2011, 80, 373–377. [Google Scholar] [CrossRef]
- Grela, E.R.; Gugała, D.; Flis, M. Influence of partial replacement of some inorganic minerals with glycine complex and vitamin D3 source on performance, slaughter traits, sensory and physico-chemical characteristics of pheasant muscles (Phasianus colchicus L.) depending on gender. Ann. Anim. Sci. 2021, 21, 245–265. [Google Scholar] [CrossRef]
- Osuch, B.; Barszcz, M.; Tomaszewska-Zaremba, D. The potential of black soldier fly (Hermetia illucens L.) larvae in chicken and swine nutrition. A review. J. Anim. Feed Sci. 2024, 33, 454–468. [Google Scholar] [CrossRef]
- Józefiak, A.; Kierończyk, B.; Rawski, M.; Mazurkiewicz, J.; Benzertiha, A.; Gobbi, P.; Nogales-Merida, S.M.; Świątkiewicz, S.; Józefiak, D. Full-fat insect meals as feed additive—The effect on broiler chicken growth performance and gastrointestinal tract microbiota. J. Anim. Feed Sci. 2018, 27, 131–139. [Google Scholar] [CrossRef]
- De Souza Vilela, J.; Andronicos, N.M.; Kolakshyapati, M.; Hilliar, M.; Sibanda, T.Z.; Andrew, N.R.; Swick, R.A.; Wilkinson, S.; Ruhnke, I. Black soldier fly larvae in broiler diets improve broiler performance and modulate the immune system. Anim. Nutr. 2021, 7, 695–706. [Google Scholar] [CrossRef]
- Huang, C.; Hernandez, C.E.; Wall, H.; Tahamtani, F.M.; Ivarsson, E.; Sun, L. Live black soldier fly (Hermetia illucens) larvae in feed for laying hens: Effects on hen gut microbiota and behaviour. Poult. Sci. 2024, 103, 103429. [Google Scholar] [CrossRef]
- Chen, L.; Sun, H.; Song, H.; Wang, G.; Ma, X.; Tu, J.; Yang, L.; Li, J.; Wang, Y.; Meng, X.; et al. Dietary inclusion of defatted black soldier fly larvae meal: Impacts on laying hen performance, egg quality, serum biomarkers, and intestinal morphology. Front. Vet. Sci. Front. Vet. Sci. 2025, 12, 1605077. [Google Scholar] [CrossRef]
- Fiorilla, E.; Gariglio, M.; Gai, F.; Zambotto, V.; Bongiorno, V.; Cappone, E.E.; Rødbotten, R.; Koga, S.; Rieder, A.; Tengstrand, E.; et al. Breaking down barriers: Live or dehydrated dietary whole black soldier fly larvae supplementation in slow-growing chickens preserves meat quality and sensory traits. Poult. Sci. 2024, 103, 104120. [Google Scholar] [CrossRef]
- Suparman Purwanti, S.; Nahariah, N. Substitution of fish meal with black soldier fly larvae (Hermetia illucens) meal to egg production and physical quality of quail (Coturnix coturnix japonica) eggs. IOP Conf. Ser. Earth Environ. Sci. 2020, 492, 012014. [Google Scholar] [CrossRef]
- Flis, M.; Czyżowski, P.; Rytlewski, G.; Grela, E.R. Insect meal as a dietary protein source for pheasant quails: Performance, carcass traits, amino acid profile and mineral contents in muscles. Animals 2024, 14, 2992. [Google Scholar] [CrossRef]
- Whiteside, M.A.; Sage, R.; Madden, J.R. Diet complexity in early life affects survival in released pheasants by altering foraging efficiency, food choice, handling skills and gut morphology. J. Anim. Ecol. 2015, 84, 1480–1489. [Google Scholar] [CrossRef]
- Secci, G.; Dabbou, S.; Lira de Medeiros, A.C.; Addeo, N.F.; Atallah, E.; Parisi, G.; Moniello, G.; Bovera, F. Low dietary inclusion levels of Tenebrio molitor larva meal slightly modify growth performance, carcass and meat traits of Japanese quail (Coturnix japonica). J. Sci. Food Agric. 2022, 102, 6578–6585. [Google Scholar] [CrossRef] [PubMed]
- Dalle Zotte, A.; Singh, Y.; Michiels, J.; Culeere, M. Black soldiers fly (Hermetia illucens) as a dietary source for laying quails: Live performance, and egg physico-chemical quality sensory profile and storage stability. Animals 2019, 9, 115. [Google Scholar] [CrossRef]
- Benzertiha, A.; Kierończyk, B.; Kołodziejski, P.; Pruszyńska-Oszmałek, E.; Rawski, M.; Józefiak, D.; Józefiak, A. Tenebrio molitor and Zophobas morio full-fat meals as functional feed additives affect broiler chickens’ growth performance and immune system traits. Poult. Sci. 2020, 99, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Lalev, M.; Hristakieva, P.; Mincheva, N.; Oblakova, M.; Ivanova, I. Insect meal as an alternative protein ingredient in broiler feed. Bulg. J. Agric. Sci. 2022, 28, 743–751. [Google Scholar]
- Loponte, R.; Nizza, S.; Bovera, F.; De Riu, N.; Fliegerova, K.; Lombardi, P.; Vassalotti, G.; Mastellone, V.; Nizza, A.; Moniello, G. Growth performance, blood profiles and carcass traits of Barbary partridge (Alectoris barbara) fed two different insect larva meals (Tenebrio molitor and Hermetia illucens). Res. Vet. Sci. 2017, 115, 183–188. [Google Scholar] [CrossRef]
- Secci, G.; Moniello, G.; Gasco, L.; Bovena, F.; Parisi, G. Barbary partridge meat quality as affected by Hermetia illucens and Tenebrio molitor larva meals in feeds. Food Res. Intern. 2018, 112, 291–298. [Google Scholar] [CrossRef] [PubMed]
- Atallah, E.; Mahayri, T.M.; Mrázek, J.; Bovena, F.; Piccolo, G.; Fliegerová, K.O.; Moniello, G. Dietary inclusion of Hermetia illucens larvae meal modifies caecal microbiota diversity and composition in barbary partridges (Alectoris barbara). Sci. Rep. 2025, 15, 25081. [Google Scholar] [CrossRef] [PubMed]
- Spranghers, T.; Michiels, J.; Vrancx, J.; Ovyn, A.; Eeckhout, M.; De Clercq, P.; De Smet, S. Gut antimicrobial effects and nutritional value of black soldier fly (Hermetia illucens L.) prepupae for weaned piglets. Anim. Feed Sci. Technol. 2018, 235, 33–42. [Google Scholar] [CrossRef]
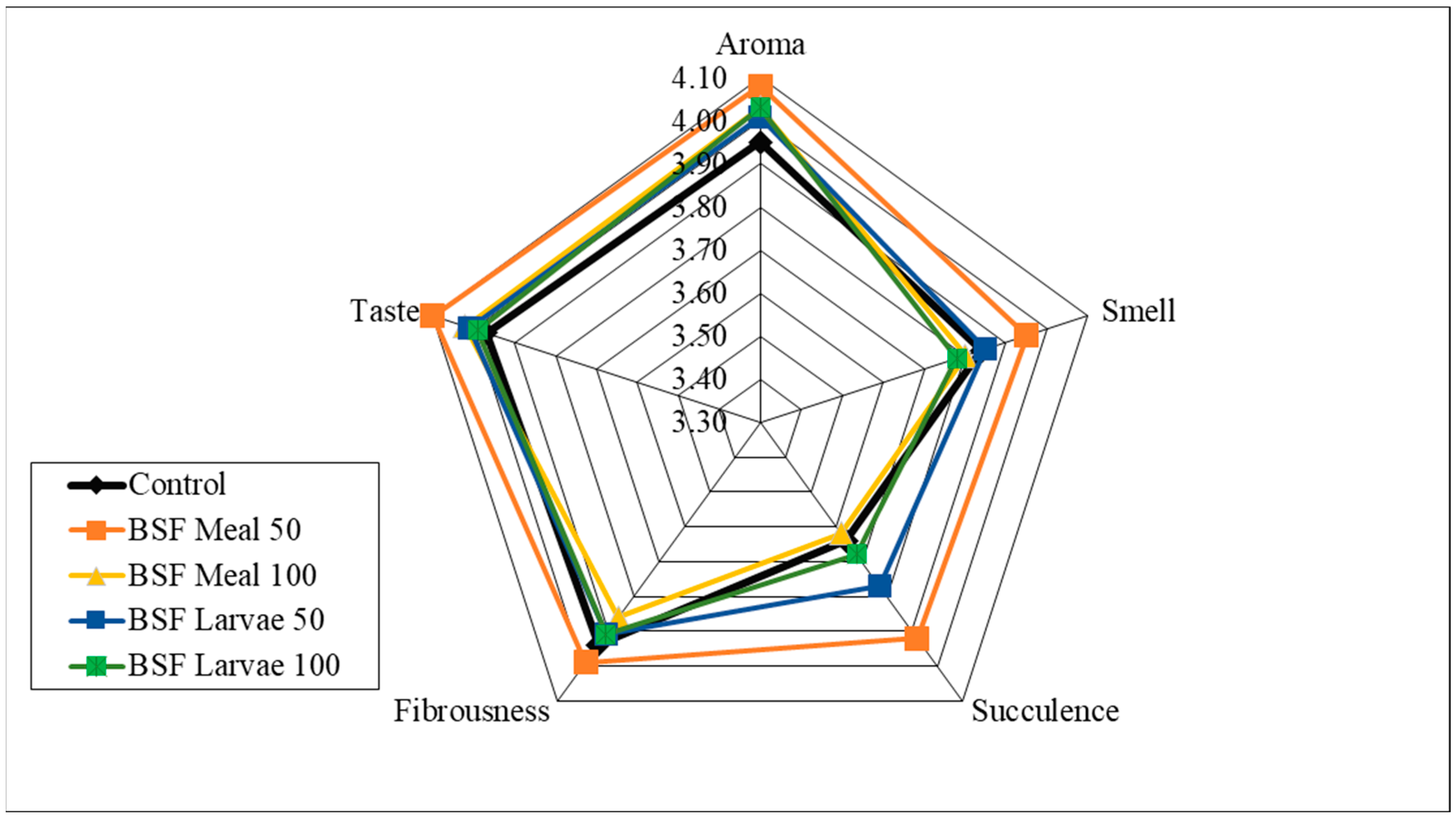
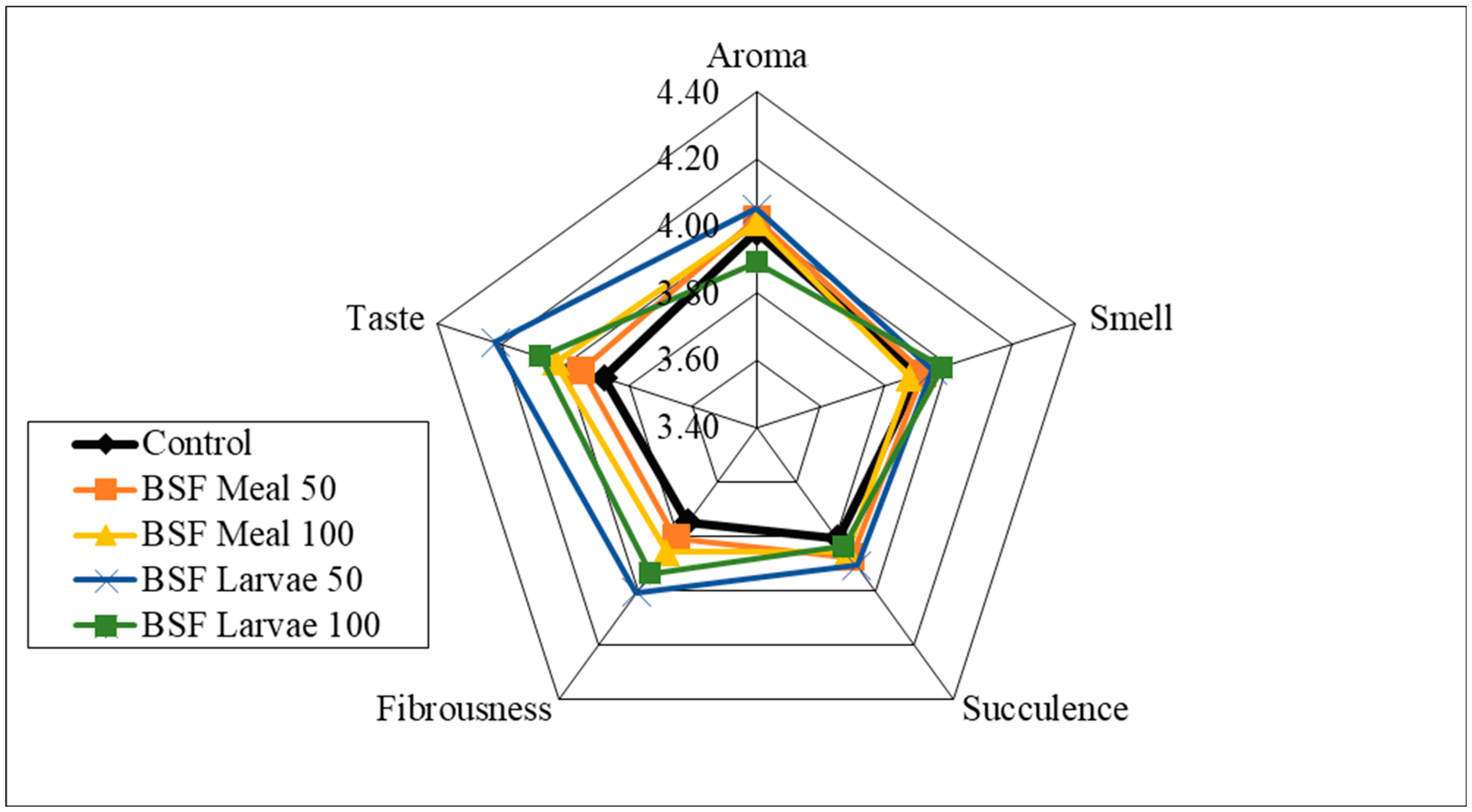
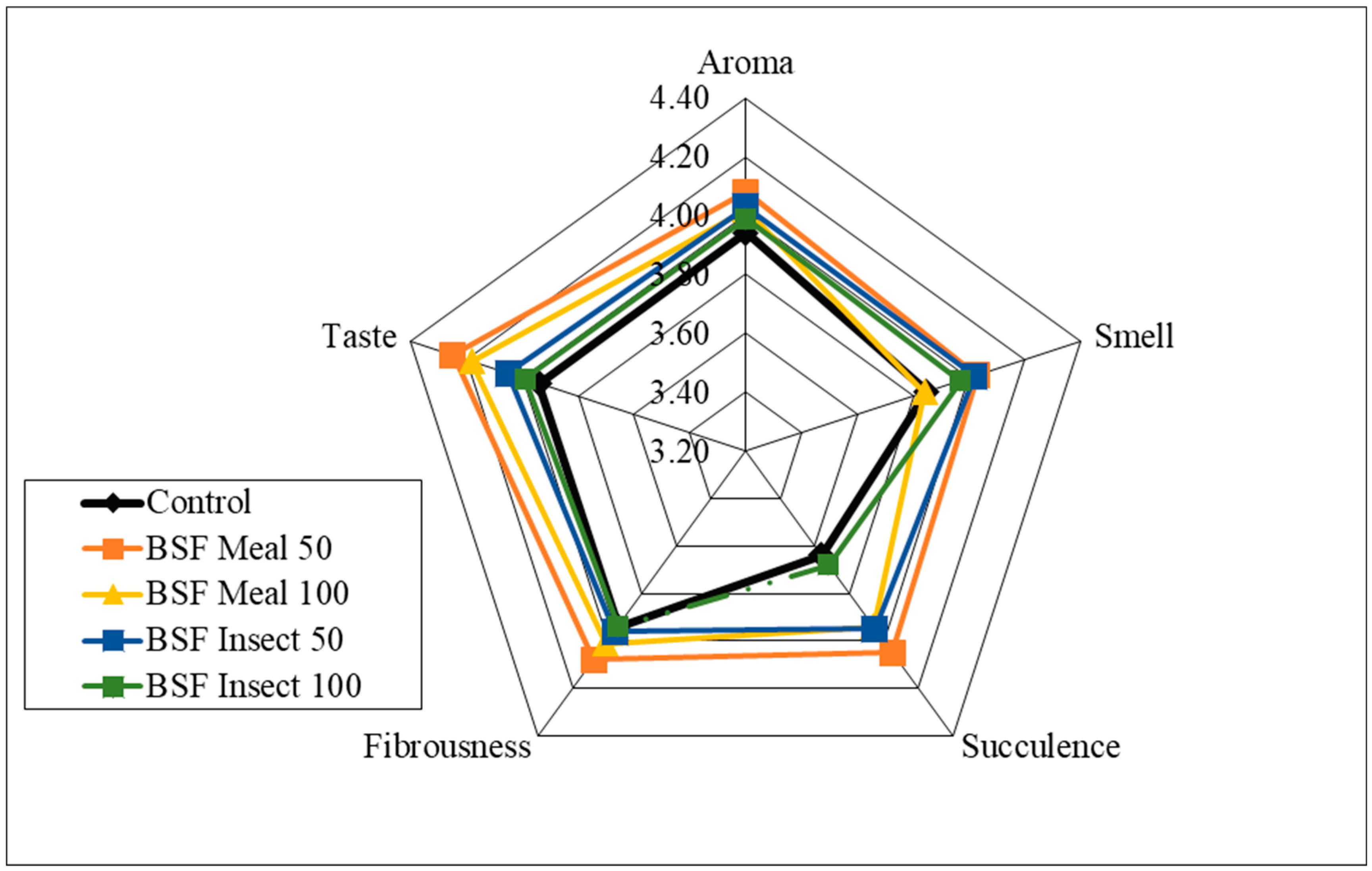
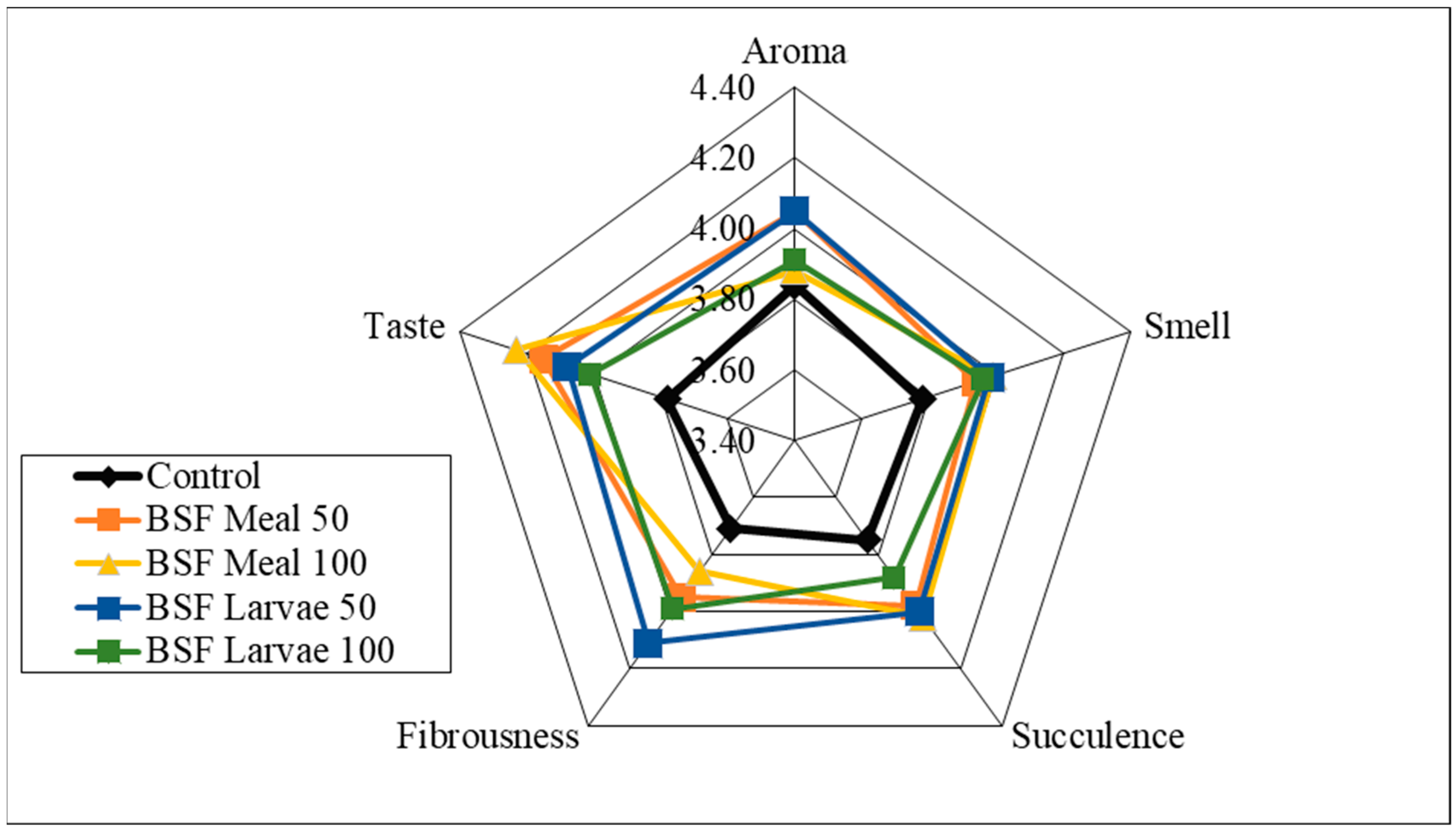
| Item | Reared (0–4 Weeks) | Grower (5–8 Weeks) Period | Finisher (9–16 Weeks) Period | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 50 HM | 100 HM | 50 HL | 100 HL | Control | 50 HM | 100 HM | 50 HL | 100 HL | ||
| Corn | 232.1 | 272.3 | 297.3 | 322.3 | 297.3 | 322.3 | 241.6 | 266.6 | 291.6 | 266.6 | 291.6 |
| Wheat | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 100.0 | 160.0 | 160.0 | 160.0 | 160.0 | 160.0 |
| Soybean meal | 280.0 | 280.0 | 140.0 | 0.0 | 140.0 | 0.0 | 250.0 | 125.0 | 0.0 | 125.0 | 0.0 |
| Garden pea | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Fish meal | 80.0 | 20.0 | 20.0 | 20.0 | 20.0 | 20.0 | 0.0 | 0.0 | 0.0 | 0.0 | 0.0 |
| Insect meal | 0.0 | 0.0 | 140.0 | 280.0 | 0.0 | 0.0 | 0.0 | 125.0 | 250.0 | 0.0 | 0.0 |
| Whole dried larvae | 0.0 | 0.0 | 0.0 | 0.0 | 140.0 | 280.0 | 0.0 | 0.0 | 0.0 | 125.0 | 250.0 |
| Linseed | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 | 40.0 |
| Sunflower meal | 80.0 | 80.0 | 80.0 | 80.0 | 80.0 | 80.0 | 80.0 | 80.0 | 80.0 | 80.0 | 80.0 |
| Soybean oil | 50.0 | 50.0 | 25.0 | 0.0 | 25.0 | 0.0 | 50.0 | 25.0 | 0.0 | 25.0 | 0.0 |
| Sorghum | 10.0 | 30.0 | 30.0 | 30.0 | 30.0 | 30.0 | 50.0 | 50.0 | 50.0 | 50.0 | 50.0 |
| Dicalcium phosphate | 16.0 | 16.0 | 16.0 | 16.0 | 16.0 | 16.0 | 17.0 | 17.0 | 17.0 | 17.0 | 17.0 |
| Calcium carbonate | 55.0 | 55.0 | 55.0 | 55.0 | 55.0 | 55.0 | 55.0 | 55.0 | 55.0 | 55.0 | 55.0 |
| Salt | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Mineral-vitamin premix | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| DL-methionine | 0.5 | 0.4 | 0.4 | 0.4 | 0.4 | 0.4 | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| L-lysine chloride | 0.9 | 0.8 | 0.8 | 0.8 | 0.8 | 0.8 | 0.6 | 0.6 | 0.6 | 0.6 | 0.6 |
| Item | Reared (0–4 Weeks) | Grower (58 Weeks) Period | Finisher (9–16 Weeks) Period | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Control | 50 HM | 100 HM | 50 HL | 100 HL | Control | 50 HM | 100 HM | 50 HL | 100 HL | ||
| Dry matter | 896.9 | 895.4 | 895.9 | 896.5 | 895.8 | 896.4 | 894.8 | 895.2 | 896.1 | 895.1 | 896.3 |
| Crude protein | 278.7 | 230.7 | 229.3 | 227.8 | 228.5 | 226.6 | 189.2 | 187.3 | 185.5 | 187.2 | 186.1 |
| Crude ash | 70.1 | 69.2 | 69.3 | 69.4 | 69.1 | 69.5 | 69.2 | 69.5 | 69.9 | 69.4 | 69.8 |
| Calcium | 26.1 | 26.3 | 26.5 | 26.9 | 26.6 | 26.8 | 26.4 | 26.5 | 26.8 | 26.6 | 26.9 |
| Total phosphorus | 7.92 | 7.81 | 7.78 | 7.73 | 7.77 | 7.71 | 7.73 | 7.71 | 7.68 | 7.71 | 7.67 |
| Ether extract | 94.2 | 82.7 | 91.3 | 97.8 | 90.9 | 98.1 | 81.4 | 87.8 | 97.6 | 88.4 | 96.9 |
| AMEn, MJ kg−1 * | 12.21 | 12.07 | 12.16 | 12.21 | 12.15 | 12.22 | 11.07 | 11.12 | 11.19 | 11.13 | 11.19 |
| Fatty acids, % of identified FA | |||||||||||
| C12:0 | 0.08 | 0.09 | 8.72 | 15.93 | 8.59 | 16.02 | 0.12 | 8.96 | 16.04 | 8.81 | 16.12 |
| C14:0 | 0.35 | 0.38 | 1.16 | 1.89 | 1.12 | 1.92 | 0.31 | 1.14 | 1.83 | 1.11 | 2.01 |
| C16:0 | 20.71 | 18.13 | 19.21 | 21.15 | 19.16 | 20.89 | 17.48 | 18.31 | 19.47 | 18.28 | 19.41 |
| C18:0 | 4.16 | 4.45 | 4.12 | 3.97 | 4.09 | 3.88 | 4.58 | 4.23 | 4.03 | 4.21 | 4.12 |
| C18:1, n-9 | 36.12 | 36.79 | 29.25 | 22.11 | 30.11 | 21.89 | 35.43 | 26.03 | 18.36 | 25.89 | 17.76 |
| C18:2, n-6 | 31.83 | 33.43 | 31.82 | 30.11 | 31.78 | 30.27 | 36.11 | 35.84 | 34.73 | 35.97 | 35.12 |
| C18:3, n-3 | 2.68 | 2.59 | 2.39 | 2.26 | 2.34 | 2.28 | 2.62 | 2.48 | 2.35 | 2.45 | 2.36 |
| Item | Feeding Groups (F) | Gender (G) | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 10) | HM 50 (n = 10) | HM 100 (n = 10) | HL 50 (n = 10) | HL 100 (n = 10) | Female (n = 10) | Male (n = 10) | F | G | F × G | |
| Body weight, g | ||||||||||
| -at start (after 4 weeks) | 189 | 197 | 194 | 191 | 191 | 184 b | 208 a | 0.431 | 0.042 | ns |
| -8 weeks | 526 | 551 | 548 | 542 | 536 | 505 b | 584 a | 0.207 | 0.025 | 0.325 |
| -12 weeks | 856 | 892 | 885 | 878 | 872 | 794 b | 968 a | 0.192 | 0.019 | 0.386 |
| -16 weeks | 1091 | 1136 | 1121 | 1142 | 1116 | 1002 b | 1254 a | 0.196 | 0.016 | 0.412 |
| ADG, g | ||||||||||
| 5–8 weeks | 11.7 | 12.7 | 12.5 | 12.3 | 12.1 | 11.1 | 12.9 | 0.204 | 0.044 | 0.386 |
| 9–12 weeks | 11.5 | 12.6 | 12.2 | 11.9 | 11.7 | 10.2 | 12.8 | 0.201 | 0.026 | 0.389 |
| 13–16 weeks | 8.6 | 9.2 | 8.8 | 8.9 | 8.9 | 7.10 | 9.80 | 0.192 | 0.018 | 0.405 |
| 5–16 weeks | 10.5 | 11.4 | 11.1 | 10.7 | 10.6 | 9.5 | 11.8 | 0.194 | 0.027 | 0.378 |
| Feed intake, g d−1 | ||||||||||
| 5–8 weeks | 40.8 | 40.1 | 40.4 | 40.6 | 40.7 | 39.0 | 41.3 | 0.406 | 0.207 | 0.528 |
| 9–12 weeks | 59.2 | 58.4 | 58.9 | 58.6 | 58.8 | 55.2 | 60.8 | 0.462 | 0.186 | 0.497 |
| 13–16 weeks | 73.2 | 72.1 | 72.6 | 72.4 | 72.6 | 69.8 | 73.7 | 0.423 | 0.193 | 0.396 |
| 5–16 weeks | 57.9 | 56.1 | 57.2 | 56.6 | 56.8 | 54.7 | 58.6 | 0.405 | 0.105 | 0.415 |
| Item | Feeding Groups (F) | Gender (G) | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 10) | 50 HM (n = 10) | 100 HM (n = 10) | 50 HL (n = 10) | 100 HL (n = 10) | Female (n = 10) | Male (n = 10) | F | G | F × G | |
| Thigh muscle mass, g | 109.4 | 113.3 | 114.8 | 113.9 | 114.1 | 98.0 b | 128.2 a | 0.264 | <0.001 | 0.437 |
| Shank muscle mass, g | 100.4 | 102.2 | 106.1 | 105.5 | 105.9 | 89.3 b | 118.7 a | 0.228 | <0.001 | 0.392 |
| Breast muscle mass, g | 216.2 | 210.7 | 212.2 | 212.0 | 211.9 | 176.7 b | 248.5 a | 0.381 | <0.001 | 0.412 |
| Head mass, g | 52.5 | 53.2 | 53.5 | 53.3 | 52.7 | 45.6 b | 60.5 a | 0.364 | <0.001 | 0.487 |
| Heart mass, g | 5.41 | 5.42 | 5.41 | 5.32 | 5.33 | 4.12 b | 6.61 a | 0.433 | <0.001 | 0.645 |
| Liver mass, g | 24.5 | 24.3 | 24.9 | 24.8 | 24.7 | 23.6 b | 25.7 a | 0.618 | 0.031 | 0.463 |
| Stomach (muscular) mass, g | 18.6 | 18.7 | 19.2 | 19.2 | 19.1 | 16.6 b | 21.3 a | 0.285 | 0.006 | 0.609 |
| Item | Feeding Groups (F) | Gender (G) | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 10) | 50 HM (n = 10) | 100 HM (n = 10) | 50 HL (n = 10) | 100 HL (n = 10) | Female (n = 10) | Male (n = 10) | F | G | F × G | |
| Cholesterol in muscles, mg/100 g: | ||||||||||
| Breast | 38.4 | 39.2 | 39.4 | 38.9 | 39.5 | 39.0 | 39.2 | 0.366 | 0.843 | 0.682 |
| Thigh | 47.9 | 48.3 | 48.8 | 48.7 | 49.1 | 48.5 | 48.6 | 0.289 | 0.875 | 0.712 |
| Serum lipid profile, mmol/L: | ||||||||||
| Total cholesterol | 4.12 | 4.19 | 4.25 | 4.16 | 4.21 | 4.11 | 4.27 | 0.203 | 0.412 | 0.612 |
| LDL | 1.83 | 1.86 | 1.88 | 1.84 | 1.80 | 1.85 | 1.83 | 0.258 | 0.568 | 0.597 |
| HDL | 1.87 | 1.92 | 1.96 | 1.91 | 2.01 | 1.87 | 1.99 | 0.185 | 0.184 | 0.465 |
| TG | 0.92 | 0.90 | 0.89 | 0.91 | 0.88 | 0.91 | 0.89 | 0.196 | 0.418 | 0.458 |
| Fatty Acids, % | Feeding Groups (F) | Gender (G) | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 10) | 50 HM (n = 10) | 100 HM (n = 10) | 50 HL (n = 10) | 100 HL (n = 10) | Female (n = 10) | Male (n = 10) | F | G | F × G | |
| C 12:0 | 0.17 | 0.45 | 0.53 | 0.44 | 0.58 | 0.38 | 0.48 | 0.001 | 0.057 | 0.431 |
| C 14:0 | 1.32 | 1.23 | 1.14 | 1.24 | 1.15 | 1.18 | 1.26 | 0.072 | 0.125 | 0.387 |
| C 14:1 | 0.07 | 0.06 | 0.05 | 0.05 | 0.05 | 0.07 | 0.05 | 0.232 | 0.152 | 0.298 |
| C 16:0 | 21.63 | 21.05 | 22.04 | 21.78 | 22.11 | 21.51 | 21.93 | 0.327 | 0.423 | 0.396 |
| C 16:1 | 3.21 | 3.14 | 3.11 | 3.28 | 3.31 | 3.15 | 3.27 | 0.278 | 0.315 | 0.458 |
| C 17:1, n-7 | 0.12 | 0.11 | 0.10 | 0.09 | 0.08 | 0.11 | 0.09 | 0.105 | 0.268 | 0.612 |
| C 18:0 | 14.56 | 13.79 | 13.94 | 13.96 | 14.07 | 13.87 | 14.25 | 0.183 | 0.217 | 0.326 |
| C 18:1, n-9 | 24.34 | 23.91 | 22.17 | 23.45 | 22.86 | 22.75 | 23.95 | 0.175 | 0.142 | 0.094 |
| C 18:1, n-7 | 2.42 | 2.41 | 2.53 | 2.38 | 2.29 | 2.46 | 2.36 | 0.347 | 0.268 | 0.543 |
| C 18-2, n-6 | 20.45 | 20.93 | 21.04 | 21.02 | 20.73 | 22.32 a | 19.34 b | 0.412 | 0.045 | 0.121 |
| C 18:3, n-3 | 0.95 b | 1.21 ab | 1.34 ab | 1.28 ab | 1.54 a | 1.14 b | 1.38 a | 0.042 | 0.039 | 0.158 |
| C 20:0 | 0.39 a | 0.29 ab | 0.21 b | 0.28 ab | 0.24 b | 0.27 | 0.29 | 0.033 | 0.312 | 0.397 |
| C 20:1, n-9 | 0.23 | 0.17 | 0.16 | 0.21 | 0.23 | 0.22 | 0.18 | 0.137 | 0.195 | 0.412 |
| C 20:2, n-6 | 0.15 | 0.14 | 0.12 | 0.14 | 0.15 | 0.16 | 0.12 | 0.379 | 0.095 | 0.312 |
| C 20:4, n-6 | 7.24 | 7.11 | 7.18 | 7.21 | 7.19 | 7.29 | 7.09 | 0.335 | 0.286 | 0.438 |
| C 20:5, n-3 | 0.21 | 0.23 | 0.25 | 0.24 | 0.26 | 0.25 | 0.23 | 0.412 | 0.524 | 0.735 |
| C 22:5, n-3 | 0.11 | 0.12 | 0.13 | 0.14 | 0.15 | 0.15 | 0.11 | 0.453 | 0.424 | 0.785 |
| C 22:6, n-3 | 0.36 | 0.39 | 0.41 | 0.40 | 0.44 | 0.41 | 0.39 | 0.346 | 0.512 | 0.658 |
| SFA | 38.07 | 36.81 | 37.86 | 37.70 | 38.15 | 37.21 | 38.21 | 0.278 | 0.296 | 0.548 |
| MUFA | 30.39 | 29.80 | 28.12 | 29.46 | 28.82 | 28.76 | 29.90 | 0.292 | 0.345 | 0.128 |
| PUFA | 29.47 | 30.13 | 30.47 | 30.43 | 30.46 | 31.72 | 28.66 | 0.326 | 0.126 | 0.387 |
| PUFA, n-3 | 1.63 c | 1.95 b | 2.13 ab | 2.06 b | 2.39 a | 1.95 | 2.11 | 0.032 | 0.189 | 0.612 |
| PUFA, n-6 | 27.84 | 28.18 | 28.34 | 28.37 | 28.07 | 29.77 a | 26.55 b | 0.147 | 0.046 | 0.386 |
| PUFA, n-6/n-3 | 17.08 a | 14.45 b | 13.31 b | 13.77 b | 11.74 c | 15.27 a | 12.58 b | 0.025 | 0.042 | 0.279 |
| Fatty Acids, % | Feeding Groups (F) | Gender (G) | p Value | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Control (n = 10) | 50 HM (n = 10) | 100 HM (n = 10) | 50 HL (n = 10) | 100 HL (n = 10) | Female (n = 10) | Male (n = 10) | F | G | F × G | |
| C 12:0 | 0.15 b | 0.36 a | 0.42 a | 0.33 a | 0.45 a | 0.36 | 0.32 | 0.001 | 0.098 | 0.426 |
| C 14:0 | 1.64 | 1.74 | 1.82 | 1.75 | 1.91 | 1.72 | 1.82 | 0.063 | 0.119 | 0.437 |
| C 14:1 | 0.12 | 0.13 | 0.15 | 0.14 | 0.16 | 0.15 | 0.13 | 0.226 | 0.327 | 0.392 |
| C 16:0 | 27.11 | 27.58 | 27.67 | 27.55 | 27.84 | 27.05 | 28.05 | 0.384 | 0.223 | 0.412 |
| C 16:1 | 2.57 | 2.43 | 2.26 | 2.42 | 2.31 | 2.47 | 2.33 | 0.168 | 0.345 | 0.487 |
| C 17:1, n-7 | 0.15 | 0.14 | 0.12 | 0.14 | 0.13 | 0.15 | 0.13 | 0.135 | 0.326 | 0.645 |
| C 18:0 | 18.24 | 17.67 | 18.35 | 18.36 | 18.71 | 18.07 | 18.47 | 0.208 | 0.314 | 0.463 |
| C 18:1, n-9 | 31.23 | 30.84 | 30.11 | 31.02 | 29.97 | 31.03 | 30.13 | 0.182 | 0.164 | 0.109 |
| C 18:1, n-7 | 1.89 | 1.78 | 1.73 | 1.68 | 1.72 | 1.79 | 1.73 | 0.369 | 0.462 | 0.654 |
| C 18-2, n-6 | 10.45 | 10.93 | 11.04 | 11.02 | 10.73 | 11.38 | 10.33 | 0.396 | 0.065 | 0.226 |
| C 18:3, n-3 | 0.64 b | 0.73 b | 0.87 ab | 0.78 b | 0.96 a | 0.84 | 0.76 | 0.041 | 0.109 | 0.352 |
| C 20:0 | 0.12 | 0.14 | 0.15 | 0.13 | 0.15 | 0.11 | 0.17 | 0.295 | 0.112 | 0.412 |
| C 20:1, n-9 | 0.19 | 0.17 | 0.18 | 0.15 | 0.17 | 0.20 | 0.14 | 0.232 | 0.091 | 0.436 |
| C 20:2, n-6 | 0.21 | 0.22 | 0.23 | 0.19 | 0.22 | 0.23 | 0.19 | 0.385 | 0.209 | 0.431 |
| C 20:4, n-6 | 2.23 a | 1.94 ab | 1.82 ab | 1.87 ab | 1.69 b | 2.06 | 1.76 | 0.034 | 0.148 | 0.134 |
| C 20:5, n-3 | 0.26 | 0.26 | 0.25 | 0.28 | 0.29 | 0.28 | 0.26 | 0.425 | 0.584 | 0.738 |
| C 22:5, n-3 | 0.15 | 0.16 | 0.15 | 0.18 | 0.19 | 0.18 | 0.16 | 0.492 | 0.422 | 0.756 |
| C 22:6, n-3 | 0.39 a | 0.38 a | 0.35 a | 0.38 a | 0.27 b | 0.37 | 0.33 | 0.041 | 0.505 | 0.651 |
| SFA | 47.26 | 47.49 | 48.41 | 48.12 | 49.06 | 47.31 | 48.83 | 0.124 | 0.209 | 0.512 |
| MUFA | 36.15 | 35.49 | 34.55 | 35.55 | 34.46 | 35.79 | 34.59 | 0.272 | 0.348 | 0.232 |
| PUFA | 14.33 | 14.62 | 14.71 | 14.7 | 14.35 | 15.34 | 13.79 | 0.463 | 0.122 | 0.379 |
| PUFA, n-3 | 1.44 | 1.53 | 1.62 | 1.62 | 1.71 | 1.67 | 1.51 | 0.124 | 0.201 | 0.513 |
| PUFA, n-6 | 12.89 | 13.09 | 13.09 | 13.08 | 12.64 | 13.67 | 12.28 | 0.195 | 0.094 | 0.506 |
| PUFA, n-6/n-3 | 8.95 a | 8.56 ab | 8.08 b | 8.07 b | 7.39 b | 8.19 | 8.13 | 0.034 | 0.448 | 0.307 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rytlewski, G.; Flis, M.; Grela, E.R. Effect of Meal and Whole Larvae of Black Soldier Fly (Hermetia illucens) on the Performance, Blood Lipid Profile, Slaughter Characteristics, Sensory Properties and Fatty Acid Composition of Pheasant (Phasianus colchicus L.) Muscles. Animals 2025, 15, 3215. https://doi.org/10.3390/ani15213215
Rytlewski G, Flis M, Grela ER. Effect of Meal and Whole Larvae of Black Soldier Fly (Hermetia illucens) on the Performance, Blood Lipid Profile, Slaughter Characteristics, Sensory Properties and Fatty Acid Composition of Pheasant (Phasianus colchicus L.) Muscles. Animals. 2025; 15(21):3215. https://doi.org/10.3390/ani15213215
Chicago/Turabian StyleRytlewski, Grzegorz, Marian Flis, and Eugeniusz R. Grela. 2025. "Effect of Meal and Whole Larvae of Black Soldier Fly (Hermetia illucens) on the Performance, Blood Lipid Profile, Slaughter Characteristics, Sensory Properties and Fatty Acid Composition of Pheasant (Phasianus colchicus L.) Muscles" Animals 15, no. 21: 3215. https://doi.org/10.3390/ani15213215
APA StyleRytlewski, G., Flis, M., & Grela, E. R. (2025). Effect of Meal and Whole Larvae of Black Soldier Fly (Hermetia illucens) on the Performance, Blood Lipid Profile, Slaughter Characteristics, Sensory Properties and Fatty Acid Composition of Pheasant (Phasianus colchicus L.) Muscles. Animals, 15(21), 3215. https://doi.org/10.3390/ani15213215






