Evaluating the Effects of Managed Free-Roaming Cat Populations on Prey Through Stable Isotope Analysis: A Pilot Study from British Columbia, Canada
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
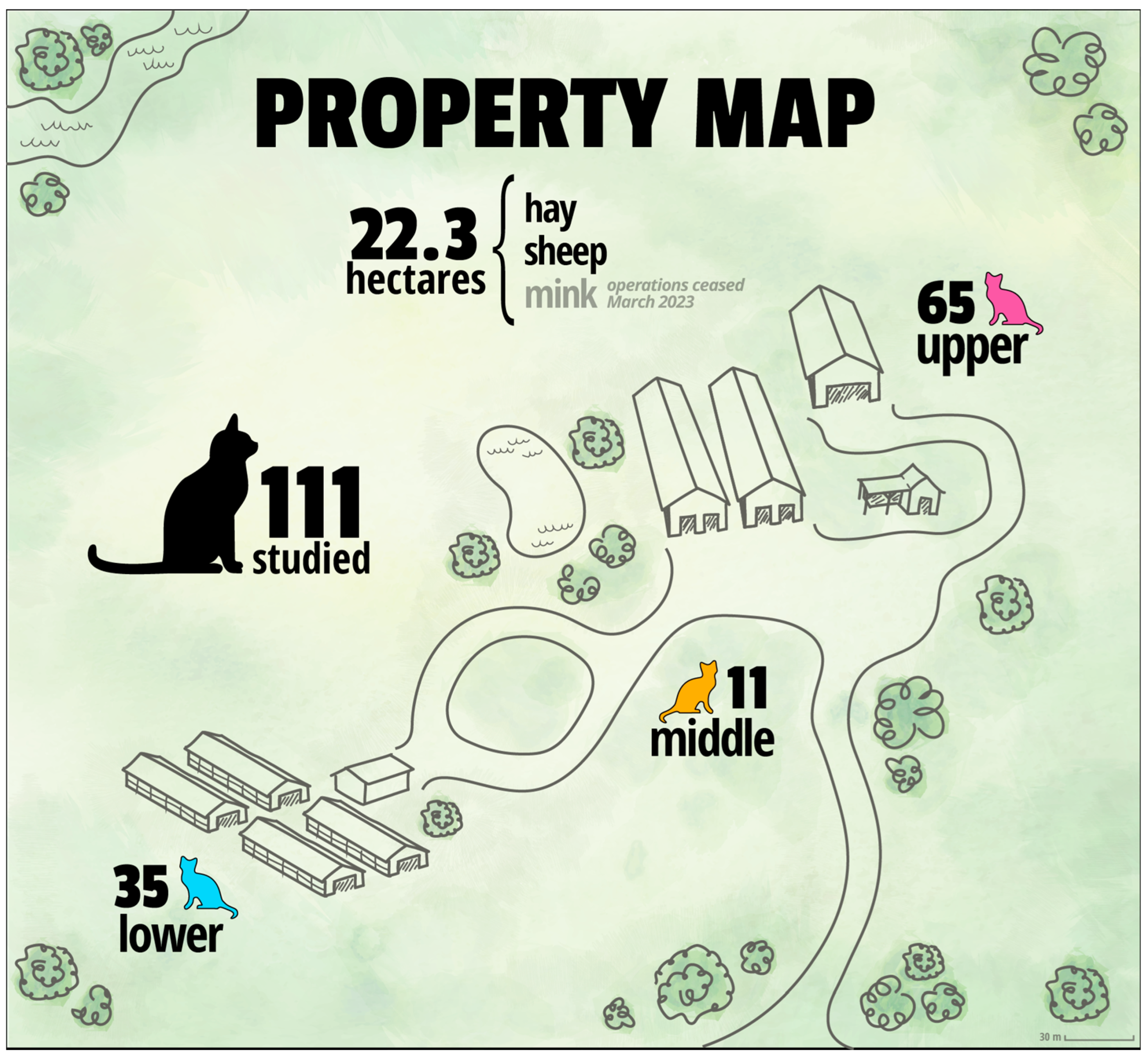
2.1. The Study Site
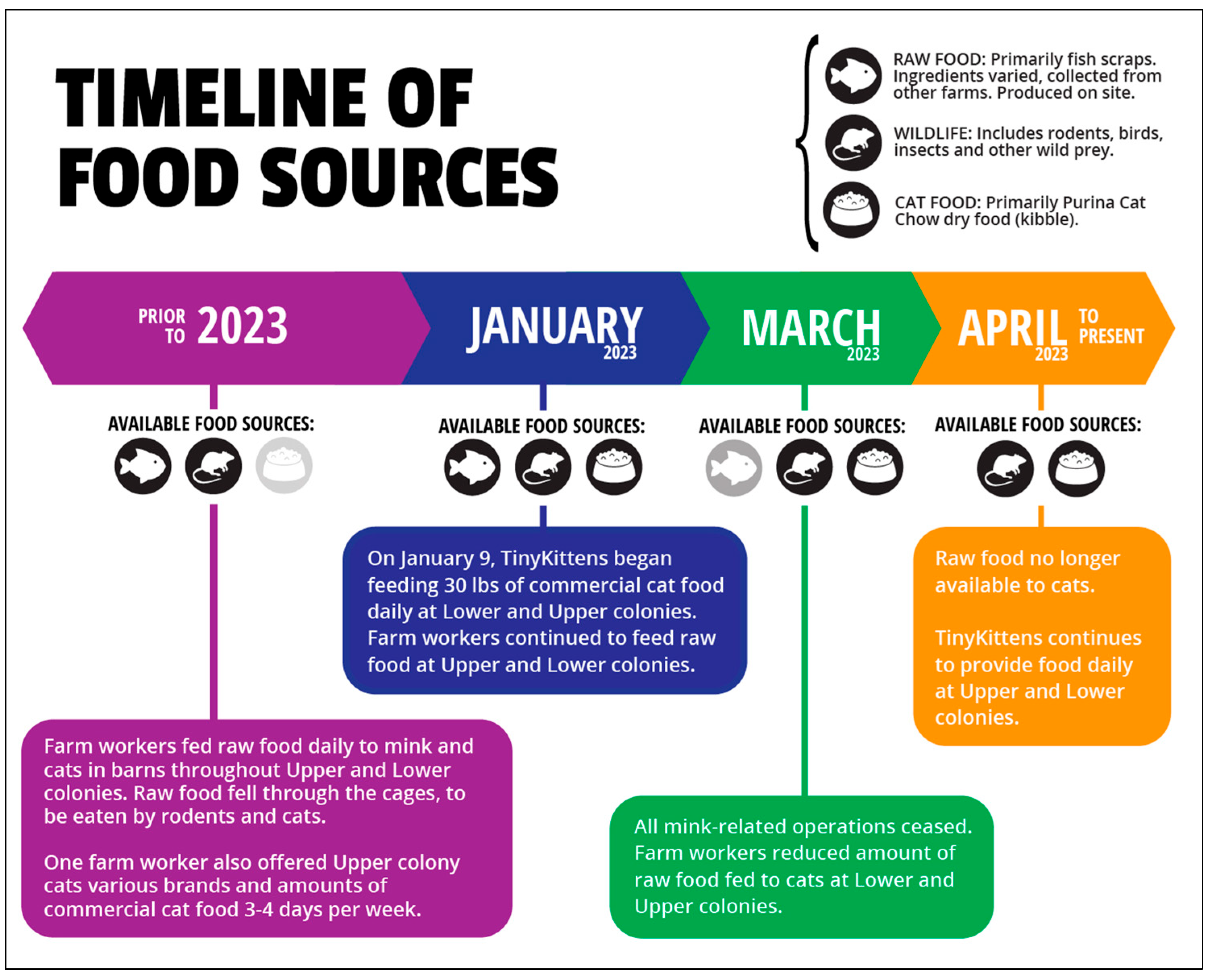
2.2. Sampling
2.3. SIA—Fur and Feathers
2.4. SIA—Bone
2.5. SIA—Cat Food
2.6. Statistical Analysis
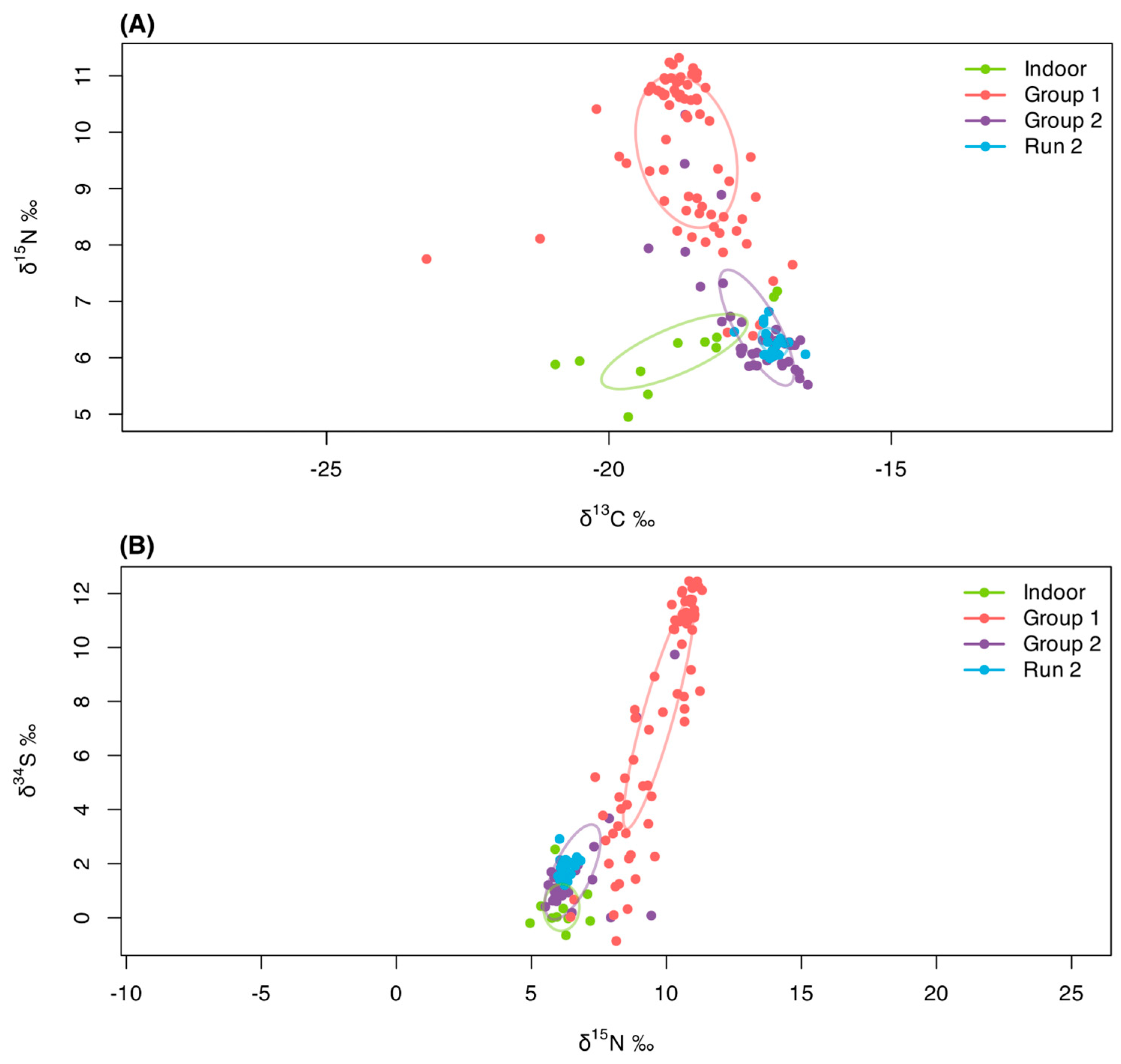
3. Results
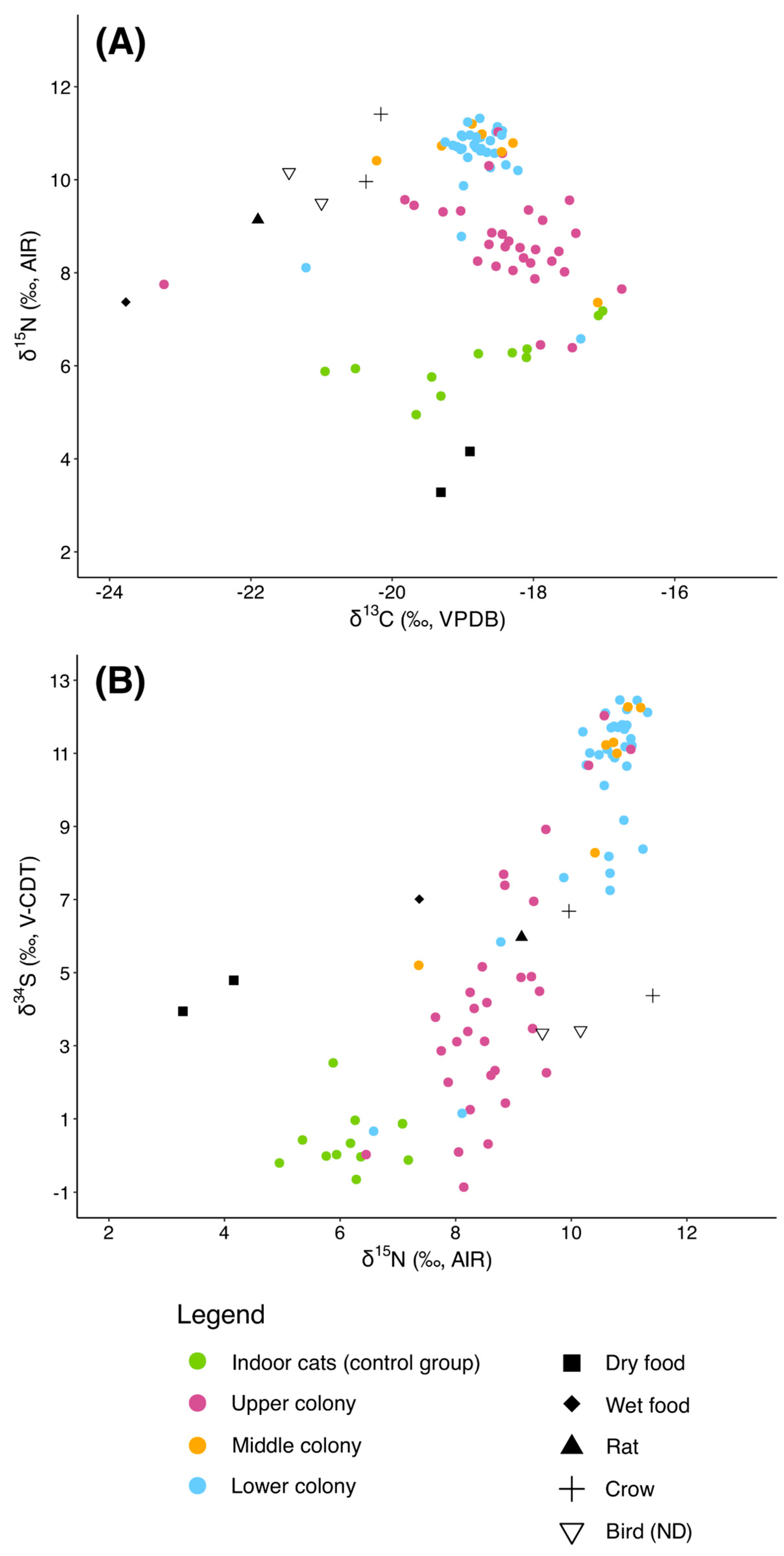
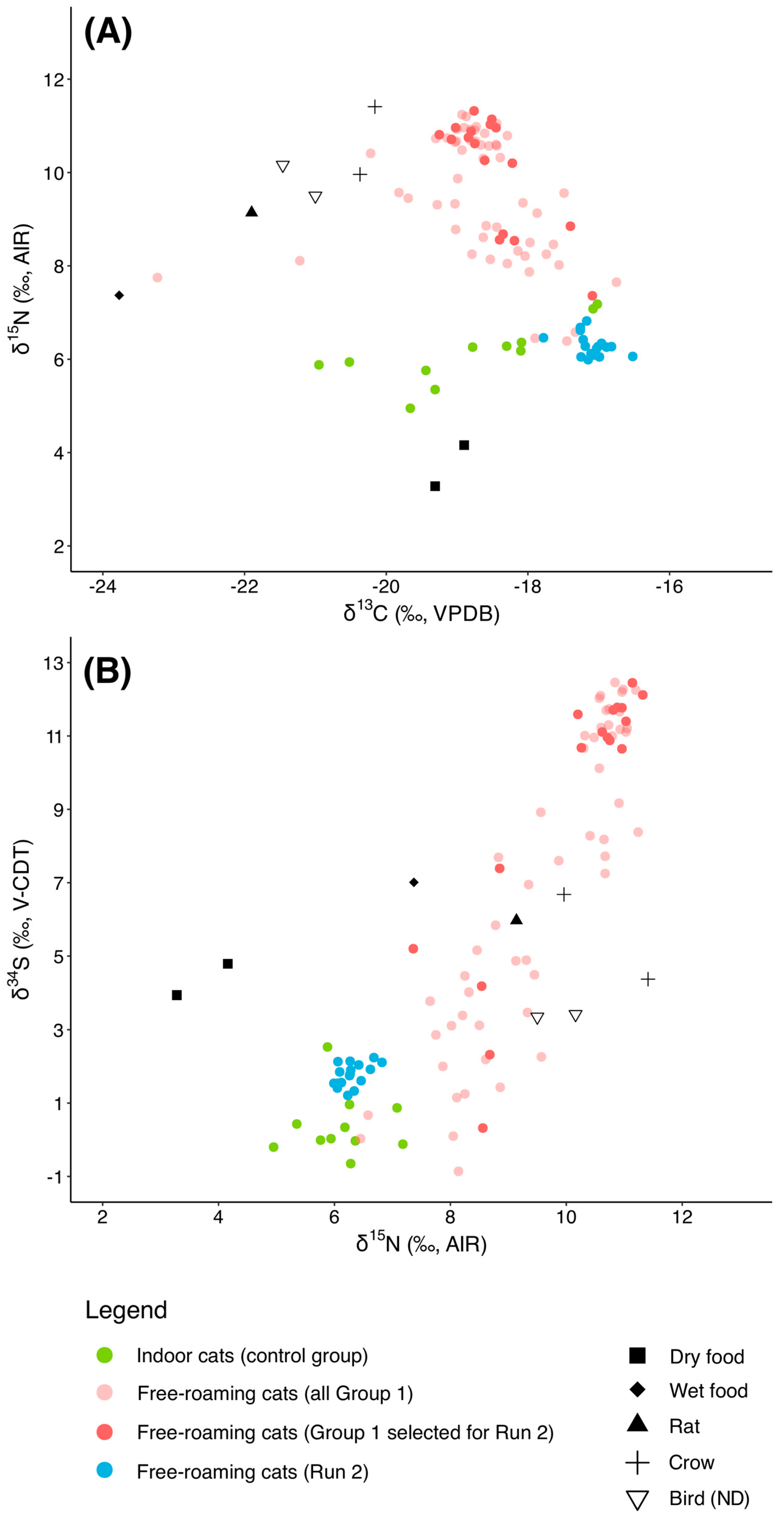
3.1. Baseline
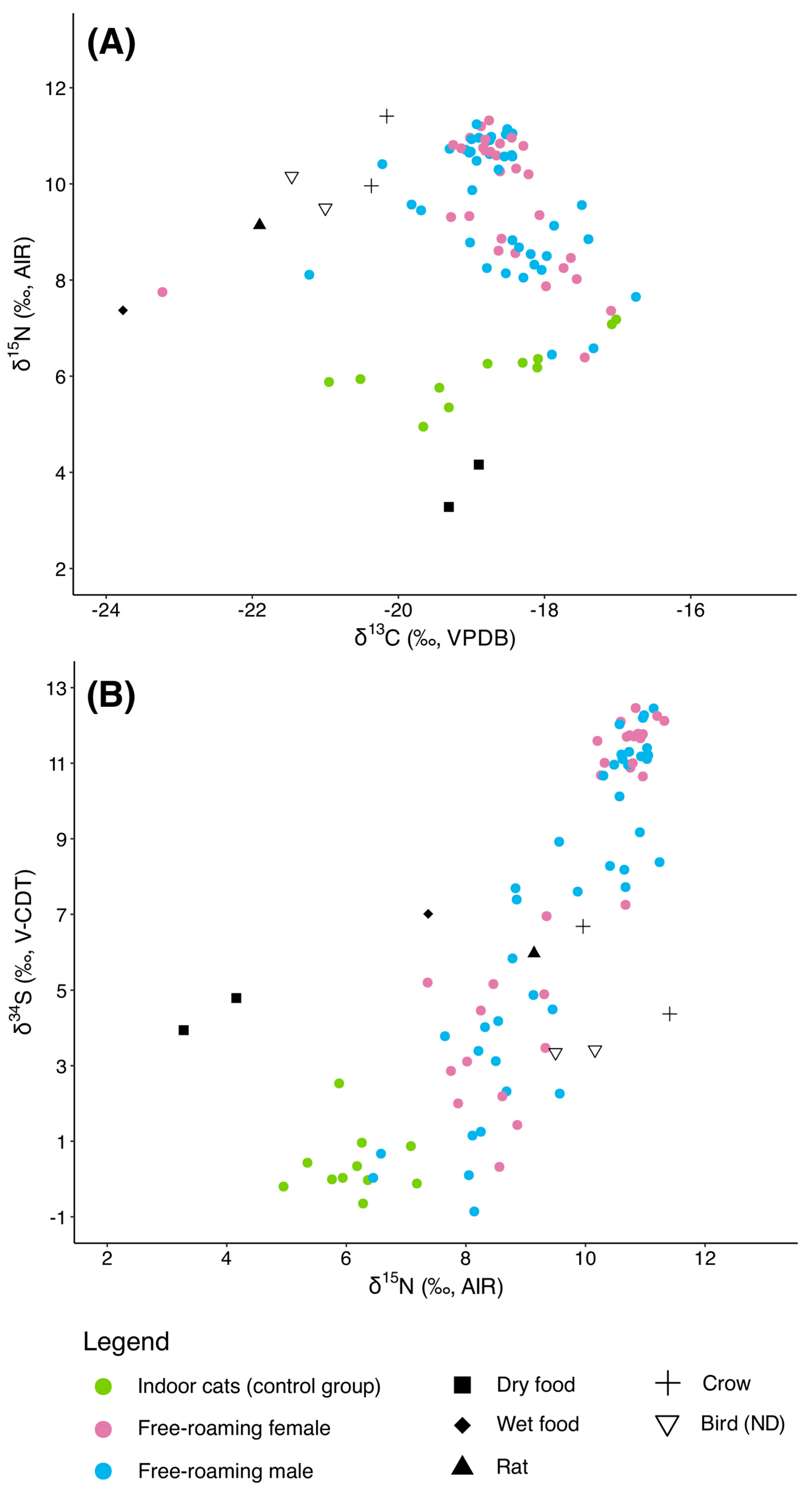
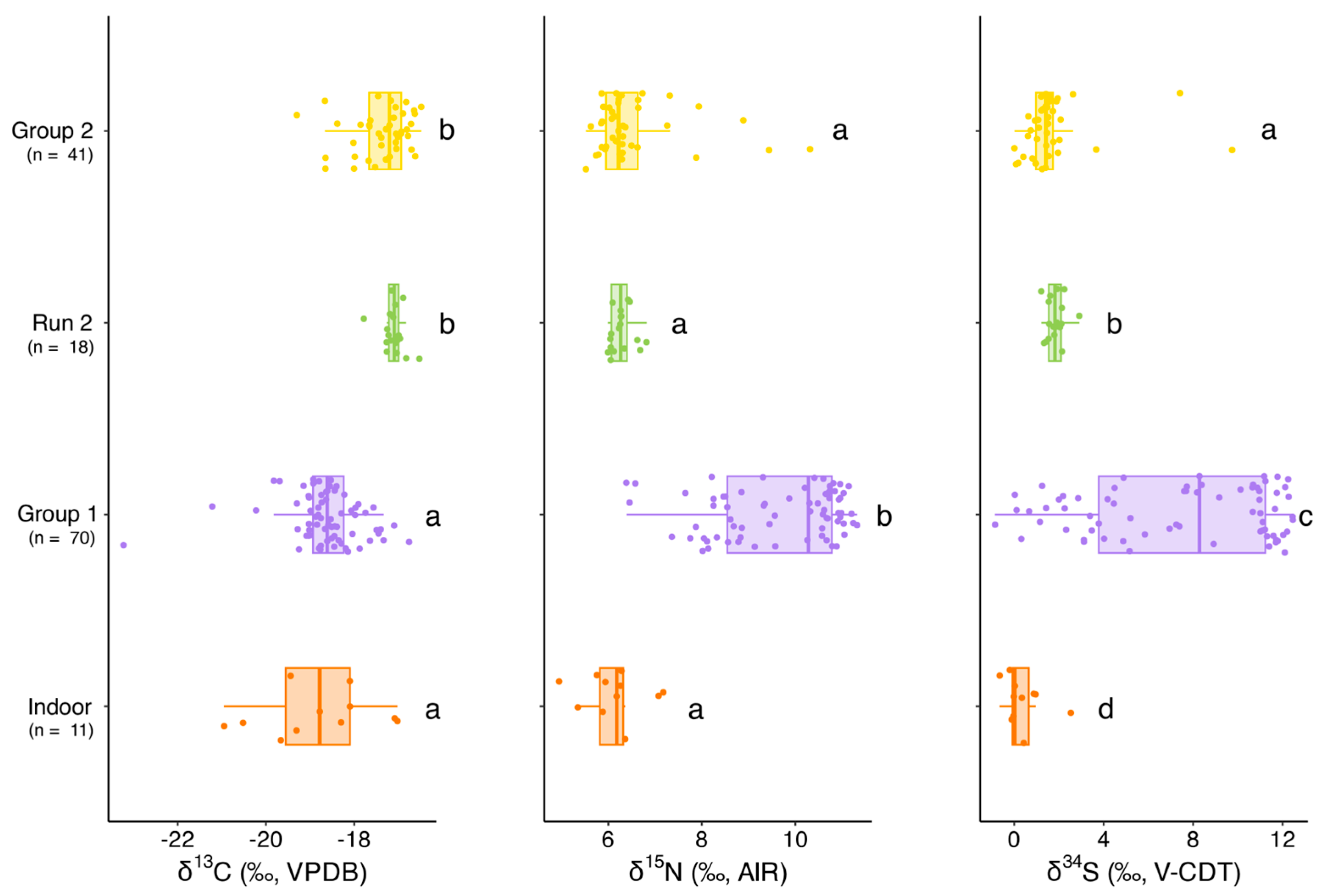
3.2. Indoor Cats
3.3. Free-Roaming Cats (Group 1)
3.3.1. Sex
3.3.2. Colonies
3.3.3. Sterilization Date
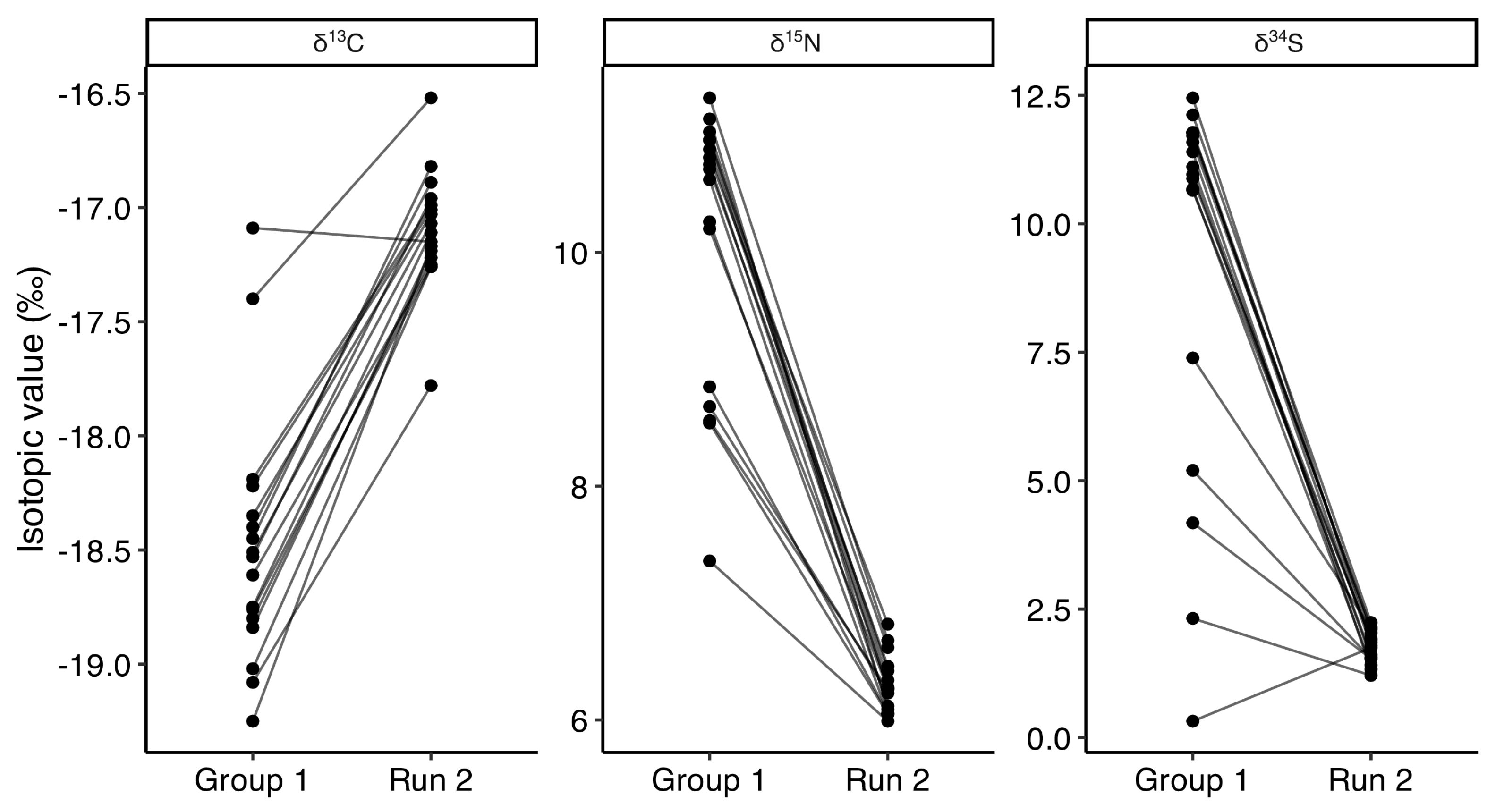
3.4. Run 2
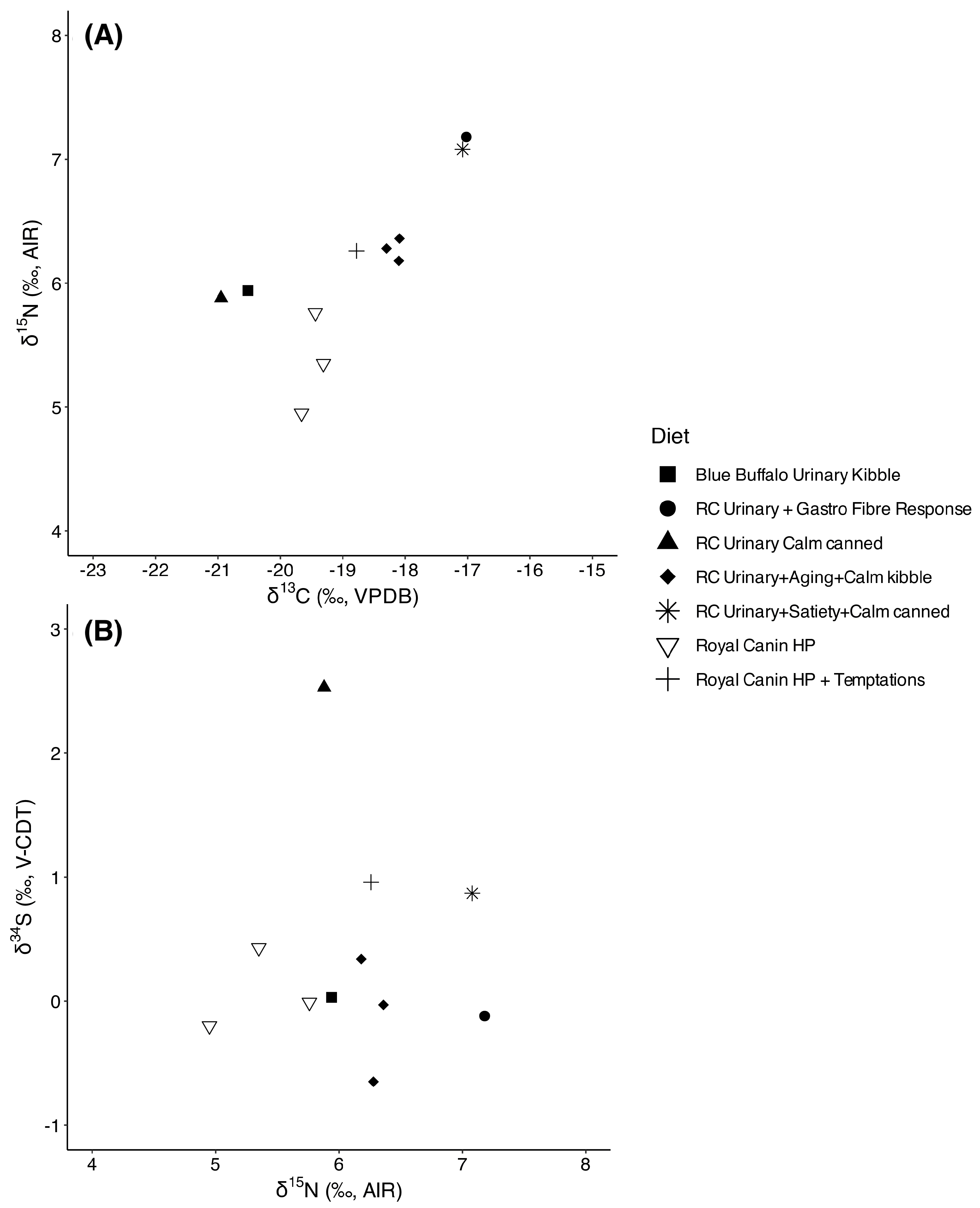
3.5. Group 2
4. Discussion
4.1. Dietary Differences Between Indoor and Free-Roaming Cats
4.2. Pre-TNR Variability: Group 1
4.3. Dietary Transitions Post-TNR: Run 2 and Group 2
4.4. Broader Implications and Ecological Relevance
4.5. Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| TNR | Trap-neuter-return |
| TKS | TinyKittens Society |
| SIA | Stable isotope analysis |
| TDF | Trophic discrimination factor |
| VPDB | Vienna Pee Dee Belemnite |
| AIR | Ambient Inhalable Reservoir |
| V-CDT | Vienna Canyon Diablo Troilite |
References
- Aeluro, S.; Buchanan, J.M.; Boone, J.D.; Rabinowitz, P.M. “State of the Mewnion”: Practices of Feral Cat Care and Advocacy Organizations in the United States. Front. Vet. Sci. 2021, 8, 791134. [Google Scholar] [CrossRef] [PubMed]
- Neal, S.M.; Wolf, P.J. A Cat Is a Cat: Attachment to Community Cats Transcends Ownership Status. J. Shelter. Med. Community Anim. Health 2023, 2. [Google Scholar] [CrossRef]
- Hughes, K.L.; Slater, M.R. Implementation of a Feral Cat Management Program on a University Campus. J. Appl. Anim. Welf. Sci. 2002, 5, 15–28. [Google Scholar] [CrossRef]
- Normand, C.M. Feral Cat Virus Infection Prevalence, Survival, Population Density, and Multi-Scale Habitat Use in an Exurban Landscape. Master’s Thesis, Arkansas Tech University, Russellville, AR, USA, 2014. [Google Scholar]
- Stoskopf, M.K.; Nutter, F.B. Analyzing approaches to feral cat management—One size does not fit all. J. Am. Vet. Med. Assoc. 2004, 225, 1361–1964. [Google Scholar] [CrossRef]
- Kreisler, R.E.; Cornell, H.N.; Levy, J.K. Decrease in Population and Increase in Welfare of Community Cats in a Twenty-Three Year Trap-Neuter-Return Program in Key Largo, FL: The ORCAT Program. Front. Vet. Sci. 2019, 6, 7. [Google Scholar] [CrossRef]
- Levy, J.K.; Gale, D.W.; Gale, L.A. Evaluation of the effect of a long-term trap-neuter-return and adoption program on a free-roaming cat population. J. Am. Vet. Med. Assoc. 2003, 222, 42–46. [Google Scholar] [CrossRef] [PubMed]
- Spehar, D.; Wolf, P. A Case Study in Citizen Science: The Effectiveness of a Trap-Neuter-Return Program in a Chicago Neighborhood. Animals 2018, 8, 14. [Google Scholar] [CrossRef]
- Spehar, D.; Wolf, P.J. Back to School: An Updated Evaluation of the Effectiveness of a Long-Term Trap-Neuter-Return Program on a University’s Free-Roaming Cat Population. Animals 2019, 9, 768. [Google Scholar] [CrossRef]
- Spehar, D.; Wolf, P.J. The Impact of Targeted Trap–Neuter–Return Efforts in the San Francisco Bay Area. Animals 2020, 10, 2089. [Google Scholar] [CrossRef] [PubMed]
- Lepczyk, C.A.; Duffy, D.C.; Bird, D.M.; Calver, M.; Cherkassky, D.; Cherkassky, L.; Dickman, C.R.; Hunter, D.; Jessup, D.; Longcore, T.; et al. A science-based policy for managing free-roaming cats. Biol. Invasions 2022, 24, 3693–3701. [Google Scholar] [CrossRef]
- Loss, S.R.; Marra, P.P. Population impacts of free-ranging domestic cats on mainland vertebrates. Front. Ecol. Environ. 2017, 15, 502–509. [Google Scholar] [CrossRef]
- Hernandez, S.M.; Loyd, K.A.T.; Newton, A.N.; Gallagher, M.C.; Carswell, B.L.; Abernathy, K.J. Activity patterns and interspecific interactions of free-roaming, domestic cats in managed Trap-Neuter-Return colonies. Appl. Anim. Behav. Sci. 2018, 202, 63–68. [Google Scholar] [CrossRef]
- Kays, R.; Feranec, R.S. Using Stable Carbon Isotopes to Distinguish Wild from Captive Wolves. Northeast. Nat. 2011, 18, 253–264. [Google Scholar] [CrossRef]
- McDonald, B.W.; Perkins, T.; Dunn, R.R.; McDonald, J.; Cole, H.; Feranec, R.S.; Kays, R. High variability within pet foods prevents the identification of native species in pet cats’ diets using isotopic evaluation. PeerJ 2020, 8, e8337. [Google Scholar] [CrossRef]
- Roth, J.D.; Hobson, K.A. Stable carbon and nitrogen isotopic fractionation between diet and tissue of captive red fox: Implications for dietary reconstruction. Can. J. Zool. 2000, 78, 848–852. [Google Scholar] [CrossRef]
- Parng, E.; Crumpacker, A.; Kurle, C.M. Variation in the stable carbon and nitrogen isotope discrimination factors from diet to fur in four felid species held on different diets. J. Mammal. 2014, 95, 151–159. [Google Scholar] [CrossRef]
- Cove, M.V.; Gardner, B.; Simons, T.R.; Kays, R.; O’Connell, A.F. Free-ranging domestic cats (Felis catus) on public lands: Estimating density, activity, and diet in the Florida Keys. Biol. Invasions 2018, 20, 333–344. [Google Scholar] [CrossRef]
- Maeda, T.; Nakashita, R.; Shionosaki, K.; Yamada, F.; Watari, Y. Predation on endangered species by human-subsidized domestic cats on Tokunoshima Island. Sci. Rep. 2019, 9, 16200. [Google Scholar] [CrossRef] [PubMed]
- Peterson, B.J.; Fry, B. Stable Isotopes in Ecosystem Studies. Annu. Rev. Ecol. Syst. 1987, 18, 293–320. [Google Scholar] [CrossRef]
- Richards, M.P. Isotope Analysis for Diet Studies. In Archaeological Science: An Introduction; Britton, K., Richards, M.P., Eds.; Cambridge University Press: Cambridge, UK, 2020; pp. 125–144. ISBN 978-0-521-19522-5. [Google Scholar]
- Deniro, M.J.; Epstein, S. Influence of diet on the distribution of nitrogen isotopes in animals. Geochim. Et Cosmochim. Acta 1981, 45, 341–351. [Google Scholar] [CrossRef]
- Post, D.M. Using stable isotopes to estimate trophic position: Models, methods, and assumptions. Ecology 2002, 83, 703–718. [Google Scholar] [CrossRef]
- Nehlich, O. The application of sulphur isotope analyses in archaeological research: A review. Earth-Sci. Rev. 2015, 142, 1–17. [Google Scholar] [CrossRef]
- Rees, C.E.; Jenkins, W.J.; Monster, J. The sulphur isotopic composition of ocean water sulphate. Geochim. Et Cosmochim. Acta 1978, 42, 377–381. [Google Scholar] [CrossRef]
- Roy, D.M.; Hall, R.; Mix, A.C.; Bonnichsen, R. Using stable isotope analysis to obtain dietary profiles from old hair: A case study from Plains Indians. Am. J. Phys. Anthropol. 2005, 128, 444–452. [Google Scholar] [CrossRef] [PubMed]
- Wilson, A.S. Hair as a Bioresource in Archaeological Study. In Hair in Toxicology: An Important Biomonitor; Tobin, D.J., Ed.; Springer: Berlin, Germany, 2005; pp. 321–345. [Google Scholar]
- Wojtaś, J.; Karpiński, M.; Czyżowski, P.; Garbiec, A.; Kaliszyk, K.; Klimas, A.; Pustuła, K.; Kurpas, K.; Parszewska, S.; Stokłosińska, K.; et al. Hair cortisol levels in cats before and during their first month at a homeless animal shelter. Med. Weter. 2024, 80, 21–24. [Google Scholar] [CrossRef]
- Baker, K.P. Hair Growth and Replacement in the Cat. Br. Vet. J. 1974, 130, 327–335. [Google Scholar] [CrossRef]
- Caut, S.; Angulo, E.; Courchamp, F. Variation in discrimination factors (Δ 15 N and Δ 13 C): The effect of diet isotopic values and applications for diet reconstruction. J. Appl. Ecol. 2009, 46, 443–453. [Google Scholar] [CrossRef]
- Gurney, K.E.B.; Classen, H.L.; Clark, R.G. Testing for effects of growth rate on isotope trophic discrimination factors and evaluating the performance of Bayesian stable isotope mixing models experimentally: A moment of truth? PLoS ONE 2024, 19, e0304495. [Google Scholar] [CrossRef]
- McCutchan, J.H.; Lewis, W.M.; Kendall, C.; McGrath, C.C. Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 2003, 102, 378–390. [Google Scholar] [CrossRef]
- Britton, K.; McManus-Fry, E.; Nehlich, O.; Richards, M.; Ledger, P.M.; Knecht, R. Stable carbon, nitrogen and sulphur isotope analysis of permafrost preserved human hair from rescue excavations (2009, 2010) at the precontact site of Nunalleq, Alaska. J. Archaeol. Sci. Rep. 2018, 17, 950–963. [Google Scholar] [CrossRef]
- O’Connell, T.C.; Hedges, R.E.M.; Healey, M.A.; Simpson, A.H.R.W. Isotopic Comparison of Hair, Nail and Bone: Modern Analyses. J. Archaeol. Sci. 2001, 28, 1247–1255. [Google Scholar] [CrossRef]
- Jackson, G.P.; An, Y.; Konstantynova, K.I.; Rashaid, A.H.B. Biometrics from the carbon isotope ratio analysis of amino acids in human hair. Sci. Justice 2015, 55, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Mora, A.; Pacheco, A.; Roberts, C.; Smith, C. Pica 8: Refining dietary reconstruction through amino acid δ 13 C analysis of tendon collagen and hair keratin. J. Archaeol. Sci. 2018, 93, 94–109. [Google Scholar] [CrossRef]
- Müldner, G.; Richards, M.P. Fast or feast: Reconstructing diet in later medieval England by stable isotope analysis. J. Archaeol. Sci. 2005, 32, 39–48. [Google Scholar] [CrossRef]
- Brown, T.A.; Nelson, D.E.; Vogel, J.S.; Southon, J.R. Improved collagen extraction by modified Longin method. Radiocarbon 1988, 30, 171–177. [Google Scholar] [CrossRef]
- Piasentier, E.; Valusso, R.; Camin, F.; Versini, G. Stable isotope ratio analysis for authentication of lamb meat. Meat Sci. 2003, 64, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Post, D.M.; Layman, C.A.; Arrington, D.A.; Takimoto, G.; Quattrochi, J.; Montaña, C.G. Getting to the fat of the matter: Models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 2007, 152, 179–189. [Google Scholar] [CrossRef]
- Jackson, A.L.; Inger, R.; Parnell, A.C.; Bearhop, S. Comparing isotopic niche widths among and within communities: SIBER-Stable Isotope Bayesian Ellipses in R: Bayesian isotopic niche metrics. J. Anim. Ecol. 2011, 80, 595–602. [Google Scholar] [CrossRef]
- Cecchetti, M.; Crowley, S.L.; Goodwin, C.E.D.; Cole, H.; McDonald, J.; Bearhop, S.; McDonald, R.A. Contributions of wild and provisioned foods to the diets of domestic cats that depredate wild animals. Ecosphere 2021, 12, e03737. [Google Scholar] [CrossRef]
- Newsome, S.D.; Bentall, G.B.; Tinker, M.T.; Oftedal, O.T.; Ralls, K.; Estes, J.A.; Fogel, M.L. Variation in δ13C and δ15N diet–vibrissae trophic discrimination factors in a wild population of California sea otters. Ecol. Appl. 2010, 20, 1744–1752. [Google Scholar] [CrossRef]
- Silva-Rodríguez, E.A.; Sieving, K.E. Influence of Care of Domestic Carnivores on Their Predation on Vertebrates: Domestic Carnivore Predation Behavior. Conserv. Biol. 2011, 25, 808–815. [Google Scholar] [CrossRef] [PubMed]

| Sample ID | Material | δ13C (‰) | δ15N (‰) | δ34S (‰) |
|---|---|---|---|---|
| 4098 | Rat bone | −21.9 | 9.1 | 6.0 |
| 4099 | Wet food | −23.8 | 7.4 | 7.0 |
| 4100 | Dry food | −19.3 | 3.3 | 3.9 |
| 4101 | Dry food | −18.9 | 4.2 | 4.8 |
| 4102 | Crow feather | −20.2 | 11.4 | 4.4 |
| 4103 | Crow feather | −20.4 | 10.0 | 6.7 |
| 4104 | Bird feather | −21.0 | 9.5 | 3.4 |
| 4105 | Bird feather | −21.5 | 10.2 | 3.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinoia, V.; Ferguson, R.; Wolf, P.J.; Carić, M.; Novak, M.; Roche, S. Evaluating the Effects of Managed Free-Roaming Cat Populations on Prey Through Stable Isotope Analysis: A Pilot Study from British Columbia, Canada. Animals 2025, 15, 3204. https://doi.org/10.3390/ani15213204
Martinoia V, Ferguson R, Wolf PJ, Carić M, Novak M, Roche S. Evaluating the Effects of Managed Free-Roaming Cat Populations on Prey Through Stable Isotope Analysis: A Pilot Study from British Columbia, Canada. Animals. 2025; 15(21):3204. https://doi.org/10.3390/ani15213204
Chicago/Turabian StyleMartinoia, Valentina, Renee Ferguson, Peter J. Wolf, Mario Carić, Mario Novak, and Shelly Roche. 2025. "Evaluating the Effects of Managed Free-Roaming Cat Populations on Prey Through Stable Isotope Analysis: A Pilot Study from British Columbia, Canada" Animals 15, no. 21: 3204. https://doi.org/10.3390/ani15213204
APA StyleMartinoia, V., Ferguson, R., Wolf, P. J., Carić, M., Novak, M., & Roche, S. (2025). Evaluating the Effects of Managed Free-Roaming Cat Populations on Prey Through Stable Isotope Analysis: A Pilot Study from British Columbia, Canada. Animals, 15(21), 3204. https://doi.org/10.3390/ani15213204





