Melatonin Alleviates the Damage of Polystyrene Microplastics to Porcine Oocytes by Reducing Oxidative Stress and Mitochondrial Damage, and Regulating Autophagy and Apoptosis Levels
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents, Consumables, and Instruments
2.2. Vitro Maturation of Oocytes and Expansion of Cumulus Cells
2.3. Cell Treatment, Parthenogenetic Activation, and In Vitro Embryo Production
2.4. Determination of Reactive Oxygen Species
2.5. Determination of Glutathione
2.6. Determination of Mitochondrial Distribution
2.7. Determination of ATP Content
2.8. Determination of Annexin-V Content
2.9. Detection of Blastocyst Apoptosis by the TUNEL Method
2.10. Immunofluorescence
2.11. Reverse Transcription–Polymerase Chain Reaction (RT-PCR)
2.12. Statistical Analysis
3. Research Results
3.1. Exposure to PS-MPs Affects the Maturation of Porcine Oocytes
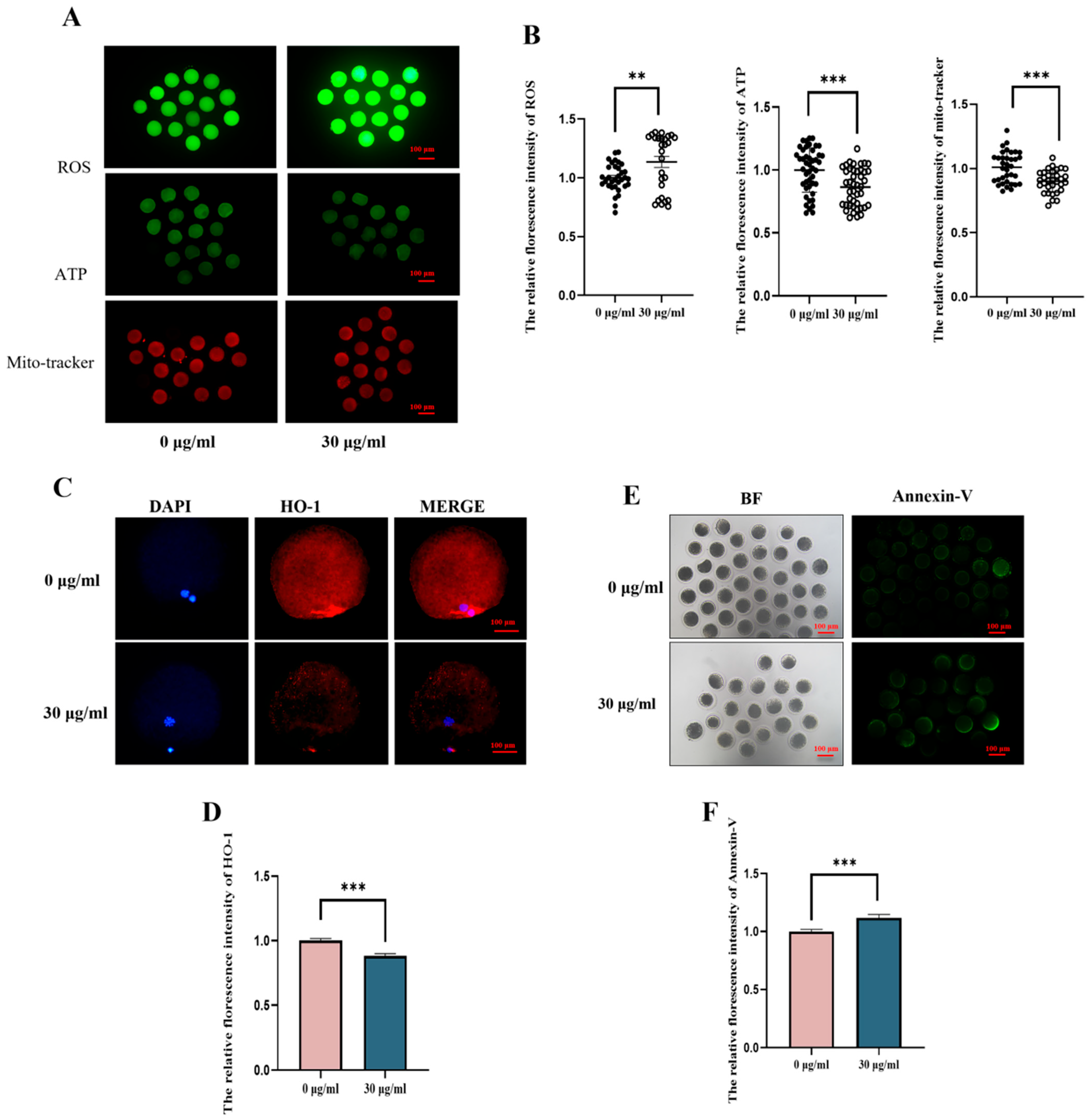
3.2. PS-MPs Exposure Affects Mitochondrial Function, Redox Balance, and Apoptotic Level of Porcine Oocytes
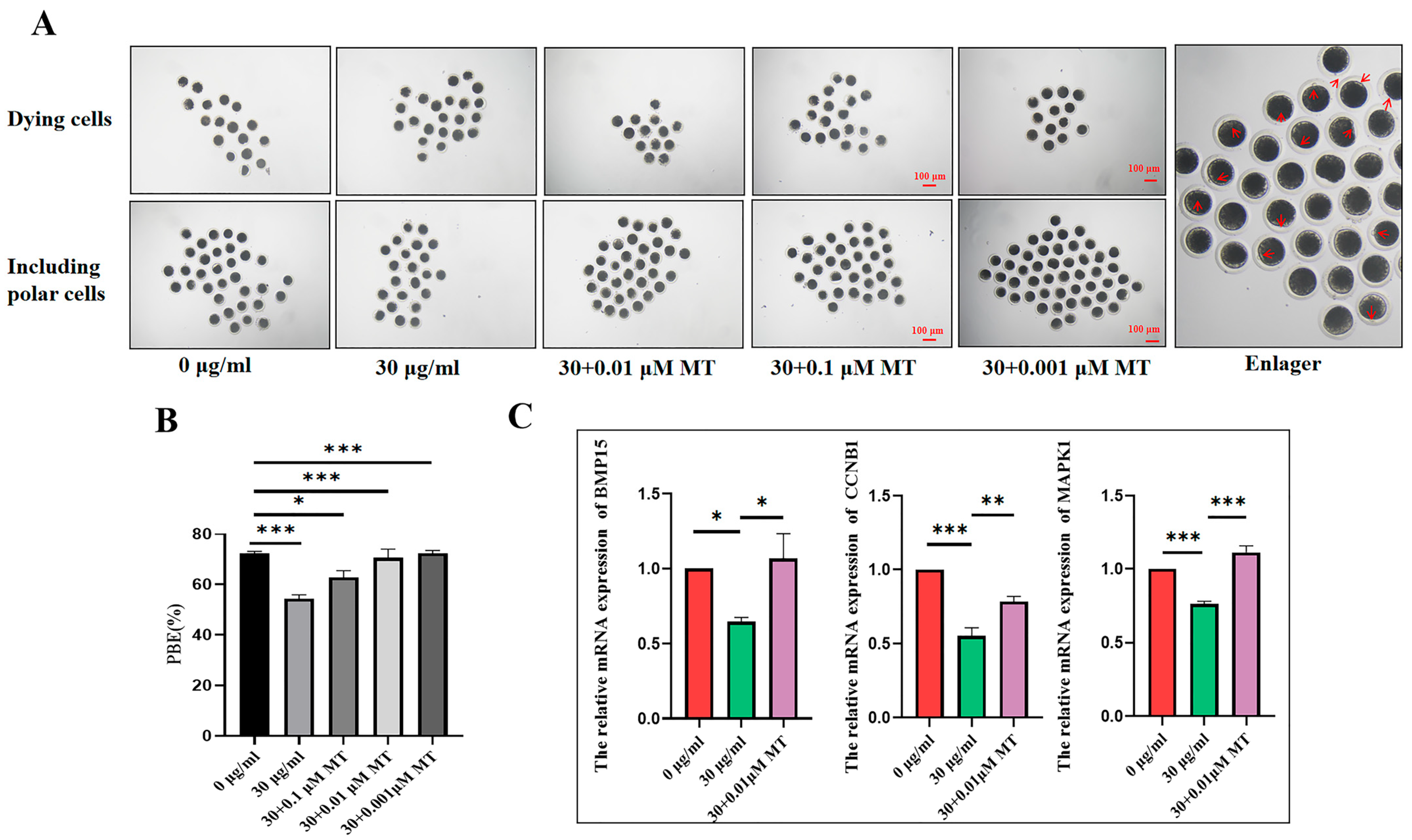
3.3. MT Can Alleviate the Damage to the Meiosis Process of Porcine Oocytes Induced by PS-MPs
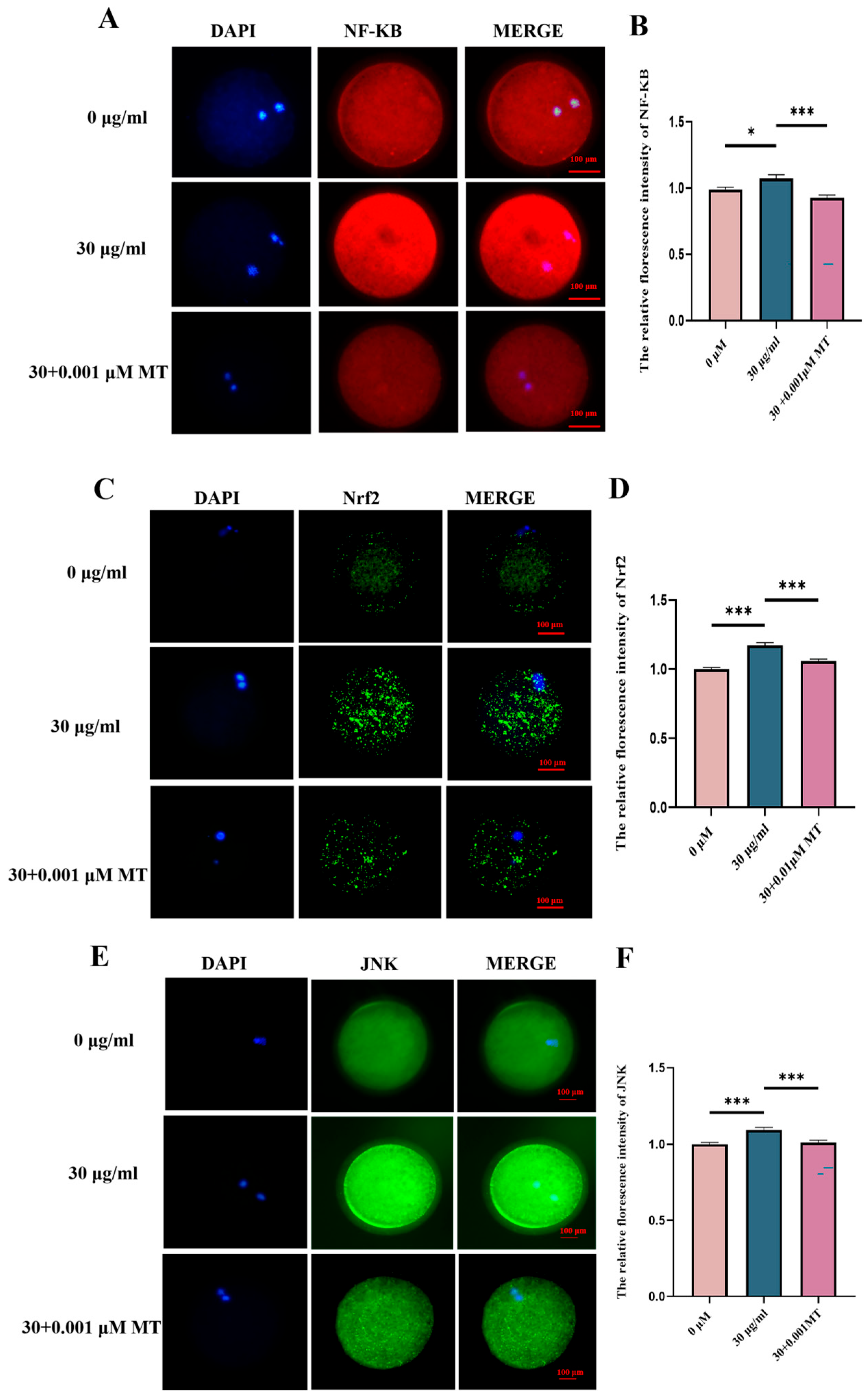
3.4. MT Can Alleviate Oxidative Stress and Mitochondrial Damage in Porcine Oocytes Induced by PS-MPs
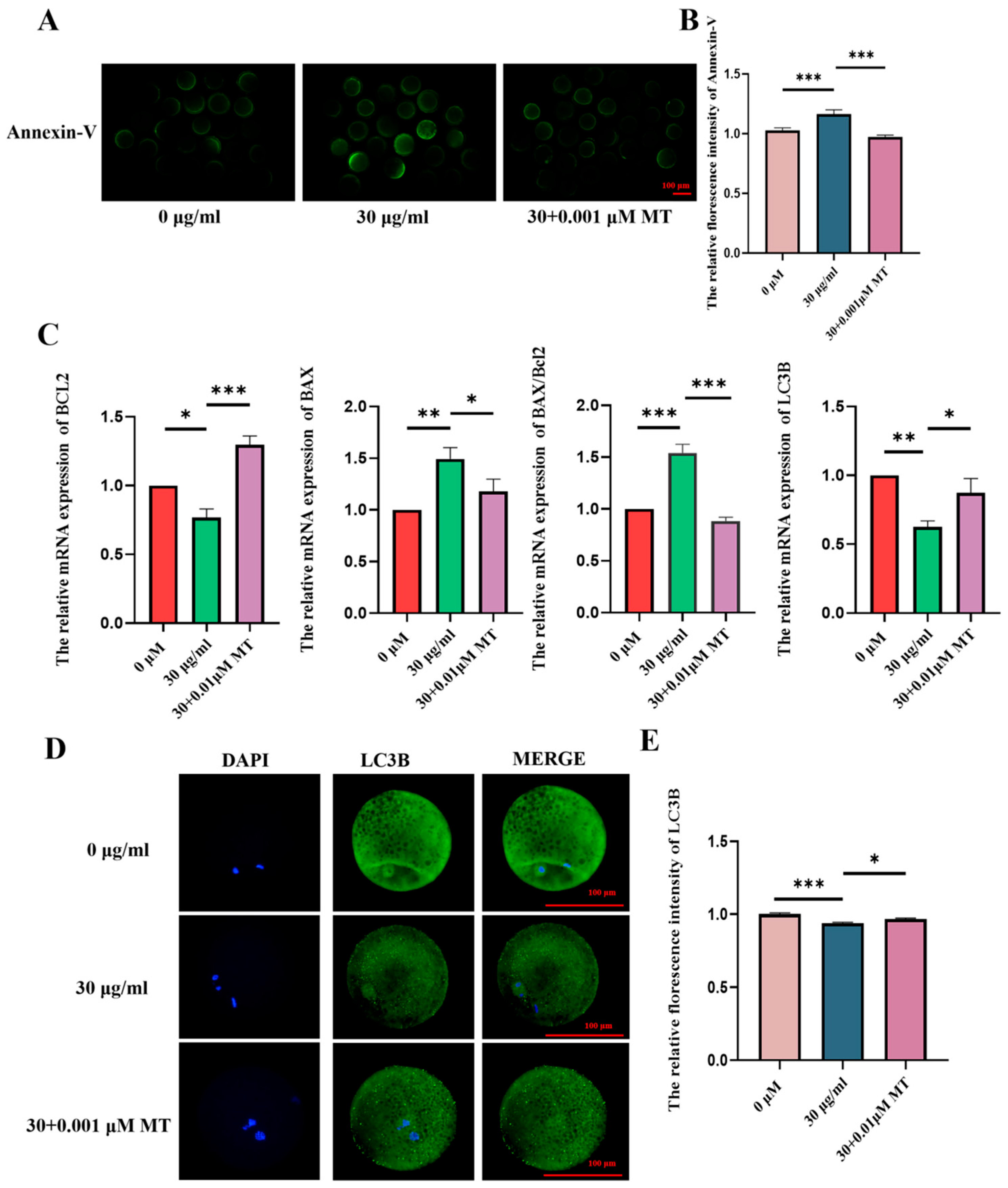
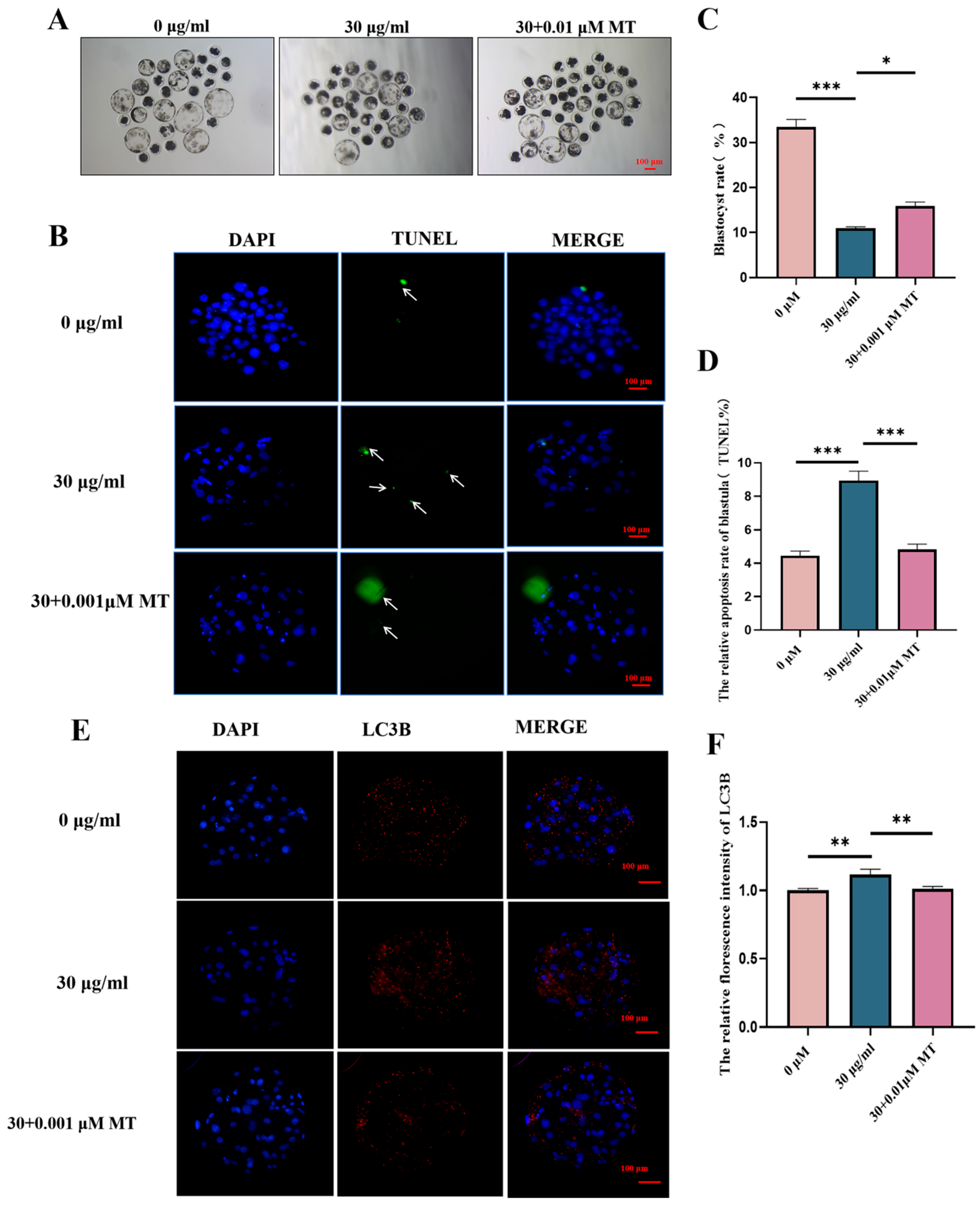
3.5. MT Can Alleviate the Apoptosis and Autophagy Disorders of Porcine Oocytes Induced by PS-MPs
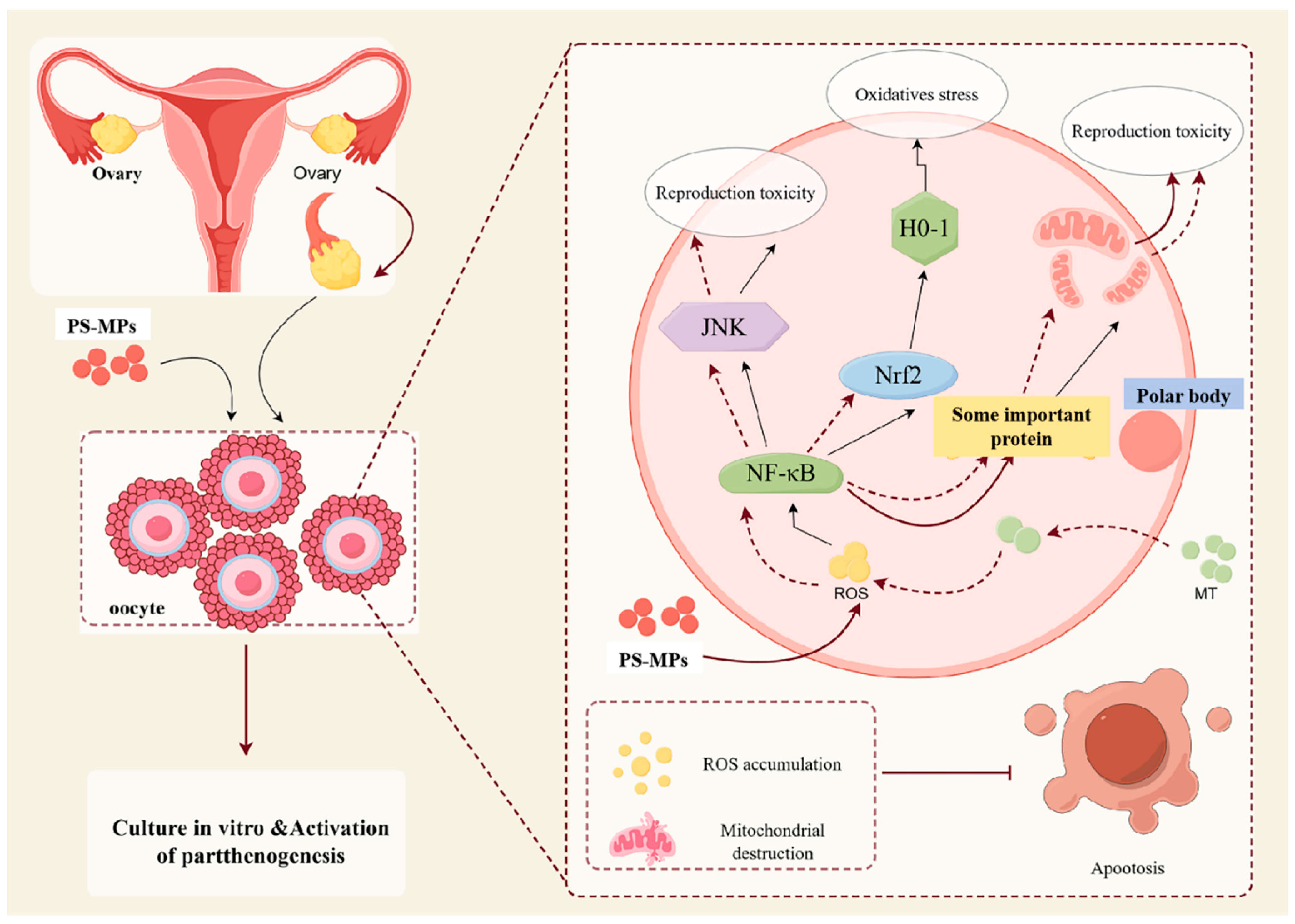
3.6. PS-MPs Cross Through the NF-KB/Nrf2/JNK MAPK Signaling Pathway to Induce Oxidative Stress, Mitochondrial Damage, and Inflammatory Responses
3.7. MT Can Alleviate the Subsequent Developmental Damage of PS-MPs on the Maturation of Porcine Oocytes—Early Embryos
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ho, B.T.; Roberts, T.K.; Lucas, S. An overview on biodegradation of polystyrene and modified polystyrene: The microbial approach. Crit. Rev. Biotechnol. 2018, 38, 308–320. [Google Scholar] [CrossRef]
- Dong, C.D.; Chen, C.W.; Chen, Y.C.; Chen, H.H.; Lee, J.S.; Lin, C.H. Polystyrene microplastic particles: In vitro pulmonary toxicity assessment. J. Hazard. Mater. 2020, 385, 121575. [Google Scholar] [CrossRef]
- Das, A. The emerging role of microplastics in systemic toxicity: Involvement of reactive oxygen species (ROS). Sci. Total Environ. 2023, 895, 165076. [Google Scholar] [CrossRef]
- Lu, H.; Yin, K.; Su, H.; Wang, D.; Zhang, Y.; Hou, L.; Li, J.B.; Wang, Y.; Xing, M. Polystyrene microplastics induce autophagy and apoptosis in birds lungs via PTEN/PI3K/AKT/mTOR. Environ. Toxicol. 2023, 38, 78–89. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Wang, D.; Yin, K.; Zhang, Y.; Lu, H.; Guo, T.; Li, J.; Zhao, H.; Xing, M. Polystyrene microplastics induce apoptosis in chicken testis via crosstalk between NF-κB and Nrf2 pathways. Comp. Biochem. Physiol. Toxicol. Pharmacol. CBP 2022, 262, 109444. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Yin, K.; Wang, D.; Wang, Y.; Lu, H.; Zhao, H.; Xing, M. Polystyrene microplastics-induced cardiotoxicity in chickens via the ROS-driven NF-κB-NLRP3-GSDMD and AMPK-PGC-1α axes. Sci. Total Environ. 2022, 840, 156727. [Google Scholar] [CrossRef] [PubMed]
- Weber, A.; Jeckel, N.; Weil, C.; Umbach, S.; Brennholt, N.; Reifferscheid, G.; Wagner, M. Ingestion and Toxicity of Polystyrene Microplastics in Freshwater Bivalves. Environ. Toxicol. Chem. 2021, 40, 2247–2260. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, Y.; Deng, Y.; Jiang, W.; Zhao, Y.; Geng, J.; Ding, L.; Ren, H. Uptake and Accumulation of Polystyrene Microplastics in Zebrafish (Danio rerio) and Toxic Effects in Liver. Environ. Sci. Technol. 2016, 50, 4054–4060. [Google Scholar] [CrossRef]
- Wu, D.; Deng, L.; Wang, T.; Du, W.; Yin, Y.; Guo, H. Aging process does not necessarily enhance the toxicity of polystyrene microplastics to Microcystis aeruginosa. Sci. Total Environ. 2023, 882, 163608. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, M.; Bao, T.T.; Lan, H. Long-term exposure to polystyrene microplastics triggers premature testicular aging. Part. Fibre Toxicol. 2023, 20, 35. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, W.; Chan, H.; Peng, J.; Zhu, P.; Li, J.; Jiang, X.; Zhang, Z.; Wang, Y.; Tan, Z.; et al. Polystyrene microplastics induce size-dependent multi-organ damage in mice: Insights into gut microbiota and fecal metabolites. J. Hazard. Mater. 2024, 461, 132503. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Meng, F.; Zhao, T.; Du, J.; Li, N.; Qiao, X.; Yao, Y.; Wu, D.; Peng, F.; Wang, D.; et al. Melatonin improves mouse oocyte quality from 2-ethylhexyl diphenyl phosphate-induced toxicity by enhancing mitochondrial function. Ecotoxicol. Environ. Saf. 2024, 280, 116559. [Google Scholar] [CrossRef]
- Malott, K.F.; Luderer, U. Toxicant effects on mammalian oocyte mitochondria. Biol. Reprod. 2021, 104, 784–793. [Google Scholar] [CrossRef]
- Zhu, Q.; Ding, D.; Yang, H.; Zou, W.; Yang, D.; Wang, K.; Zhang, C.; Chen, B.; Ji, D.; Hao, Y.; et al. Melatonin Protects Mitochondrial Function and Inhibits Oxidative Damage against the Decline of Human Oocytes Development Caused by Prolonged Cryopreservation. Cells 2022, 11, 4018. [Google Scholar] [CrossRef]
- Fan, L.; Guan, F.; Ma, Y.; Zhang, Y.; Li, L.; Sun, Y.; Cao, C.; Du, H.; He, M. N-Acetylcysteine improves oocyte quality through modulating the Nrf2 signaling pathway to ameliorate oxidative stress caused by repeated controlled ovarian hyperstimulation. Reprod. Fertil. Dev. 2022, 34, 736–750. [Google Scholar] [CrossRef]
- Zhan, C.; Cao, X.; Zhang, T.; Guo, J.; Xu, G.; Wang, H.; Yang, W.; Yang, L.; Che, D.; Lu, W.; et al. Melatonin protects porcine oocyte from copper exposure potentially by reducing oxidative stress potentially through the Nrf2 pathway. Theriogenology 2022, 193, 1–10. [Google Scholar] [CrossRef]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.J.; Kim, Y.K. The role of LC3B in autophagy as an RNA-binding protein. Autophagy 2023, 19, 1028–1030. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Fleisher, T.A. Apoptosis. Ann. Allergy Asthma Immunol. 1997, 78, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Qin, J.; Pan, B.; Qazi, I.H.; Ye, J.; Fang, Y.; Zhou, G. Oxidative Stress and Oocyte Cryopreservation: Recent Advances in Mitigation Strategies Involving Antioxidants. Cells 2022, 11, 3573. [Google Scholar] [CrossRef]
- Chen, X.; Li, X.; Zhang, W.; He, J.; Xu, B.; Lei, B.; Wang, Z.; Cates, C.; Rousselle, T.; Li, J. Activation of AMPK inhibits inflammatory response during hypoxia and reoxygenation through modulating JNK-mediated NF-κB pathway. Metab. Clin. Exp. 2018, 83, 256–270. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, H.; Wang, J.; Li, F.; Li, X.; Li, T.; Wang, C.; Li, L.; Peng, R.; Liu, L.; et al. Annexin A5 ameliorates traumatic brain injury-induced neuroinflammation and neuronal ferroptosis by modulating the NF-ĸB/HMGB1 and Nrf2/HO-1 pathways. Int. Immunopharmacol. 2023, 114, 109619. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Zhuan, Q.; Zhang, L.; Meng, L.; Fu, X.; Hou, Y. Polystyrene microplastics induced female reproductive toxicity in mice. J. Hazard. Mater. 2022, 424, 127629. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Yan, M.; Pan, C.; Liu, Z.; Sha, X.; Jiang, C.; Li, L.; Pan, M.; Li, D.; Han, X.; et al. Chronic exposure to polystyrene microplastics induced male reproductive toxicity and decreased testosterone levels via the LH-mediated LHR/cAMP/PKA/StAR pathway. Part. Fibre Toxicol. 2022, 19, 13. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Deng, T.; Duan, J.; Xie, J.; Yuan, J.; Chen, M. Exposure to polystyrene microplastics causes reproductive toxicity through oxidative stress and activation of the p38 MAPK signaling pathway. Ecotoxicol. Environ. Saf. 2020, 190, 110133. [Google Scholar] [CrossRef]
- Yong, W.; Ma, H.; Na, M.; Gao, T.; Zhang, Y.; Hao, L.; Yu, H.; Yang, H.; Deng, X. Roles of melatonin in the field of reproductive medicine. Biomed. Pharmacother. 2021, 144, 112001. [Google Scholar] [CrossRef]
- Nadri, P.; Zahmatkesh, A.; Bakhtari, A. The potential effect of melatonin on in vitro oocyte maturation and embryo development in animals. Biol. Reprod. 2024, 111, 529–542. [Google Scholar] [CrossRef]
- He, Y.; Yu, T.; Li, H.; Sun, Q.; Chen, M.; Lin, Y.; Dai, J.; Wang, W.; Li, Q.; Ju, S. Polystyrene nanoplastic exposure actives ferroptosis by oxidative stress-induced lipid peroxidation in porcine oocytes during maturation. J. Anim. Sci. Biotechnol. 2024, 15, 117. [Google Scholar] [CrossRef]
- Afreen, V.; Hashmi, K.; Nasir, R.; Saleem, A.; Khan, M.I.; Akhtar, M.F. Adverse health effects and mechanisms of microplastics on female reproductive system: A descriptive review. Environ. Sci. Pollut. Res. Int. 2023, 30, 76283–76296. [Google Scholar] [CrossRef]
- Chatterjee, A.; Maity, S.; Banerjee, S.; Dutta, S.; Adhikari, M.; Guchhait, R.; Biswas, C.; De, S.; Pramanick, K. Toxicological impacts of nanopolystyrene on zebrafish oocyte with insight into the mechanism of action: An expression-based analysis. Sci. Total Environ. 2022, 830, 154796. [Google Scholar] [CrossRef] [PubMed]
- Hou, B.; Wang, F.; Liu, T.; Wang, Z. Reproductive toxicity of polystyrene microplastics: In vivo experimental study on testicular toxicity in mice. J. Hazard. Mater. 2021, 405, 124028. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Hou, B.; Zhang, Y.; Wang, Z. Determination of Biological and Molecular Attributes Related to Polystyrene Microplastic-Induced Reproductive Toxicity and Its Reversibility in Male Mice. Int. J. Environ. Res. Public Health 2022, 19, 14093. [Google Scholar] [CrossRef] [PubMed]
- An, R.; Wang, X.; Yang, L.; Zhang, J.; Wang, N.; Xu, F.; Hou, Y.; Zhang, H.; Zhang, L. Polystyrene microplastics cause granulosa cells apoptosis and fibrosis in ovary through oxidative stress in rats. Toxicology 2021, 449, 152665. [Google Scholar] [CrossRef]
- Russo, M.; Forte, G.; Montanino Oliva, M.; Laganà, A.S.; Unfer, V. Melatonin and Myo-Inositol: Supporting Reproduction from the Oocyte to Birth. Int. J. Mol. Sci. 2021, 22, 8433. [Google Scholar] [CrossRef] [PubMed]
- El Gazzar, W.B.; Sliem, R.E.; Bayoumi, H.; Nasr, H.E.; Shabanah, M.; Elalfy, A.; Radwaan, S.E.; Gebba, M.A.; Mansour, H.M.; Badr, A.M.; et al. Melatonin Alleviates Intestinal Barrier Damaging Effects Induced by Polyethylene Microplastics in Albino Rats. Int. J. Mol. Sci. 2023, 24, 13619. [Google Scholar] [CrossRef]
- Zeng, M.; Zhan, C.; Li, Y.; Liao, H.; Liu, W.; Chen, G.; Wang, J. Melatonin prevents the transgenerational toxicity of nanoplastics in zebrafish (Danio rerio). Sci. Total Environ. 2024, 953, 176043. [Google Scholar] [CrossRef]
- Missawi, O.; Jeddou, I.B.; Venditti, M.; Zitouni, N.; Zaouali, M.A.; Abdennebi, H.B.; Messaoudi, I.; Reiter, R.J.; Minucci, S.; Banni, M. Environmental microplastic accumulation exacerbates liver ischemia-reperfusion injury in rat: Protective effects of melatonin. Sci. Total Environ. 2023, 860, 160155. [Google Scholar] [CrossRef]
- Xue, Y.; Cheng, X.; Ma, Z.Q.; Wang, H.P.; Zhou, C.; Li, J.; Zhang, D.L.; Hu, L.L.; Cui, Y.F.; Huang, J.; et al. Polystyrene nanoplastics induce apoptosis, autophagy, and steroidogenesis disruption in granulosa cells to reduce oocyte quality and fertility by inhibiting the PI3K/AKT pathway in female mice. J. Nanobiotechnol. 2024, 22, 460. [Google Scholar] [CrossRef]
- Morgan, M.J.; Liu, Z.G. Crosstalk of reactive oxygen species and NF-κB signaling. Cell Res. 2011, 21, 103–115. [Google Scholar] [CrossRef]
- Xu, F.; Xu, J.; Xiong, X.; Deng, Y. Salidroside inhibits MAPK, NF-κB, and STAT3 pathways in psoriasis-associated oxidative stress via SIRT1 activation. Redox Rep. Commun. Free Radic. Res. 2019, 24, 70–74. [Google Scholar] [CrossRef]
- Shengchen, W.; Jing, L.; Yujie, Y.; Yue, W.; Shiwen, X. Polystyrene microplastics-induced ROS overproduction disrupts the skeletal muscle regeneration by converting myoblasts into adipocytes. J. Hazard. Mater. 2021, 417, 125962. [Google Scholar] [CrossRef] [PubMed]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Nakano, H.; Nakajima, A.; Sakon-Komazawa, S.; Piao, J.H.; Xue, X.; Okumura, K. Reactive oxygen species mediate crosstalk between NF-kappaB and JNK. Cell Death Differ. 2006, 13, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Zhou, P.; Xu, K.; Chen, T.; Jiao, J.; Wei, H.; Yang, X.; Xu, W.; Wan, W.; Xiao, J. Metformin induces cell cycle arrest, apoptosis and autophagy through ROS/JNK signaling pathway in human osteosarcoma. Int. J. Biol. Sci. 2020, 16, 74–84. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Xiong, S.; Jing, Q.; van Gestel, C.A.M.; van Straalen, N.M.; Roelofs, D.; Sun, L.; Qiu, H. Maternal exposure to polystyrene nanoparticles retarded fetal growth and triggered metabolic disorders of placenta and fetus in mice. Sci. Total Environ. 2023, 854, 158666. [Google Scholar] [CrossRef]
- Yang, D.; Zhu, J.; Zhou, X.; Pan, D.; Nan, S.; Yin, R.; Lei, Q.; Ma, N.; Zhu, H.; Chen, J.; et al. Polystyrene micro- and nano-particle coexposure injures fetal thalamus by inducing ROS-mediated cell apoptosis. Environ. Int. 2022, 166, 107362. [Google Scholar] [CrossRef]
- Wan, S.; Wang, X.; Chen, W.; Xu, Z.; Zhao, J.; Huang, W.; Wang, M.; Zhang, H. Polystyrene Nanoplastics Activate Autophagy and Suppress Trophoblast Cell Migration/Invasion and Migrasome Formation to Induce Miscarriage. ACS Nano 2024, 18, 3733–3751. [Google Scholar] [CrossRef]
- Zaffagnini, G.; Cheng, S.; Salzer, M.C.; Pernaute, B.; Duran, J.M.; Irimia, M.; Schuh, M.; Böke, E. Mouse oocytes sequester aggregated proteins in degradative super-organelles. Cell 2024, 187, 1109–1126.e1121. [Google Scholar] [CrossRef]


| Genes | Forward Primers (5′ to 3′) | Reverse Primers (5′ to 3′) |
|---|---|---|
| GAPDH | GTCGGTTGTGGATCTGACCT | TTGACGAAGTGGTCGTTGAG |
| PTX3 | TGTGTGGGTGGTGGCTTTGATG | TGGGGCTGAATCTCTGTGACTCC |
| GDF9 | CAGTCAGCTGAAGTGGGACA | TGGATGATGTTCTGCACCAT |
| ERK1 | CCTCCAACCTGCTCATCAACAC | ACATATTCCGTCAAGAAGCCAGTG |
| MAPK1 | GGCTGTTCCCAAATGCTGACTC | CCTGCTCTACTTCAATCCTCTTGTG |
| CAT | AGCCAGTGACCAGATGAAGCATTG | ATGTCGTGTGTGACCTCAAAGTAGC |
| GPX | CTGGTCGTGCTCGGCTTCC | GCCTGGTCGGACGTACTTGAG |
| SOD1 | CTCTCGGGAGACCATTCCATCATTG | TCCACCTCTGCCCAAGTCATCTG |
| BAX | TGCCTCAGGATGCATCTACC | AAGTAGAAAAGCGCGACCAC |
| BCL2 | AGGGCATTCAGTGACCTGAC | CGATCCGACTCACCAATACC |
| CDK1 | GGGCACTCCCAATAATGAAGT | GTTCTTGATACAACGTGTGGGAA |
| BMP15 | CCTCCATCCTTTCCAAGTCA | GTGTAGTACCCGAGGGCAGA |
| LC3B | GTGTAGTACCCGAGGGCAGA | TTTGGTAGGATGCTGCTCTCG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, H.-M.; Peng, H.-L.; Huang, C.-M.; Zhang, J.-T.; Li, Y.-H.; Lin, Z.-L.; Cao, Q.-L.; Xu, Y.-N. Melatonin Alleviates the Damage of Polystyrene Microplastics to Porcine Oocytes by Reducing Oxidative Stress and Mitochondrial Damage, and Regulating Autophagy and Apoptosis Levels. Animals 2025, 15, 3163. https://doi.org/10.3390/ani15213163
Huang H-M, Peng H-L, Huang C-M, Zhang J-T, Li Y-H, Lin Z-L, Cao Q-L, Xu Y-N. Melatonin Alleviates the Damage of Polystyrene Microplastics to Porcine Oocytes by Reducing Oxidative Stress and Mitochondrial Damage, and Regulating Autophagy and Apoptosis Levels. Animals. 2025; 15(21):3163. https://doi.org/10.3390/ani15213163
Chicago/Turabian StyleHuang, Hui-Mei, Hui-Lin Peng, Chu-Man Huang, Jun-Tong Zhang, Ying-Hua Li, Zi-Li Lin, Qi-Long Cao, and Yong-Nan Xu. 2025. "Melatonin Alleviates the Damage of Polystyrene Microplastics to Porcine Oocytes by Reducing Oxidative Stress and Mitochondrial Damage, and Regulating Autophagy and Apoptosis Levels" Animals 15, no. 21: 3163. https://doi.org/10.3390/ani15213163
APA StyleHuang, H.-M., Peng, H.-L., Huang, C.-M., Zhang, J.-T., Li, Y.-H., Lin, Z.-L., Cao, Q.-L., & Xu, Y.-N. (2025). Melatonin Alleviates the Damage of Polystyrene Microplastics to Porcine Oocytes by Reducing Oxidative Stress and Mitochondrial Damage, and Regulating Autophagy and Apoptosis Levels. Animals, 15(21), 3163. https://doi.org/10.3390/ani15213163




