1. Introduction
Plastic pollution has become widespread worldwide in recent years [
1]. Plastics are accumulating in the environment at a rate of 31.9 million tons annually, with projections indicating that over 33 billion tons will have accumulated globally by 2050 [
2]. Polystyrene (PS), a synthetic polymer compound that serves as a common raw material for plastics, is widely used in both consumer and industrial applications owing to its thermal stability, ease of manufacturing, and low cost [
3]. Plastics can undergo progressive fragmentation into microplastics (MPs, less than 5 mm in diameter) or even smaller particles, known as nanoparticles (NPs, less than 100 nm in diameter). Furthermore, NPs are smaller than MPs and have a higher likelihood of crossing biological barriers that cause damage to the organism [
4]. Therefore, in this study, we have focused on polystyrene nanoplastics (NPs) because of their ability to exert negative effects on aquatic animals and plants by accumulating in various animal organs and plant roots, thus indirectly posing a threat to animal health [
5,
6,
7].
M/NPs are emerging environmental contaminants of great concern because of their ubiquitous distribution in air, soil, water, and food. Poultry are exposed to NPs through contaminated feed, water, and environmental ingestion [
8]. The gastrointestinal tract, therefore, serves as the primary route of exposure, through which the gut microbial community is inevitably affected [
7,
9]. The intestinal microbiota plays a vital role in the physiological system of poultry, with significant effects encompassing host metabolism, immune function, health maintenance, and growth and development. These microorganisms play a crucial role in host growth and health through their functional contributions to digestion and absorption processes, with the intestine being one of the major organs where these processes occur [
10,
11]. The digestive tract is the main route of NPs exposure. Previous studies have found that NPs can affect the abundance and composition of the gut microbiota [
12,
13]. In aquatic models (e.g., zebrafish), NPs exposure disrupts gut microbiota composition and impairs carbohydrate and amino acid metabolism [
14].
The pervasive presence of plastic pollution has raised alarms over its potential to infiltrate the food chain. The ability of NPs to traverse biological barriers and accumulate in animal tissues—as evidenced by their detection in human placenta [
15]—poses a direct concern for the safety of animal-derived foods. This risk is particularly relevant to poultry production, as recent evidence confirming the presence of polystyrene microplastics in broiler feces underscores a tangible route of exposure [
16]. As a vertebrate species occupying a high ecological niche, chickens and their products, such as eggs, are nutrient-rich and essential parts of daily life, playing a crucial role in food safety. Given that chickens and their eggs constitute a nutrient-rich staple of the global diet, contamination of these products could represent a significant pathway for human ingestion of plastic particles. While initial findings confirm exposure, the functional consequences of NPs ingestion on avian health and product quality remain poorly understood.
Therefore, this study was designed to bridge this knowledge gap by systematically investigating the effects of polystyrene NPs on chicken intestinal morphology and the cecal microbiota, assessing the impact of this exposure on a suite of egg quality parameters, and analyzing the correlative relationships between these microbial shifts and egg quality metrics.
2. Materials and Methods
2.1. Ethics Statement
All animal experiments were approved by the Animal Ethics Committee of the Jiangsu Institute of Poultry Science (Jiangsu, China; Approval No. 202411001). Euthanasia was carried out adhering to the American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals (2020 edition), fulfilling institutional and national ethical standards for the humane use of animals in research.
2.2. NPs Characterization
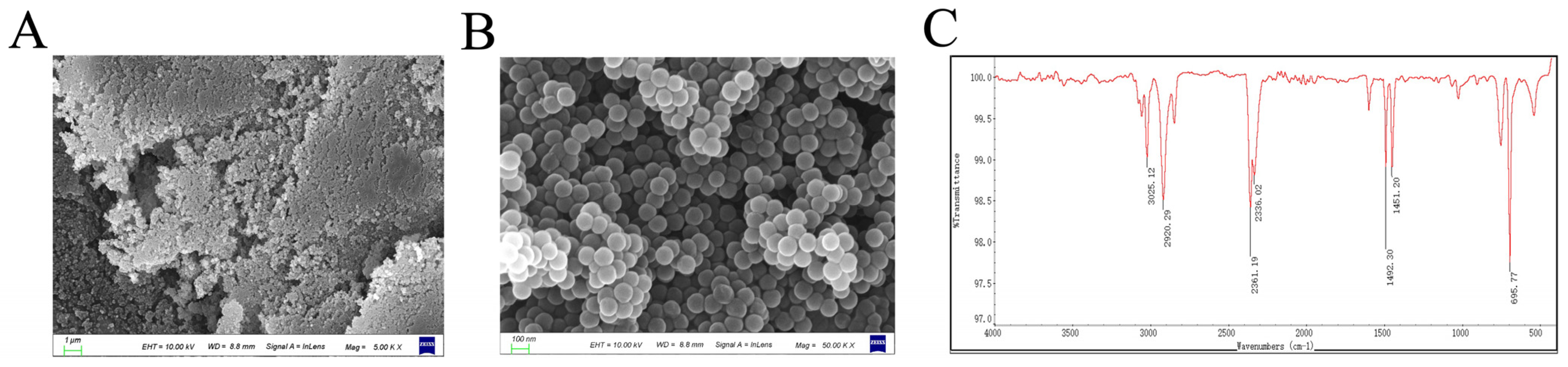
Monodisperse NPs with a nominal diameter of 100 nm and a concentration of 25 mg/mL were procured from Tianjin Base-line ChromTech Research Centre (Tianjin, China). The stock suspension was diluted with double-distilled water (DDW) and subjected to sonication (40 kHz, 85% power, 10 min) in a water bath sonicator before each experiment to ensure homogeneity of dispersion. ATR-FTIR spectroscopic analysis of the NPs was conducted on a Nicolet iS50 spectrometer (Thermo Fisher Scientific, Waltham, MA, USA) to determine their chemical characteristics. For morphological analysis, the NPs were examined using scanning electron microscopy (SEM; Gemini SEM 300, Oberkochen, Germany), which allowed direct visualization of NPs morphology, size, and dispersion state at the nanoscale, confirming both primary particle size and the absence of large aggregates.
2.3. Experimental Animals
A total of 60 green-shelled laying hens (120 days old) underwent a 7-day acclimation period under standardized environmental conditions. All birds were housed in individual cages under a standardized lighting schedule of 16h of light and 8h of dark, with light intensity maintained at approximately 50 lux. The temperature was maintained at 24 ± 1 °C and the relative humidity at 55 ± 5%. Birds had ad libitum access to feed and water throughout the experimental period. The birds were randomly allocated into two experimental groups (
n = 30 per group). Based on evidence that the aquatic environment serves as a major route of microplastic exposure in avian species, the exposure concentration used in this study was selected to reflect environmentally relevant levels as reported in previous research [
17]. One group received a basal diet (for detailed composition, see
Table S1) supplemented with 100 nm NPs at a concentration of 10 mg/kg feed, while the control group received the same basal diet without NPs. The experimental period spanned 120 days, during which feed and water were provided ad libitum. After a 12 h fasting period, all birds were humanely euthanized by CO
2 asphyxiation. During dissection, cecal and fecal contents were collected. Tissue samples designated for histopathological examination were immediately fixed in 4% paraformaldehyde, and those intended for microbial analysis were flash-frozen in liquid nitrogen and stored at −80 °C until DNA extraction.
2.4. Ex Vivo Fluorescence Imaging
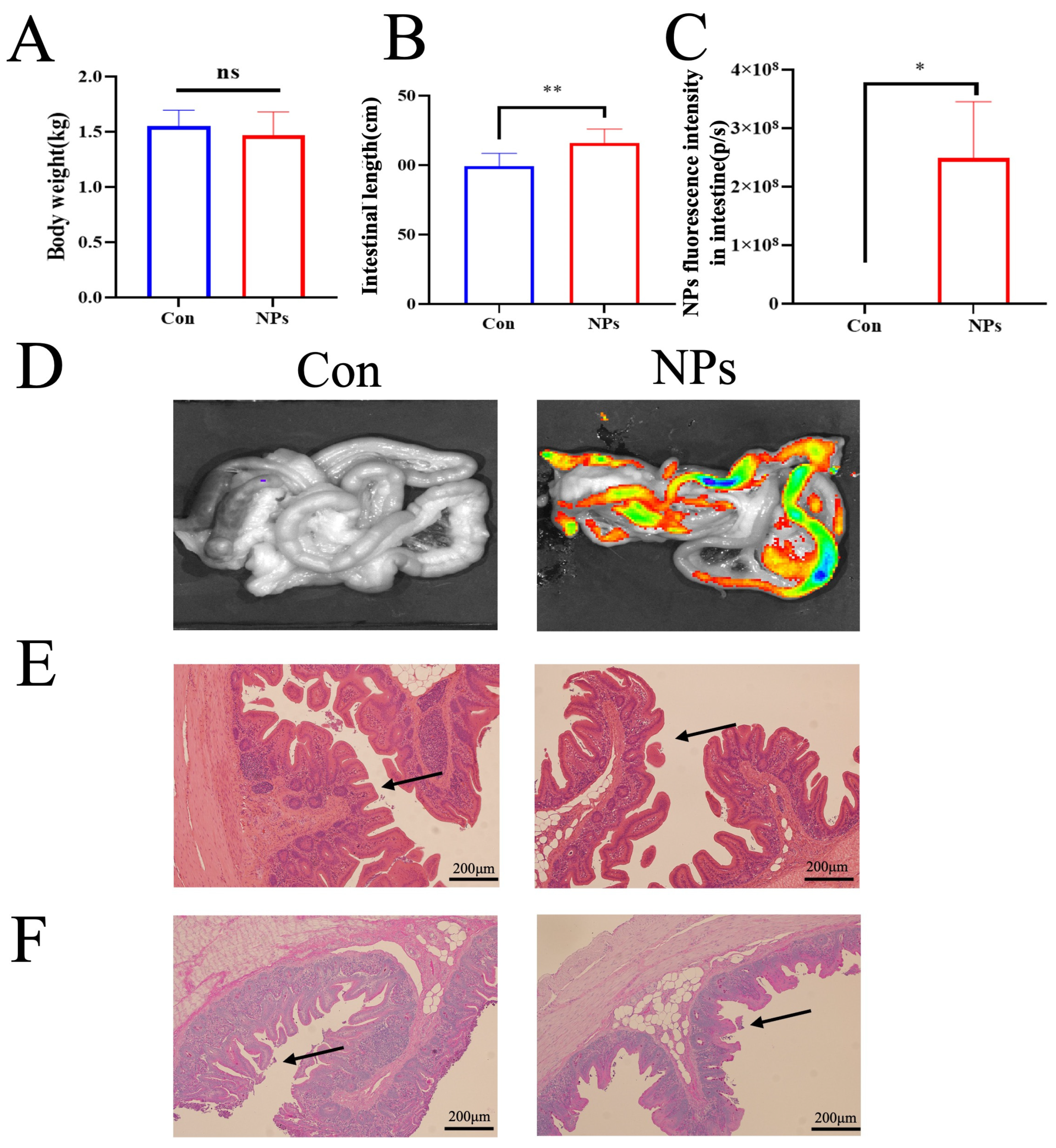
Fluorescent imaging was performed on chickens using an IVIS Lumina III in vivo imaging system (PerkinElmer, Shelton, CT, USA). The acquisition parameters were set to an excitation wavelength of 580 nm and an emission wavelength of 620 nm. Following image acquisition, fluorescence intensity was quantified using Living Image Software 4.5.2 (PerkinElmer, USA). Regions of interest (ROIs) were manually delineated over the abdominal area, which was selected as the primary region for NPs accumulation based on gastrointestinal tract anatomy. The total radiant efficiency ([p/s]/[μW/cm2]) was calculated for each ROI. To ensure accuracy, background fluorescence signals obtained from the same-sized ROIs in control animals not exposed to NPs were subtracted from all measurements.
2.5. Hematoxylin and Eosin Staining
All chicken intestines were surgically removed, trimmed, and immersed in a fixative solution containing 4% paraformaldehyde for 24 h, by established protocols. After discarding the fixative, the tissue was cleaned, allowed to dry naturally using a gradient of ethanol concentration, turned transparent using a xylene solution, and then impregnated with wax. Sections of the tissue were attached to slides using a microtome. HE was used to stain the cell nucleus and cytoplasm of tissue wax specimens directly sectioned at a thickness of 5 µm. Every specimen was examined and documented utilizing a Leica light microscope DMI3000B (Leica, Wetzlar, Germany) outfitted with a digital camera.
2.6. Periodic Acid Schiff Staining
Tissue sections were treated with periodic acid solution (5 g/L in distilled water) for 5 min, followed by rinsing with tepid distilled water for 5 min. Subsequently, slides were stained with Schiff reagent for 5 min and counterstained with hematoxylin for 2 s. After staining, sections were dehydrated through an ethanol series, cleared in xylene, and mounted. For microscopic analysis of goblet cells in chicken intestinal tissues, the stained sections were examined using a Leica DMI3000B system (Wetzlar, Germany).
2.7. Measurement of Egg Quality Parameters
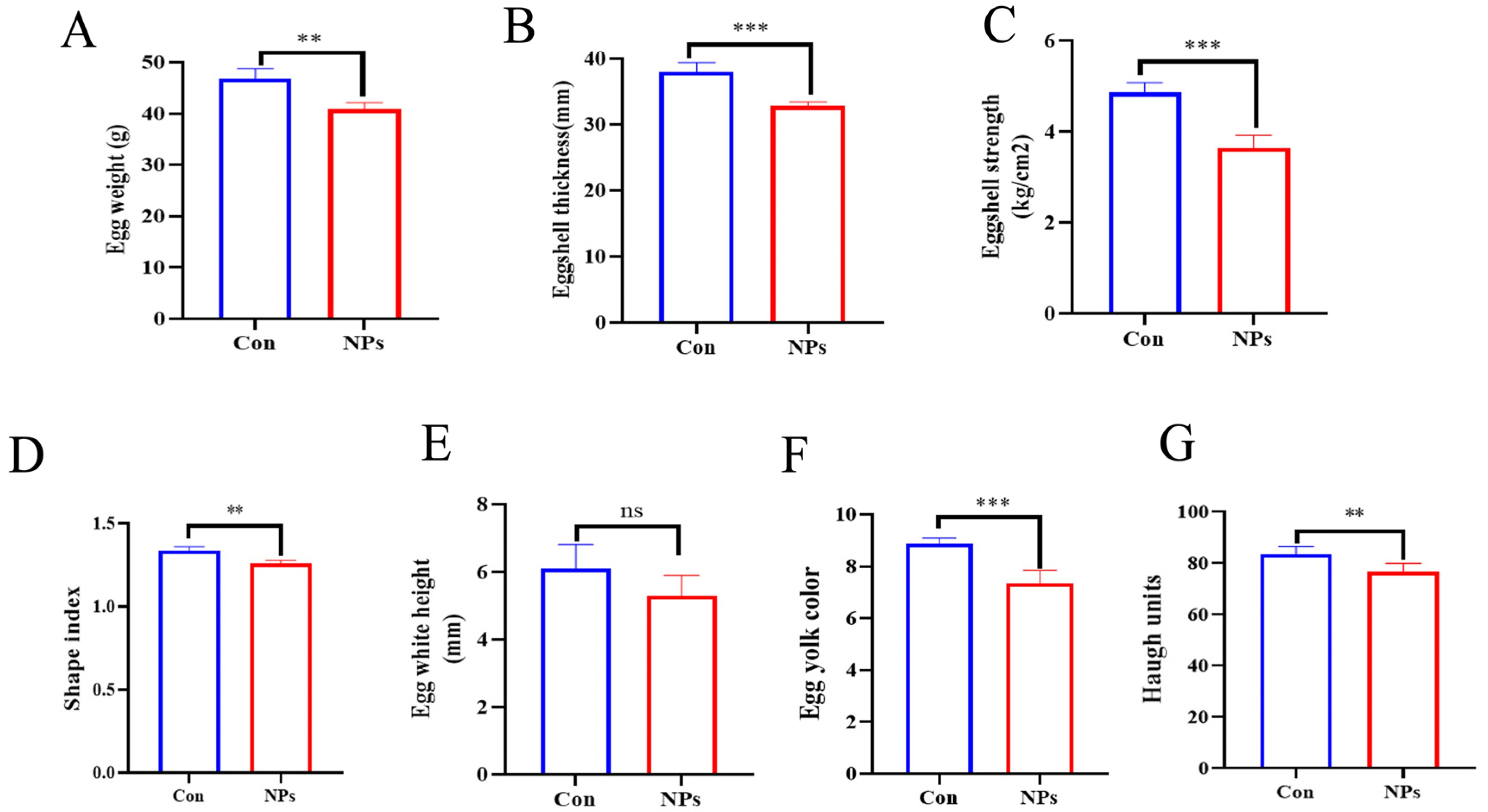
Egg quality on study day 120 was evaluated. Six eggs from each group were collected within 24 h for analysis. Parameters were measured using the following methods and instruments. Eggshell thickness was measured using the ETG-1061A instrument (Robotmation, Tokyo, Japan) with measurements taken at three positions (blunt end, equatorial region, and sharp end) and the average value calculated. Egg shape index was determined using a Digimatic caliper (0–200 mm; Guilin Guanglu, Guilin, China) by measuring the maximum vertical diameter and maximum horizontal diameter, with the index calculated as (maximum vertical diameter/maximum horizontal diameter) × 100. Eggshell strength was assessed using the EFG-0502 tester (Robotmation, Japan) by applying force to the equatorial region of the egg until fracture occurred. Egg white height, yolk color, and Haugh units were collectively measured using the EMT-7300 multi-functional tester (Robotmation, Japan). The Haugh unit was calculated using the standard formula HU = 100 × log (H − 1.7 W^0.37 + 7.6), where H is the albumen height (mm) and W is the egg weight (g).
2.8. 16S rRNA Sequencing
Fecal samples were collected from the chickens in each group. According to the manufacturer’s protocols, total microbial genomic DNA was extracted from the fecal samples using the TIANamp Soil DNA Kit (TianGen, Beijing, China). The bacterial primers 341F (5′-CCTAYGGGRBGCASCAG-3′) and 806R (5′-GGACTACNNGGGTATCTAAT-3′) were used to amplify the V3–V4 hypervariable regions of the 16S rRNA gene. All PCR reactions were performed using 15 μL of Phusion High-Fidelity PCR Master Mix, 0.2 μM of each forward and reverse primer, and approximately 10 ng of template DNA. Thermal cycling consisted of an initial denaturation at 98 °C for 1 min, followed by 30 cycles of denaturation at 98 °C for 10 s, annealing at 50 °C for 30 s, elongation at 72 °C for 30 s, and a final extension at 72 °C for 5 min. The PCR products were purified using a magnetic bead-based method.
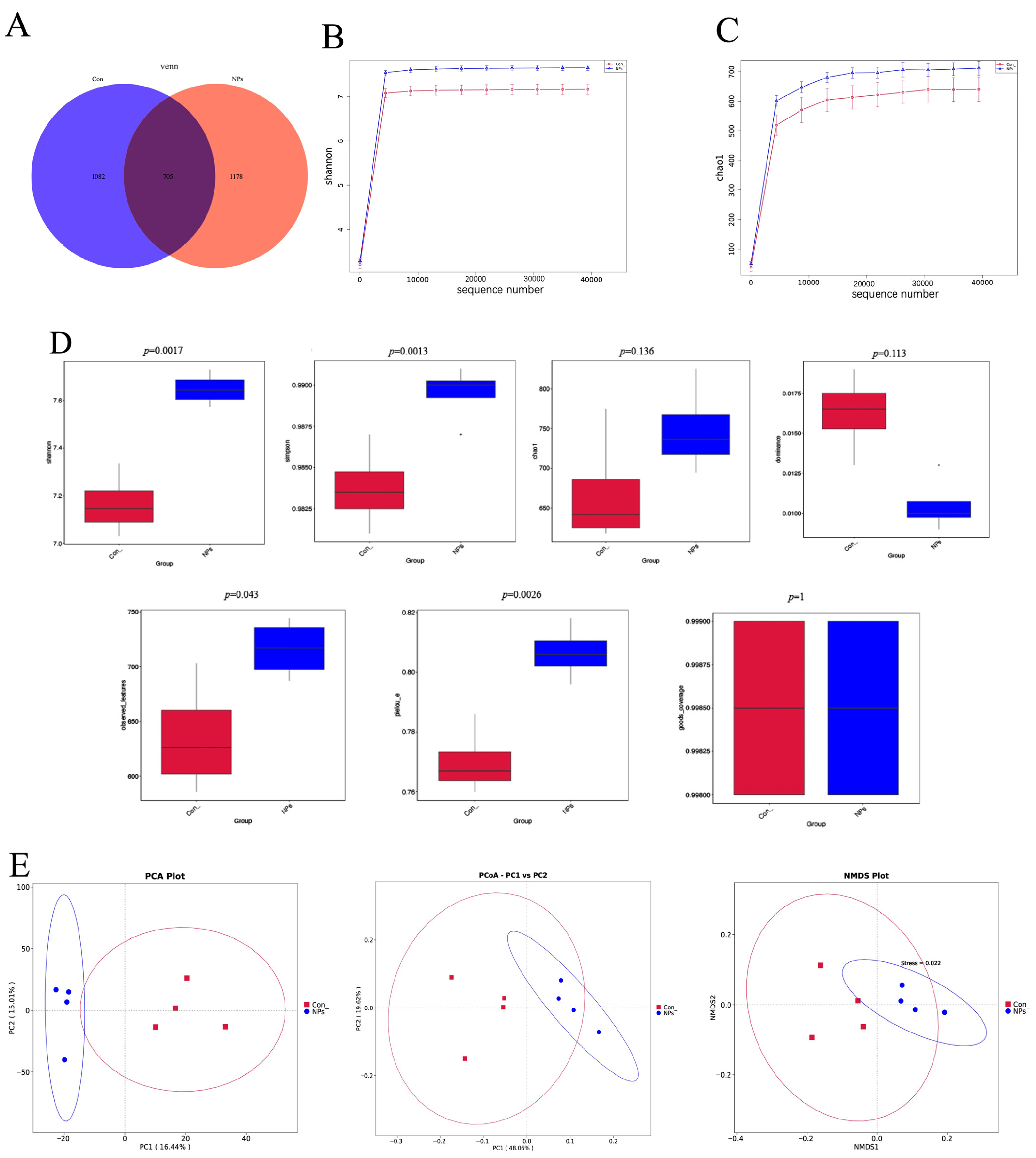
The amplicon libraries were sequenced on an Illumina NovaSeq 6000 platform (Illumina, San Diego, CA, USA) using a 2 × 250 bp paired-end configuration. Raw sequencing data were processed using QIIME 2 (version 2023.9). Sequence denoising, including quality filtering, dereplication, chimera removal, and paired-end read merging, was performed with the DADA2 plugin, which generated amplicon sequence variants (ASVs) as the final features. For subsequent diversity analyses, all samples were rarefied to a depth of 30,000 sequences per sample to ensure even sampling depth. Both α-diversity (Shannon, Simpson, Chao1, and observed features) and β-diversity (weighted and unweighted UniFrac, Bray–Curtis) metrics were calculated based on this rarefied ASV table.
To analyze the diversity, richness, and uniformity of the communities in the sample, alpha diversity was calculated from 7 indices in QIIME2(version 202202), including Observed_otus, Chao1, Shannon, Simpson, Dominance, and Pielou_e. Three indices were selected to identify community richness: Observed_otus—the number of observed species (
https://www.osgeo.cn/scikit-bio/generated/skbio.diversity.alpha.observed_otus.html (accessed on 15 June 2025)); Chao—the Chao1 estimator (
https://www.osgeo.cn/scikit-bio/generated/skbio.diversity.alpha.chao1.html#skbio.diversity.alpha.chao1 (accessed on 15 June 2025)); and Dominance—the dominance index (
https://www.osgeo.cn/scikit-bio/generated/skbio.diversity.alpha.dominance.html#skbio.diversity.alpha.dominance (accessed on 15 June 2025)). Two indices were used to identify community diversity: Shannon—the Shannon index (
https://www.osgeo.cn/scikit-bio/generated/skbio.diversity.alpha.shannon.html#skbio.diversity.alpha.shannon (accessed on 15 June 2025)) and Simpson—the Simpson index (
https://www.osgeo.cn/scikit-bio/generated/skbio.diversity.alpha.simpson.html#skbio.diversity.alpha.simpson (accessed on 15 June 2025)). One index was used to calculate sequencing depth: coverage—the Good’s coverage (
https://www.osgeo.cn/scikit-bio/generated/skbio.diversity.alpha.goods_coverage.html#skbio.diversity.alpha.goods_coverage (accessed on 15 June 2025)); One index was used to estimate species evenness: Pielou_e—Pielou’s evenness index (
https://www.osgeo.cn/scikit-bio/generated/skbio.diversity.alpha.pielou_e.html#skbio.diversity.alpha.pielou_e (accessed on 15 June 2025)). To evaluate the richness of the microbial community and sample size, a species accumulation boxplot can be used to visualize data, which is performed with the vegan package in R software.
Beta diversity was evaluated through cluster analysis. Before clustering, principal component analysis (PCA) was employed for dimensionality reduction of the original variables using the ade4 and ggplot2 packages in R software (version 4.0.3). Principal coordinates analysis (PCoA) was subsequently performed to extract principal coordinates and visualize complex, multidimensional data. A distance matrix based on weighted or unweighted UniFrac metrics was constructed and projected onto a new set of orthogonal axes, where the first principal coordinate represents the maximum variance, followed by successively lower variances in subsequent coordinates. All PCoA visualizations were generated using the ade4 and ggplot2 packages in R software. Non-metric multidimensional scaling (NMDS) was also implemented for data dimension reduction. Like the PCoA, NMDS also uses the distance matrix, but it emphasizes the numerical rank instead. The distance between sample points on the diagram can only reflect the rank information rather than the numerical differences. NMDS analysis was implemented using the R software with the ade4 and ggplot2 packages.
2.9. Quantification and Statistical Analysis
Statistical analyses were performed using SPSS (version 22.0; IBM, Armonk, NY, USA) and R software (version 4.3.1). The normality of all data was examined using the Kolmogorov–Smirnov test. For comparisons between the two groups, data conforming to a normal distribution were analyzed using Student’s t-test; results are expressed as the means ± standard deviation. p < 0.05 indicates a significant difference. Spearman’s correlation analysis was used to examine relationships between specific microbial genera and egg quality parameters. The Mann–Whitney U test was used for the predictive analysis of gut microbiota functions.
4. Discussion
Plastic pollution has become an environmental problem that cannot be ignored. In aquatic environments, plastic debris causes entanglement and ingestion hazards to marine life, while NPs can accumulate in sediment and water columns, disrupting nutrient cycles and food webs. Current research has primarily focused on its impacts on aquatic organisms and plants [
14], with limited investigation into its accumulation and toxicological effects in terrestrial species, particularly in the context of the food chain. Chickens, like humans, are exposed to NPs through contaminated feed, mimicking real-world exposure routes such as leaching from food packaging (e.g., plastic bags, tea bags, etc.) or bioaccumulation in agricultural products [
18,
19]. This study offers new insights into the effects of NPs on poultry intestinal health and gut microbiota. We provide compelling evidence that NPs cause gut microbiota dysbiosis, impair metabolic function, and reduce egg quality. These findings expand research on plastic particle toxicity from aquatic systems to terrestrial food-producing animals and have important implications for animal health and food safety.
SEM and FTIR analyses confirmed the successful synthesis of monodisperse NPs with uniform spherical shape (100 nm) and characteristic polystyrene chemical bonds. The uniform dispersion and clear edges of the NPs suggest minimal aggregation, which is vital for assessing their biological interactions. We therefore sought to determine the in vivo distribution and toxicological consequences of these NPs within the intestinal tract. Increasing evidence has suggested that MPs can accumulate in the gut and cause intestinal damage. For example, MPs caused oxidative damage and inflammation in the gut, and destroyed all kinds of intestinal barriers, including physical barriers, chemical barriers, microbiological barriers, and immune barriers [
20]. Our findings demonstrate that 100 nm NPs preferentially accumulate in intestinal tissues, disrupt villus architecture, and decrease the number of mucin-producing goblet cells. However, previous studies have mainly focused on the hazardous effect of NPs on the healthy intestine, while the responses of the intestine to NP upon certain kinds of stress may differ from those of healthy intestines [
18,
21]. This physical damage to the intestinal tract is expected to significantly alter the gut microbiome, thereby impacting the resident microbial community.
The gut microbiota is a complex and dynamic microecosystem that is essential for food metabolism, nutrient absorption, and maintaining intestinal barrier integrity. The natural stability of the gut microbiota results from interactions and plasticity within the microbial community [
22]. However, certain factors, especially MPs, can disrupt the intestinal environment and impair the survival of the microbiota [
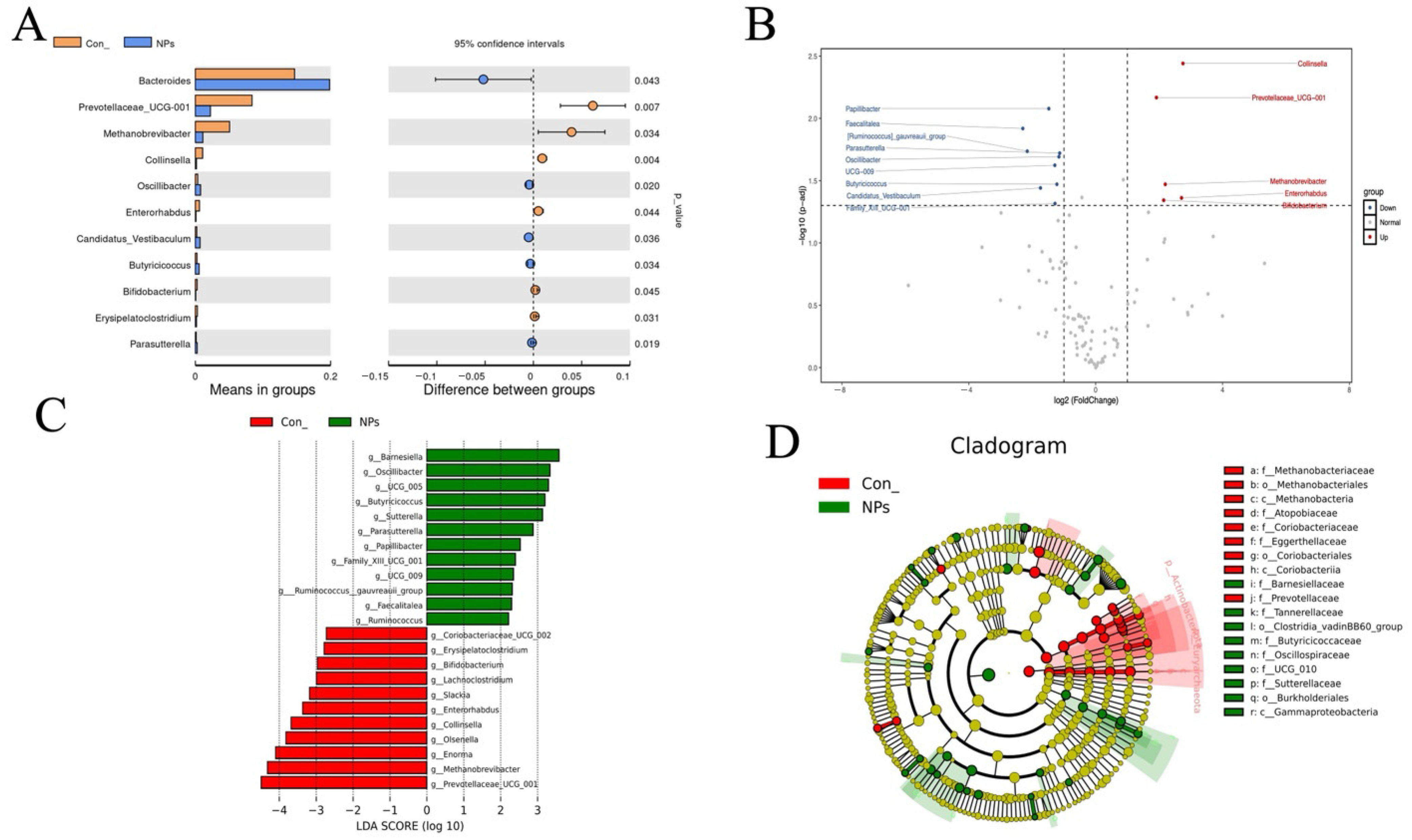
23]. The total OTU count corresponds to microbial species richness, while the Shannon and Simpson indices characterize microbial diversity within samples. The Chao1 index reflects community richness by estimating total species abundance. Our 16S rRNA sequencing results demonstrate that NPs exposure significantly alters the cecal microbiota composition in chickens, consistent with previous reports of microbial community disruption following MPs exposure [
24,
25]. The observed reduction in shared feature sequences (30.7%) between the Con and NPs groups provides direct evidence for NPs-induced microbial restructuring, supporting findings by Deng et al. regarding MP-associated dysbiosis [
24]. These microbial alterations may have functional consequences, as maintenance of gut microbial homeostasis is known to be critical for proper intestinal function, including nutrient absorption and barrier maintenance [
26,
27]. The observed microbial shifts could therefore contribute to increased intestinal permeability and metabolic disturbances, though further research is needed to establish direct causal relationships in avian models. Meanwhile, this may also be one of the reasons for the decreasing growth performance of chickens during exposure to NPs. In addition, we observed significant changes in the major components of the gut microbiota between the groups. These results demonstrate that gut microbial homeostasis is strongly influenced by NPs.
Having established these broad changes in microbial community structure, we then used network analysis to identify the specific keystone taxa and interactions most affected by NPs exposure. Network analysis showing Bacteroides and
Faecalibacterium as high-connectivity hubs is particularly noteworthy, as these taxa are known to play essential roles in maintaining microbial community stability and host metabolic health [
28]. The preservation of
Faecalibacterium abundance despite considerable community restructuring may indicate a compensatory mechanism to sustain butyrate production, which is vital for colonocyte health and anti-inflammatory effects. However, the significant reduction in other beneficial taxa such as
Butyricicoccus and
Ruminococcus suggests a potential impairment of microbial functions related to fiber fermentation and short-chain fatty acid production. The identification of 17 significant microbial biomarkers through LEfSe analysis provides strong evidence for the selective pressure exerted by NPs on gut microbiota composition. These findings support the idea that MPs exposure not only directly impacts microbial abundance but also disrupts the complex network of microbial interactions responsible for maintaining gut homeostasis.
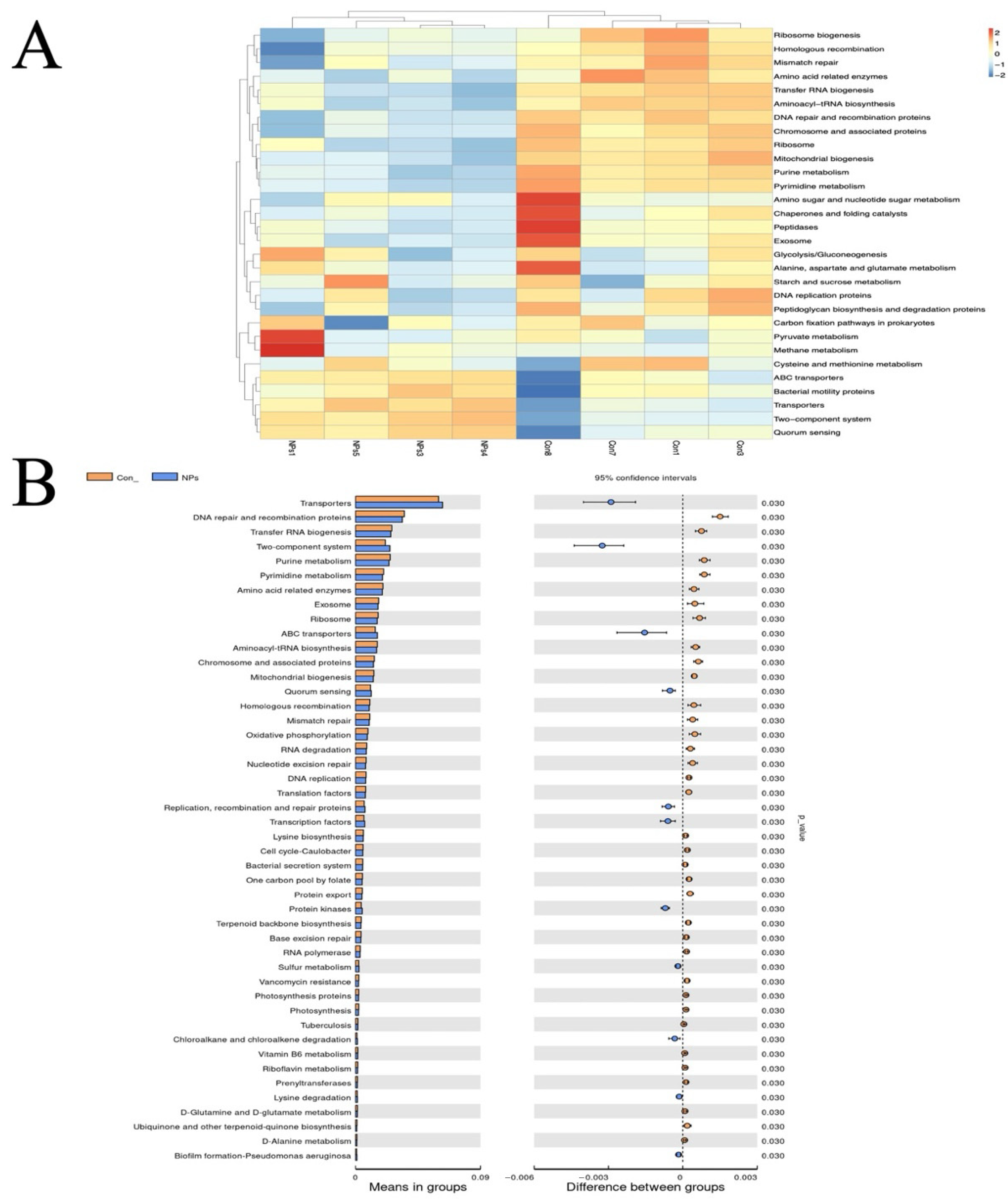
To determine the functional implications of this disrupted microbial network, we performed functional prediction based on 16S rRNA gene sequencing data using the Tax4Fun tool [
29]. The observed alterations in predicted metabolic pathways may be functionally linked to the specific taxonomic shifts reported previously. Notably, the predicted impairments in carbohydrate and amino acid metabolism pathways could be partially attributed to the reduction in
Prevotellaceae_UCG-001, a taxonomic group recognized for its involvement in polysaccharide degradation. The concurrent predicted downregulation of ABC transporters is also consistent with a potential decrease in nutrient absorption capacity [
30]. Meanwhile, the predicted upregulation of DNA repair pathways and two-component systems suggests an activated microbial stress response, which appears to correlate with the enrichment of
Bacteroides, a genus often observed to exhibit resilience under stress conditions [
31,
32]. Furthermore, the predicted differential abundance of genes related to ribosomal proteins and tRNA biogenesis indicates a potential restructuring of microbial protein synthesis, which might contribute to the observed community-wide taxonomic changes. These metabolic disruptions likely contribute to the compromised intestinal barrier function and reduced nutrient utilization efficiency observed in NPs-exposed poultry [
33], highlighting the need for further investigation into targeted interventions to maintain microbial homeostasis in agricultural systems.
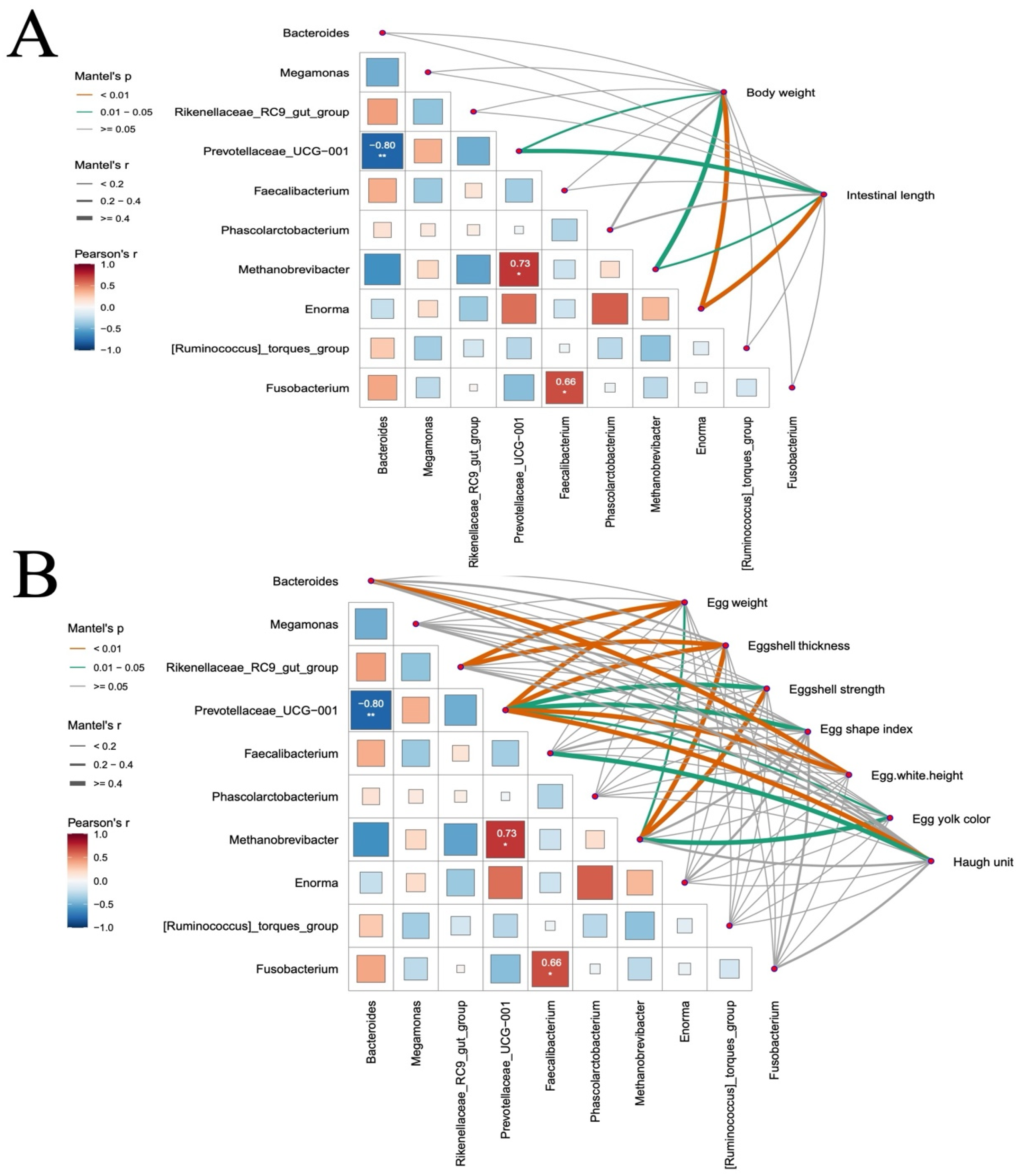
To directly link these predicted functional impairments to the observed decline in egg quality, we performed correlation analysis between microbial taxa and egg quality parameters. Our results identify the decline of
Prevotellaceae_UCG-001, a bacterial genus whose abundance correlated positively with all egg quality metrics, as a key mediator in the pathway through which NPs exposure compromises egg quality. This genus represents primary degraders of dietary fiber, which are responsible for generating short-chain fatty acids (SCFAs) through fermentation. In laying hens, SCFAs serve as essential energy substrates and provide critical carbon skeletons for vitellogenesis (yolk formation) and eggshell biomineralization [
34]. Furthermore, the increased abundance of
Bacteroides and its positive correlation with albumen height may indicate a metabolic shift towards enhanced utilization of intestinal mucins or dietary proteins [
35]. Additionally, the strong positive correlations between
Methanobrevibacter and eggshell quality metrics suggest that NPs may disrupt microbial cross-feeding interactions, particularly hydrogen transfer, thereby impairing overall metabolic efficiency [
36]. Collectively, these findings indicate that NPs induce a comprehensive decline in egg quality by disrupting a core functional network of gut microbiota that is essential for host nutrient utilization.
Collectively, our findings underscore the pivotal role of gut microbiota in poultry health and production. Accumulating evidence implies the intestinal microbiota of laying hens is a potential mediator to improve prevalent issues in terms of egg quality decline in the late phase of laying production [
33]. Our results demonstrate that NPs exposure initiates a pathological cascade from intestinal damage to systemic productivity loss in chickens. Gut barrier disruption and microbial dysbiosis directly drive multifaceted egg quality impairment: Eggshell integrity deterioration aligns with suppressed
Methanobrevibacter abundance, as evidenced by positive correlations with strength metrics. Yolk/albumen quality decline corresponds to
Faecalibacterium depletion and
Bacteroides enrichment. These findings establish gut microbiota as a pivotal regulator of NPs-induced productivity loss, providing a foundation for microbial homeostasis-targeted interventions.
In conclusion, this study provides clear evidence that NPs induce intestinal damage and microbial dysbiosis in broilers, characterized by reduced Bacteroides abundance and compromised egg quality. Bifidobacterium depletion correlates with impaired nutrient absorption efficiency, while elevated Escherichia-Shigella abundance associates with proinflammatory responses and growth reduction. These alterations establish NPs-induced gut microbiota disruption as a primary driver of egg quality decline and systemic productivity loss in poultry systems.