Simple Summary
A one-year study of Odontobutis potamophila in Nansi Lake found that this species matures at one year and around 73.6 mm, spawns primarily from March to June (peaking in May), and depends on individuals aged two years and older for more than 80% of total egg production. To promote sustainable populations, we recommend implementing a seasonal fishing closure from March to June, establishing a minimum catch size of 80 mm, and utilizing peak GSI values in May to optimize hatchery operations. These measures aid in conserving this ecologically significant benthic fish during crucial reproductive phases.
Abstract
Odontobutis potamophila, a small benthic carnivorous fish endemic to the Yangtze River basin, holds considerable ecological and commercial value. However, overfishing and habitat degradation have led to a severe decline in its wild population. A lack of quantitative reproductive data has further hampered effective conservation and resource management. To address this, we conducted monthly sampling, collecting a total of 894 individuals from Nansi Lake between August 2017 and July 2018. By integrating gonadal histological staging, gonadosomatic index (GSI) analysis, logistic regression, and fecundity assessments, we provide a foundational understanding of the species’ reproductive biology. The annual sex ratio was 1.06:1, with a temporary female bias in April (2.14:1) shifting due to male nest-guarding behavior. Both sexes reached maturity at one year and approximately 73.6 mm in length. Spawning occurred from March to June, peaking in May (GSI = 28.92%). Absolute fecundity ranged 2306 ± 1430 eggs and correlated positively with body size and age, while relative fecundity stabilized after age two. Individuals aged two years and older contributed over 80% of total egg production, reflecting a strategy of early maturation with high reproductive output at older ages. This study aims to systematically understand the reproductive biology of O. potamophila. These results support science-based measures such as Covering the entire window from gonadal maturation to fry dispersal, an annual fish ban established from March to June, a minimum catch size of 80 mm, and improved broodstock management for aquaculture and conservation efforts aimed at this and related benthic fishes in shallow lake ecosystems.
1. Introduction
Reproductive biology is fundamental to understanding fish life history, linking individual physiology with population level strategies. It plays a critical role in determining a population’s resilience and regenerative capacity under environmental stress [1,2]. Imbalances in sex ratios, impaired gonadal development, delayed maturation, and reduced reproductive output can interact with density-dependent mechanisms, potentially leading to population instability or collapse [3,4,5,6,7,8]. External factors such as temperature, nutrition, hydrology, and fishing pressure regulate these traits through neuroendocrine pathways, energy allocation trade-offs, and behavioral adaptations [6,9,10,11,12,13]. For economically significant species, reproductive parameters directly influence artificial breeding outcomes, including induction, fertilization, and seedling survival rates, as well as the economic efficiency and genetic gains in selective breeding programs [14,15,16]. Insufficient reproductive data often perpetuates a cycle of overfishing, population decline, and unsuccessful restocking, exacerbated by suboptimal practices in spawning timing, sex ratios, or nutrition [17,18]. Genetic diversity alone cannot break this cycle; only by filling the gaps in reproductive biology data can precise conservation strategies be developed. Thus, detailed investigation of species-specific reproductive traits is crucial for deciphering adaptive responses to environmental change and for scientifically guiding population management, conservation efforts, and aquaculture development.
Nansi Lake, a key storage lake in Shandong Province for the South-to-North Water Diversion Project’s East Route, spans approximately 1266 km2 and represents a typical shallow lake ecosystem in northern China [19,20]. The lake’s pronounced annual water-level variations promote the development of diverse habitats, including shallow shoals and wetlands. These structurally complex microenvironments support abundant benthic, planktonic, and macrophyte communities, which in turn sustain a rich fish assemblage and contribute to stable fishery yields [21,22].
The dark sleeper, Odontobutis potamophila is widely distributed in the slow-flowing or shallow waters of the eastern Chinese freshwater systems [23,24]. This benthic ambush predator feeds mainly on crustaceans, small fish, and aquatic insects. It demonstrates strong physiological adaptations, such as broad temperature tolerance, resilience to low-oxygen conditions, and rapid growth, contributing to its high economic value and market appeal [24,25,26,27,28,29,30]. These characteristics make it a promising species for aquaculture development [25,26]. Although previous studies have focused on genetic diversity, chromosome karyotypes, and early developmental morphology in certain regional populations [24,27,28,29,30], the reproductive biology of this species, especially in shallow lake habitats, remains poorly understood [23,25]. Key reproductive parameters such as sex ratio, annual gonadal development, size at first maturity, and fecundity have not been quantitatively studied. The absence of these critical data hinders the optimization of artificial breeding, particularly in determining induced-spawning timing and broodstock ratios, thereby limiting large-scale seed production and conservation efforts.
Based on year-round monthly surveys in Nansi Lake, this study integrated histological staging, gonadosomatic index dynamics, and logistic maturation modeling to systematically quantify sex ratio, gonadal development, size at first maturity, and reproductive capacity of O. potamophila. The results confirm a shallow-water adaptation strategy characterized by small body size, early maturation, and high reproductive investment, elucidating the species’ reproductive tactics and energy allocation mechanisms in shallow lake ecosystems. The findings provide directly applicable biological benchmarks for establishing fish ban periods, setting harvestable size limits, and implementing broodstock management.
2. Materials and Methods
2.1. Study Area
Nansi Lake (116°34′–117°21′ E, 34°27′–35°20′ N) consists of four interconnected sub-lakes: Nanyang, Dushan, Zhaoyang, and Weishan. The lake has a mean depth of approximately 1.5 m, though areas south of Weishan Island reach around 3 m. Oriented north–south, the lake is divided by a dam structure into two main sections: the upper lake, which is 67 km long with an area of 602 km2, and the lower lake, extending 58 km in length and covering 664 km2 (Figure 1). Sampling was conducted monthly at fixed stations in Nansi Lake Han Zhuang Waters from August 2017 to July 2018, with the exception of February 2018 due to ice cover. All sampling was conducted in situ on the same day without any artificial heating or cooling of the water bodies. Environmental indicators were also maintained in their natural state, accurately reflecting the regulatory effects of natural temperature and environment on gonadal maturation. The fish were captured using benthic trap nets, each measuring 15 m in length and 0.6 m in width with a mesh size of 4 mm. The nets were set in the evening and retrieved after 12 h [31]. The samples were transported on ice to the laboratory for further processing and analysis.
Figure 1.
Study area—Nansi Lake.
2.2. Sample Processing
Each month, the standard length (SL, 0.1 mm) and body weight (BW, 0.01 g) of all collected individuals were measured. Age was determined via otolith analysis, with the study focusing exclusively on reproductively active individuals [23]. Sex was initially identified based on the genital pore, followed by comprehensive dissection and macroscopic examination of the gonads. Developmental stages of the gonads were classified according to external features including color, size, yolk deposition, and degree of spermatid filling [32,33] (Table 1). Sex was determined by the presence of gonads at stage II or later. Supplementary criteria included abdominal protrusion and genital pore morphology during the breeding season. Morphological staging revealed that early-stage gonads are minute; they gradually enlarge as development proceeds. At maturity, ovaries occupy most of the body cavity with eggs visible to the naked eye, whereas testes appear as yellowish-white, densely vascularised bands. After ovulation or spermiation, the gonads rapidly shrink and become flaccid, showing a marked reduction in volume [3,34,35]. The gonads (gonad weight, WG) were weighed, and the empty weight (net weight, WN) was measured after the removal of internal organs and gonads to compute the gonadosomatic index (GSI) [36]. Since we use eviscerated weight for calculations, the GSI values obtained here will be elevated:
Based on the length-age data from January to June, each 10 mm of body length was grouped together, and the SL50% method was applied to calculate the maturity ratio of each group [37]. The initial sexual maturity length of male and female individuals was determined by fitting the Logistic curve equation:
In the formula: Pi is the body length group, SLi is the percentage of gonadal maturity; m is the maturity growth coefficient (The estimated values obtained by fitting the logistic curve equation were 0.09 for males and 0.03 for females); SL50% is the body length at 50% sexual maturity [38].
Ovaries from mature females (gonadal stages IV and V) were used to estimate absolute fecundity and relative fecundity. After confirming that the left and right ovaries were similar in size, approximately 0.3 g of oocytes were sub-sampled from the anterior, middle, and posterior sections of the right ovary. The total number of oocytes (n) in the sub-sample was counted under a dissecting microscope with visible light inspection (Olympus, SZX16, manufactured by Olympus Corporation, Tokyo, Japan, 2× magnification). The oocytes were then thoroughly mixed, weighed (oocyte weight, Wo), and preserved in 5% formaldehyde. Absolute fecundity (AF) was calculated using the following formula [36]:
In the formula: n is the number of eggs in sub-sample.
Relative fecundity (RF) was derived as

Table 1.
Macroscopic characteristics of gonadal developmental stages of Odontobutis potamophila.
Table 1.
Macroscopic characteristics of gonadal developmental stages of Odontobutis potamophila.
| Stages | Female | Male |
|---|---|---|
| I | Semi-transparent ribbon-like, extremely small, closely attached to both sides of the abdominal membrane on the back, male and female cannot be distinguished. | |
| II | Strip-shaped, slightly enlarged compared to stage I, but with no significant changes. | Linear or thin ribbon-like, more enlarged than stage I, with inconspicuous blood vessels. |
| III | Sac-shaped, with small white particles visible inside, slightly yellowish in color. | Strip-like, pale yellow, with obvious blood vessels. |
| IV | Oocytes clearly visible, yellowish-white in color, with thick, white ovarian walls. | Flat strip-like, with almost equal width at both ends, slightly yellowish. |
| V | Ovarian walls very thin and soft, with evidently enlarged oocytes, yellowish-white in color. Light pressure on the abdomen can release free oocytes [39]. | Flat ribbon-like, with very obvious crisscrossing blood vessels, Upon slight pressure applied to the anterior cloacal region, milky-white semen can be expelled [40]. |
| VI | The ovaries show reduced volume and softened tissue, with visible capillary congestion. | The testicles appear flaccid, with a noticeable reduction in volume. |
2.3. Data Processing and Analysis
The χ2-test was employed to assess whether the observed sex ratio of O. potamophila deviated significantly from the theoretical 1:1 ratio. One-way ANOVA was applied to examine differences in body length and weight between sexes, as well as monthly variations in the gonadosomatic index (GSI) of females. The relationships between absolute fecundity and both body length and weight were analyzed using linear regression. Data are expressed as mean ± standard deviation (mean ± SD). All datasets were tested for normality, and non-parametric alternatives were employed when heterogeneous variance was observed. However, since the raw data for body weight in both genders did not satisfy the assumption of normal distribution, and remained non-normally distributed even after log transformation, nonparametric tests were selected. Analyses and graphing were performed with SPSS 19.0 and Origin Pro 8.0, respectively, with significance set at p < 0.05.
3. Results
3.1. Sex Ratio, Body Length, and Body Weight
A total of 894 O. potamophila specimens were collected, comprising 433 females, 408 males, and 53 individuals whose sex could not be determined, yielding a female:male ratio of 1.06:1. The χ2-test indicated no significant deviation from the expected 1:1 ratio (p > 0.05). However, monthly analysis revealed a significant higher proportion of females in April (sex ratio = 2.14, p < 0.05), while no significant differences were observed in other months (Table 2).

Table 2.
Monthly ratio of female to male (F:M) for Odontobutis potamophila collected from Nansi Lake from August 2017 to July 2018.
A total of 644 individuals were dissected, consisting 294 males, 297 females, and 53 individuals of undetermined sex. Several fish of unidentified sex were primarily juveniles; since their gonads were in stage II or earlier at the time of dissection, sex could not be determined. Specimens with body lengths of 80–90 mm and body weights of 10–20 g were most abundant, while as larger individuals were relatively uncommon (Figure 2). Owing to significant departures from normality (Kolmogorov–Smirnov test, p < 0.05), sexual differences in body size were evaluated with a Mann–Whitney U test. Males averaged 91.03 ± 14.97 mm in length and 23.72 ± 15.60 g in mass, whereas females averaged 92.21 ± 17.73 mm and 21.51 ± 11.28 g, respectively; neither length (p = 0.926) nor weight (p = 0.316) differed significantly between sexes. To rigorously examine sexual dimorphism, comparisons should be made between male and female individuals within the same age group. Detailed comparisons can be conducted after obtaining age data through otolith identification [23].
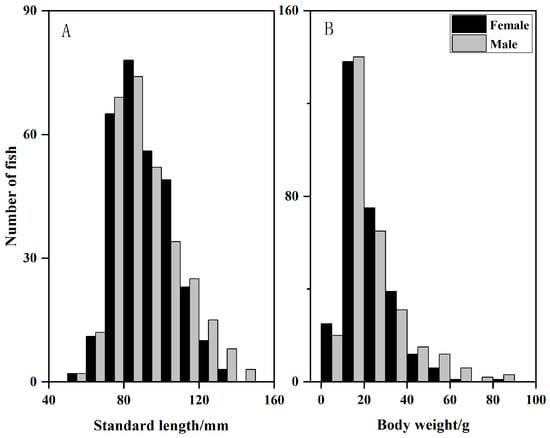
Figure 2.
Frequency distributions of standard length (A) and body weight (B) for Odontobutis potamophila sampled from Nansi Lake.
3.2. Sexual Development and Breeding Season
Through visual observation and based on their gonadal development characteristics, it can be determined that the gonadal development of O. potamophila exhibits seasonal fluctuations, with females serving as representative examples. From August to September, ovaries are predominantly in stage II; from October to December, stage III gradually increases; and by January of the following year, stage IV gonads are observed. Between March and June, stages IV, V, and VI constitute the majority, while a small proportion of ovaries remain at stage VI as late as July. Males display a similar developmental pattern, indicating synchronous reproductive cyclical between sexes (Figure 3). Concurrently, annual monitoring of the female gonadosomatic index (GSI) revealed values ranging from 0.03% to 28.92%. The GSI increased gradually from August to December, followed by a rapid linear rise beginning in March, peaking in May, and declining sharply to its lowest point after spawning in June and July. These findings indicate that the breeding season of O. potamophila in Nansi Lake spans from March to June, with reproductive activity reaching its peak in May (Figure 4).
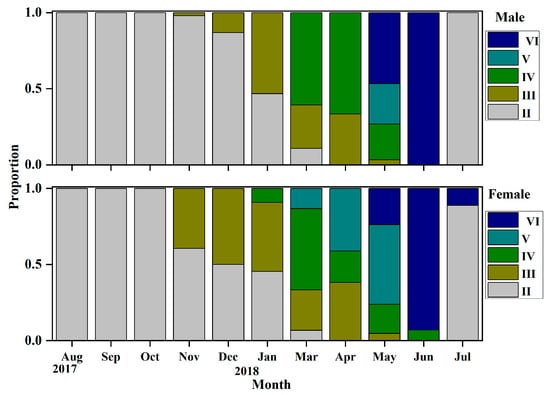
Figure 3.
Monthly gonadal maturity stages of male and female Odontobutis potamophila in Nansi Lake (August 2017—July 2018).
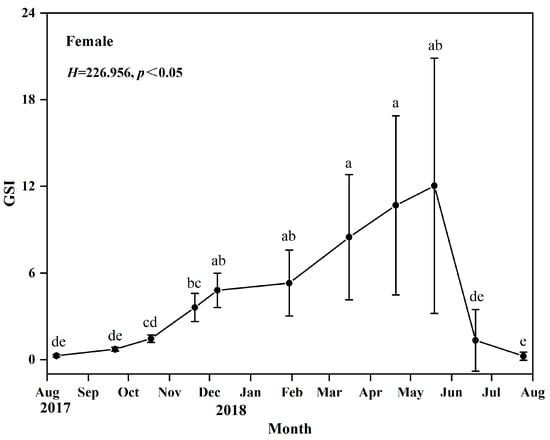
Figure 4.
Monthly variation in the gonadosomatic index (GSI) of female Odontobutis potamophila in Nansi Lake (August 2017—July 2018) (different letters mean that there existed a significant difference).
3.3. Initial Sexual Maturity Body Length
Based on the relationship between the sex ratio of sexually mature individuals and body length in O. potamophila, Logistic curve equations were fitted to estimate the initial sexual maturity body lengths. For females, the equation was (R2 = 0.47, n = 136) and for males, (R2 = 0.89, n = 126). The initial sexual maturity body lengths were determined to be 73.59 mm for females and 73.66 mm for males (Figure 5).
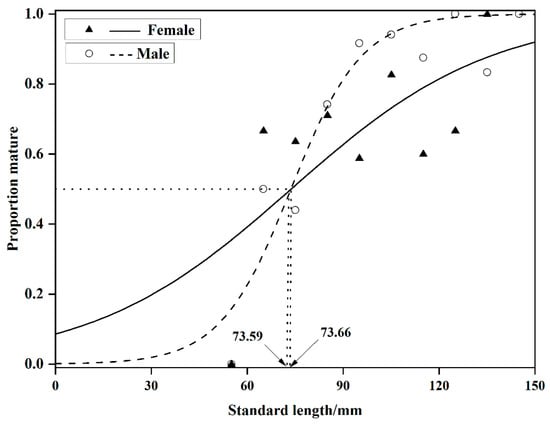
Figure 5.
Proportion of mature Odontobutis potamophila by 10 mm size intervals in Nansi Lake (August 2017—July 2018).
3.4. Fecundity
A total of 59 individuals were used to assess reproductive capacity, with body lengths ranging from 66.08 to 135.96 mm and weights from 8.40 to 85.32 g. The absolute fecundity (AF) of O. potamophila ranged from 643 to 8904 eggs, with a mean (±SD) of 2306 ± 1430 eggs. Relative fecundity (RF) varied from 31.84 to 186.36 eggs/g, averaging 91.20 ± 40.09 eggs/g. Analysis across age groups showed that 1-year-old individuals had a mean AF of 1819 ± 839 eggs and a mean RF of 94.87 ± 43.10 eggs/g. Among 2-year-old individuals, mean AF was 3176 ± 1319 eggs and mean RF was 81.89 ± 31.92 eggs/g. Three-year-old individuals exhibited a mean AF of 6006 ± 4097 eggs and a mean RF of 84.07 ± 28.69 eggs/g.
The relationships between absolute fecundity (AF) and body length (SL) or body weight (BW) were described by the following equations:
Absolute fecundity increased with standard length and body weight, showing progressively greater gains from age-1 to age-3 (Figure 6).
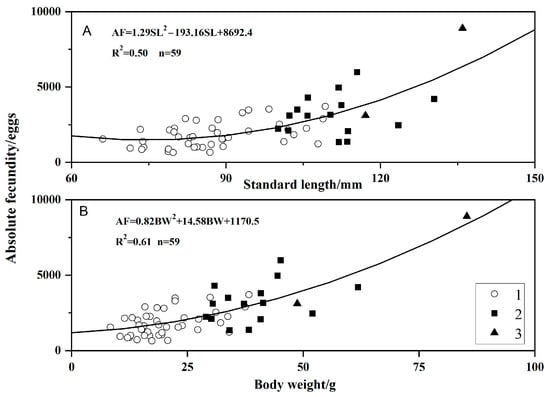
Figure 6.
Relationships of absolute fecundity to standard length (A) and body weight (B) by age class in Odontobutis potamophila from Nansi Lake (August 2017–July 2018) (open circle, age 1; black squares, age 2; black triangles, age 3).
4. Discussion
4.1. Sex Ratio and Gonadal Coordination Pattern: Reproductive Characteristics Driven by Male Nest-Guarding Behavior
The annual sex ratio of O. potamophila in Nansi Lake did not deviate significantly from 1:1 (χ2 = 0.372, p > 0.05), which is consistent with the typical pattern observed in successfully reproducing populations of bony fishes [41,42]. However, notable temporal fluctuations occurred during the breeding season. In April, the female:male ratio was significantly elevated (F:M = 2.14, p < 0.05), while in May, the ratio shifted in favor of males (F:M = 0.74). Such variations in sex ratio may arise from multiple factors including differential growth rates, mortality, longevity, seasonal influences, and potential sex reversal [43,44]. In this population, the transient skew is likely linked to male reproductive behavior, specifically nest guarding [45]. By mid-to-late March, males establish nests within vacant clam shells or rock crevices. Females are attracted to these nests through visual and chemical cues, deposit their eggs, and then quickly depart to participate in additional spawning events [45,46]. In contrast, males remain to guard the eggs. This brood-guarding behavior reduces male activity ranges and increases their concealment, thereby decreasing their susceptibility to capture by fishing gear [46]. Meanwhile, females exhibit broader foraging movements to meet the high energetic demands of vitellogenesis, leading to their higher catch rates during the early reproductive period.
This nest-guarding behavior has been shown to enhance egg hatching success and maximize the reproductive investment of females during the breeding season. Gonadal developmental stages and gonadosomatic index (GSI) values revealed a unimodal annual reproductive cycle. From August onward, ovaries were predominantly at Stage II, accompanied by the lowest GSI values of the year. The proportion of Stage III ovaries gradually increased thereafter, with a concurrent gradual rise in GSI. The first appearance of Stage IV ovaries in January of the following year indicated the full initiation of vitellogenesis [47,48,49]. During the subsequent spring (March to June), the proportion of individuals at Stages IV–V increased rapidly, reaching a peak in May. This period coincided with the highest GSI values (exceeding 10%), reflecting final gonadal maturation and concentrated gamete release [50]. By July, most ovaries had regressed to Stage VI, accompanied by a sharp decline in GSI, marking the end of the annual reproductive cycle. Increasing day length in spring has been shown to elevate metabolic rates and stimulate gonadal endocrine activity, thereby supplying the energy and hormonal support necessary for final oocyte maturation [51,52]. Conversely, declining water temperatures in autumn suppress the pituitary–gonadal axis, leading to the cessation of reproductive activity [53,54]. The synchronous peak in GSI and the high prevalence of Stages IV–V gonads provide independent physiological validation of the breeding period identified by histological staging [55]. This alignment further underscores the spatiotemporal coordination between male nest-guarding behavior and female reproductive investment. Therefore, the current fishing moratorium from March to June, coupled with targeted protection of nest-guarding males aged two years and older, is expected to enhance female reproductive success and preserve the benefits of male parental care. These measures are likely to significantly support the sustainable recruitment of O. potamophila in Nansi Lake.
4.2. Cooperative Regulation of Sexual Maturity Threshold and Reproductive Output
In this study the initial sexual maturity body lengths for male and female O. potamophila in Nansi Lake were determined to be 73.59 mm and 73.66 mm, respectively, with a difference of less than 0.1 mm. It is widely recognized that body size, rather than age, serves as the primary indicator of sexual maturation in fishes [56]. Despite reaching maturity at a similar size threshold, the two sexes exhibit markedly divergent developmental trajectories. Below this size threshold, the proportion of mature females consistently exceeds that of males. Once the threshold is surpassed, however, males undergo rapid gonadal development, achieving maturation over a significantly shorter cycle compared to females (Figure 5). Histological observations reveal that females initiate maturation earlier, with a high prevalence of Stage III ovaries beginning in November, and some individuals retaining Stage VI features as late as July the following year. In contrast, males predominantly remain at Stage II from July to October, but accelerate rapidly into Stages III–IV after November (Figure 3). This “female-initiated, male-accelerated” maturation strategy minimizes inter-sexual timing differences in gonad development, thereby facilitating synchronized spawning and enhancing reproductive efficiency.
The absolute fecundity (AF) of O. potamophila in Nansi Lake ranged from 643 to 8904 eggs, with a mean value of 2306 ± 1430 eggs. Relative fecundity (RF) varied from 31.84 to 186.36 eggs/g, averaging 91.20 ± 40.09 eggs/g. Compared to the Taihu Lake population (RF = 87 eggs/g), the RF in Nansi Lake is slightly higher, yet it remains lower than that of the Jiangxi River system (RF = 121 eggs/g). Notably, the AF in Nansi Lake is the lowest among the three regions, suggesting that intraspecific variation in reproductive output is influenced by regional differences in prey availability and environmental conditions [23,24,25,26]. Furthermore, the AF of the wild population in Nansi Lake was significantly higher than that reported for eco-friendly aquaculture populations (2 years old: 2570 ± 943 eggs; 1 year old: 1157 ± 666 eggs) [57]. This disparity implies that dietary diversity and nutritional quality directly affect energy allocation to gonad development [58]. Natural selection factors such as predation pressure, disease prevalence, and resource competition may drive wild populations to adopt a strategy of high reproductive investment to compensate for adult mortality under environmental stress, thereby maintaining population replenishment over evolutionary time [59,60,61].
The age-dependent pattern reveals a clear energy allocation trade-off, characterized by an exponential increase in absolute fecundity with age: 1819 ± 839 eggs in 1-year-old individuals, 3176 ± 1319 eggs in 2-year-olds, and 6006 ± 4097 eggs in 3-year-olds. Linear modeling indicated a strong positive correlation between absolute fecundity and both body weight (R2 = 0.61) and body length (R2 = 0.50, p < 0.01), confirming that energy reserves are a primary factor driving increased egg production. Relative fecundity rose rapidly between ages 1 and 2, but stabilized and slightly declined thereafter, suggesting that older individuals prioritize metabolic maintenance over further increasing reproductive output per unit weight. This pattern aligns with the principle of “isometric growth–allometric reproduction” [62,63]. Therefore, implementing selective fishing regulations that protect mature female O. potamophila is expected to enhance overall reproductive efficiency and contribute to population stability in Nansi Lake.
4.3. Reproduction Parameter-Oriented Resource Management Pathways and Future Prospects
O. potamophila exhibits a reproductive strategy characterized by early maturation at a small body size and high reproductive output, with notable sexual dimorphism in developmental timing. Vitellogenesis in females commences prior to reaching 73.59 mm standard length (SL), and individuals aged two years or older account for more than 80% of the total egg production. Absolute fecundity increased exponentially with body weight, indicating a life history tactic that combines early maturation with sustained high reproductive investment in later life stages, a key adaptation for population sustainability. Ecologically, this strategy maximizes reproductive output while minimizing structural size costs [61]. Management efforts should therefore prioritize three key interventions: a seasonal fishing ban during critical reproductive periods, enforcement of a minimum catch size to protect immature individuals, and protection of feeding habitats to ensure adequate nutrition for broodstock.
The fishing ban should be implemented throughout the entire period from gonadal maturation to fry dispersal, specifically from March to June annually. This interval corresponds to the critical window during which the average daily water temperature in the lake increases from 18 °C to 26 °C. Concurrently, the current minimum catch size should be raised from 60 mm to 80 mm SL, which corresponds to the average body length of two-year-old females, thereby preserving highly reproductive individuals. Upgrading fishing gear to enforce this regulation is both practical and feasible [64]. In artificial breeding programs, the peak gonadosomatic index (GSI) in May can serve as a reference for scheduling spawning batches. Water temperatures of 22–26 °C are recommended for induction, and broodstock should receive enhanced nutrition, including feed with ≥45% protein and high levels of highly unsaturated fatty acids (HUFA), starting 60 days prior to the spawning period [49]. Looking forward, integrating reproductive parameters with environmental monitoring data to develop population dynamic models will enable annual resource forecasts and support precision quota management. Such an approach will provide scientific and managerial foundations for the sustainable utilization of O. potamophila. Moreover, advance prediction of annual catch potential and optimization of quota allocation can further quantify this species’ supply potential and economic value in the edible fish market.
Nevertheless, several uncertainties remain to be addressed. It is unclear how rising water temperatures may reduce the reproductive window, or how male nesting behavior responds to fishing pressure, and the impact of food availability on the spawning period of this species as a shallow-water benthic predator. Long-term data are needed to assess these impacts. Future work should develop integrated models combining climate, reproduction, food supply, and fishing factors to evaluate population resilience under different management strategies. Such approaches will improve resource recovery efforts for O. potamophila.
5. Conclusions
Based on a study of 894 O. potamophila from Nansi Lake (2017–2018), this research reveals key reproductive traits. The sex ratio averaged 1.06:1, but peaked at 2.14:1 (female:male) in April. Both sexes matured at one year and approximately 73.6 mm standard length. Spawning occurred from March to June, peaking in May when the gonadosomatic index reached 28.92%. Fecundity ranged from 643 to 8904 eggs and increased with size and age. Fish aged two years or older produced over 80% of all eggs, although relative fecundity stabilized after age two, indicating a shift toward growth rather than reproduction in older individuals. These findings support the “early maturation and high productivity” reproductive strategy of O. potamophila. The results provide a scientific basis for setting fishing closures, adjusting size limits, and improving broodstock management to aid conservation of this and similar benthic species. However, the model does not incorporate the potential negative effects of warming rates and extreme hydrological events on egg hatching rates. Future research should validate the robustness of management assumptions under global warming scenarios using long-term data.
Author Contributions
Conceptualization, M.X. and S.Z.; methodology, S.Z. and B.L.; software, M.X. and L.L.; validation, M.W., J.W. and R.L.; formal analysis, M.X. and S.Z.; investigation, M.X.; resources, L.Z.; data curation, S.Z.; writing—original draft preparation, M.X. and S.Z.; writing—review and editing, M.W., J.W. and L.Z.; visualization, S.Z.; supervision, L.Z.; project administration, L.Z.; funding acquisition, L.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Technology Project of Hubei Province (grant number 2022BBA009), the earmarked fund for China Agriculture Research System (CARS-46), the Project of Gehu Lake Fisheries Administration Committee Office of Jiangsu Province (grant number 2021-0039), the Science and Technology Project of Huai’an City, Jiangsu Province (grant number HAB202377, HAB2024099), Central Public-interest Scientific Institution Basal Research Fund, CAFS (No. 2023TD61) and Central Public-interest Scientific Institution Basal Research Fund (No. YFI202415).
Institutional Review Board Statement
The study was conducted in accordance with the Institutional Animal Care and Use Committee of the Yangtze River Fisheries Research Institute, Chinese Academy of Fishery Sciences (approval No. YFI2024XM01, 15 August 2024).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Appreciate for field sampling assistance provided by Kun Li, Kun Xiao and Haoran Liu.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Labeille, M.; Devaux, A.; Lefrançois, E.; Abbaci, K.; Santos, R.; Besnard, A.; Bony, S.; Lareyre, J.-J.; Teichert, N. Variation in reproductive strategies of two amphidromous gobies of the West Indies: Sicydium plumieri and Sicydium punctatum. Hydrobiologia 2024, 851, 4567–4584. [Google Scholar] [CrossRef]
- Chen, X.; Liu, B.; Lin, D. Sexual maturation, reproductive habits, and fecundity of fish. In Biology of Fishery Resources; Springer Nature: Singapore, 2022; pp. 113–142. [Google Scholar]
- Bezerra, V.M.; Reis, G.P.A.; de Melo, C.L.; Menezes, W.F.; dos Santos, B.D.; Ferreira, M.P.; Pires, D.C.; da Costa, F.F.B.; Ramírez, J.F.P.; Teixeira, J.P.; et al. A long-term high temperature on young Nile tilapia females affects its urogenital papilla morphology and future reproductive performance. Aquaculture 2025, 595, 741666. [Google Scholar] [CrossRef]
- Chen, L.; Wang, S.; Duan, X.; Cao, X.; Wang, S.; Fu, M.; Fan, Y.; Jia, Y.; Du, Q.; Chang, Z. Activation or inhibition of NF-κB from the juvenile stage results in skewed sex ratios in common carp (Cyprinus carpio L.). Aquaculture 2024, 580, 740316. [Google Scholar] [CrossRef]
- Li, P.; Liu, J.; Lu, W.; Sun, S.; Wang, J. Age, growth, reproduction and mortality of Xenocypris argentea (Günther, 1868) in the lower reaches of the Tangwang River, China. PeerJ 2024, 12, e16673. [Google Scholar] [CrossRef]
- Haag, W.R. The role of fecundity and reproductive effort in defining life-history strategies of North American freshwater mussels. Biol. Rev. 2013, 88, 745–766. [Google Scholar] [CrossRef] [PubMed]
- Nash, J.P.; Kime, D.E.; Van der Ven, L.T.M.; Wester, P.W.; Brion, F.; Maack, G.; Stahlschmidt-Allner, P.; Tyler, C.R. Long-Term Exposure to Environmental Concentrations of the Pharmaceutical Ethynylestradiol Causes Reproductive Failure in Fish. Environ. Health Perspect. 2004, 112, 1725–1733. [Google Scholar] [CrossRef]
- Le Bris, A.; Pershing, A.J.; Hernandez, C.M.; Mills, K.E.; Sherwood, G.D. Modelling the effects of variation in reproductive traits on fish population resilience. ICES J. Mar. Sci. 2015, 72, 2590–2599. [Google Scholar] [CrossRef]
- Spaet, J.L.Y.; Berumen, M.L. Fish market surveys indicate unsustainable elasmobranch fisheries in the Saudi Arabian Red Sea. Fish. Res. 2015, 161, 356–364. [Google Scholar] [CrossRef]
- Pérez-Atehortúa, M.; Hernández, A.J.; Risopatrón, J.; Farías, J.; Villalobos, E.F.; Valdebenito, I. Effect of diet composition on maturation rate of female Atlantic salmon (Salmo salar) during gonadal maturation. Aquaculture 2024, 582, 740513. [Google Scholar] [CrossRef]
- Zhou, X.J.; Xie, C.X.; Huo, B.; Duan, Y.J.; Yang, X.; Ma, B.S. Reproductive biology of Schizothorax waltoni (cyprinidae: Schizothoracinae) in the yarlung zangbo river in Tibet, China. Environ. Biol. Fishes 2014, 98, 597–609. [Google Scholar] [CrossRef]
- McKeon, C.M.; Buckley, Y.M.; Moriarty, M.; Lundy, M.; Kelly, R. Increased signal of fishing pressure on community life-history traits at larger spatial scales. Glob. Ecol. Biogeogr. 2024, 33, e13815. [Google Scholar] [CrossRef]
- Chaparro-Pedraza, P.C.; de Roos, A.M. Environmental change effects on life-history traits and population dynamics of anadromous fishes. Anim. Ecol. 2019, 88, 1178–1190. [Google Scholar] [CrossRef]
- Lowerre-Barbieri, S.K.; Brown-Peterson, N.J.; Wyanski, D.M.; Moncrief-Cox, H.E.; Kolmos, K.J.; Menendez, H.S.; Barnett, B.K.; Friess, C. A unified framework and terminology for reproductive traits integral to understanding fish population productivity. Mar. Coastal Fish 2023, 15, e10276. [Google Scholar] [CrossRef]
- Lavin, C.P.; Jones, G.P.; Williamson, D.H.; Harrison, H.B. Minimum size limits and the reproductive value of numerous, young, mature female fish. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2021, 288, 20202714. [Google Scholar] [CrossRef]
- Thomas, L.; Underwood, J.N.; Adam, A.A.S.; Richards, Z.T.; Dugal, L.; Miller, K.J.; Gilmour, J.P. Contrasting patterns of genetic connectivity in brooding and spawning corals across a remote atoll system in northwest Australia. Coral Reefs 2020, 39, 55–60. [Google Scholar] [CrossRef]
- Jin, S.; Jiao, Y.; Li, J.; Xu, Z.; Xu, Y.; Zou, M.; Ding, J.; Li, X.; Wang, Z.; Wang, M.; et al. Length-based stock assessment for Procambarus clarkii aquaculture management in China: An alarming of ongoing recruitment overfishing. Aquaculture 2024, 579, 740182. [Google Scholar] [CrossRef]
- Moon, S.Y.; Baeck, G.W.; Jung, J.H.; Choi, H.; Kim, C.; Koo, M.S.; Park, J.-H. Spatiotemporal distribution and reproductive biology of the brown croaker (Miichthys miiuy) in the southwestern waters of Korea. Front. Mar. Sci. 2024, 11, 1416771. [Google Scholar] [CrossRef]
- Chen, L.; Shi, Y.; Wang, S.; Sun, M.; Wang, M.; Ren, X.; Gao, Z.; Zhou, Y.; Zhang, J.; Zhuang, W.; et al. Temperature and phosphorus: The main environmental factors affecting the seasonal variation of soil bacterial diversity in Nansi Lake Wetland. Front. Microbiol. 2023, 14, 1169444. [Google Scholar] [CrossRef]
- Wang, T. Analysis on the area evolution and causes of Nansi Lake in History. AIP Conf. Proc. 2022, 2474, 020014. [Google Scholar] [CrossRef]
- Divya, K.R.; Zhao, S.; Chen, Y.; Cheng, F.; Zhang, L.; Qin, J.; Arunjith, T.S.; Schmidt, V.B.; Xie, S. A comparison of zooplankton assemblages in Nansi Lake and Hongze Lake, potential influences of the East Route of the South-to-North Water Transfer Project, China. J. Oceanol. Limnol. 2020, 39, 623–636. [Google Scholar] [CrossRef]
- Wang, P.; Wang, C. Numerical Model for Flow Through Submerged Vegetation Regions in a Shallow Lake. J. Hydrodyn. 2011, 23, 170–178. [Google Scholar] [CrossRef]
- Xiang, M.; Liu, H.; Li, B.; Guo, H.; Zhu, C.; Wang, M.; Wang, J.; Xie, S.; Zhang, L. Otolith annulus validation and population dynamics of dark sleeper Odontobutis potamophila: Insights for sustainable fisheries management. Front. Mar. Sci. 2025, 11, 1528582. [Google Scholar] [CrossRef]
- Li, Q.; Wang, X.; Liu, Z.-Z. Isolation and characterization of polymorphic microsatellite markers for the river sleeper (Odontobutis potamophila). Conserv. Genet. Resour. 2014, 7, 251–253. [Google Scholar] [CrossRef]
- Cao, J.F.; Yang, X.F.; Yang, R.B.; Wei, K.J. Length-weight relationships and biological data of Odontobutis sinensis (Wu, Chen & Chong, 2002) from Liangzi Lake, China. Appl. Ichthyol. 2015, 31, 798–799. [Google Scholar]
- Ren, Y.; Xie, D.B.; Li, B.; Liu, Y.R.; Hu, S.D.; Liu, H.Z.; Shi, Y.B.; Zhu, S.H. Influence of water temperature, habitat complexity and light on the predatory performance of the dark sleeper Odontobutis potamophila (Günther, 1861). Freshw. Ecol. 2020, 35, 367–378. [Google Scholar] [CrossRef]
- Jia, Y.; Zheng, J.; Liu, S.; Li, F.; Chi, M.; Cheng, S.; Gu, Z. A chromosome-level genome assembly of the dark sleeper Odontobutis potamophila. Genome Biolo. Evol. 2021, 13, evaa271. [Google Scholar] [CrossRef]
- Zhu, F.; Zhang, L.J.; Yin, S.W.; Zhang, H.W.; Hou, X.-Y.; Hu, Y.L.; Luo, J. Genetic diversity and variation in wild populations of dark sleeper (Odontobutis potamophila) in China inferred with microsatellite markers. Biochem. Syst. Ecol. 2014, 57, 40–47. [Google Scholar] [CrossRef]
- Wang, P.P.; Ren, M.; Chen, S.Q.; Yin, S.W.; Zhao, C.; Zhang, H.Y.; Li, X.R.; Cao, Q.Q.; Zhou, G.Q. Characterization and development of 56 EST-SSR markers derived from the transcriptome of Odontobutis potamophila. Genet. Mol. Res. 2017, 16, gmr16029129. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, H.; Zhang, Y.; Zhu, F.; Hou, X.; Hu, Y.; Yin, S. Development and characterization of 42 novel polymorphic microsatellite markers for Odontobutis potamophila from EST sequences. Conserv. Genet. Resour. 2014, 6, 469–472. [Google Scholar] [CrossRef]
- Guo, Z.Q.; Cucherousset, J.; Lek, S.; Li, Z.; Zhu, F.; Tang, J.; Liu, J. Comparative study of the reproductive biology of two congeneric and introduced goby species: Implications for management strategies. Hydrobiologia 2013, 709, 89–99. [Google Scholar] [CrossRef]
- Nikolsky, G.V. Ecology of Fishes; Academic Press: Cambridge, MA, USA, 1963; p. 404. [Google Scholar]
- Zhao, X.Q. Reproductive System Development and Reproduction Behavior in Odontobutis potamophila (GUnther); East China Normal University: Shanghai, China, 2006. [Google Scholar]
- Zhu, Q.; Han, C.; Liu, S.; Ouyang, H.; Liu, D.; Zhang, Z.; Huang, J.; Han, L.; Li, S.; Li, G.; et al. Development and gene expression analysis of gonad during 17α-methyltestosterone-induced sex reversal in mandarin fish (Siniperca chuatsi). Aquac. Rep. 2022, 23, 101049. [Google Scholar] [CrossRef]
- Cole, K.S. Patterns of reproductive morphology in the genus Gobiodon (Teleostei: Gobiidae). Environ. Biol. Fishes 2011, 92, 323–335. [Google Scholar] [CrossRef]
- Zhang, F.; Song, W.; Yang, R.; Jin, C.; Xie, Y.; Shen, Y.; Gao, X.; Sun, H.; Nie, T.; Yue, X.; et al. Semen promotes oocyte development in Sebastes schlegelii elucidating ovarian development dynamics in live-bearing fish. iScience 2024, 27, 109193. [Google Scholar] [CrossRef]
- Cejko, B.I.; Fopp-Bayat, D.; Kujawa, R. Effect of anti-adhesive supplement doses on sperm motility characteristics in the European whitefish (Coregonus lavaretus). Anim. Reprod. Sci. 2024, 262, 107423. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, P.; Bentes, L.; Coelho, R.; Correia, C.; Gonçalves, J.M.S.; Lino, P.G.; Ribeiro, J.; Erzini, K. Age and growth, mortality, reproduction and relative yield per recruit of the bogue, Boops boops Linné, 1758 (Sparidae), from the Algarve (south of Portugal) longline fishery. J. Appl. Ichthyol. 2006, 22, 345–352. [Google Scholar] [CrossRef]
- Caill-Milly, N.; Sanchez, F.; Benito, D.; Ruiz, P.; Izagirre, U.; Briaudeau, T. Assessment of size at first maturity for Ruditapes philippinarum from Arcachon Bay (French Atlantic coast): New insights for fishery management. Estuar Coast. Shelf Sci. 2023, 285, 108321. [Google Scholar] [CrossRef]
- Zhao, S.S.; Cheng, F.; Hou, G.; Hu, Z.Y.; Xie, S.G. Opportunistic-tended life history traits of Siniperca kneri in the Three Gorges Reservoir, China: Potential responses to impoundment. J. Oceanol. Limnol. 2019, 37, 694–705. [Google Scholar] [CrossRef]
- Freitas, T.M.d.S.; Almeida, V.H.d.C.; Montag, L.F.d.A.; Rocha, R.M.d.; Fontoura, N.F. Seasonal changes in the gonadossomatic index, allometric condition factor and sex ratio of an auchenipterid catfish from eastern Amazonia. Neotrop. Ichthyol. 2011, 9, 839–847. [Google Scholar] [CrossRef]
- Hamazaki, K.; Miura, O. Highly biased sex ratios in the twelve species of the freshwater snail genus Semisulcospira in and around Lake Biwa. J. Mollus. Stud. 2024, 90, eyae011. [Google Scholar] [CrossRef]
- Geffroy, B.; Douhard, M. The adaptive sex in stressful environments. Trends Ecol. Evol. 2019, 34, 628–640. [Google Scholar] [CrossRef] [PubMed]
- Geffroy, B.; Wedekind, C. Effect of global warming on sex ratio in fishes. J. Fish Biol. 2020, 97, 596–606. [Google Scholar] [CrossRef]
- Hellmann, J.K.; Keagy, J.; Carlson, E.R.; Kempfer, S.; Bell, A.M. Predator-induced transgenerational plasticity of parental care behavior in male three-spined stickleback fish across two generations. Proc. R. Soc. Lond. Ser. B Biol. Sci. 2024, 291, 20232582. [Google Scholar]
- Peterson, M.I.; Kitano, S. Spawning season and nest guarding behavior of invasive smallmouth bass (Micropterus dolomieu) in a Japanese Lake. Ecol. Res. 2022, 37, 598–608. [Google Scholar] [CrossRef]
- Butler, J.M.; Maruska, K.P. Noise during mouthbrooding impairs maternal care behaviors and juvenile development and alters brain transcriptomes in the African cichlid fish Astatotilapia burtoni. Genes Brain Behav. 2021, 20, e12692. [Google Scholar] [CrossRef]
- Pham, H.Q.; Le, H.M. Seasonal changes in three indices of gonadal maturation in male golden rabbitfish (Siganus guttatus): Implications for artificial propagation. Fish Physiol. Biochem. 2020, 46, 1111–1120. [Google Scholar] [CrossRef]
- Reading, B.J.; Andersen, L.K.; Ryu, Y.W.; Mushirobira, Y.; Todo, T.; Hiramatsu, N. Oogenesis and Egg Quality in Finfish: Yolk Formation and Other Factors Influencing Female Fertility. Fishes 2018, 3, 45. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Takebe, T.; Nakachi, M.; Kawabata, Y.; Teruya, K.; Soyano, K. Histological description and endocrine regulation of ovarian maturation in wild and captive white-streaked grouper Epinephelus ongus. Aquacult. Rep. 2023, 33, 101865. [Google Scholar] [CrossRef]
- Acharyya, A.; Das, J.; Hasan, K.N. Rhythmicity in testicular melatonin and its correlation with the dynamics of spermatogenic cells in an annual reproductive cycle of Clarias batrachus under natural photo-thermal conditions. Theriogenology 2023, 208, 15–27. [Google Scholar] [CrossRef]
- Vinanthi Rajalakshmi, K.S.; Liu, W.C.; Balamuralikrishnan, B.; Meyyazhagan, A.; Sattanathan, G.; Pappuswamy, M.; Joseph, K.S.; Paari, K.A.; Lee, J.W. Cadmium as an Endocrine Disruptor That Hinders the Reproductive and Developmental Pathways in Freshwater Fish: A Review. Fishes 2023, 8, 589. [Google Scholar] [CrossRef]
- Brown, M.L.; Kasiga, T.; Spengler, D.E.; Clapper, J.A. Reproductive cycle of northern largemouth bass Micropterus salmoides salmoides. J. Exp. Zool. Part A 2019, 331, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Duhamel, G.; Kock, K.H.; Balguerias, E.; Hureau, J.C. Reproduction in fish of the Weddell Sea. Polar Biol. 1993, 13, 193–200. [Google Scholar] [CrossRef]
- Fontoura, N.F.; Ceni, G.; Braun, A.S.; Marques, C.d.S. Defining the reproductive period of freshwater fish species using the Gonadosomatic Index: A proposed protocol applied to ten species of the Patos Lagoon basin. Neotrop. Ichthyol. 2018, 16, e170006. [Google Scholar] [CrossRef]
- Rojo, J.H.; Boy, C.C. Life-history traits in the southernmost landlocked population of the fish Galaxias maculatus. Polar Biol. 2022, 45, 1093–1103. [Google Scholar] [CrossRef]
- Xiang, M.; Li, L.; Xu, H.; Li, B.; Guo, H.; Wang, M.; Wang, J.; Xin, W.; Xie, S.G.; Zhu, X.M.; et al. Imitative Ecological Breeding Technology of Odontobutis potamophlia. Acta Hydrobiol. Sin. 2024, 48, 1385–1392. [Google Scholar]
- Huang, Y.F.; Peng, L.P.; Chen, L.Y. Characteristics of fecundity and oocyte diameter of Hemiculter leucisculus during the spawning season in the lower reaches of the Yuanshui River in Hunan Province. Acta Hydrobiol. Sin. 2021, 45, 97–105. [Google Scholar]
- Dasgupta, P.; Halder, S.; Dari, D.; Nabeel, P.; Vajja, S.S.; Nandy, B. Evolution of a novel female reproductive strategy in Drosophila melanogaster populations subjected to long-term protein restriction. Evolution 2022, 76, 1836–1848. [Google Scholar] [CrossRef]
- Kornis, M.S.; Weidel, B.C.; Vander Zanden, M.J. Divergent life histories of invasive round gobies (Neogobius melanostomus) in lake Michigan and its tributaries. Ecol. Freshw. Fish 2017, 26, 563–574. [Google Scholar] [CrossRef]
- Evans-Powell, R.T.; Hesp, S.A.; Denham, A.; Beckley, L.E. Implications of big, old, fat, fecund, female fish (BOFFFFs) for the reproductive potential of a demersal teleost stock. Fish. Res. 2024, 272, 106934. [Google Scholar] [CrossRef]
- Anderson, D.M.; Gillooly, J.F. Predicting egg size across temperatures in marine teleost fishes. Fish Fish. 2020, 21, 1027–1033. [Google Scholar] [CrossRef]
- Cooper, W.T.; Barbieri, L.R.; Murphy, M.D.; Lowerre-Barbieri, S.K. Assessing stock reproductive potential in species with indeterminate fecundity: Effects of age truncation and size-dependent reproductive timing. Fish. Res. 2013, 138, 31–41. [Google Scholar] [CrossRef]
- Florisson, J.H.; Tweedley, J.R.; Walker, T.H.E.; Chaplin, J.A. Reef vision: A citizen science program for monitoring the fish faunas of artificial reefs. Fish. Res. 2018, 206, 296–308. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).