Thiamine Diphosphate Supplementation as a Heat-Stress Mitigation Strategy for Hair Male and Female Lambs in Feedlot: Physiological Responses, Growth Performance, and Carcass Traits
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site and Experimental Period
2.2. Animals and Pre-Experimental Handling
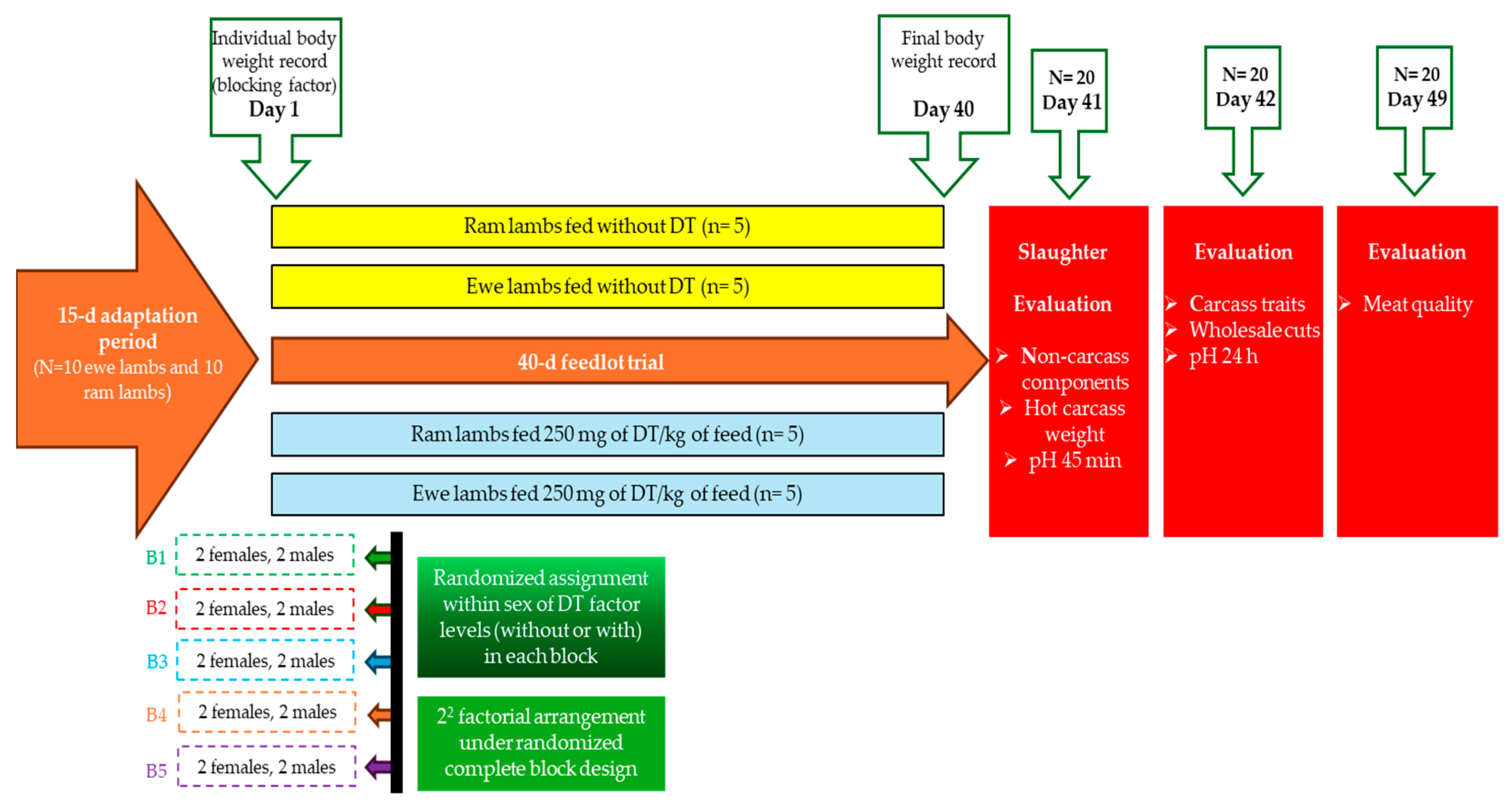
2.3. Experimental Design and Handling
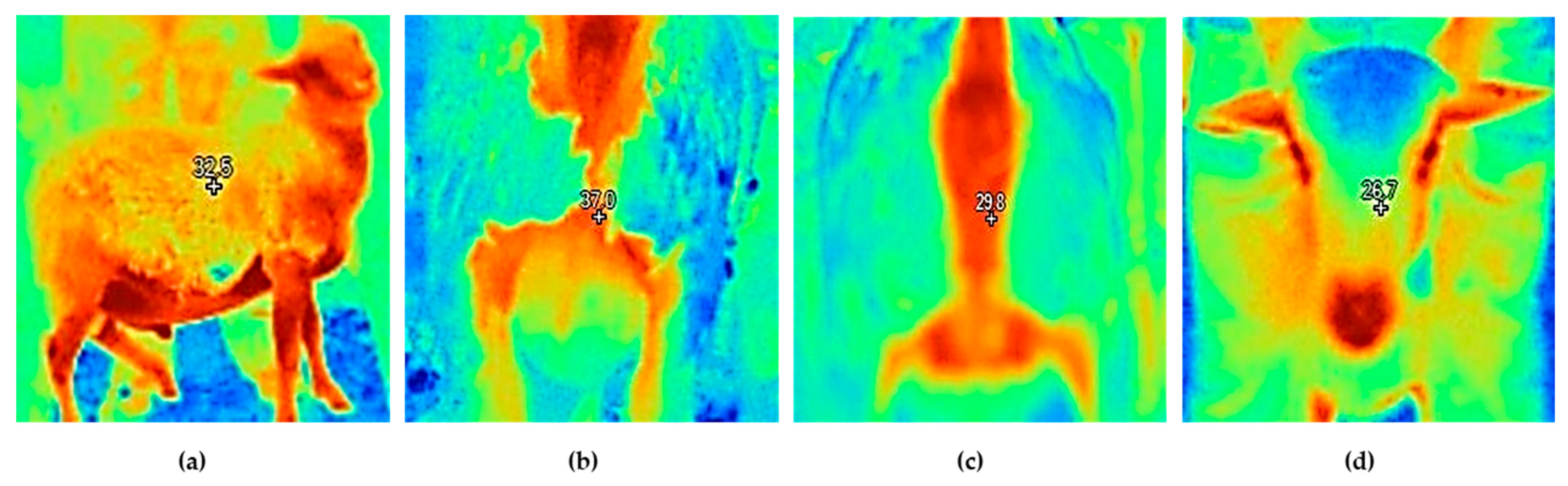
2.4. Climatic and Physiological Variables
2.5. Feedlot Performance
2.6. Carcass Traits, Non-Carcass Components, and Wholesale Cut
2.7. Meat Quality
2.8. Statistical Analysis
3. Results
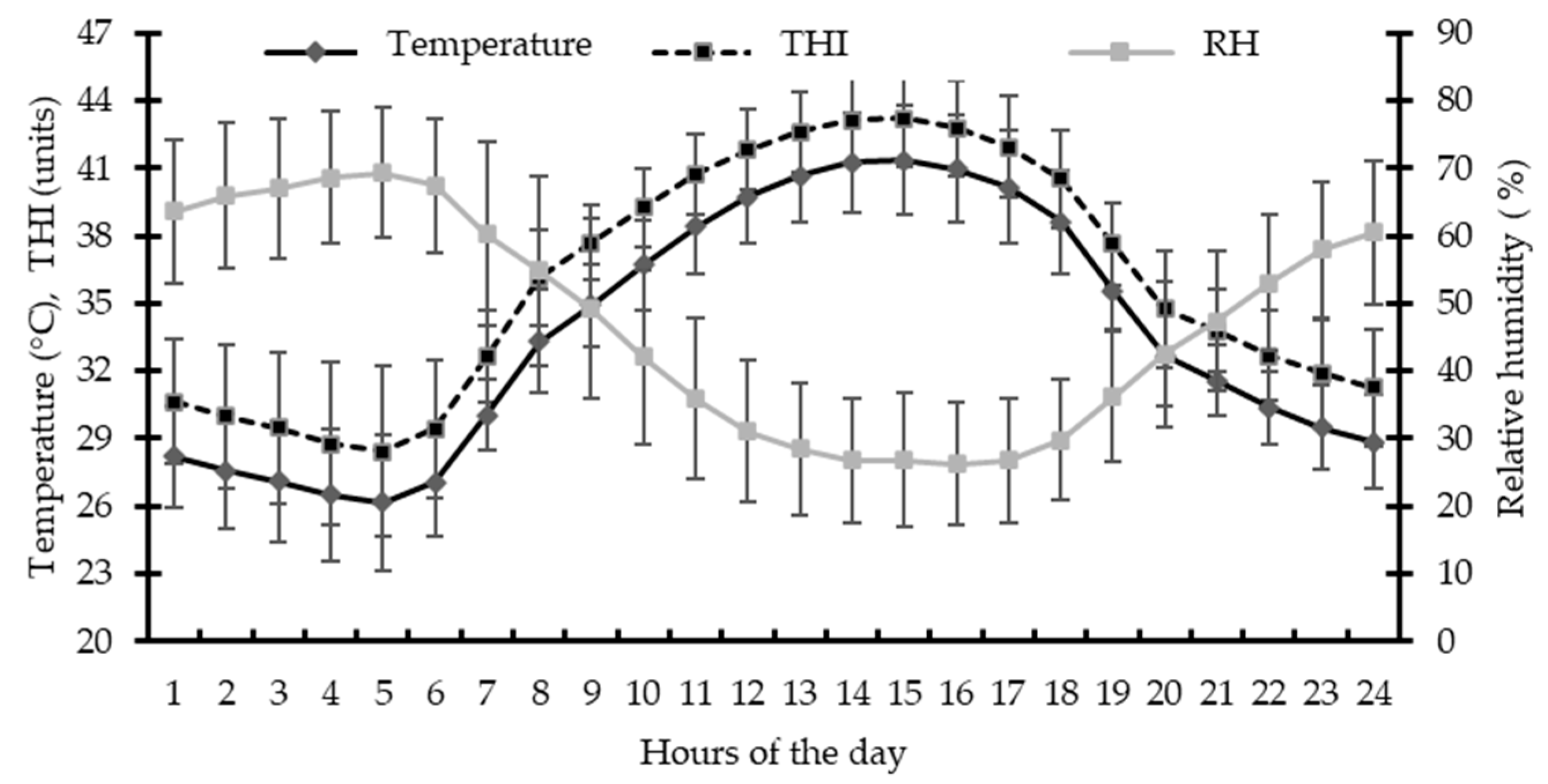
3.1. Environmental Conditions
3.2. Effects of Thiamine Diphosphate
3.3. Effects of Gender
4. Discussion
4.1. Environmental Conditions
4.2. Effects of Thiamine Diphosphate
4.3. Effects of Gender
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vicente Pérez, R.; Macías Cruz, U.; Avendaño Reyes, L.; Correa Calderón, A.; López Baca, M.D.l.Á.; Lara Rivera, A.L. Impacto Del Estrés Por Calor En La Producción de Ovinos de Pelo. Revisión. Rev. Mex. Cienc. Pecu. 2020, 11, 205–222. [Google Scholar] [CrossRef]
- Macías-Cruz, U.; Avendaño-Reyes, L.; Álvarez-Valenzuela, F.D.; Torrentera-Olivera, N.G.; Meza-Herrera, C.; Mellado-Bosque, M.; Correa-Calderón, A. Growth and Carcass Characteristics of Ewe Lambs Treated with Zilpaterol Hydrochloride during Spring and Summer. Rev. Mex. Cienc. Pecu. 2013, 4, 1–12. Available online: https://cienciaspecuarias.inifap.gob.mx/index.php/Pecuarias/article/view/2822/2367 (accessed on 10 August 2025).
- Macías-Cruz, U.; Saavedra, O.R.; Correa-Calderón, A.; Mellado, M.; Torrentera, N.G.; Chay-Canul, A.; López-Baca, M.A.; Avendaño-Reyes, L. Feedlot Growth, Carcass Characteristics and Meat Quality of Hair Breed Male Lambs Exposed to Seasonal Heat Stress (Winter vs. Summer) in an Arid Climate. Meat Sci. 2020, 169, 108202. [Google Scholar] [CrossRef] [PubMed]
- Nicolás-López, P.; Macías-Cruz, U.; Mellado, M.; Correa-Calderón, A.; Meza-Herrera, C.A.; Avendaño-Reyes, L. Growth Performance and Changes in Physiological, Metabolic and Hematological Parameters Due to Outdoor Heat Stress in Hair Breed Male Lambs Finished in Feedlot. Int. J. Biometeorol. 2021, 65, 1451–1459. [Google Scholar] [CrossRef]
- Serra, M.; Mollace, R.; Ritorto, G.; Ussia, S.; Altomare, C.; Tavernese, A.; Preianò, M.; Palma, E.; Muscoli, C.; Mollace, V.; et al. A Systematic Review of Thiamine Supplementation in Improving Diabetes and Its Related Cardiovascular Dysfunction. Int. J. Mol. Sci. 2025, 26, 3932. [Google Scholar] [CrossRef]
- Manzetti, S.; Zhang, J.; van der Spoel, D. Thiamin Function, Metabolism, Uptake, and Transport. Biochemistry 2014, 53, 821–835. [Google Scholar] [CrossRef]
- Hrubša, M.; Siatka, T.; Nejmanová, I.; Vopršalová, M.; Krčmová, L.K.; Matoušová, K.; Javorská, L.; Macáková, K.; Mercolini, L.; Remião, F.; et al. Biological Properties of Vitamins of the B-Complex, Part 1: Vitamins B1, B2, B3, and B5. Nutrients 2022, 14, 484. [Google Scholar] [CrossRef]
- Mann, G.; Mora, S.; Madu, G.; Adegoke, O.A.J. Branched-Chain Amino Acids: Catabolism in Skeletal Muscle and Implications for Muscle and Whole-Body Metabolism. Front. Physiol. 2021, 12, 702826. [Google Scholar] [CrossRef]
- Kaźmierczak-Barańska, J.; Halczuk, K.; Karwowski, B.T. Thiamine (Vitamin B1)—An Essential Health Regulator. Nutrients 2025, 17, 2206. [Google Scholar] [CrossRef]
- Kalyesubula, M.; Mopuri, R.; Asiku, J.; Rosov, A.; Yosefi, S.; Edery, N.; Bocobza, S.; Moallem, U.; Dvir, H. High-Dose Vitamin B1 Therapy Prevents the Development of Experimental Fatty Liver Driven by Overnutrition. DMM Dis. Models Mech. 2021, 14, dmm048355. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, P.; Li, P.; Elsabagh, M.; Cheng, L.; Chen, H.; Feng, Y.; Li, Z.; Xu, M. Dietary Thiamine Supplementation Modulates Ruminal Microbiota and Partly Restores Lactation Performance in Lactating Hu Ewes under Heat-Stress Conditions. Anim. Feed. Sci. Technol. 2024, 318, 116119. [Google Scholar] [CrossRef]
- McManus, C.M.; Lucci, C.M.; Maranhão, A.Q.; Pimentel, D.; Pimentel, F.; Rezende Paiva, S. Response to Heat Stress for Small Ruminants: Physiological and Genetic Aspects. Livest. Sci. 2022, 263, 105028. [Google Scholar] [CrossRef]
- Neville, B.W.; Schauer, C.S.; Karges, K.; Gibson, M.L.; Thompson, M.M.; Kirschten, L.A.; Dyer, N.W.; Berg, P.T.; Lardy, G.P. Effect of Thiamine Concentration on Animal Health, Feedlot Performance, Carcass Characteristics, and Ruminal Hydrogen Sulfide Concentrations in Lambs Fed Diets Based on 60% Distillers Dried Grains plus Solubles. J. Anim. Sci. 2010, 88, 2444–2455. [Google Scholar] [CrossRef] [PubMed]
- Clarke, S.D.; Clarke, I.J.; Rao, A.; Cowley, M.A.; Henry, B.A. Sex Differences in the Metabolic Effects of Testosterone in Sheep. Endocrinology 2012, 153, 123–131. [Google Scholar] [CrossRef]
- Gallo, C.; Tarumán, J.; Larrondo, C. Main Factors Affecting Animal Welfare and Meat Quality in Lambs for Slaughter in Chile. Animals 2018, 8, 165. [Google Scholar] [CrossRef]
- Schumacher, M.; Delcurto-Wyffels, H.; Thomson, J.; Boles, J. Fat Deposition and Fat Effects on Meat Quality—A Review. Animals 2022, 12, 1550. [Google Scholar] [CrossRef]
- van Wettere, W.H.E.J.; Kind, K.L.; Gatford, K.L.; Swinbourne, A.M.; Leu, S.T.; Hayman, P.T.; Kelly, J.M.; Weaver, A.C.; Kleemann, D.O.; Walker, S.K. Review of the Impact of Heat Stress on Reproductive Performance of Sheep. J. Anim. Sci. Biotechnol. 2021, 12, 26. [Google Scholar] [CrossRef]
- Macías-Cruz, U.; Gastélum, M.A.; Álvarez, F.D.; Correa, A.; Díaz, R.; Meza-Herrera, C.A.; Mellado, M.; Avendaño-Reyes, L. Effects of Summer Heat Stress on Physiological Variables, Ovulation and Progesterone Secretion in Pelibuey Ewes under Natural Outdoor Conditions in an Arid Region. Anim. Sci. J. 2016, 87, 354–360. [Google Scholar] [CrossRef]
- Barragán, A.L.; Avendaño-Reyes, L.; Mellado-Bosque, M.; Meza-Herrera, C.A.; Vicente-Pérez, R.; Castañeda, V.J.; Díaz-Molina, R.; Macías-Cruz, U. Seasonal Heat Stress Compromises Testicular Thermoregulation and Semen Quality of Dorper Rams Raised in a Desert Climate. J. Therm. Biol. 2023, 118, 103737. [Google Scholar] [CrossRef]
- Theusme, C.; Avendaño-Reyes, L.; Macías-Cruz, U.; Correa-Calderón, A.; García-Cueto, R.O.; Mellado, M.; Vargas-Villamil, L.; Vicente-Pérez, A. Climate Change Vulnerability of Confined Livestock Systems Predicted Using Bioclimatic Indexes in an Arid Region of México. Sci. Total Environ. 2021, 751, 141779. [Google Scholar] [CrossRef]
- NOM-051-ZOO-1995. Available online: https://www.gob.mx/senasica/documentos/nom-051-zoo-1995 (accessed on 10 August 2025).
- NOM-062-ZOO-1999. Available online: https://www.gob.mx/cms/uploads/attachment/file/203498/NOM-062-ZOO-1999_220801.pdf (accessed on 10 August 2025).
- NOM-033-SAG/ZOO-2014. Available online: https://www.gob.mx/cms/uploads/attachment/file/133499/4.-_NORMA_OFICIAL_MEXICANA_NOM-033-SAG-ZOO-2014.pdf (accessed on 10 August 2025).
- NRC. Nutrient Requirements of Small Ruminants; National Academies Press: Washington, DC, USA, 2007; Volume 9, ISBN 978-0-309-10213-1. [Google Scholar]
- NRC. Nutrient Requirements of Sheep; National Academy of Press: Washington, DC, USA, 1985; ISBN 0309035961. [Google Scholar]
- Marai, I.F.M.; El-Darawany, A.A.; Fadiel, A.; Abdel-Hafez, M.A.M. Physiological Traits as Affected by Heat Stress in Sheep-A Review. Small Rumin. Res. 2007, 71, 1–12. [Google Scholar] [CrossRef]
- Avendaño-Reyes, L.; Macías-Cruz, U.; Álvarez-Valenzuela, F.D.; Águila-Tepato, E.; Torrentera-Olivera, N.G.; Soto-Navarro, S.A. Effects of Zilpaterol Hydrochloride on Growth Performance, Carcass Characteristics, and Wholesale Cut Yield of Hair-Breed Ewe Lambs Consuming Feedlot Diets under Moderate Environmental Conditions. J. Anim. Sci. 2011, 89, 4188–4194. [Google Scholar] [CrossRef]
- Sutton, D.S.; Ellis, M.; Lan, Y.; McKeith, F.K.; Wilson, E.R. Influence of Slaughter Weight and Stress Gene Genotype on the Water-Holding Capacity and Protein Gel Characteristics of Three Porcine Muscles. Meat Sci. 1997, 46, 173–180. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, R.C.M.; Araújo Furtado, D.; Ribeiro, N.L.; Silva, R.d.S.; de Oliveira, A.G.; da Costa Silva, J.A.P.; da Silva, M.R.; Mascarenhas, N.M.H.; Cavalcanti, C.R.; Junqueira Ayres, G.D.; et al. Thermoneutral Temperature and Thermal Stress of Sheep Creole: Physiological and Ingestive Behaviour. J. Appl. Anim. Res. 2024, 52, 2404113. [Google Scholar] [CrossRef]
- Silva, R.d.S.; Furtado, D.A.; Ribeiro, N.L.; Neto, J.P.L.; Rodrigues, R.C.M.; Oliveira, A.G.d.; Silva, J.A.P.d.C.; Silva, M.R.d.; Mascarenhas, N.M.H.; Marques, J.I.; et al. Physiological Variables and Estimates of Heat Exchange in Sheep Kept at Thermoneutral and Thermal Stress Temperatures. Small Rumin. Res. 2024, 237, 107320. [Google Scholar] [CrossRef]
- Macías-Cruz, U.; López-Baca, M.A.; Vicente, R.; Mejía, A.; Álvarez, F.D.; Correa-Calderón, A.; Meza-Herrera, C.A.; Mellado, M.; Guerra-Liera, J.E.; Avendaño-Reyes, L. Effects of Seasonal Ambient Heat Stress (Spring vs. Summer) on Physiological and Metabolic Variables in Hair Sheep Located in an Arid Region. Int. J. Biometeorol. 2016, 60, 1279–1286. [Google Scholar] [CrossRef]
- Sejian, V.; Krishnan, G.; Bagath, M.; Vaswani, S.; Pragna, P.; Aleena, J.; Lees, A.M.; Maurya, V.P.; Bhatta, R. Measurement of Severity of Heat Stress in Sheep. In Sheep Production Adapting to Climate Change; Springer: Singapore, 2017; pp. 307–318. [Google Scholar]
- Vinnai, B.Á.; Arianti, R.; Fischer-Posovszky, P.; Wabitsch, M.; Fésüs, L.; Kristóf, E. The Importance of Thiamine Availability in the Thermogenic Competency of Human Adipocytes. Mol. Cell. Endocrinol. 2025, 599, 112483. [Google Scholar] [CrossRef]
- Yasmin, F.; Ali, S.H.; Naeem, A.; Savul, S.; Afridi, M.S.I.; Kamran, N.; Fazal, F.; Khawer, S.; Savul, I.S.; Najeeb, H.; et al. Current Evidence and Future Perspectives of the Best Supplements for Cardioprotection: Have We Reached the Final Chapter for Vitamins? Rev. Cardiovasc. Med. 2022, 23, 381. [Google Scholar] [CrossRef]
- Goma, A.A. Thiamine Deficiency and Brain Degeneration in Calves. Anim. Sci. Cases 2025, ascs20250005. [Google Scholar] [CrossRef]
- Shvareva, N.; Kaplanski, J.; Abramovich, L.; Sod-Moriah, U.A. Testosterone Modifies Response to Chronic Heat Exposure in Rats. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 1998, 120, 575–578. [Google Scholar] [CrossRef]
- Alsaad, K.M.; Dhahi, J.H.; Tawfiq, S.I. Polioencephalomalacia Caused by Thiamine Deficiency in Sheep of Basrah Province, Iraq. Egypt. J. Vet. Sci. 2020, 51, 73–81. [Google Scholar] [CrossRef]
- Hakim, A.M. The Induction and Reversibility of Cerebral Acidosis in Thiamine Deficiency. Ann. Neurol. 1984, 16, 673–679. [Google Scholar] [CrossRef]
- Kalyesubula, M.; Mopuri, R.; Rosov, A.; Van Bommel, G.; Dvir, H. Metabolic Effects of Vitamin B1 Therapy under Overnutrition and Undernutrition Conditions in Sheep. Nutrients 2021, 13, 3463. [Google Scholar] [CrossRef] [PubMed]
- Rosas Aragón, J. Respuesta Productiva, Costo de Producción, Calidad de la Canal y de la Carne de Toretes Suplementados con Difosfato de Tiamina y β-Agonistas. Master’s Thesis, Universidad Autónoma Chapingo, Texcoco, Mexico, 2018. [Google Scholar]
- Silzell, S.A.; Hellwig, D.H.; Kegley, E.B.; Coffey, K.P.; Beers, K.; Daniels, L.B. Effects of Supplemental Thiamin on Growth Performance and Immune Function in Stressed Stocker Cattle1. J. Appl. Anim. Res. 2002, 22, 145–156. [Google Scholar] [CrossRef]
- Hulbert, L.E.; Carroll, J.A.; Ballou, M.A.; Burdick, N.C.; Dailey, J.W.; Caldwell, L.C.; Loyd, A.N.; Vann, R.C.; Welsh, T.H.; Randel, R.D. Sexually Dimorphic Stress and Pro-Inflammatory Cytokine Responses to an Intravenous Corticotropin-Releasing Hormone Challenge of Brahman Cattle Following Transportation. Innate Immun. 2013, 19, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Iddriss, A.R.I.; Rahim, A.A. Heat Tolerance in Djallonke Sheep under Guinea Savannah Conditions. Trop. Agric. 2018, 95, 274–283. [Google Scholar]
- Abbaya, H.Y.; Philimon, Y.; Elihu, A.; Lawal, A.U.; Lumboyi, I.A. Species, Age and Sex Effect on Thermoregulatory Parameters of Animals in Hot Season of Mubi. J. Biol. Genet. Res. 2022, 8, 1–12. [Google Scholar] [CrossRef]
- Fernández-Peña, C.; Reimúndez, A.; Viana, F.; Arce, V.M.; Señarís, R. Sex Differences in Thermoregulation in Mammals: Implications for Energy Homeostasis. Front. Endocrinol. 2023, 14, 1093376. [Google Scholar] [CrossRef]
- Macías-Cruz, U.; Álvarez-Valenzuela, F.D.; Rodríguez-García, J.; Correa-Calderón, A.; Torrentera-Olivera, N.G.; Molina Ramírez, L.; Avendaño-Reyes, L. Growth and Carcass Traits in Pure Pelibuey Lambs and Crosses F1 with Dorper and Katahdin Breeds in Confinement. Arch. Med. Vet. 2010, 42, 147–154. [Google Scholar] [CrossRef]
- Tadeu, M.; Cardoso, M.; Vieira Landim, A.; Louvandini, H.; Mcmanus, C. Performance and Carcass Quality in Three Genetic Groups of Sheep in Brazil. Rev. Bras. Zootec. 2013, 42, 734–742. [Google Scholar] [CrossRef]
- Muñoz-Osorio, G.A.; Aguilar-Caballero, A.J.; Sarmiento-Franco, L.A.; Wurzinger, M.; Sandoval-Castro, C.A. Effect of Two Housing Systems and Sex on Productive Performance of Lamb during the Fattening. Arch. Zootec. 2020, 268, 494–498. [Google Scholar] [CrossRef]
- Landim, A.V.; Roriz, N.D.; Silveira, R.M.F.; Vega, W.H.O.; Costa, H.H.A.; de Sousa, L.C.O.; Alves, G.C.; Ferreira, J.; Mourão, G.B. Sheep Meat Production in the Brazilian Semi-Arid Region: Crossing between Indigenous Breeds. Trop. Anim. Health Prod. 2021, 53, 510. [Google Scholar] [CrossRef] [PubMed]
- de Vargas Junior, F.M.; Martins, C.F.; dos Santos Pinto, G.; Ferreira, M.B.; de Almeida Ricardo, H.; Leão, A.G.; Fernandes, A.R.M.; Teixeira, A. The Effect of Sex and Genotype on Growth Performance, Feed Efficiency, and Carcass Traits of Local Sheep Group Pantaneiro and Texel or Santa Inês Crossbred Finished on Feedlot. Trop. Anim. Health Prod. 2014, 46, 869–875. [Google Scholar] [CrossRef] [PubMed]
- Barragán Sierra, A.; Avendaño-Reyes, L.; Rivera, J.A.H.; Vicente-Pérez, R.; Correa-Calderón, A.; Mellado, M.; Meza-Herrera, C.A.; MacÍas-Cruz, U. Thermoregulation and Reproductive Responses of Rams under Heat Stress. Review. Rev. Mex. Cienc. Pecu. 2021, 12, 910–931. [Google Scholar] [CrossRef]
- Lefaucheur, L.A. A Second Look into Fibre Typing—Relation to Meat Quality. Meat Sci. 2010, 84, 257–270. [Google Scholar] [CrossRef]
- De Lima Jùnior, D.; de Carvalho, F.; Da Silva, F.; do N Rangel, A.; Novaes, L.; Difante, G. Intrinsic Factors Affecting Sheep Meat Quality: A Review. Rev. Colomb. Cien Pecu. 2016, 29, 3–15. [Google Scholar] [CrossRef]
- Lee, J.H.; Wildeus, S.; Lemma, B.B.; Kouakou, B. Carcass and Meat Quality Characteristics of Purebred (Hair) and Crossbred (Wool × Hair) Sheep Lambs Grazing Fescue Pasture as Influenced by Breed Type, Sex, and Supplementation. J. Appl. Anim. Res. 2024, 52, 1–14. [Google Scholar] [CrossRef]
- Corazzin, M.; Del Bianco, S.; Bovolenta, S.; Piasentier, E. Carcass Characteristics and Meat Quality of Sheep and Goat. In More than Beef, Pork and Chicken—The Production, Processing, and Quality Traits of Other Sources of Meat for Human Diet; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Ponnampalam, E.N.; Hopkins, D.L.; Bruce, H.; Li, D.; Baldi, G.; Bekhit, A.E. Causes and Contributing Factors to “Dark Cutting” Meat: Current Trends and Future Directions: A Review. Compr. Rev. Food Sci. Food Saf. 2017, 16, 400–430. [Google Scholar] [CrossRef]
- Maggiolino, A.; Forte, L.; Landi, V.; Pateiro, M.; Lorenzo, J.M.; De Palo, P. Enhancement of Culled Ewes’ Meat Quality: Effects of Aging Method and Time. Food Chem. X 2024, 23, 101687. [Google Scholar] [CrossRef]
| Ingredients 1 | % Mixed Basis | Chemical Composition 2 | % DM Basis |
|---|---|---|---|
| Alfalfa hay | 12.5 | Dry matter | 90.5 |
| Wheat straw | 15.0 | Crude protein | 16.2 |
| Ground wheat grain | 60.0 | Ether extract | 1.50 |
| Soybean meal | 10.0 | Crude fiber | 8.30 |
| Mineral-vitamin premix | 2.00 | Acid detergent fiber | 18.0 |
| Calcium bicarbonate | 0.50 | Neutral detergent fiber | 25.7 |
| Ash | 7.80 | ||
| Calculates net energy | Mcal/kg DM | ||
| Maintenance | 1.83 | ||
| Gain 3 | 1.06 |
| Items 2 | Thiamine Diphosphate 1 | Gender | p-Values | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Without | With | SEM | Male | Females | SEM | TD | G | H | TD × G | TD × H | G × H | |
| RT (°C) | 40.2 | 40.1 | 0.09 | 40.1 | 40.2 | 0.09 | 0.79 | 0.83 | <0.01 | 0.37 | 0.49 | 0.75 |
| RR (bpm) | 142 | 144 | 2.27 | 137 a | 149 b | 1.27 | 0.58 | <0.01 | <0.01 | 0.33 | 0.67 | 0.27 |
| Body surface temperatures (°C) | ||||||||||||
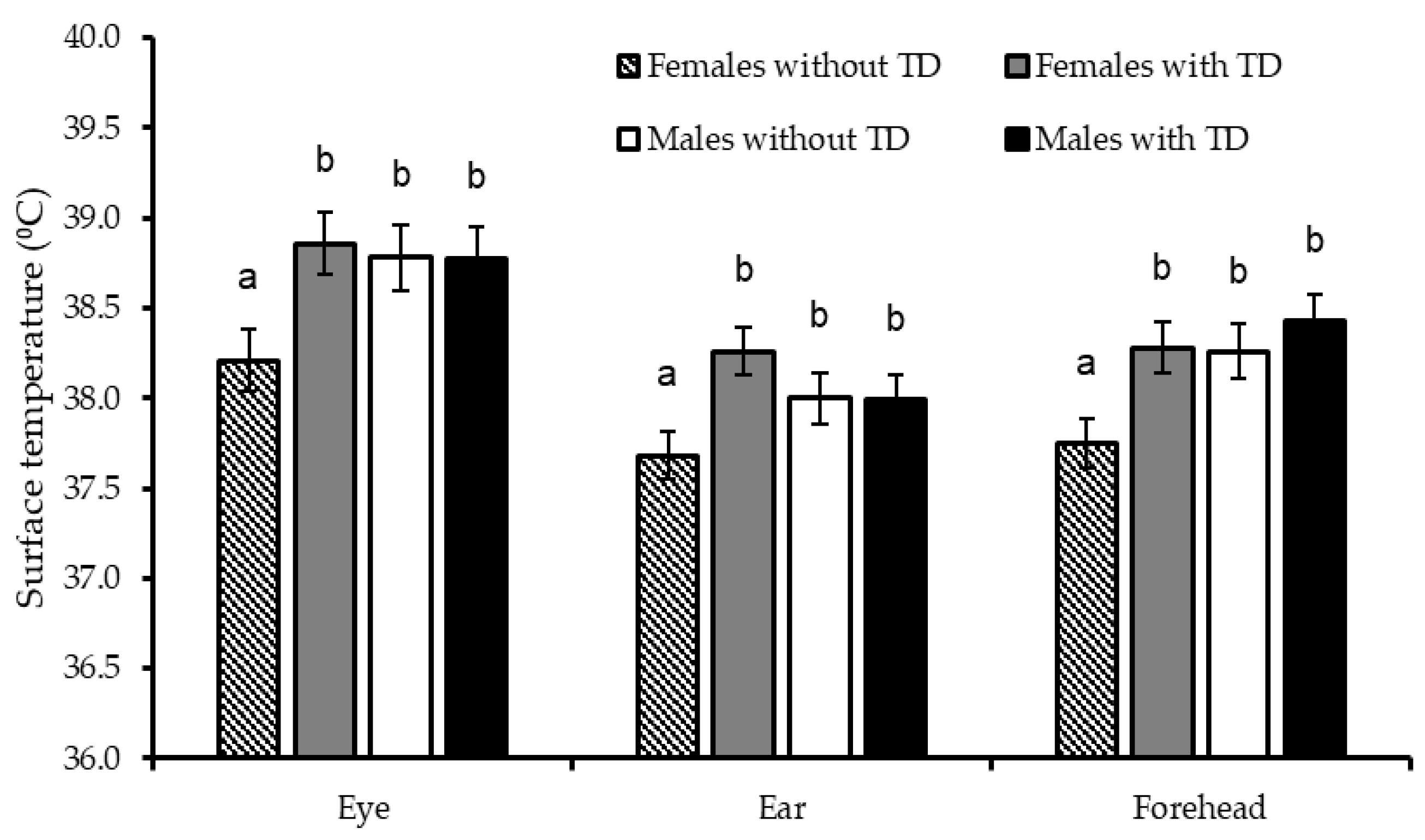
| Eye | 38.5 | 38.8 | 0.12 | 38.8 | 38.5 | 0.13 | 0.09 | 0.18 | <0.01 | 0.04 | 0.52 | 0.11 |
| Ear | 37.9 | 38.1 | 0.09 | 38.0 | 37.7 | 0.10 | 0.07 | 0.91 | <0.01 | 0.05 | 0.86 | 0.12 |
| Muzzle | 36.7 | 36.7 | 0.12 | 36.8 | 36.7 | 0.12 | 0.61 | 0.66 | <0.01 | 0.49 | 0.31 | 0.49 |
| Forehead | 38.0 a | 38.4 b | 0.10 | 38.3 b | 38.0 a | 0.10 | 0.04 | 0.04 | <0.01 | 0.05 | 0.34 | 0.20 |
| Nostrils | 37.9 | 38.1 | 0.11 | 38.2 b | 37.8 a | 0.11 | 0.18 | 0.04 | <0.01 | 0.32 | 0.18 | 0.27 |
| Neck | 37.6 a | 38.0 b | 0.12 | 37.9 | 37.7 | 0.12 | 0.04 | 0.17 | <0.01 | 0.54 | 0.54 | 0.11 |
| Shoulder | 37.9 a | 38.4 b | 0.15 | 38.3 | 38.0 | 0.15 | 0.05 | 0.19 | <0.01 | 0.52 | 0.44 | 0.12 |
| Loin | 37.6 a | 38.0 b | 0.13 | 38.0 | 37.7 | 0.13 | 0.05 | 0.08 | <0.01 | 0.17 | 0.65 | 0.12 |
| Ribs | 37.9 | 38.2 | 0.15 | 38.3 b | 37.8 a | 0.15 | 0.09 | 0.04 | <0.01 | 0.24 | 0.65 | 0.11 |
| Belly | 37.9 | 38.3 | 0.17 | 38.3 | 37.8 | 0.17 | 0.12 | 0.08 | <0.01 | 0.46 | 0.33 | 0.11 |
| Flank | 37.3 | 37.7 | 0.15 | 37.6 | 37.4 | 0.15 | 0.07 | 0.20 | <0.01 | 0.22 | 0.35 | 0.11 |
| Rump | 37.5 a | 37.9 b | 0.12 | 37.8 | 37.6 | 0.12 | 0.02 | 0.08 | <0.01 | 0.27 | 0.65 | 0.12 |
| Leg | 37.3 | 37.8 | 0.20 | 37.8 | 37.3 | 0.20 | 0.11 | 0.12 | <0.01 | 0.64 | 0.33 | 0.14 |
| Fore limb | 37.2 a | 37.7 b | 0.11 | 37.6 | 37.3 | 0.11 | 0.01 | 0.08 | <0.01 | 0.12 | 0.39 | 0.20 |
| Hind limb | 37.3 | 37.6 | 0.13 | 37.6 | 37.3 | 0.13 | 0.09 | 0.12 | <0.01 | 0.60 | 0.33 | 0.28 |
| Anal | 39.2 a | 39.7 b | 0.11 | 39.6 b | 39.2 a | 0.11 | <0.01 | 0.02 | <0.01 | 0.37 | 0.56 | 0.67 |
| Vulva | 38.2 a | 38.9 b | 0.09 | --- | --- | --- | <0.01 | --- | <0.01 | --- | 0.18 | --- |
| Perineum | 37.9 a | 38.7 b | 0.13 | --- | --- | --- | <0.01 | --- | <0.01 | --- | 0.59 | --- |
| Testicles | 33.8 a | 35.4 b | 0.25 | --- | --- | --- | 0.01 | --- | <0.01 | --- | 0.34 | --- |
| Items 2 | Thiamine Diphosphate 1 | Gender | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Without | With | SEM | Males | Females | SEM | TD | G | TD × G | |
| Initial weight (kg) | 31.5 | 31.5 | 0.40 | 31.6 | 31.4 | 0.40 | 0.94 | 0.70 | 0.92 |
| Final weight (kg) | 40.2 | 40.5 | 0.67 | 42.2 b | 38.5 a | 0.67 | 0.70 | <0.01 | 0.58 |
| Daily weight gain (g/d) | 223 | 235 | 12.4 | 261 b | 183 a | 12.6 | 0.58 | <0.01 | 0.43 |
| Total weight gain (kg) | 8.76 | 8.99 | 0.41 | 10.6 b | 7.09 a | 0.41 | 0.58 | <0.01 | 0.43 |
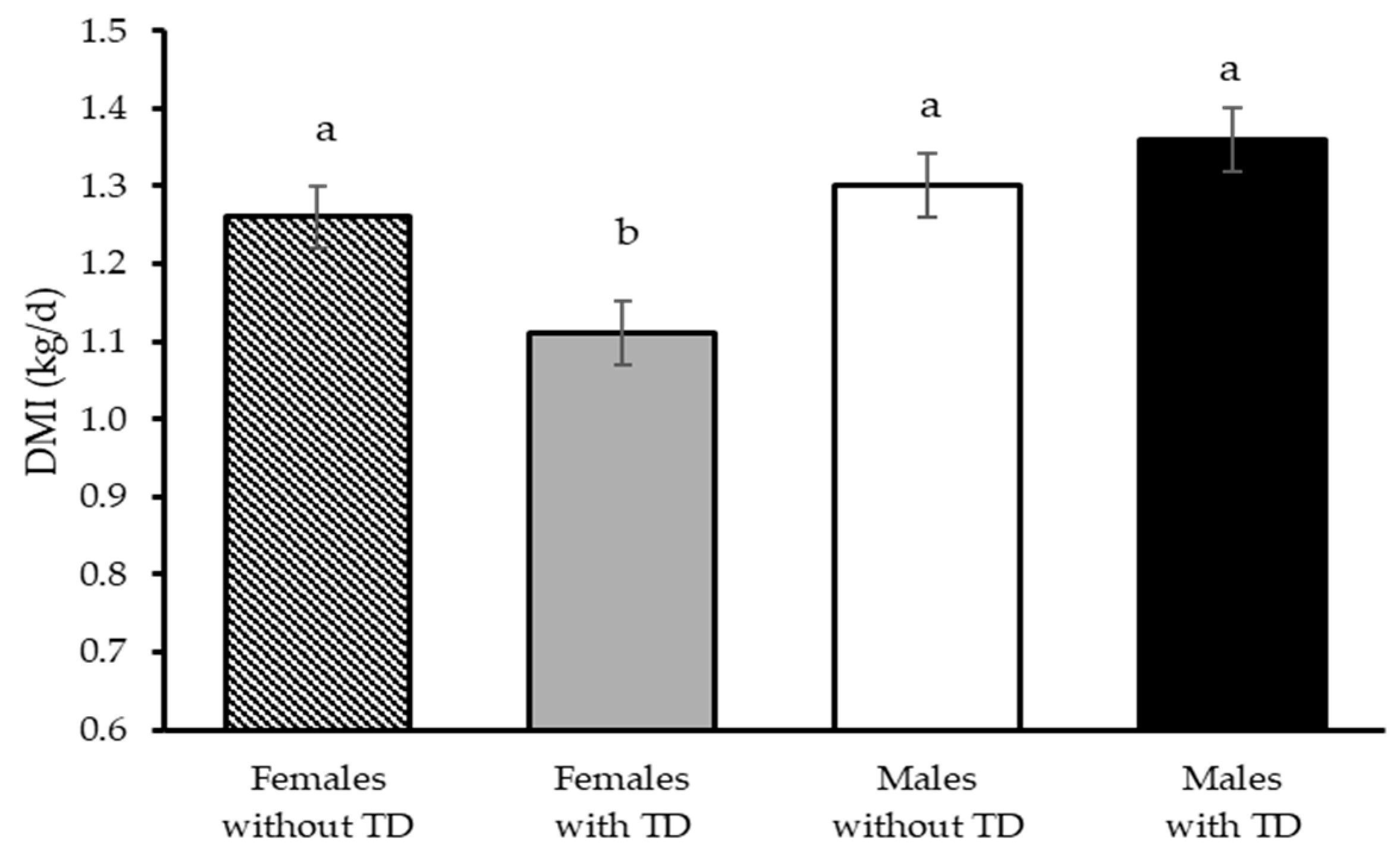
| Dry matter intake (kg/d) | 1.28 | 1.24 | 0.03 | 1.33 b | 1.19 a | 0.03 | 0.28 | <0.01 | 0.02 |
| Feed efficiency (g/kg) | 174 | 189 | 7 | 197 b | 153 a | 11.5 | 0.30 | <0.01 | 0.65 |
| Items 2 | Thiamine Diphosphate 1 | Gender | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Without | With | SEM | Males | Females | SEM | TD | G | TD × G | |
| Empty live weight (kg) | 33.7 | 34.1 | 0.71 | 35.1 b | 32.7 a | 0.71 | 0.71 | 0.04 | 0.70 |
| Hot carcass weight (kg) | 18.9 | 19.1 | 0.44 | 19.4 | 18.6 | 0.44 | 0.80 | 0.24 | 0.83 |
| Cold carcass weight (kg) | 17.9 | 18.5 | 0.47 | 18.7 | 17.6 | 0.48 | 0.42 | 0.15 | 0.77 |
| Dressing (%) | 48.9 | 48.5 | 0.38 | 47.7 a | 49.7 b | 0.38 | 0.57 | <0.01 | 0.53 |
| LT area (cm2) | 17.9 | 18.2 | 1.41 | 18.6 | 17.4 | 1.43 | 0.86 | 0.57 | 0.36 |
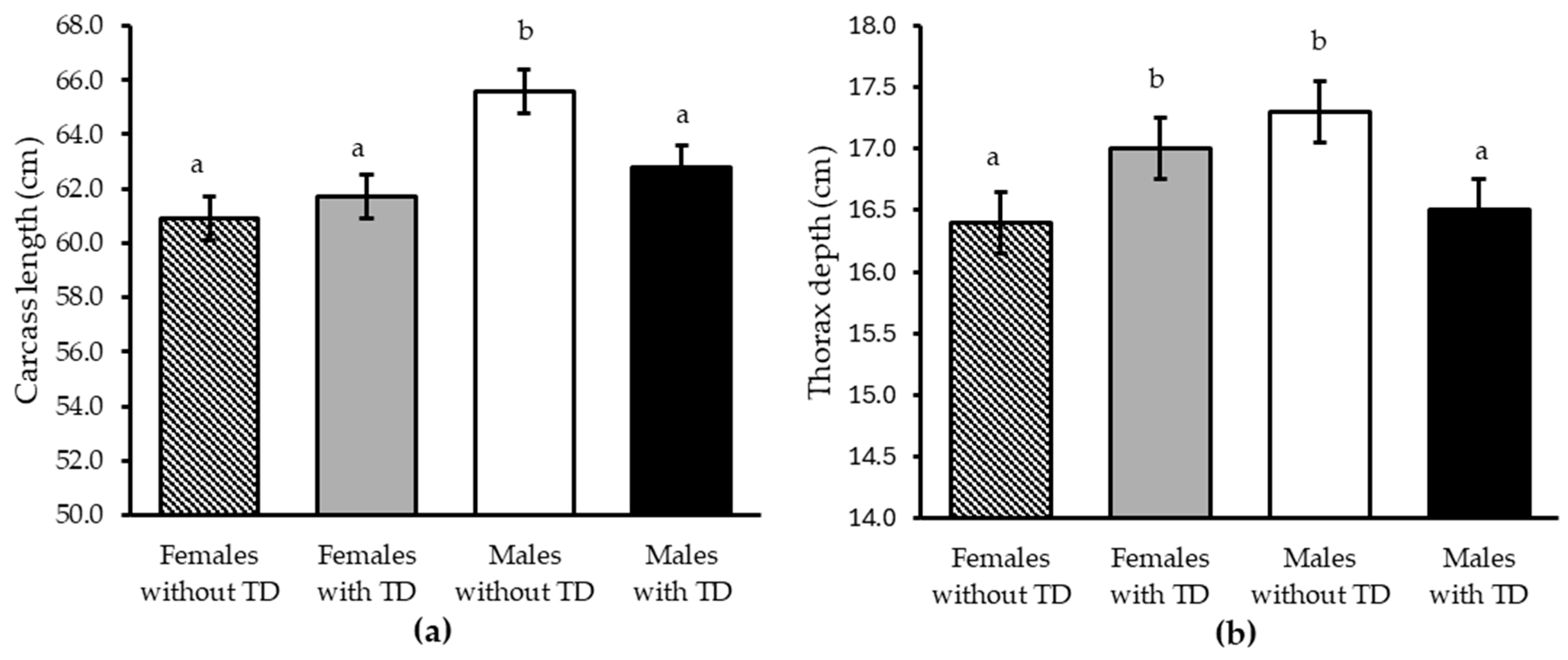
| Carcass length (cm) | 63.3 | 62.3 | 0.63 | 64.2 b | 61.3 a | 0.64 | 0.25 | 0.01 | 0.05 |
| Thorax depth (cm) | 16.8 | 16.7 | 0.17 | 16.9 | 16.7 | 0.17 | 0.73 | 0.47 | 0.02 |
| Chest circumference (cm) | 67.6 | 68.3 | 0.67 | 68.8 | 67.1 | 0.68 | 0.44 | 0.11 | 0.15 |
| Leg length (cm) | 32.2 | 31.4 | 0.78 | 32.5 | 31.0 | 0.79 | 0.49 | 0.24 | 0.71 |
| Leg circumference (cm) | 42.8 | 43.0 | 0.73 | 42.2 | 43.5 | 0.74 | 0.91 | 0.26 | 0.69 |
| Body fat | |||||||||
| Backfat thickness (mm) | 3.00 | 2.82 | 0.40 | 1.80 a | 4.02 b | 0.40 | 0.76 | <0.01 | 0.69 |
| KPH (%) | 2.25 | 2.27 | 0.11 | 1.96 a | 2.56 b | 0.11 | 0.90 | <0.01 | 0.74 |
| Omental (%) | 2.98 | 3.19 | 0.17 | 2.87 | 3.30 | 0.17 | 0.39 | 0.10 | 0.50 |
| Mesenteric (%) | 2.30 b | 1.80 a | 0.14 | 1.69 a | 2.41 b | 0.14 | 0.03 | <0.01 | 0.67 |
| Items 2 | Thiamine Diphosphate 1 | Gender | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Without | With | SEM | Males | Females | SEM | TD | G | TD × G | |
| Head (%) | 5.55 | 5.74 | 0.17 | 6.10 b | 5.19 a | 0.17 | 0.44 | <0.01 | 0.51 |
| Blood (%) | 4.45 | 4.39 | 0.12 | 4.55 | 4.30 | 0.12 | 0.72 | 0.17 | 0.56 |
| Feet (%) | 2.60 | 2.52 | 0.04 | 2.61 | 2.51 | 0.04 | 0.14 | 0.08 | 0.19 |
| Skin (%) | 11.3 | 11.4 | 0.31 | 11.2 | 11.5 | 0.31 | 0.90 | 0.52 | 0.90 |
| Kidney (%) | 0.31 | 0.30 | 0.01 | 0.32 b | 0.29 a | 0.01 | 0.35 | 0.04 | 0.75 |
| Lung (%) | 1.51 | 1.42 | 0.07 | 1.50 | 1.44 | 0.07 | 0.18 | 0.77 | 0.82 |
| Liver (%) | 2.16 | 2.12 | 0.05 | 2.20 | 2.09 | 0.05 | 0.50 | 0.14 | 0.47 |
| Heart (%) | 0.40 | 0.40 | 0.01 | 0.39 | 0.41 | 0.01 | 0.76 | 0.36 | 0.85 |
| Spleen (%) | 0.23 | 0.23 | 0.04 | 0.23 | 0.23 | 0.04 | 0.95 | 0.98 | 0.38 |
| Empty GT (%) | 5.46 | 5.41 | 0.14 | 5.64 | 5.22 | 0.14 | 0.33 | 0.22 | 0.62 |
| Testicles (%) | 1.73 | 1.85 | 0.04 | --- | --- | --- | 0.23 | --- | --- |
| Items 2 | Thiamine Diphosphate 1 | Gender | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Without | With | SEM | Males | Females | SEM | TD | G | TD × G | |
| Forequarter (%) | 52.9 | 52.6 | 0.62 | 53.6 | 51.9 | 0.63 | 0.81 | 0.10 | 0.84 |
| Neck (%) | 3.68 | 3.49 | 0.13 | 3.77 | 3.41 | 0.14 | 0.35 | 0.10 | 0.25 |
| Shoulder (%) | 31.0 | 31.6 | 0.41 | 32.5 b | 30.2 a | 0.42 | 0.35 | <0.01 | 0.88 |
| Loin (%) | 8.11 | 7.43 | 0.43 | 7.76 | 7.77 | 0.44 | 0.29 | 0.99 | 0.81 |
| Ribs (%) | 10.0 | 10.1 | 0.38 | 9.54 | 10.6 | 0.39 | 0.92 | 0.10 | 0.98 |
| Hindquarter (%) | 47.1 | 47.3 | 0.62 | 46.4 | 48.1 | 0.63 | 0.81 | 0.10 | 0.84 |
| Leg (%) | 33.2 | 33.5 | 0.47 | 33.3 | 33.5 | 0.48 | 0.67 | 0.82 | 0.77 |
| Plain loin (%) | 8.96 | 8.90 | 0.40 | 8.33 | 9.53 | 0.40 | 0.92 | 0.06 | 0.99 |
| Breast and flank (%) | 4.96 | 4.93 | 0.18 | 4.80 | 5.08 | 0.18 | 0.69 | 0.21 | 0.36 |
| Items 2 | Thiamine Diphosphate 1 | Gender | p-Values | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Without | With | SEM | Males | Females | SEM | TD | G | TD × G | |
| pH postmortem | |||||||||
| 45 min | 6.42 | 6.43 | 0.04 | 6.36 a | 6.49 b | 0.04 | 0.85 | 0.04 | 0.40 |
| 24 h | 5.89 | 5.87 | 0.02 | 5.94 b | 5.82 a | 0.02 | 0.70 | 0.01 | 0.44 |
| Meat aged for 7 d | |||||||||
| pH | 5.62 | 5.53 | 0.04 | 5.62 | 5.54 | 0.04 | 0.16 | 0.23 | 0.82 |
| Redness (a*) | 21.9 | 22.0 | 0.25 | 22.4 b | 21.6 a | 0.25 | 0.80 | 0.05 | 0.11 |
| Yellowness (b*) | 7.02 | 7.46 | 0.46 | 7.47 | 7.01 | 0.46 | 0.50 | 0.51 | 0.41 |
| Lightness (L*) | 41.2 | 41.7 | 0.73 | 42.3 | 40.7 | 0.74 | 0.66 | 0.17 | 0.51 |
| Hue angle (H*) | 17.8 | 18.8 | 0.85 | 18.6 | 18.0 | 0.87 | 0.45 | 0.64 | 0.54 |
| Chroma (C*) | 23.1 | 23.3 | 0.35 | 23.7 | 22.7 | 0.35 | 0.61 | 0.09 | 0.15 |
| WHC (%) | 90.3 | 89.7 | 0.73 | 90.9 | 89.2 | 0.74 | 0.54 | 0.14 | 0.83 |
| WBSF (N) | 37.6 | 40.2 | 1.51 | 38.9 | 39.0 | 1.51 | 0.26 | 0.95 | 0.27 |
| Cooking loss (%) | 22.1 | 22.2 | 2.02 | 21.2 | 23.1 | 2.05 | 0.99 | 0.53 | 0.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Macías-Cruz, U.; Castillo Cristóbal, G.; Avendaño-Reyes, L.; López-Baca, M.d.l.Á.; Roque-Jiménez, J.A.; Mellado, M.; Meza-Herrera, C.A.; Vicente-Pérez, R.; López-Romero, M.; Rivero-Pérez, N. Thiamine Diphosphate Supplementation as a Heat-Stress Mitigation Strategy for Hair Male and Female Lambs in Feedlot: Physiological Responses, Growth Performance, and Carcass Traits. Animals 2025, 15, 3143. https://doi.org/10.3390/ani15213143
Macías-Cruz U, Castillo Cristóbal G, Avendaño-Reyes L, López-Baca MdlÁ, Roque-Jiménez JA, Mellado M, Meza-Herrera CA, Vicente-Pérez R, López-Romero M, Rivero-Pérez N. Thiamine Diphosphate Supplementation as a Heat-Stress Mitigation Strategy for Hair Male and Female Lambs in Feedlot: Physiological Responses, Growth Performance, and Carcass Traits. Animals. 2025; 15(21):3143. https://doi.org/10.3390/ani15213143
Chicago/Turabian StyleMacías-Cruz, Ulises, German Castillo Cristóbal, Leonel Avendaño-Reyes, María de los Ángeles López-Baca, José A. Roque-Jiménez, Miguel Mellado, César A. Meza-Herrera, Ricardo Vicente-Pérez, Marisol López-Romero, and Nallely Rivero-Pérez. 2025. "Thiamine Diphosphate Supplementation as a Heat-Stress Mitigation Strategy for Hair Male and Female Lambs in Feedlot: Physiological Responses, Growth Performance, and Carcass Traits" Animals 15, no. 21: 3143. https://doi.org/10.3390/ani15213143
APA StyleMacías-Cruz, U., Castillo Cristóbal, G., Avendaño-Reyes, L., López-Baca, M. d. l. Á., Roque-Jiménez, J. A., Mellado, M., Meza-Herrera, C. A., Vicente-Pérez, R., López-Romero, M., & Rivero-Pérez, N. (2025). Thiamine Diphosphate Supplementation as a Heat-Stress Mitigation Strategy for Hair Male and Female Lambs in Feedlot: Physiological Responses, Growth Performance, and Carcass Traits. Animals, 15(21), 3143. https://doi.org/10.3390/ani15213143








