Gene Expression of Feed Intake-Regulating Peptides in the Gut–Brain Axis of Laying Hens Housed Under Two Different Egg Production Systems
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
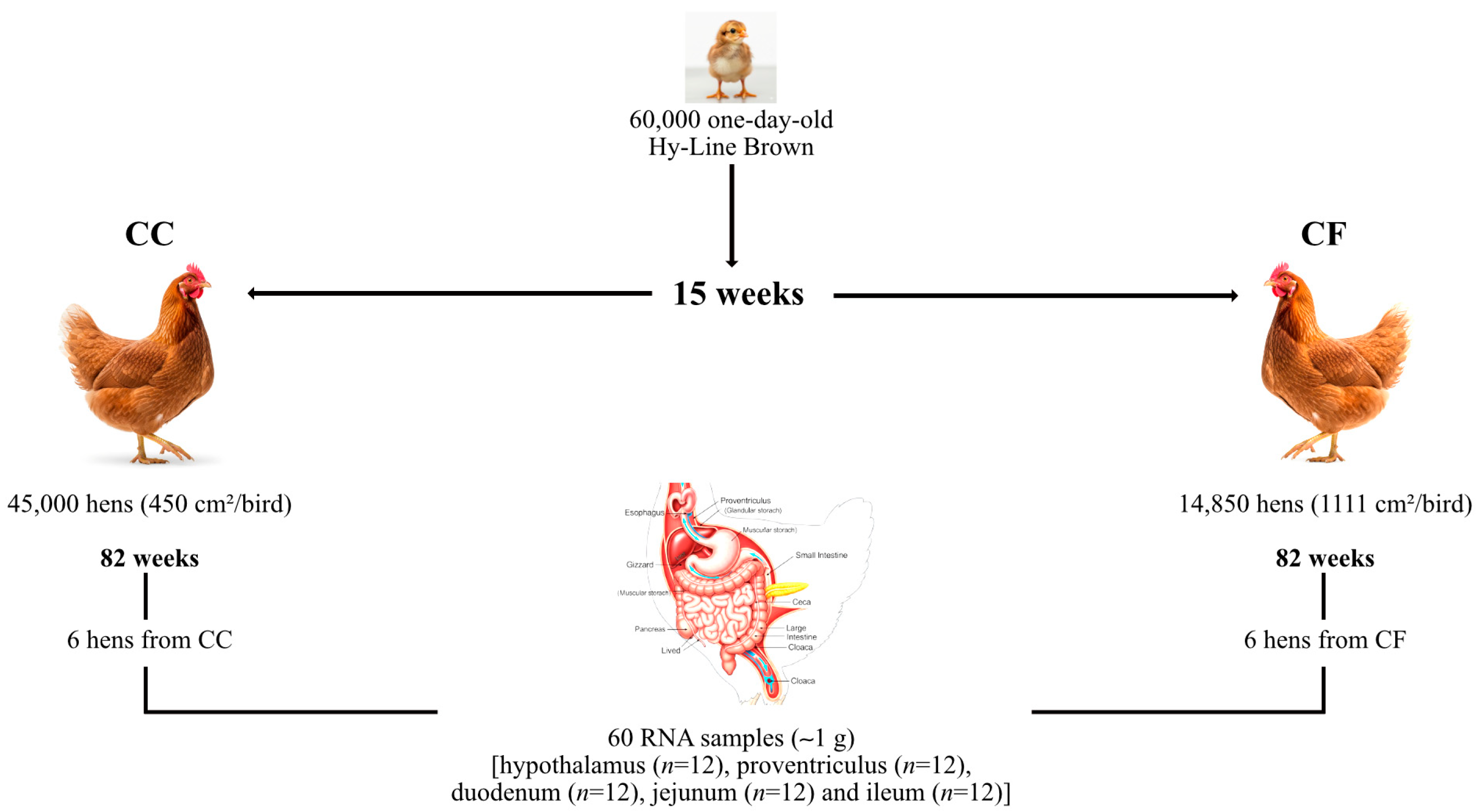
2.1. Animals and Management
2.2. Sample Collection
2.3. Samples, RNA Extraction, cDNA Synthesis, and Endpoint PCR
2.4. Quantitative Polymerase Chain Reaction (qPCR)
2.5. Selection of Reference Genes
| Type | Gene | Primer Sequences (5′–3′) | Product Size (bp) | Reference | |
|---|---|---|---|---|---|
| Feeding regulator | GHRL | F | CTGGCTGGCTCTAGTTTTT | 107 | This study |
| R | CAAAAGCTTTCTGTGCCT | ||||
| Ghsr | F | TTTGTCCTCTTCTACCTGA | 125 | ||
| R | CTGGAGAGTCTTTTCTTTG | ||||
| NPY | F | TGCTGACTTTCGCCTTGTCG | 148 | [16] | |
| R | GTGATGAGGTTGATGTAGTGCC | ||||
| AGRP | F | CATCCTCACCTCGGACCTCA | 163 | ||
| R | CAGGGCCATCTGATCCAAGTCT | ||||
| POMC | F | CGCTACGGCGGCTTCA | 88 | ||
| R | TCTTGTAGGCGCTTTTGACGAT | ||||
| CCK | F | TTCTCTGTCCTAGGAAAC | 168 | ||
| R | GTACTCGTATTCTTCAGCAC | ||||
| CART | F | CAGAGGTGCCGGTGTTGAG | 140 | ||
| R | TTCCCATAGCGAGCCCCCA | ||||
| CRH | F | AGCAGCCCGATTTCTTCCCT | 86 | ||
| R | CAACAACTCGGCGGAGGCTT | ||||
| MC4R | F | CAAGCGTGTAGGGGTCATCA | 101 | ||
| R | CAGATGATGACAACGCTGCTG | ||||
| MC1R | F | GCCCTTCTTCTTCCACCTCAT | 218 | ||
| R | GCTCCGGAAGGCATAGATCA | ||||
| MC5R | F | TCCATTCTTCCTCCATCTCATCC | 157 | ||
| R | CTTCCTCATTTCCTGGCTACG | ||||
| Reference | GAPDH | F | GAGGGTAGTGAAGGCTGCTG | 113 | [32] |
| R | CATCAAAGGTGGAGGAATGG | ||||
| ACTB | F | GCCCCCAAAGTTCTACAAT | 110 | ||
| R | AGGCGAGTAACTTCGTGTA | ||||
| 18S | F | CGAAAGCATTTGCCAAGAAT | 98 | ||
| R | GGCATCGTTTATGGTCGG | ||||
| YWHAZ | F | AGGAGCCGAGCTGTCCAATG | 85 | ||
| R | CTCCAAGATGACCTACGGGCTC | ||||
| HMBS | F | GGCTGGGAGAATCGCATAGG | 131 | ||
| R | TCCTGCAGGGCAGATACCAT | ||||
2.6. mRNA Expression
2.7. Statistical Analysis
2.8. Production Parameters Context
3. Results
3.1. Reference Gene Expression Stability
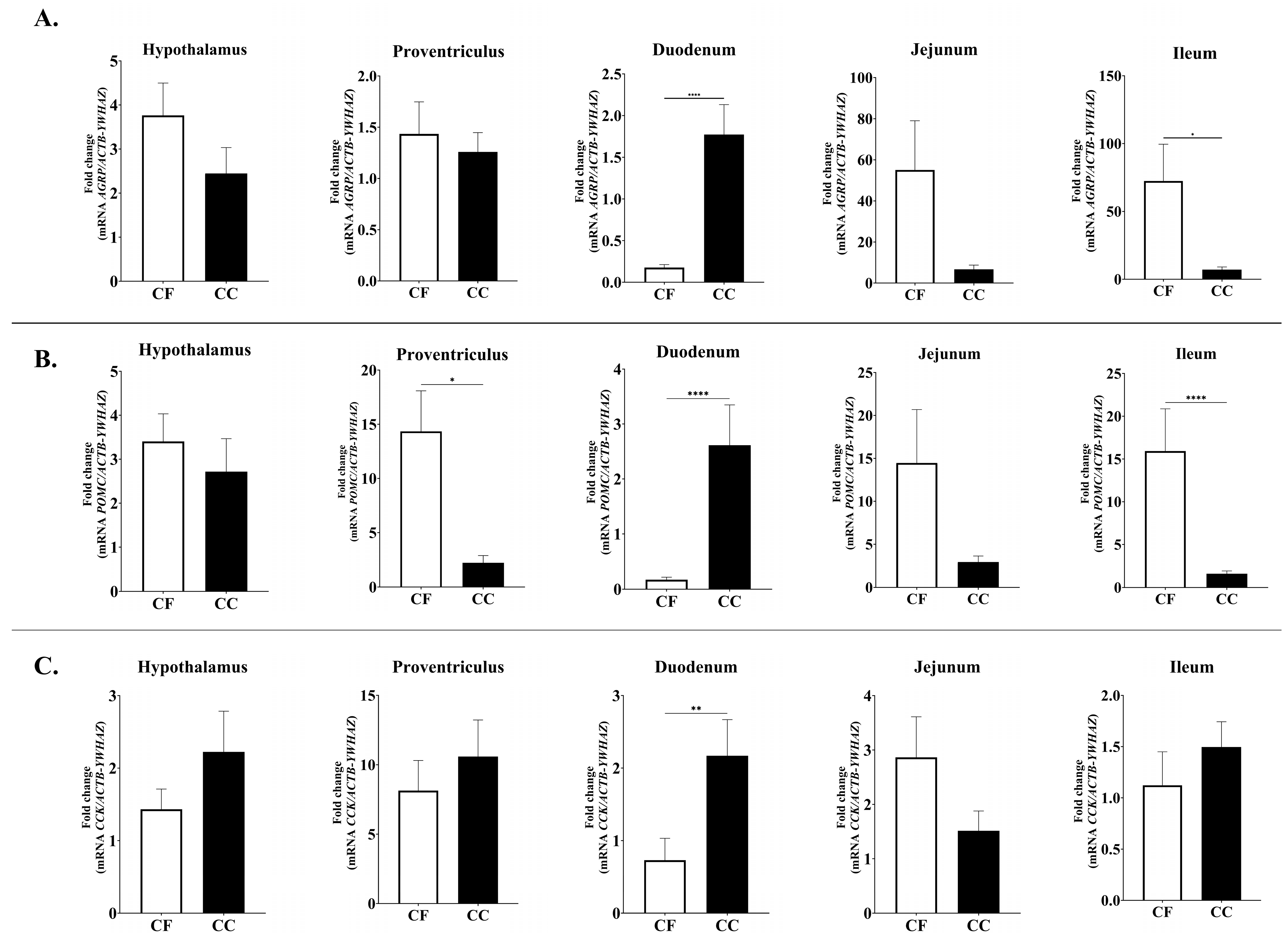
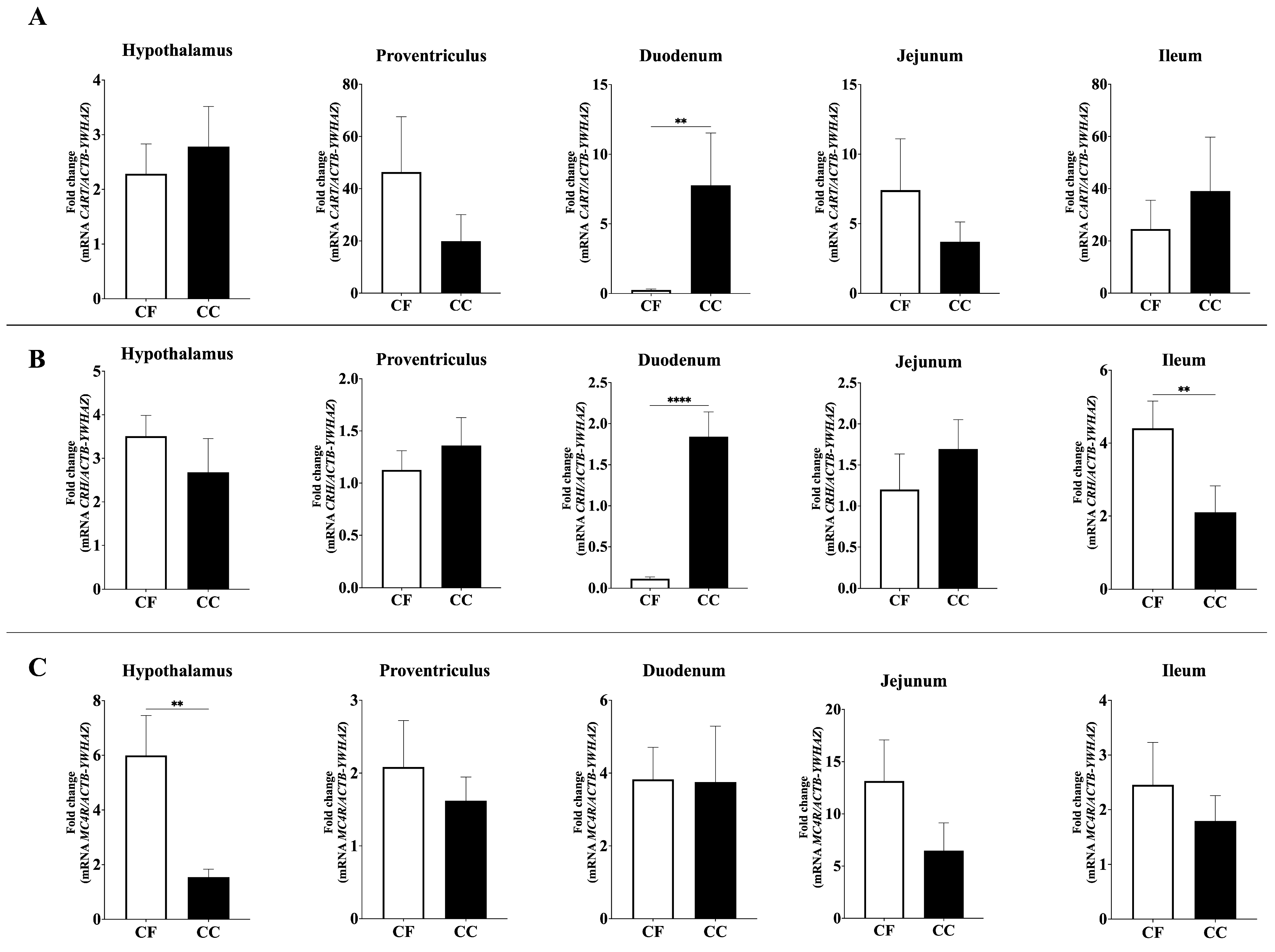
3.2. Expression of Feed Intake Regulatory Peptides
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CC | Conventional Cage |
| CF | Cage Free |
References
- Tona, G.O.; Tona, G.O. Current and Future Improvements in Livestock Nutrition and Feed Resources. In Animal Husbandry and Nutrition; Intech: London, UK, 2018. [Google Scholar] [CrossRef]
- Korver, D.R. Review: Current Challenges in Poultry Nutrition, Health, and Welfare. Animal 2023, 17, 100755. [Google Scholar] [CrossRef]
- Hartcher, K.M.; Jones, B. The Welfare of Layer Hens in Cage and Cage-Free Housing Systems. Worlds Poult. Sci. J. 2017, 73, 767–781. [Google Scholar] [CrossRef]
- Duncan, I.J.H. The Pros and Cons of Cages. Worlds Poult. Sci. J. 2001, 57, 386–390. [Google Scholar] [CrossRef]
- de Luna, M.C.T.; Yang, Q.; Agus, A.; Ito, S.; Idrus, Z.; Iman, R.H.S.; Jattuchai, J.; Lane, E.; Nuggehalli, J.; Hartcher, K.; et al. Cage Egg Producers’ Perspectives on the Adoption of Cage-Free Systems in China, Japan, Indonesia, Malaysia, Philippines, and Thailand. Front. Vet. Sci. 2022, 9, 1038362. [Google Scholar] [CrossRef]
- El Jeni, R.; Dittoe, D.K.; Olson, E.G.; Lourenco, J.; Seidel, D.S.; Ricke, S.C.; Callaway, T.R. An Overview of Health Challenges in Alternative Poultry Production Systems. Poult. Sci. 2021, 100, 101173. [Google Scholar] [CrossRef] [PubMed]
- Caputo, V.; Staples, A.J.; Tonsor, G.T.; Lusk, J.L. Egg Producer Attitudes and Expectations Regarding the Transition to Cage-Free Production: A Mixed-Methods Approach. Poult. Sci. 2023, 102, 103058. [Google Scholar] [CrossRef] [PubMed]
- Volyanskaya, A.R.; Akberdin, I.R.; Kulyashov, M.A.; Yevshin, I.S.; Romanov, M.N.; Shagimardanova, E.I.; Gusev, O.A.; Kolpakov, F.A. A Bird’s-Eye Overview of Molecular Mechanisms Regulating Feed Intake in Chickens—With Mammalian Comparisons. Anim. Nutr. 2024, 17, 61–74. [Google Scholar] [CrossRef]
- Song, Z.; Liu, L.; Yue, Y.; Jiao, H.; Lin, H.; Sheikhahmadi, A.; Everaert, N.; Decuypere, E.; Buyse, J. Fasting Alters Protein Expression of AMP-Activated Protein Kinase in the Hypothalamus of Broiler Chicks (Gallus Gallus Domesticus). Gen. Comp. Endocrinol. 2012, 178, 546–555. [Google Scholar] [CrossRef]
- Liu, L.; Song, Z.; Sheikhahmadi, A.; Jiao, H.; Lin, H. Effect of Corticosterone on Gene Expression of Feed Intake Regulatory Peptides in Laying Hens. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2012, 162, 81–87. [Google Scholar] [CrossRef]
- Kim, D.-H.; Lee, Y.-K.; Lee, S.-D.; Kim, S.-H.; Lee, K.-W. Physiological and Behavioral Responses of Laying Hens Exposed to Long-Term High Temperature. J. Therm. Biol. 2021, 99, 103017. [Google Scholar] [CrossRef]
- Kitazawa, T.; Kaiya, H. Regulation of Gastrointestinal Motility by Motilin and Ghrelin in Vertebrates. Front. Endocrinol. 2019, 10, 278. [Google Scholar] [CrossRef]
- Yu, Y.; Zhang, Y.H.; Xu, Z.Y.; Liu, T.Y.; Wang, Q.X.; Ou, C.B.; Ma, J.Y. Effects of IBDV Infection on Expression of Ghrelin and Ghrelin-Related Genes in Chicken. Poult. Sci. 2019, 98, 119–127. [Google Scholar] [CrossRef]
- Saneyasu, T. Recent Research on Mechanisms of Feeding Regulation in Chicks. J. Poult. Sci. 2024, 61, 2024012. [Google Scholar] [CrossRef]
- Campderrich, I.; Nazar, F.N.; Wichman, A.; Marin, R.H.; Estevez, I.; Keeling, L.J. Environmental Complexity: A Buffer against Stress in the Domestic Chick. PLoS ONE 2019, 14, e0210270. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Liu, L.; Sheikhahmadi, A.; Jiao, H.; Lin, H. Effect of Heat Exposure on Gene Expression of Feed Intake Regulatory Peptides in Laying Hens. J. Biomed. Biotechnol. 2012, 2012, 484869. [Google Scholar] [CrossRef]
- Dunlavey, C.J. Introduction to the Hypothalamic-Pituitary-Adrenal Axis: Healthy and Dysregulated Stress Responses, Developmental Stress and Neurodegeneration. J. Undergrad. Neurosci. Educ. 2018, 16, R59. [Google Scholar]
- Özentürk, U.; Yildiz, A. Comparison of Performance Parameters, Stress, and Immunity Levels of Native Andcommercial Layers Reared in Different Cage Densities in Turkey. Turk J. Vet. Anim. Sci. 2021, 45, 1052–1064. [Google Scholar] [CrossRef]
- Hofmann, T.; Schmucker, S.S.; Bessei, W.; Grashorn, M.; Stefanski, V. Impact of Housing Environment on the Immune System in Chickens: A Review. Animals 2020, 10, 1138. [Google Scholar] [CrossRef] [PubMed]
- Herrera-Sánchez, M.P.; Rodríguez-Hernández, R.; Rondón-Barragán, I.S. Stress-Related Gene Expression in Liver Tissues from Laying Hens Housed in Conventional Cage and Cage-Free Systems in the Tropics. Vet. Med. Int. 2024, 2024, 4107326. [Google Scholar] [CrossRef]
- Herrera-Sánchez, M.P.; Rodríguez-Hernández, R.; Rondón-Barragán, I.S. Comparative Transcriptome Analysis of Hens’ Livers in Conventional Cage vs. Cage-Free Egg Production Systems. Vet. Med. Int. 2025, 2025, 3041254. [Google Scholar] [CrossRef]
- Qaid, M.M.; Al-Garadi, M.A. Protein and amino acid metabolism in poultry during and after heat stress: A review. Animals 2021, 11, 1167. [Google Scholar] [CrossRef]
- Gao, Z.; Zheng, J.; Xu, G. Molecular Mechanisms and Regulatory Factors Governing Feed Utilization Efficiency in Laying Hens: Insights for Sustainable Poultry Production and Breeding Optimization. Int. J. Mol. Sci. 2025, 26, 6389. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Mao, Z.; Xuan, L.; Ma, G.; Wu, Y.; Xu, G. Residual Feed Intake in Late-Laying Hens: Immune Function, Metabolic Efficiency, and Feed Utilization Dynamics. Front. Vet. Sci. 2025, 12, 1624978. [Google Scholar] [CrossRef] [PubMed]
- Upadhaya, S.D.; Kim, I.H. The Impact of Weaning Stress on Gut Health and the Mechanistic Aspects of Several Feed Additives Contributing to Improved Gut Health Function in Weanling Piglets-A Review. Animals 2021, 11, 2418. [Google Scholar] [CrossRef]
- Mazzoni, M.; Zampiga, M.; Clavenzani, P.; Lattanzio, G.; Tagliavia, C.; Sirri, F. Effect of Chronic Heat Stress on Gastrointestinal Histology and Expression of Feed Intake-Regulatory Hormones in Broiler Chickens. Animal 2022, 16, 100600. [Google Scholar] [CrossRef]
- Jahan, A.A.; Dao, T.H.; Morgan, N.K.; Crowley, T.M.; Moss, A.F. Effects of AM/PM Diets on Laying Performance, Egg Quality, and Nutrient Utilisation in Free-Range Laying Hens. Appl. Sci. 2024, 14, 2163. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, R.; Rondón-Barragán, I.S.; Oviedo-Rondón, E.O. Egg Quality, Yolk Fatty Acid Profiles from Laying Hens Housed in Conventional Cage and Cage-Free Production Systems in the Andean Tropics. Animals 2024, 14, 168. [Google Scholar] [CrossRef]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate Normalization of Real-Time Quantitative RT-PCR Data by Geometric Averaging of Multiple Internal Control Genes. Genome Biol. 2002, 3, research0034-1. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.L.; Jensen, J.L.; Falck Ørntoft, T. Normalization of Real-Time Quantitative Reverse Transcription-PCR Data: A Model-Based Variance Estimation Approach to Identify Genes Suited for Normalization, Applied to Bladder and Colon Cancer Data Sets. Cancer Res. 2004, 64, 5245–5250. [Google Scholar] [CrossRef]
- Pfaffl, M.W.; Tichopad, A.; Prgomet, C.; Neuvians, T.P. Determination of Stable Housekeeping Genes, Differentially Regulated Target Genes and Sample Integrity: BestKeeper—Excel-Based Tool Using Pair-Wise Correlations. Biotechnol. Lett. 2004, 26, 509–515. [Google Scholar] [CrossRef]
- Rodríguez-Hernández, R.; Oviedo-Rondón, E.O.; Rondón-Barragán, I.S. Identification of Reliable Reference Genes for Expression Studies in the Magnum of Laying Hens Housed in Cage and Cage-free Systems. Vet. Med. Sci. 2021, 7, 1890–1898. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2-ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Baylan, M.; Kursun, K.; Abdallah, N.; Celik, L.B.; Yenilmez, F.; Kutay, H. The Effect of Housing Systems on the Growth, Egg Production, Overall Egg Weight and Egg Quality Traits of a New Turkish Laying Hen Hybrid, Akbay. Braz. J. Poult. Sci. 2024, 26, eRBCA-2024-1924. [Google Scholar] [CrossRef]
- Sharma, M.K.; McDaniel, C.D.; Kiess, A.S.; Loar, R.E.; Adhikari, P. Effect of Housing Environment and Hen Strain on Egg Production and Egg Quality as Well as Cloacal and Eggshell Microbiology in Laying Hens. Poult. Sci. 2022, 101, 101595. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Ma, R.; Qi, R.; Li, H.; Li, J.; Liu, W.; Wan, Y.; Liu, Z.; Li, S.; Chang, X.; et al. Study on the Changing Patterns of Production Performance of Laying Hens and Their Relationships with Environmental Factors in a Large-Scale Henhouse. Poult. Sci. 2024, 103, 104185. [Google Scholar] [CrossRef]
- Arulnathan, V.; Turner, I.; Bamber, N.; Ferdous, J.; Grassauer, F.; Doyon, M.; Pelletier, N. A systematic review of potential productivity, egg quality, and animal welfare implications of extended lay cycles in commercial laying hens in Canada. Poult. Sci. 2024, 103, 103475. [Google Scholar] [CrossRef]
- Richards, M.P.; Proszkowiec-Weglarz, M. Mechanisms Regulating Feed Intake, Energy Expenditure, and Body Weight in Poultry. Poult. Sci. 2007, 86, 1478–1490. [Google Scholar] [CrossRef]
- Xu, B.; Xie, X. Neurotrophic factor control of satiety and body weight. Nat. Rev. Neurosci. 2016, 17, 282–292. [Google Scholar] [CrossRef]
- Gao, Z.; Zheng, C.; Mao, Z.; Zheng, J.; Liu, D.; Xu, G. In-depth investigation of the mechanisms of high and low residual feed intake regulating hens during the late laying period via liver and gut microbiota. bioRxiv 2024. preprint. [Google Scholar] [CrossRef]
- Młynarska, E.; Bojdo, K.; Bulicz, A.; Frankenstein, H.; Gąsior, M.; Kustosik, N.; Rysz, J.; Franczyk, B. Obesity as a Multifactorial Chronic Disease: Molecular Mechanisms, Systemic Impact, and Emerging Digital Interventions. Curr. Issues Mol. Biol. 2025, 47, 787. [Google Scholar] [CrossRef]
- Wada, R.; Sakata, I.; Kaiya, H.; Nakamura, K.; Hayashi, Y.; Kangawa, K.; Sakai, T. Existence of ghrelin-immunopositive and-expressing cells in the proventriculus of the hatching and adult chicken. Regul. Pept. 2003, 111, 123–128. [Google Scholar] [CrossRef]
- Lu, V.B.; Gribble, F.M.; Reimann, F. Nutrient-induced cellular mechanisms of gut hormone secretion. Nutrients 2021, 13, 883. [Google Scholar] [CrossRef] [PubMed]
- Honda, K. Peripheral Regulation of Food Intake in Chickens: Adiposity Signals, Satiety Signals and Others. Worlds Poult. Sci. J. 2021, 77, 301–312. [Google Scholar] [CrossRef]
- Furuse, M.; Yamane, H.; Tomonaga, S.; Tsuneyoshi, Y.; Denbow, D.M. Neuropeptidergic Regulation of Food Intake in the Neonatal Chick: A Review. J. Poult. Sci. 2007, 44, 349–356. [Google Scholar] [CrossRef]
- Richards, M.P. Genetic Regulation of Feed Intake and Energy Balance in Poultry. Poult. Sci. 2003, 82, 907–916. [Google Scholar] [CrossRef] [PubMed]
- Sekeroglu, A.; Sarica, M.; Demir, E.; Ulutas, Z.; Tilki, M.; Saatci, M.; Omed, H. Effects of Different Housing Systems on Some Performance Traits and Egg Qualities of Laying Hens. J. Anim. Vet. Adv. 2010, 9, 1739–1744. [Google Scholar] [CrossRef]
- Al-Zghoul, M.B.; Alliftawi, A.R. ed S.; Saleh, K.M.M.; Jaradat, Z.W. Expression of Digestive Enzyme and Intestinal Transporter Genes during Chronic Heat Stress in the Thermally Manipulated Broiler Chicken. Poult. Sci. 2019, 98, 4113–4122. [Google Scholar] [CrossRef]
- Delporte, C. Structure and Physiological Actions of Ghrelin. Science 2013, 2013, 518909. [Google Scholar] [CrossRef]
- Choi, J.; Lee, J.; Kim, W.K. Changes in Gene and Protein Expression Related to Feed Intake and Thermoregulation in Broilers Challenged with Different Doses of Mixed Eimeria spp. Poult. Sci. 2025, 104, 105481. [Google Scholar] [CrossRef]
- Madonna, M.E.; Schurdak, J.; Yang, Y.K.; Benoit, S.; Millhauser, G.L. Agouti-Related Protein Segments Outside of the Receptor Binding Core Are Required for Enhanced Short- and Long-Term Feeding Stimulation. ACS Chem. Biol. 2012, 7, 395–402. [Google Scholar] [CrossRef]
- Dunn, I.C.; Wilson, P.W.; D’Eath, R.B.; Boswell, T. Hypothalamic Agouti-related Peptide MRNA Is Elevated during Natural and Stress-induced Anorexia. J. Neuroendocr. 2015, 27, 681–691. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Mehlkop, O.; Scharn, A.; Nolte, H.; Klemm, P.; Henschke, S.; Steuernagel, L.; Sotelo-Hitschfeld, T.; Kaya, E.; Wunderlich, C.M.; et al. Nutrient-Sensing AgRP Neurons Relay Control of Liver Autophagy during Energy Deprivation. Cell Metab. 2023, 35, 786–806.e13. [Google Scholar] [CrossRef]
- Wang, C.; Zhou, W.; He, Y.; Yang, T.; Xu, P.; Yang, Y.; Cai, X.; Wang, J.; Liu, H.; Yu, M.; et al. AgRP Neurons Trigger Long-Term Potentiation and Facilitate Food Seeking. Transl. Psychiatry 2021, 11, 1–17. [Google Scholar] [CrossRef]
- Liu, K.; Wen, Y.Y.; Liu, H.H.; Cao, H.Y.; Dong, X.Y.; Mao, H.G.; Yin, Z.Z. POMC Gene Expression, Polymorphism, and the Association with Reproduction Traits in Chickens. Poult. Sci. 2020, 99, 2895–2901. [Google Scholar] [CrossRef]
- Takeda, M.; Ohkubo, T. Identification of Hypothalamic Genes in Associating with Food Intake during Incubation Behavior in Domestic Chicken. Anim. Sci. J. 2019, 90, 1293–1302. [Google Scholar] [CrossRef]
- Gerets, H.H.J.; Peeters, K.; Arckens, L.; Vandesande, F.; Berghman, L.R. Sequence and Distribution of Pro-Opiomelanocortin in the Pituitary and the Brain of the Chicken (Gallus Gallus). J. Comp. Neurol. 2000, 417, 250–262. [Google Scholar] [CrossRef]
- Debold, C.R.; Nicholson, W.E.; Orth, D.N. Immunoreactive Proopiomelanocortin (POMC) Peptides and POMC-like Messenger Ribonucleic Acid Are Present in Many Rat Nonpituitary Tissues. Endocrinology 1988, 122, 2648–2657. [Google Scholar] [CrossRef] [PubMed]
- Fallahsharoudi, A.; De Kock, N.; Johnsson, M.; Ubhayasekera, S.J.K.A.; Bergquist, J.; Wright, D.; Jensen, P. Domestication Effects on Stress Induced Steroid Secretion and Adrenal Gene Expression in Chickens. Sci. Rep. 2015, 5, 15345. [Google Scholar] [CrossRef]
- Byerly, M.S.; Simon, J.; Lebihan-Duval, E.; Duclos, M.J.; Cogburn, L.A.; Porter, T.E. Effects of BDNF, T3, and Corticosterone on Expression of the Hypothalamic Obesity Gene Network in Vivo and in Vitro. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 296, R1180–R1189. [Google Scholar] [CrossRef] [PubMed]
- Svihus, B. Function of the Digestive System. J. Appl. Poult. Res. 2014, 23, 306–314. [Google Scholar] [CrossRef]
- Hadinia, S.H.; Carneiro, P.R.O.; Fitzsimmons, C.J.; Bédécarrats, G.Y.; Zuidhof, M.J. Post-Photostimulation Energy Intake Accelerated Pubertal Development in Broiler Breeder Pullets. Poult. Sci. 2020, 99, 2215–2229. [Google Scholar] [CrossRef]
- Tachibana, T.; Tsutsui, K. Neuropeptide Control of Feeding Behavior in Birds and Its Difference with Mammals. Front. Neurosci. 2016, 10, 228263. [Google Scholar] [CrossRef] [PubMed]
- Miyasaka, K.; Funakoshi, A. Cholecystokinin and Cholecystokinin Receptors. J. Gastroenterol. 2003, 38, 1–13. [Google Scholar] [CrossRef]
- Schwartz, G.J. The Role of Gastrointestinal Vagal Afferents in the Control of Food Intake: Current Prospects. Nutrition 2000, 16, 866–873. [Google Scholar] [CrossRef]
- Gozen, O.; Balkan, B.; Yararbas, G.; Koylu, E.O.; Kuhar, M.J.; Pogun, S. Sex Differences in the Regulation of Cocaine and Amphetamine-regulated Transcript Expression in Hypothalamic Nuclei of Rats by Forced Swim Stress. Synapse 2007, 61, 561–568. [Google Scholar] [CrossRef] [PubMed]
- Boswell, T.; Dunn, I.C. Regulation of Agouti-Related Protein and pro-Opiomelanocortin Gene Expression in the Avian Arcuate Nucleus. Front. Endocrinol. 2017, 8, 257776. [Google Scholar] [CrossRef]
- Tao, Y.X. The Melanocortin-4 Receptor: Physiology, Pharmacology, and Pathophysiology. Endocr. Rev. 2010, 31, 506–543. [Google Scholar] [CrossRef] [PubMed]
- Aboelhassan, D.M.; Darwish, H.R.; Mansour, H.; Abozaid, H.; Ghaly, I.S.; Radwan, H.A.; Hassan, E.R.; Farag, I.M. Polymorphisms and Expressions of ADSL, MC4R and CAPN1 Genes and Their Effects on Economic Traits in Egyptian Chicken Breeds. Mol. Biol. Rep. 2024, 51, 4. [Google Scholar] [CrossRef]
- Li, C.Y.; Li, H. Association of MC4R Gene Polymorphisms with Growth and Body Composition Traits in Chicken. Asian-Australas. J. Anim. Sci. 2006, 19, 763–768. [Google Scholar] [CrossRef]
- Kubota, S.; Vandee, A.; Keawnakient, P.; Molee, W.; Yongsawatdikul, J.; Molee, A. Effects of the MC4R, CAPN1, and ADSL Genes on Body Weight and Purine Content in Slow-Growing Chickens. Poult. Sci. 2019, 98, 4327–4337. [Google Scholar] [CrossRef]
- Herrera-Sánchez, M.P.; Lozano-Villegas, K.J.; Rondón-Barragán, I.S.; Rodríguez-Hernández, R. Identification of Reference Genes for Expression Studies in the Liver and Spleen of Laying Hens Housed in Cage and Cage-Free Systems. Open Vet. J. 2023, 13, 270–277. [Google Scholar]
- Gu, Y.; Tang, S.; Wang, Z.; Cai, L.; Lian, H.; Shen, Y.; Zhou, Y. A Pan-Cancer Analysis of the Prognostic and Immunological Role of β-Actin (ACTB) in Human Cancers. Bioengineered 2021, 12, 6166–6185. [Google Scholar] [CrossRef]
- Bahadoran, S.; Dehghani Samani, A.; Hassanpour, H. Effect of Heat Stress on the Gene Expression of Ion Transporters/Channels in the Uterus of Laying Hens during Eggshell Formation. Stress 2018, 21, 51–58. [Google Scholar] [CrossRef]
- Chapman, J.R.; Waldenström, J. With Reference to Reference Genes: A Systematic Review of Endogenous Controls in Gene Expression Studies. PLoS ONE 2015, 10, e0141853. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.B.; Shen, X.; Li, X.J.; Tian, Y.B.; Ouyang, H.J.; Huang, Y.M. Reference Gene Selection for Expression Studies in the Reproductive Axis Tissues of Magang Geese at Different Reproductive Stages under Light Treatment. Sci. Rep. 2021, 11, 7573. [Google Scholar] [CrossRef] [PubMed]
- Hassanpour, H.; Bahadoran, S.; Farhadfar, F.; Fallahi Chamali, Z.; Nazari, H.; Kaewduangta, W. Identification of Reliable Reference Genes for Quantitative Real-Time PCR in Lung and Heart of Pulmonary Hypertensive Chickens. Poult. Sci. 2018, 97, 4048–4056. [Google Scholar] [CrossRef]
- Jérôme, M.; Paudel, H.K. 14-3-3ζ Regulates Nuclear Trafficking of Protein Phosphatase 1α (PP1α) in HEK-293 Cells. Arch. Biochem. Biophys. 2014, 558, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Wan, R.P.; Liu, Z.G.; Huang, X.F.; Kwan, P.; Li, Y.P.; Qu, X.C.; Ye, X.G.; Chen, F.Y.; Zhang, D.W.; He, M.F.; et al. YWHAZ Variation Causes Intellectual Disability and Global Developmental Delay with Brain Malformation. Hum. Mol. Genet. 2023, 32, 462–472. [Google Scholar] [CrossRef]
| Tissue | geNorm | BestKeeper | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CC | CF | CC-CF | CC | CF | CC-CF | |||||||||
| Ranking | M Value | Ranking | M Value | Ranking | M Value | Ranking | SD | Ranking | SD | Ranking | SD | |||
| Proventriculus | 1 | 18S | 1.8 | ACTB | 1.0 | ACTB | 1.5 | 1 | ACTB | 0.7 | ACTB | 0.8 | ACTB | 0.7 |
| 2 | HMBS | 1.8 | HMBS | 1.0 | YWHAZ | 1.5 | 2 | YWHAZ | 1 | YWHAZ | 1 | YWHAZ | 1 | |
| 3 | ACTB | 2.4 | YWHAZ | 1.4 | 18S | 2.5 | 3 | GAPDH | 2.5 | HMBS | 1 | 18S | 2.5 | |
| 4 | YWHAZ | 2.8 | GAPDH | 2.1 | HMBS | 2.8 | 4 | 18S | 2.6 | 18S | 2.3 | GAPDH | 2.5 | |
| 5 | GAPDH | 3.5 | 18S | 2.7 | GAPDH | 3.3 | 5 | HMBS | 3.1 | GAPDH | 2.6 | HMBS | 2.7 | |
| Duodenum | 1 | YWHAZ | 0 | YWHAZ | 0.5 | YWHAZ | 0.3 | 1 | ACTB | 2.0 | ACTB | 0.8 | ACTB | 1.8 |
| 2 | GAPDH | 0 | GAPDH | 0.5 | GAPDH | 0.3 | 2 | HMBS | 3.4 | 18S | 1.6 | YWHAZ | 3.1 | |
| 3 | 18S | 2.5 | ACTB | 1.9 | ACTB | 2.4 | 3 | YWHAZ | 3.7 | YWHAZ | 2.5 | GAPDH | 3.1 | |
| 4 | ACTB | 3.4 | 18S | 2.8 | 18S | 3.0 | 4 | GAPDH | 3.7 | GAPDH | 2.5 | 18S | 3.2 | |
| 5 | HMBS | 4.5 | HMBS | 3.5 | HMBS | 3.9 | 5 | 18S | 4.3 | HMBS | 3.1 | HMBS | 3.5 | |
| Ileum | 1 | YWHAZ | 1.0 | YWHAZ | 1.1 | YWHAZ | 1.1 | 1 | ACTB | 0.8 | ACTB | 0.6 | ACTB | 1.0 |
| 2 | GAPDH | 1.0 | GAPDH | 1.1 | GAPDH | 1.1 | 2 | 18S | 0.8 | 18S | 1.6 | 18S | 1.6 | |
| 3 | ACTB | 1.6 | ACTB | 1.7 | ACTB | 1.6 | 3 | YWHAZ | 1.8 | YWHAZ | 1.8 | YWHAZ | 2 | |
| 4 | 18S | 2.2 | 18S | 2.5 | 18S | 2.3 | 4 | HMBS | 2.3 | GAPDH | 2.3 | GAPDH | 2.6 | |
| 5 | HMBS | 2.7 | HMBS | 3.1 | HMBS | 2.8 | 5 | GAPDH | 2.8 | HMBS | 2.6 | HMBS | 2.8 | |
| Jejunum | 1 | YWHAZ | 0.8 | YWHAZ | 0.5 | YWHAZ | 0.6 | 1 | ACTB | 1.5 | 18S | 1 | 18S | 1.4 |
| 2 | GAPDH | 0.8 | GAPDH | 0.5 | GAPDH | 0.6 | 2 | 18S | 0.1 | ACTB | 1.6 | ACTB | 1.5 | |
| 3 | ACTB | 1.7 | ACTB | 1.5 | ACTB | 1.5 | 3 | YWHAZ | 2.5 | HMBS | 2.8 | HMBS | 2.7 | |
| 4 | 18S | 2.6 | 18S | 2.3 | 18S | 2.4 | 4 | HMBS | 2.6 | YWHAZ | 3.3 | YWHAZ | 2.9 | |
| 5 | HMBS | 3.2 | HMBS | 2.8 | HMBS | 2.9 | 5 | GAPDH | 2.8 | GAPDH | 3.6 | GAPDH | 3.3 | |
| Tissue | NormFinder | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CC | CF | CC-CF | The Best Combination of Two Genes | ||||||
| Ranking | Stability Value | Ranking | Stability Value | Ranking | Stability Value | Genes | Stability Value | ||
| Proventriculus | 1 | ACTB | 0.775 | ACTB | 0.516 | ACTB | 0.772 | ACTB-YWHAZ | 0.399 |
| 2 | YWHAZ | 1.108 | HMBS | 0.516 | YWHAZ | 1.016 | |||
| 3 | 18S | 2.604 | YWHAZ | 0.532 | HMBS | 2.925 | |||
| 4 | HMBS | 3.476 | GAPDH | 3.505 | 18S | 2.961 | |||
| 5 | GAPDH | 4.32 | 18S | 3.58 | GAPDH | 3.743 | |||
| Duodenum | 1 | ACTB | 1.781 | ACTB | 1.084 | ACTB | 0.795 | ACTB-YWHAZ | 0.838 |
| 2 | 18s | 2.205 | 18S | 1.929 | 18S | 1.983 | |||
| 3 | YWHAZ | 3.184 | GAPDH | 3.055 | GAPDH | 2.98 | |||
| 4 | GAPDH | 3.184 | YWHAZ | 3.077 | YWHAZ | 2.984 | |||
| 5 | HMBS | 5.82 | HMBS | 4.3 | HMBS | 4.878 | |||
| Ileum | 1 | ACTB | 0.753 | ACTB | 0.917 | ACTB | 0.811 | ACTB-YWHAZ | 0.537 |
| 2 | YWHAZ | 1.576 | 18S | 0.1886 | 18S | 1.751 | |||
| 3 | 18S | 1.803 | YWHAZ | 2.248 | YWHAZ | 1.841 | |||
| 4 | GAPDH | 2.723 | GAPDH | 3.004 | GAPDH | 2.766 | |||
| 5 | HMBS | 3.212 | HMBS | 3.7 | HMBS | 3.384 | |||
| Jejunum | 1 | ACTB | 0.931 | ACTB | 0.931 | ACTB | 0.888 | ACTB-YWHAZ | 0.929 |
| 2 | YWHAZ | 2.192 | YWHAZ | 1.994 | YWHAZ | 2.026 | |||
| 3 | 18S | 2.33 | 18S | 2.071 | 18S | 2.1 | |||
| 4 | GAPDH | 3.006 | GAPDH | 2.481 | GAPDH | 2.634 | |||
| 5 | HMBS | 3.738 | HMBS | 3.295 | HMBS | 3.474 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lozano-Villegas, K.J.; Herrera-Sánchez, M.P.; Rondón-Barragán, I.S.; Rodríguez-Hernández, R. Gene Expression of Feed Intake-Regulating Peptides in the Gut–Brain Axis of Laying Hens Housed Under Two Different Egg Production Systems. Animals 2025, 15, 3127. https://doi.org/10.3390/ani15213127
Lozano-Villegas KJ, Herrera-Sánchez MP, Rondón-Barragán IS, Rodríguez-Hernández R. Gene Expression of Feed Intake-Regulating Peptides in the Gut–Brain Axis of Laying Hens Housed Under Two Different Egg Production Systems. Animals. 2025; 15(21):3127. https://doi.org/10.3390/ani15213127
Chicago/Turabian StyleLozano-Villegas, Kelly Johanna, María Paula Herrera-Sánchez, Iang Schroniltgen Rondón-Barragán, and Roy Rodríguez-Hernández. 2025. "Gene Expression of Feed Intake-Regulating Peptides in the Gut–Brain Axis of Laying Hens Housed Under Two Different Egg Production Systems" Animals 15, no. 21: 3127. https://doi.org/10.3390/ani15213127
APA StyleLozano-Villegas, K. J., Herrera-Sánchez, M. P., Rondón-Barragán, I. S., & Rodríguez-Hernández, R. (2025). Gene Expression of Feed Intake-Regulating Peptides in the Gut–Brain Axis of Laying Hens Housed Under Two Different Egg Production Systems. Animals, 15(21), 3127. https://doi.org/10.3390/ani15213127









