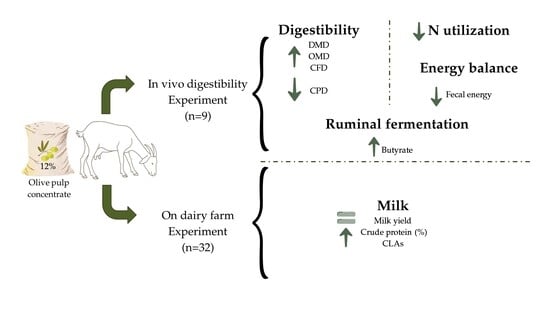
Inclusion of Novel Olive Pulp: Impacts on Nutrient Digestibility, Rumen Fermentation, and Dairy Goat Performance
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Experiments
2.3. Experimental Diets
2.4. Experimental Procedure
2.4.1. Experiment 1: In Vivo Trial for the Evaluation of Nutrient Digestibility, N Utilization and Energy Balance from the Inclusion of OP in Goat Feed
2.4.2. Experiment 2: On-Farm Trial for the Evaluation of the Effect of the Inclusion of OP in the Diet of Dairy Goats on the Evolution of Live Weight, Milk Yield, Composition, and Fatty Acid Profile
2.5. Sample Analysis, Calculations and Statistical Analysis
2.5.1. Chemical Composition of the Diets
2.5.2. Determination of the Ruminal Fermentation Profile (SCFA and N-NH3)
2.5.3. Purine Derivatives and Creatinine in Urine
2.5.4. Calculations and Statistical Analysis
- Digestible N (g/kg0.75) = (N intake − N in feces)/Metabolic weight.
- N balance (g/kg0.75) = (Digestible N − N in urine)/Metabolic weight.
- Digestible energy (DE) (MJ/kg0.75) = (Energy intake − Energy in feces)/Metabolic weight.
- Metabolizable energy (ME) (MJ/kg0.75) = (DE − Energy in urine − Methane energy)/Metabolic weight.
3. Results
3.1. Experiment 1: In Vivo Trial for the Evaluation of Nutrient Digestibility, N Utilization and Energy Balance from the Inclusion of OP in Goat Feed
3.2. Experiment 2: On-Farm Trial to Evaluate the Effects of the Inclusion of OP in Dairy Goat Feeding: Animal Performance, Milk Yield, Composition and Fat Lipid Profile
3.2.1. Animal Performance
3.2.2. Milk Yield, Composition and Fatty Acid Profile
4. Discussion
4.1. Chemical Composition of OP
4.2. Digestibility Assay, N Utilization and Energy Balance
4.3. Ruminal Fermentation Parameters
4.4. Milk Production, Crude Composition and Fatty Acid Profile
4.5. Suitability of the Use of OP for the Dairy Goat Sector
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABP | Agroindustrial by-products |
| ADF | Acid detergent fiber |
| ADL | Acid detergent lignin |
| CF | Crude fat |
| CLA | Conjugated linoleic acid |
| CP | Crude protein |
| CTL | Diet of control group |
| DDGS | Distiller’s dried grains with solubles. |
| DDVOP | Dried destoned virgin olive pomace |
| DE | Digestible energy |
| DHA | Docosahexaenoic acid |
| DIM | Days in milk |
| DM | Dry matter |
| DPA | Docosapentaenoic acid |
| EPA | Eicosapentaenoic acid |
| FAO | Food and Agriculture Organization |
| FPCM | Fat-protein corrected milk |
| GE | Gross energy |
| GEI | Gross energy intake |
| IPCC | Intergovernmental Panel on Climate Change |
| LCA | Life-cycle assessment |
| ME | Metabolizable energy |
| MUFA | Monounsaturated fatty acids |
| N | Nitrogen |
| NDF | Neutral detergent fiber |
| NSC | Non-structural carbohydrates |
| OBP | Olive by-products |
| OM | Organic matter |
| OP | Olive pulp |
| OPC | Olive pulp concentrate with 12% DM of inclusion of OP |
| OPD | Olive pulp diet including OPC |
| PUFA | Polyunsaturated fatty acids |
| SCFA | Short-chain fatty acids |
| SEM | Standard error of mean |
| SFA | Saturated fatty acids |
| VA | Vaccenic acid |
References
- FAO. The Future of Food and Agriculture: Trends and Challenges. 2017. Available online: https://openknowledge.fao.org/items/ede32306-aeec-4891-9fe6-7e4f2fd93143 (accessed on 19 September 2025).
- Scotto, A.L. Impatto Ambientale dei Rifiuti e Degli Sprechi Agroalimentari in Europa e in Italia. Ph.D. Thesis, Alma Mater Studiorum Università di Bologna, Bologna, Italy, 26 July 2012. [Google Scholar]
- Pérez Barbería, F.J. The Ruminant: Life History and Digestive Physiology of a Symbiotic Animal. In Sustainable and Environmentally Friendly Dairy Farms; SpringerBriefs in Applied Sciences and Technology; Springer International Publishing: Cham, Switzerland, 2020; pp. 19–45. [Google Scholar] [CrossRef]
- Arco-Pérez, A.; Ramos-Morales, E.; Yáñez-Ruiz, D.R.; Abecia, L.; Martín-García, A.I. Nutritive evaluation and milk quality of including of tomato or olive by-products silages with sunflower oil in the diet of dairy goats. Anim. Feed. Sci. Technol. 2017, 232, 57–70. [Google Scholar] [CrossRef]
- Lopreiato, V.; Ferronato, G.; Amato, A.; Cavallo, C.; Trevisi, E.; Llobat, L.; Chiofalo, V.; Liotta, L. Effects of dietary supplementation with olive cake enriched in polyphenols on growth, rumen fermentation, and metabolic status of finishing Limousine bulls. Ital. J. Anim. Sci. 2025, 24, 174–181. [Google Scholar] [CrossRef]
- MAPA. Resumen Anual de la Alimentación. 2022. Available online: https://www.mapa.gob.es/es/alimentacion/temas/consumo-tendencias/panel-de-consumo-alimentario/resumen-anual-de-la-alimentacion/ (accessed on 19 September 2025).
- Tzamaloukas, O.; Neofytou, M.C.; Simitzis, P.E. Application of Olive By-Products in Livestock with Emphasis on Small Ruminants: Implications on Rumen Function, Growth Performance, Milk and Meat Quality. Animals 2021, 11, 531. [Google Scholar] [CrossRef]
- El Otmani, S.; Chebli, Y.; Chentouf, M.; Hornick, J.-L.; Cabaraux, J.-F. Effects of Olive Cake and Cactus Cladodes as Alternative Feed Resources on Goat Milk Production and Quality. Agriculture 2021, 11, 3. [Google Scholar] [CrossRef]
- Fernandez Mayer, A. Actualización sobre el uso de orujos de aceituna en alimentación animal para carne y leche = Olive pomace use on animal feeding for meat and milk production: A review. Rev Foro Aliment. Nutr. Salud 2021, 3, 31–39. [Google Scholar]
- Ghanbari, R.; Anwar, F.; Alkharfy, K.M.; Gilani, A.-H.; Saari, N. Valuable Nutrients and Functional Bioactives in Different Parts of Olive (Olea europaea L.)—A Review. Int. J. Mol. Sci. 2012, 13, 3291–3340. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; Gonzálvez, J.; García, D.; Cegarra, J. Agrochemical characterisation of “alperujo”, a solid by-product of the two-phase centrifugation method for olive oil extraction. Bioresour. Technol. 2004, 91, 195–200. [Google Scholar] [CrossRef]
- Sanchez-Garcia, A.M.; Huelva, M.R.; Martin-Garcia, A.I. Evaluación y caracterización in vitro de la pulpa de aceituna como ingrediente en piensos de caprino lechero. C3-Bioeconomy Circ. Sustain. Bioeconomy 2024, 5, 69–86. [Google Scholar] [CrossRef]
- Benincasa, C.; Pellegrino, M.; Veltri, L.; Claps, S.; Fallara, C.; Perri, E. Dried Destoned Virgin Olive Pomace: A Promising New By-Product from Pomace Extraction Process. Molecules 2021, 26, 4337. [Google Scholar] [CrossRef] [PubMed]
- Romero-Huelva, M.; Ramos-Morales, E.; Molina-Alcaide, E. Nutrient utilization, ruminal fermentation, microbial abundances, and milk yield and composition in dairy goats fed diets including tomato and cucumber waste fruits. J. Dairy Sci. 2012, 95, 6015–6026. [Google Scholar] [CrossRef] [PubMed]
- Molina-Alcaide, E.; Yáñez-Ruiz, D.R. Potential use of olive by-products in ruminant feeding: A review. Anim. Feed. Sci. Technol. 2008, 147, 247–264. [Google Scholar] [CrossRef]
- MAPA. Evolución de los Precios. 2025. Available online: https://www.mapa.gob.es/es/agricultura/temas/producciones-agricolas/cultivos-herbaceos/cereales/evolucion-de-los-precios-de-los-principales-cereales (accessed on 17 October 2025).
- Aguilera, J.F.; Prieto, C.; Fonollá, J. Protein and energy metabolism of lactating Granadina goats. Br. J. Nutr. 1990, 63, 165–175. [Google Scholar] [CrossRef]
- Belanche, A.; Palma-Hidalgo, J.M.; Nejjam, I.; Jiménez, E.; Martín-García, A.I.; Yáñez-Ruiz, D.R. Inoculation with rumen fluid in early life as a strategy to optimize the weaning process in intensive dairy goat systems. J. Dairy Sci. 2020, 103, 5047–5060. [Google Scholar] [CrossRef] [PubMed]
- Aguilera, J.F. Aportaciones al conocimiento de la nutrición energética de pequeños rumiantes, con particular referencia al ganado caprino. Archivos de Zootecnia 2001, 50, 8. [Google Scholar]
- Folch, J.; Lees, M.; Sloane Stanley, G.H. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957, 226, 497–509. [Google Scholar] [CrossRef]
- Latimer, G.W., Jr. (Ed.) Official Methods of Analysis, 22nd ed.; AOAC International: Gaithersburg, MD, USA, 2023; Available online: https://www.aoac.org/official-methods-of-analysis/ (accessed on 19 September 2025).
- Van Soest, P.J.; Robertson, J.B.; Lewis, B.A. Methods for dietary fiber, neutral detergent fiber, and nonstarch polysaccharides in relation to animal nutrition. J. Dairy Sci. 1991, 74, 3583–3597. [Google Scholar] [CrossRef]
- Pardo, Z.; Palma-Hidalgo, J.M.; Sánchez-García, A.M.; Martín-García, A.I. Chemical composition and in vitro nutritional assessment of watermelon (Citrullus lanatus) plant silage as a forage option for Murciano-Granadina goats. PLoS ONE 2025, 20, e0323553. [Google Scholar] [CrossRef]
- Weatherburn, M.W. Phenol-hypochlorite reaction for determination of ammonia. Anal. Chem. 1967, 39, 971–974. [Google Scholar] [CrossRef]
- Mancilla-Leytón, J.M.; Morales-Jerrett, E.; Delgado-Pertiñez, M.; Mena, Y. Fat- and protein-corrected milk formulation to be used in the life-cycle assessment of Mediterranean dairy goat systems. Livest. Sci. 2021, 253, 104697. [Google Scholar] [CrossRef]
- Marcos, C.N.; Carro, M.D.; Fernández Yepes, J.E.; Haro, A.; Romero-Huelva, M.; Molina-Alcaide, E. Effects of agroindustrial by-product supplementation on dairy goat milk characteristics, nutrient utilization, ruminal fermentation, and methane production. J. Dairy Sci. 2020, 103, 1472–1483. [Google Scholar] [CrossRef]
- Marcos, C.N.; Chávez, S.; Blas, C.; Molina-Alcaide, E.; Ranilla, M.J.; Carro, M.D. Chemical composition and in vitro rumen fermentation of crude olive cake and olive extracts. Options Méditerranéennes Ser. A 2019, 123, 123–128. [Google Scholar]
- Molina Alcaide, E.; Yáñez Ruiz, D.; Moumen, A.; Martín García, I. Chemical composition and nitrogen availability for goats and sheep of some olive by-products. Small Rumin. Res. 2003, 49, 329–336. [Google Scholar] [CrossRef]
- Hassan, M.; Belanche, A.; Jiménez, E.; Rivelli, I.; Martín-García, A.I.; Margolles, A.; Yáñez-Ruiz, D.R. Evaluation of the nutritional value and presence of minerals and pesticides residues in agro-industrial by-products to replace conventional ingredients of small ruminant diets. Small Rumin. Res. 2023, 229, 107117. [Google Scholar] [CrossRef]
- Yáñez-Ruiz, D.R.; Molina-Alcaide, E. A comparative study of nutrients utilization, alkaline phosphatase activity and creatinine concentration in the serum of sheep and goats fed diets based on olive leaves. J. Anim. Physiol. Anim. Nutr. 2008, 92, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Arco, A.; Yáñez-Ruiz, D.R.; Martín-García, A.I. Is it safe using olive and green-house agroindustrial by-products in dairy goats feeding ? Options Méditerranéennes Ser. A 2016, 115, 275–279. [Google Scholar]
- Correddu, F.; Lunesu, M.F.; Buffa, G.; Atzori, A.S.; Nudda, A.; Battacone, G.; Pulina, G. Can Agro-Industrial By-Products Rich in Polyphenols be Advantageously Used in the Feeding and Nutrition of Dairy Small Ruminants? Animals 2020, 10, 131. [Google Scholar] [CrossRef]
- Obeidat, B.S.; Thomas, M.G. Growth Performance, Blood Metabolites and Carcass Characteristics of Black Goat Kids Fed Diets Containing Olive Cake. Animals 2024, 14, 272. [Google Scholar] [CrossRef] [PubMed]
- IPCC. IPCC Guidelines for National Greenhouse Gas In-Ventories; IGES: Hayama, Japan, 2006; Available online: http://www.ipcc-nggip.iges.or.jp/ (accessed on 19 September 2025).
- Martín García, A.I.; Yáñez Ruiz, D.R.; Moumen, A.; Molina Alcaide, E. Effect of polyethylene-glycol on the chemical composition and nutrient availability of olive (Olea europaea var. Europaea) by-products. Anim. Feed. Sci. Technol. 2004, 114, 159–177. [Google Scholar] [CrossRef]
- Formato, M.; Vastolo, A.; Piccolella, S.; Calabrò, S.; Cutrignelli, M.I.; Zidorn, C.; Pacifico, S. Castanea sativa Mill. Leaf: UHPLC-HR MS/MS Analysis and Effects on In Vitro Rumen Fermentation and Methanogenesis. Molecules 2022, 27, 8662. [Google Scholar] [CrossRef]
- Zhang, J.; Bu, L.; Liu, Y.; Huo, W.; Xia, C.; Pei, C.; Liu, Q. Dietary supplementation of sodium butyrate enhances lactation performance by promoting nutrient digestion and mammary gland development in dairy cows. Anim. Nutr. 2023, 15, 137–148. [Google Scholar] [CrossRef]
- Pallara, G.; Buccioni, A.; Pastorelli, R.; Minieri, S.; Mele, M.; Rapaccini, S.; Messini, A.; Pauselli, M.; Servili, M.; Giovannetti, L.; et al. Effect of stoned olive pomace on rumen microbial communities and polyunsaturated fatty acid biohydrogenation: An in vitrostudy. BMC Vet. Res. 2014, 10, 271. [Google Scholar] [CrossRef] [PubMed]
- Keles, G.; Yildiz-Akgul, F.; Kocaman, V. Performance and milk composition of dairy goats as affected by the dietary level of stoned olive cake silages. Asian-Australas. J. Anim. Sci. 2017, 30, 363–369. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Castellani, F.; Vitali, A.; Bernardi, N.; Marone, E.; Palazzo, F.; Grotta, L.; Martino, G. Dietary supplementation with dried olive pomace in dairy cows modifies the composition of fatty acids and the aromatic profile in milk and related cheese. J. Dairy Sci. 2017, 100, 8658–8669. [Google Scholar] [CrossRef] [PubMed]
- Buccioni, A.; Serra, A.; Minieri, S.; Mannelli, F.; Cappucci, A.; Benvenuti, D.; Rapaccini, S.; Conte, G.; Mele, M. Milk production, composition, and milk fatty acid profile from grazing sheep fed diets supplemented with chestnut tannin extract and extruded linseed. Small Rumin. Res. 2015, 130, 200–207. [Google Scholar] [CrossRef]
- Taormina, V.M.; Unger, A.L.; Schiksnis, M.R.; Torres-Gonzalez, M.; Kraft, J. Branched-Chain Fatty Acids—An Underexplored Class of Dairy-Derived Fatty Acids. Nutrients 2020, 12, 2875. [Google Scholar] [CrossRef]
- Liang, J.; Zhou, Q.; Kwame Amakye, W.; Su, Y.; Zhang, Z. Biomarkers of dairy fat intake and risk of cardiovascular disease: A systematic review and meta analysis of prospective studies. Crit. Rev. Food Sci. Nutr. 2018, 58, 1122–1130. [Google Scholar] [CrossRef]
- Khaw, K.-T.; Friesen, M.D.; Riboli, E.; Luben, R.; Wareham, N. Plasma phospholipid fatty acid concentration and incident coronary heart disease in men and women: The EPIC-Norfolk prospective study. PLoS Med. 2012, 9, e1001255. [Google Scholar] [CrossRef]
- Or-Rashid, M.M.; Odongo, N.E.; McBride, B.W. Fatty acid composition of ruminal bacteria and protozoa, with emphasis on conjugated linoleic acid, vaccenic acid, and odd-chain and branched-chain fatty acids1. J. Anim. Sci. 2007, 85, 1228–1234. [Google Scholar] [CrossRef]
- Fievez, V.; Colman, E.; Castro-Montoya, J.M.; Stefanov, I.; Vlaeminck, B. Milk odd- and branched-chain fatty acids as biomarkers of rumen function—An update. Anim. Feed. Sci. Technol. 2012, 172, 51–65. [Google Scholar] [CrossRef]
- Badawy, S.; Liu, Y.; Guo, M.; Liu, Z.; Xie, C.; Marawan, M.A.; Ares, I.; Lopez-Torres, B.; Martínez, M.; Maximiliano, J.-E.; et al. Conjugated linoleic acid (CLA) as a functional food: Is it beneficial or not? Food Res. Int. 2023, 172, 113158. [Google Scholar] [CrossRef]
- Basiricò, L.; Morera, P.; Dipasquale, D.; Tröscher, A.; Serra, A.; Mele, M.; Bernabucci, U. Conjugated linoleic acid isomers strongly improve the redox status of bovine mammary epithelial cells (BME-UV1). J. Dairy Sci. 2015, 98, 7071–7082. [Google Scholar] [CrossRef] [PubMed]
- Cappucci, A.; Alves, S.P.; Bessa, R.J.B.; Buccioni, A.; Mannelli, F.; Pauselli, M.; Viti, C.; Pastorelli, R.; Roscini, V.; Serra, A.; et al. Effect of increasing amounts of olive crude phenolic concentrate in the diet of dairy ewes on rumen liquor and milk fatty acid composition. J. Dairy Sci. 2018, 101, 4992–5005. [Google Scholar] [CrossRef]
- Molina-Alcaide, E.; Morales-García, E.Y.; Martín-García, A.I.; Ben Salem, H.; Nefzaoui, A.; Sanz-Sampelayo, M.R. Effects of partial replacement of concentrate with feed blocks on nutrient utilization, microbial N flow, and milk yield and composition in goats. J. Dairy Sci. 2010, 93, 2076–2087. [Google Scholar] [CrossRef]
- Aro, A.; Antoine, J.M.; Pizzoferrato, L.; Reykdal, O.; van Poppel, G. Trans Fatty Acids in Dairy and Meat Products from 14 European Countries: The TRANSFAIR Study. J. Food Compos. Anal. 1998, 11, 150–160. [Google Scholar] [CrossRef]
- Pipoyan, D.; Stepanyan, S.; Stepanyan, S.; Beglaryan, M.; Costantini, L.; Molinari, R.; Merendino, N. The Effect of Trans Fatty Acids on Human Health: Regulation and Consumption Patterns. Foods 2021, 10, 2452. [Google Scholar] [CrossRef]
- Ianni, A.; Innosa, D.; Oliva, E.; Bennato, F.; Grotta, L.; Saletti, M.A.; Pomilio, F.; Sergi, M.; Martino, G. Effect of olive leaves feeding on phenolic composition and lipolytic volatile profile in goat milk. J. Dairy Sci. 2021, 104, 8835–8845. [Google Scholar] [CrossRef]
- Litrenta, F.; Cincotta, F.; Russo, N.; Cavallo, C.; Caggia, C.; Amato, A.; Lopreiato, V.; Merlino, M.; Verzera, A.; Randazzo, C.L.; et al. Feeding Cows with Olive Cake Enriched in Polyphenols Improves the Sustainability and Enhances the Nutritional and Organoleptic Features of Fresh Caciocavallo Cheese. Foods 2024, 13, 3320. [Google Scholar] [CrossRef]
- Leparmarai, P.T.; Sinz, S.; Kunz, C.; Liesegang, A.; Ortmann, S.; Kreuzer, M.; Marquardt, S. Transfer of total phenols from a grapeseed-supplemented diet to dairy sheep and goat milk, and effects on performance and milk quality. J. Anim. Sci. 2019, 97, 1840–1851. [Google Scholar] [CrossRef]
- Valenti, B.; Luciano, G.; Morbidini, L.; Rossetti, U.; Codini, M.; Avondo, M.; Priolo, A.; Bella, M.; Natalello, A.; Pauselli, M. Dietary Pomegranate Pulp: Effect on Ewe Milk Quality During Late Lactation. Animals 2019, 9, 283. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Ying, H.; Stefanovski, D.; Shurson, G.C.; Chen, T.; Wang, Z.; Yin, Y.; Zheng, H.; Nakaishi, T.; Li, J.; et al. Food waste used as a resource can reduce climate and resource burdens in agrifood systems. Nat. Food 2025, 6, 478–490. [Google Scholar] [CrossRef] [PubMed]
| Concentrates | ||||
|---|---|---|---|---|
| Ingredients (% DM) | OP | Oat Hay | CTL | OPC |
| Bran | 17.0 | 15.0 | ||
| Maize grain | 20.0 | 17.6 | ||
| Soya (47% CP) | 19.6 | 17.3 | ||
| OP | - | 12.0 | ||
| Soybean hull | 11.4 | 10.0 | ||
| Maize DDGS | 6.42 | 5.65 | ||
| Sugarbeet pulp | 9.96 | 8.77 | ||
| Barley | 4.55 | 4.00 | ||
| Wheat | 7.48 | 6.59 | ||
| Additives 1 | 3.61 | 3.18 | ||
| Total | 100 | 100 | ||
| Nutrients (% DM) | ||||
| DM | 91.5 | 86.1 | 88.4 | 89.2 |
| OM | 88.2 | 81.0 | 93.2 | 93.4 |
| NFC | 21.2 | 12.7 | 32.9 | 40.5 |
| CP | 9.87 | 14.4 | 19.5 | 17.2 |
| CF | 7.90 | 2.40 | 4.03 | 4.03 |
| NDF | 49.2 | 51.5 | 36.7 | 31.7 |
| ADF | 35.9 | 26.9 | 17.3 | 15.0 |
| ADL | 24.5 | 5.01 | 4.98 | 5.37 |
| GE (MJ/kg DM) | 21.0 | 15.8 | 17.9 | 18.6 |
| Minerals (%) | ||||
| Ca | 1.05 | 2.44 | 0.75 | 0.71 |
| K | 3.15 | 1.54 | 0.94 | 0.98 |
| Mg | 0.40 | 0.21 | 0.32 | 0.22 |
| Na | 0.14 | 0.53 | 0.48 | 0.31 |
| P | 0.27 | 0.20 | 0.47 | 0.38 |
| S | 0.14 | 0.29 | 0.26 | 0.20 |
| Essential trace minerals (mg/kg) | ||||
| Al | 1012 | 2032 | 182 | 205 |
| Cr | 16.4 | 5.33 | 2.85 | 2.62 |
| Cu | 26.7 | 24.7 | 8.55 | 7.79 |
| Fe | 718 | 1276 | 282 | 237 |
| Mn | 56.8 | 100 | 143 | 91.8 |
| Ni | 12.8 | 4.19 | 2.95 | 2.81 |
| Si | 533 | 98.1 | 347 | 337 |
| V | 0.93 | 2.69 | 0.01 | <0.01 |
| Zn | 30.9 | 49.2 | 179 | 129 |
| Non-essential trace minerals (mg/kg) | ||||
| Sr | 48.0 | 185 | 22.0 | 23.9 |
| Ti | 2.39 | 3.73 | 1.61 | 1.64 |
| Concentrate cost (EUR/ton) | 246.8 | 228.9 | ||
| Concentrates | ||||
|---|---|---|---|---|
| Fatty Acids (g/100 g) | OP | Oat Hay | CTL | OPC |
| C8 | 0.063 | 0.144 | - | 0.066 |
| C10 | 0.778 | 1.41 | 0.237 | 0.344 |
| C11 | - | - | 0.142 | 0.176 |
| C12 | - | 1.05 | 0.582 | 0.142 |
| C14 | 0.282 | 2.50 | 0.992 | 0.466 |
| C14:1 | 2.19 | - | - | 0.500 |
| C15 | 0.236 | - | - | - |
| C15:1 | - | 0.287 | - | - |
| C16 | 16.2 | 29.9 | 23.4 | 18.6 |
| C16:1 | 1.27 | 1.00 | 0.254 | 0.452 |
| C16:1 | 2.22 | 8.80 | 1.67 | 2.51 |
| C17 | 4.10 | - | - | 0.950 |
| C17:1 | 0.224 | - | - | - |
| C18 | 6.68 | 18.0 | 5.53 | 7.29 |
| C18:1n9c | 50.4 | 8.86 | 24.9 | 24.4 |
| C18:2n6c | 12.0 | 12.3 | 37.8 | 39.6 |
| C18:3n6 γ | 0.051 | - | - | - |
| C18:3n3 α | 1.08 | 14.5 | 3.44 | 3.87 |
| C20 | 0.535 | - | - | - |
| C20:2 | 0.241 | 1.06 | 0.975 | 0.304 |
| C21 | 1.29 | - | - | 0.306 |
| C20:3n3 | 0.172 | 0.254 | 0.090 | 0.076 |
| Summary | ||||
| SFA | 30.1 | 52.9 | 30.9 | 28.3 |
| MUFA | 56.3 | 18.9 | 26.8 | 27.9 |
| PUFA | 13.6 | 28.1 | 42.3 | 43.9 |
| Diets | ||||
|---|---|---|---|---|
| CTL | OPD | SEM | p-Value | |
| Metabolic weight (kg0.75) | 14.1 | 14.2 | 0.213 | 0.814 |
| Intake (g DM/day) | ||||
| Oat hay | 270 | 227 | 17.3 | 0.224 |
| Concentrate | 619 | 619 | 2.70 | 0.972 |
| Intake (g DM/kg0.75) | 63.4 | 59.7 | 1.05 | 0.084 |
| Nutrient intake (g/day) | ||||
| DM | 889 | 846 | 17.7 | 0.235 |
| OM | 770 | 735 | 15.6 | 0.274 |
| CP | 166 | 139 | 4.41 | <0.001 |
| CF | 30.3 | 28.7 | 0.497 | 0.115 |
| NDF | 349 | 297 | 11.4 | 0.016 |
| ADF | 180 | 157 | 5.24 | 0.025 |
| GE (MJ/day) | 14.8 | 14.4 | 0.306 | 0.517 |
| Apparent digestibility (%) | ||||
| DMD | 68.8 | 71.2 | 0.545 | 0.028 |
| OMD | 68.4 | 70.8 | 0.550 | 0.026 |
| CPD | 73.4 | 70.7 | 0.555 | 0.012 |
| NDFD | 51.4 | 50.9 | 1.32 | 0.876 |
| ADFD | 49.2 | 49.3 | 1.05 | 0.985 |
| CFD | 83.4 | 85.9 | 0.599 | 0.024 |
| N utilization (g/kg0.75) | ||||
| N intake | 1.89 | 1.57 | 0.049 | <0.001 |
| N excretion | 1.44 | 1.24 | 0.033 | <0.001 |
| N balance 1 | 0.487 | 0.326 | 0.031 | 0.042 |
| Urine N | 0.939 | 0.781 | 0.028 | 0.001 |
| Fecal N | 0.503 | 0.458 | 0.011 | 0.066 |
| Digestible N 2 | 1.39 | 1.11 | 0.041 | <0.001 |
| Digestible N/N intake (%) | 73.4 | 70.7 | 0.557 | 0.012 |
| N balance/Digestible N (%) | 32.2 | 29.0 | 1.88 | 0.418 |
| Energy balance (MJ/kg0.75) | ||||
| GEI | 1.06 | 1.02 | 0.017 | 0.161 |
| Fecal energy | 0.352 | 0.313 | 0.009 | 0.018 |
| Urine energy | 0.041 | 0.040 | 0.001 | 0.986 |
| DE 3 | 0.704 | 0.704 | 0.013 | 0.696 |
| Methane energy 5 | 0.073 | 0.073 | 0.001 | 0.696 |
| ME 4 | 0.591 | 0.592 | 0.011 | 0.692 |
| DE/GEI (%) | 66.7 | 69.2 | 0.627 | 0.094 |
| ME/GEI (%) | 55.9 | 58.1 | 0.559 | 0.157 |
| Creatine and purine derivatives in urine (µM) | ||||
| Creatine | 4736 | 4298 | 207 | 0.304 |
| Allantoin | 6380 | 6162 | 256 | 0.684 |
| Hipoxanthine | 642 | 568 | 38.1 | 0.347 |
| Xanthine | 18.4 | 16.6 | 1.30 | 0.515 |
| Uric acid | 397 | 346 | 47.7 | 0.613 |
| Total | 7437 | 7093 | 297 | 0.578 |
| Diets | ||||
|---|---|---|---|---|
| CTL | OPD | SEM | p-Value | |
| Total SCFA (µM) | 61.0 | 57.6 | 2.64 | 0.540 |
| Molar proportions (mol/100 mol) | ||||
| Acetate | 60.9 | 59.9 | 0.840 | 0.554 |
| Propionate | 24.1 | 22.6 | 0.950 | 0.444 |
| Isobutyrate | 1.08 | 1.07 | 0.076 | 0.959 |
| Butyrate | 11.3 | 13.5 | 0.490 | 0.020 |
| Isovalerate | 0.900 | 0.920 | 0.070 | 0.918 |
| Valerate | 1.69 | 1.70 | 0.070 | 0.900 |
| Acetate: Propionate | 2.54 | 2.82 | 0.130 | 0.305 |
| N-NH3 (mg/100 mL) | 2.81 | 1.72 | 0.402 | 0.187 |
| Diets | ||||
|---|---|---|---|---|
| Body Weight (kg) | CTL | OPD | SEM | p-Value |
| Day 0 | 52.0 | 55.2 | 1.14 | 0.168 |
| Day 30 | 54.7 | 57.8 | 1.46 | 0.192 |
| Diets | ||||
|---|---|---|---|---|
| CTL | OPD | SEM | p-Value | |
| Milk yield (g/d) | 2160 | 1920 | 104 | 0.269 |
| CF | 120 | 109 | 5.15 | 0.318 |
| Lactose | 102 | 88.3 | 4.98 | 0.151 |
| CP | 80.7 | 78.7 | 3.66 | 0.789 |
| Composition (%) | ||||
| CF | 5.65 | 5.86 | 0.140 | 0.465 |
| CP | 3.79 | 4.17 | 0.090 | 0.036 |
| Lactose | 4.76 | 4.58 | 0.030 | 0.008 |
| Total solids (CP + CF + Lactose) | 14.2 | 14.7 | 0.213 | 0.255 |
| FPCM (g/day) | 2282 | 2113 | 0.994 | 0.411 |
| Somatic cells count (×103/mL) | 1503 | 1740 | 359 | 0.746 |
| Antioxidant activity | ||||
| Total polyphenols (mg/kg milk) | 44.6 | 37.5 | 1.05 | 0.003 |
| ABTS (µmol/kg milk) | 1361 | 1301 | 47.6 | 0.540 |
| Diets | ||||
|---|---|---|---|---|
| Fatty Acid (wt%) | CTL | OPD | SEM | p-Value |
| C4 | 1.93 | 1.99 | 0.046 | 0.499 |
| C6 | 1.99 | 2.06 | 0.048 | 0.498 |
| C8 | 2.32 | 2.35 | 0.042 | 0.772 |
| C10 | 8.10 | 9.02 | 0.179 | 0.009 |
| C11 | 0.172 | 0.186 | 0.006 | 0.205 |
| C12 | 4.74 | 5.38 | 0.088 | <0.001 |
| C13 | 0.119 | 0.131 | 0.005 | 0.245 |
| C14 | 9.53 | 9.72 | 0.147 | 0.528 |
| C14:1 | 0.299 | 0.315 | 0.007 | 0.261 |
| C15 | 0.699 | 1.01 | 0.047 | <0.001 |
| C15:1 | 0.137 | 0.215 | 0.010 | <0.001 |
| C16 | 29.9 | 28.4 | 0.316 | 0.020 |
| C16:1 | 1.52 | 1.66 | 0.043 | 0.121 |
| C17 | 0.405 | 0.839 | 0.045 | <0.001 |
| C17:1 | 0.247 | 0.448 | 0.027 | <0.001 |
| C18 | 7.88 | 8.09 | 0.200 | 0.622 |
| C18:1n9t | 0.679 | 0.693 | 0.025 | 0.779 |
| C18:1n11t | 1.21 | 1.30 | 0.031 | 0.136 |
| C18:1n9c | 21.5 | 19.6 | 0.347 | 0.004 |
| C18:2n6t | 0.219 | 0.189 | 0.010 | 0.145 |
| C18:2n6c | 3.08 | 2.79 | 0.083 | 0.079 |
| C18:3n6 γ | 0.070 | 0.069 | 0.004 | 0.833 |
| C18:3n3 α | 0.23.9 | 0.552 | 0.032 | <0.001 |
| C20 | 0.767 | 0.586 | 0.032 | 0.003 |
| 9c-11t CLA | 0.235 | 0.346 | 0.014 | <0.001 |
| 9t-11t CLA | 0.066 | 0.112 | 0.007 | <0.001 |
| 10t12c CLA/9c-11cCLA | 0.104 | 0.149 | 0.007 | <0.001 |
| C20:1n9 | 0.558 | 0.554 | 0.011 | 0.859 |
| C20:2 | 0.111 | 0.106 | 0.003 | 0.397 |
| C21 | 0.035 | 0.055 | 0.003 | 0.003 |
| C20:3n6 | 0.121 | 0.131 | 0.003 | 0.053 |
| C20:4n6 | 0.271 | 0.228 | 0.003 | 0.035 |
| C20:3n3 | 0.089 | 0.071 | 0.004 | <0.001 |
| C22 | 0.106 | 0.092 | 0.004 | 0.078 |
| C22:1n9 | 0.119 | 0.108 | 0.003 | 0.152 |
| C20:5n3 (EPA) | 0.123 | 0.119 | 0.017 | 0.445 |
| C22:2 | 0.044 | 0.061 | 0.003 | 0.001 |
| C23 | 0.026 | 0.031 | 0.002 | 0.216 |
| C24:0 | 0.002 | 0.009 | 0.000 | <0.001 |
| C24:1 | 0.003 | 0.012 | 0.001 | <0.001 |
| C22:5n3 (DPA) | 0.188 | 0.166 | 0.006 | 0.060 |
| C22:6n3 (DHA) | 0.084 | 0.081 | 0.003 | 0.646 |
| Summary | ||||
| SFA | 68.7 | 69.9 | 0.401 | 0.121 |
| MUFA | 26.3 | 24.9 | 0.372 | 0.061 |
| PUFA | 5.04 | 5.18 | 0.085 | 0.418 |
| Trans FA | 2.09 | 2.05 | 0.066 | 0.733 |
| MUFA/PUFA | 5.27 | 4.84 | 0.100 | 0.033 |
| PUFA/MUFA | 0.19 | 0.21 | 0.004 | 0.031 |
| CLA | 0.40 | 0.62 | 0.024 | <0.001 |
| Atherogenicity index | 2.22 | 2.34 | 0.044 | 0.198 |
| Ratio Omega 6/omega 3 | 6.19 | 4.00 | 0.245 | <0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-García, A.M.; Romero-Huelva, M.; Pino-López, N.; Jiménez-Romero, I.; Rosillo-Lozano, J.A.; Martín-García, A.I. Inclusion of Novel Olive Pulp: Impacts on Nutrient Digestibility, Rumen Fermentation, and Dairy Goat Performance. Animals 2025, 15, 3128. https://doi.org/10.3390/ani15213128
Sánchez-García AM, Romero-Huelva M, Pino-López N, Jiménez-Romero I, Rosillo-Lozano JA, Martín-García AI. Inclusion of Novel Olive Pulp: Impacts on Nutrient Digestibility, Rumen Fermentation, and Dairy Goat Performance. Animals. 2025; 15(21):3128. https://doi.org/10.3390/ani15213128
Chicago/Turabian StyleSánchez-García, Alberto Manuel, Manuel Romero-Huelva, Noemí Pino-López, Isabel Jiménez-Romero, José Antonio Rosillo-Lozano, and Antonio Ignacio Martín-García. 2025. "Inclusion of Novel Olive Pulp: Impacts on Nutrient Digestibility, Rumen Fermentation, and Dairy Goat Performance" Animals 15, no. 21: 3128. https://doi.org/10.3390/ani15213128
APA StyleSánchez-García, A. M., Romero-Huelva, M., Pino-López, N., Jiménez-Romero, I., Rosillo-Lozano, J. A., & Martín-García, A. I. (2025). Inclusion of Novel Olive Pulp: Impacts on Nutrient Digestibility, Rumen Fermentation, and Dairy Goat Performance. Animals, 15(21), 3128. https://doi.org/10.3390/ani15213128