1. Introduction
Extremely high temperatures have become more common in recent years due to global warming. Livestock are exposed to multiple stressors caused by climate change, which may exacerbate thermal stress and thereby reduce productivity and profitability through declines in feed efficiency, growth rates, and reproduction rates [
1]. Heat stress can impair optimal production through multiple mechanisms, such as negatively affecting key productive parameters including milk yield, growth, and reproduction [
2]. The rumen, the primary forestomach of ruminants, serves as a vital digestive organ that plays a critical role in nutrient metabolism. However, heat stress is known to adversely affect rumen functionality. During heat stress, blood flow to the rumen epithelium is reduced, and the acid–base homeostasis is altered [
3]. The end result is ruminal acidosis because of compromised buffering capacity [
4], which may impair rumen functionality and damage the rumen epithelium’s integrity. In addition, heat stress causes the imbalance of the rumen microbial community and affects rumen peristalsis [
5]. These heat stress-induced alterations in rumen motility and microbiota negatively impact feed digestibility and fermentation efficiency [
6]. Thus, some feed additives have been investigated for their action on the animal physiology to cope with heat stress in livestock.
Pogostemon cablin Benth., commonly referred to as patchouli, serves as a key ingredient in various renowned traditional Chinese medicinal formulations. Notable examples include its application in Huoxiang Zhengqi Koufuye (oral liquid preparations) and Baoji Pian (tablet formulations). At present, the analysis of and research on the chemical composition of patchouli mainly focus on the volatile oil, which is also known as
Pogostemon cablin essential oil (PEO) [
7]. The antioxidant and anti-inflammatory activities of PEO have been reported [
8]. For over 2000 years,
Pogostemon cablin has been renowned for its “dispelling heat and dampness” and is recognized as a cure for heat stress. Currently, the Chinese patent drug Huoxiang Zhengqi San, which contains patchouli as its primary component, is widely used in clinical settings for the treatment of heat stroke [
9]. In animal husbandry, El-Zaiat and Abdalla [
10] found that PEO could modulate the ruminal fermentation pattern and function as a ruminal modifier due to its abatement potential on methanogenesis. Furthermore,
Pogostemon cablin finds application in traditional Chinese medicine (TCM) to alleviate gastrointestinal disorders [
11]. Liu et al. [
12] showed that the Huoxiang Zhengqi Oral Liquid enhances the ultrastructure of intestinal epithelial cells, upregulates the expression of zonula occludens-1 (ZO-1) and occludin, repairs the colonic epithelial barrier, and reduces intestinal permeability. These results suggested that PEO exerts modulatory effects on ruminal fermentation and stabilizes the rumen environment in ruminants. However, limited studies have focused on its effects on heat stress in animals, and the specific regulatory mechanisms and action pathways remain unclear. In our previous study, our team systematically evaluated the effects of dietary supplementation with PEO at four different concentrations (0, 50, 100, and 150 mg/kg) on heat-stressed beef cattle. The results indicated that a dosage of 50 mg/kg yielded the most significant improvements in growth performance and immune function. Furthermore, PEO at 50 mg/kg was found to help restore the rumen epithelial barrier [
13]. Therefore, the current study was designed to further investigate the effects and mechanisms of this pre-identified optimal dose. The study aimed to investigate the effects of PEO on nutrient digestibility, rumen antioxidant capacity, and rumen epithelial integrity. Additionally, we sought to investigate and elucidate on the mechanisms by which PEO alleviates heat stress in beef cattle and identify the pathways through which patchouli oil protects rumen epithelial health.
4. Discussion
The THI, which combines the effects of temperature and humidity, has been widely used for decades to assess heat stress in cattle. Hahn et al. [
19] defined heat stress severity based on THI ranges: normal, THI < 74; alert, 74 ≤ THI < 79; danger, 79 ≤ THI < 84; and emergency, THI ≥ 84. As confirmed by environmental data from the same trial [
13], the temperature–humidity index (THI) persistently exceeded 79 for the duration of the study. This confirms that the test cattle were subjected to continuous heat stress. In TCM,
Pogostemon cablin has been historically used to treat gastrointestinal disorders, alleviate summer heat syndrome, regulate dampness, and stimulate appetite [
20]. However, the anti-heat stress mechanisms of PEO in livestock remain poorly understood, warranting further studies to elucidate the underlying molecular pathways.
Nutrients play a pivotal role in ruminant physiology, providing energy substrates, supporting growth, reproduction and production, while maintaining systemic homeostasis. Improving the digestibility of nutrients is conducive to improving the growth performance of animals. However, under heat stress, nutrient absorption is significantly reduced across species; in cattle, dry matter intake may decline by approximately 30% [
21]. Consistent with this, Gao et al. [
22] reported that heat stress suppresses feed intake and nutrient digestibility in dairy cows. Notably, in a dose-finding and production performance study conducted by our research team under identical heat stress conditions, supplementation with 50 mg/kg PEO not only showed a strong trend toward increased average daily dry matter intake (ADMI) but also significantly improved the average daily gain (ADG) in beef cattle [
13]. The gastrointestinal tract is the main site for nutrient absorption, and its integrity is of crucial importance for the absorption of nutrients. Studies suggest that
Pogostemon cablin is a medicinal plant for treating gastrointestinal symptoms and has demonstrated protective effects on the gastrointestinal tract [
23,
24]. Moreover, Huoxiang Zhengqi Oral Liquid has been found to effectively regulate gastrointestinal motility in a two-way manner [
11]. By normalizing motility, it likely promotes a more stable ruminal environment, which in turn provides sufficient fermentation time. Thus, these factors may improve the digestion and absorption of nutrients. According to the findings of this study, supplemental 50 mg/kg PEO increased CP and NDF digestibility, and there was also a slight increase in ADF digestibility. These findings corroborate the research findings mentioned above. The enhanced nutrient digestibility observed in the present study by our team provides a physiological explanation for the improved growth performance documented in our companion study [
13]. Furthermore, PEO supplementation enhanced the activity of cellulase in the rumen. The rumen is a digestive organ unique to ruminants. The higher digestive enzyme activity in the rumen indicates that the fiber in feed can be degraded more effectively, which is consistent with the NDF digestibility result. The decline in growth performance of ruminants under heat stress is attributed not only to reduced feed intake but also to diminished carbon and energy supply from microbial fermentation [
25]. Ruminants lack endogenous fiber-digesting enzymes; instead, they rely on complex microbial communities (consortia) to produce polysaccharide-degrading enzymes with enzymatic activity directly dependent on microbial composition and functionality [
26,
27]. The enzymatic activity of rumen fluid is a direct manifestation of the microbial metabolic activities. Our results indicated that 50 mg/kg dietary supplementation with PEO enhanced the activity of digestive enzymes in the rumen. Rumen microorganisms digest feed through the action of the enzymes they produce. Based on this, we infer that rumen microbes and enzymes collectively degrade proteins and starches into VFA through synergistic interactions, compensating for energy deficits caused by heat stress-induced hypophagia and improving nutrient utilization efficiency.
The ruminal epithelium, a metabolically active tissue, is prone to oxidative stress due to endogenous reactive oxygen species (ROS) generated during intensive metabolic processes [
28]. Heat stress is one of the many factors that causes oxidative stress in vivo. Under heat stress, endogenous free radical production and ROS accumulation increase, while antioxidant capacity declines in animals [
29]. The excessive accumulation of free radical can induce unsaturated fatty acids to produce MDA in cells, and MDA promotes cell death by disintegrating cells [
30]. In this investigation, PEO treatment enhanced the T-AOC and GSH-Px activity as well as the T-SOD in rumen tissue, concurrently reducing MDA levels. This indicates a potentiation of the local antioxidant defense system within the rumen. The antioxidant capacity of PEO is likely attributable to its primary bioactive component, patchouli alcohol (PA). This is supported by an in vitro study demonstrating that PA alleviates heat shock-induced oxidative stress in intestinal epithelial cells by activating the Nrf2–Keap1 pathway and reducing MDA accumulation [
31]. Although direct evidence of PEO’s anti-heat stress effects in ruminants remains limited, its well-documented antioxidant and antimicrobial properties [
8,
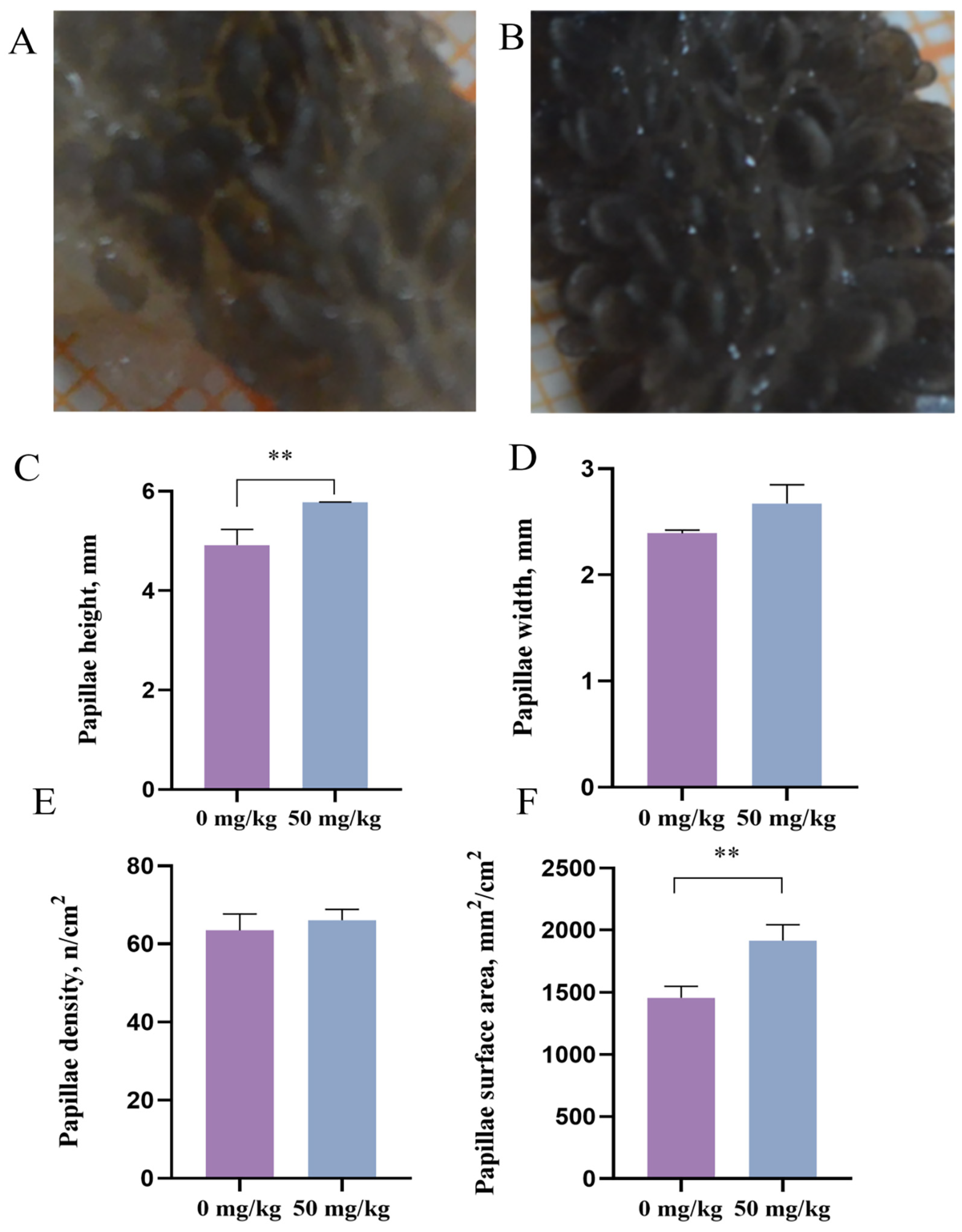
32] support its potential to neutralize free radicals and protect rumen epithelial integrity. Consistent with this mechanism, PEO supplementation increased the ruminal papillary length, width and surface area in heat-stressed cattle. These morphological parameters (papillary length and width) are established biomarkers of rumen mucosal development [
33,
34]. Previous studies have demonstrated that heat stress reduces feed intake in dairy cows, thereby limiting fermentable substrates available for ruminal microbes and ultimately decreasing ruminal papillary width, perimeter and surface area [
35]. These findings suggest that PEO may ameliorate heat stress-induced rumen epithelial damage. Increased papillary dimensions (height and width) directly expand the absorptive surface area of the rumen epithelium [
36], which aligns with our observed enhancement in nutrient digestibility. A similar finding showed that dietary supplementation with plant essential oils enhances ruminal digestive efficiency in beef cattle by promoting papillary growth and modulating microbiota–epithelial interactions [
37].
In this study, the addition of PEO increased propionate and VFA contents. Elevated ruminal propionate production is strongly correlated with improved growth performance in ruminants [
38]. The enhancements in rumen fermentation efficiency and nutrient digestibility observed in this study provide a physiological explanation for the significant improvements in average daily gain reported in our companion dose-finding study using the same PEO supplementation level (50 mg/kg) under heat stress [
13]. In ruminants, both acetate butyrate and propionate, the primary energy substrates for ruminants, stimulate ruminal epithelial development. VFA (acetate, propionate, butyrate) modulate key signaling and metabolic pathways—including PPARγ and mTOR signaling pathways and propionate metabolism—via the regulation of genes (e.g., HMGCS2, PPARG, ECHS1, and RNF152) [
39]. These pathway modifications drive morphological adaptations in the rumen epithelium, such as increased papillary length and width (as observed herein). Wang et al. [
40] further demonstrated that elevated VFA concentrations enhance ruminal maturation during early postnatal stages. This suggests that in addition to enhancing the protective mechanism by increased oxidation resistance, PEO improved epithelial morphology by a trophic effect through increasing propionate concentration, further promoting the improvement of nutrient digestibility. Wang et al. [
38] investigated the effects of traditional Chinese medicine compounds containing
Pogostemon cablin on rumen fermentation via an in vitro gas production technique. Their results demonstrated that traditional Chinese medicine compounds supplementation reduced acetate molar proportion, increased propionate molar proportion, and maintained stable ruminal pH—which are findings consistent with our study. Ruminal NH
3-N concentration reflects dietary protein degradation efficiency, as NH
3-N serves as the primary nitrogen source for MCP synthesis by rumen microbiota [
41]. In this study, dietary supplementation with PEO reduced the ruminal NH
3-N concentration in beef cattle. This suggests that PEO may enhance the incorporation of nitrogen into microbial biomass, as NH
3-N is a primary precursor for microbial protein (MCP) synthesis. Although the observed increase in MCP concentration was not statistically significant, the numerical increase, coupled with the significant reduction in NH
3-N, indicates a potential for improved nitrogen utilization efficiency that warrants further investigation. The observed reduction in ruminal NH
3-N concentration with PEO supplementation aligns with the well-documented effects of other plant essential oils. Jahani-Azizabadi et al. [
42] reported that essential oils rich in phenolic compounds (e.g., thymol in thyme, cinnamaldehyde in cinnamon) significantly suppress in vitro ruminal NH
3-N concentration, which is a finding corroborated by subsequent research [
43] where dietary supplementation with plant essential oils reduced NH
3-N levels in goats. Although PEO is characterized by its high content of sesquiterpenoids (e.g., PA) rather than phenolics, it belongs to the same broader class of phytogenic compounds known for their antimicrobial potency. The shared ability of these chemically distinct yet functionally analogous essential oils to lower NH
3-N suggests a common physiological outcome: the selective modulation of rumen microbiota to inhibit hyper-ammonia-producing microbes. Thus, our results demonstrate that PEO, like other bioactive essential oils, enhances NH
3-N utilization efficiency during heat stress, thereby optimizing the substrate available for microbial protein synthesis.
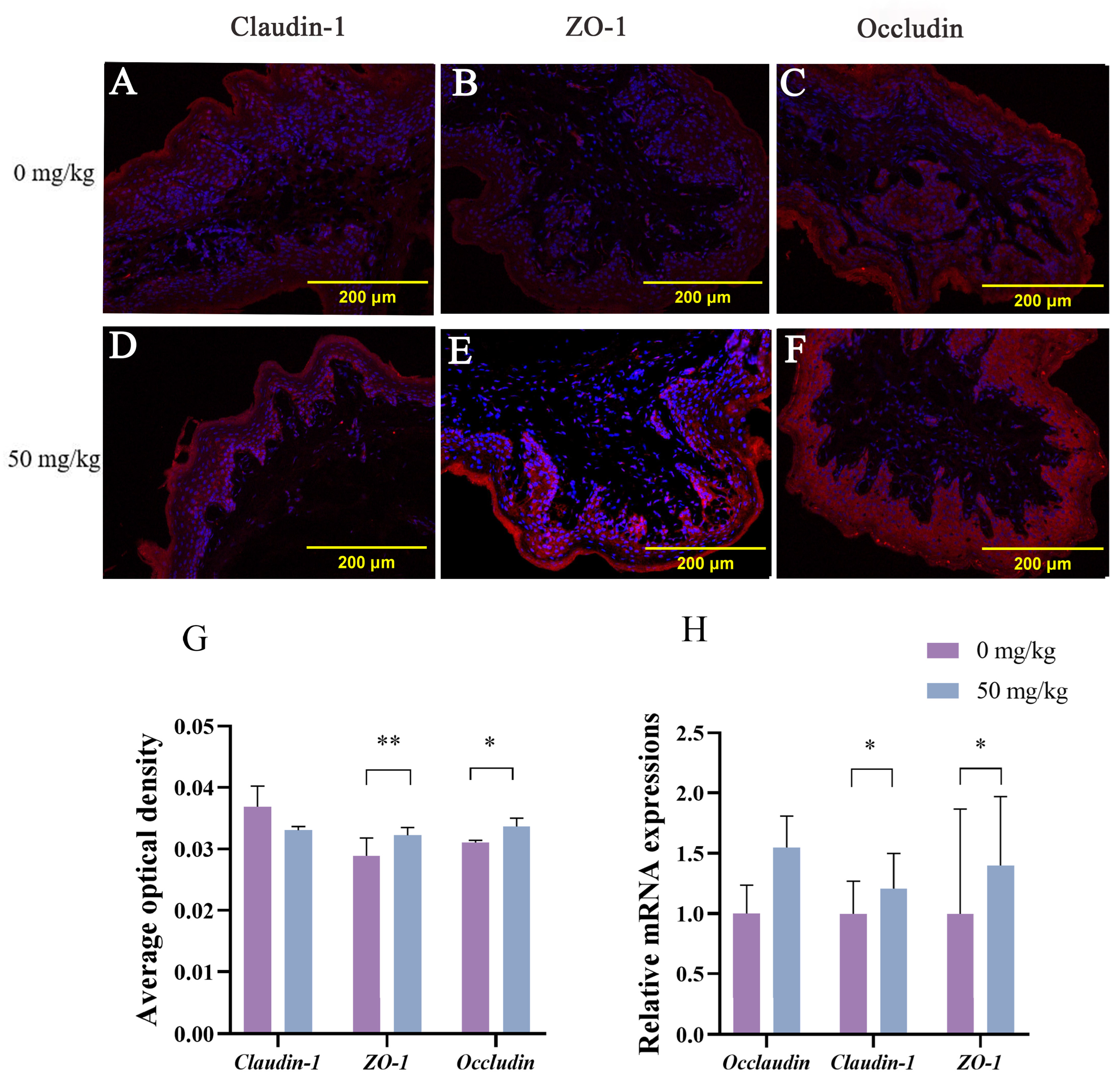
The tight junction proteins play a key role in maintaining intercellular adhesion and the physical barrier function of the rumen epithelium [
44]. In this study, dietary supplementation with 50 mg/kg PEO significantly upregulated mRNA expression and elevated the average optical density values of ZO-1 and occludin in rumen tissues. Optical density values, quantified via semiquantitative fluorescence signal analysis, directly reflect tight junction protein expression intensity. Immunofluorescence localization revealed distinct tight junction distribution patterns: ZO-1, claudin-1 and occludin exhibited irregular and discontinuous localization in the control group; all proteins displayed continuous, linear distribution along epithelial junctions in the patchouli oil group. As core components of tight junction complexes, ZO-1 and occludin are critical for barrier maintenance. Previous studies confirmed that heat stress compromises rumen epithelial integrity and downregulates tight junction gene expression [
45]. Tight junction dysfunction increases intestinal permeability, predisposing to inflammation and gastrointestinal pathologies [
46]. Importantly, the protective effect of PEO’s primary bioactive component, PA, on tight junction proteins and intestinal barrier integrity has been well documented. Studies have demonstrated that PA significantly upregulates the expression of ZO-1, ZO-2, claudin-1, and occludin in intestinal epithelial models while concurrently reducing pro-inflammatory cytokines and promoting mucosal repair [
47]. Emerging evidence indicates that certain active constituents of Chinese herbal medicines possess prebiotic-like effects that alleviate inflammation and benefit the gut epithelial environment [
48,
49]. Supporting this, a study demonstrated that PEO and its derived compounds exert significant prebiotic-like effects in the C57BL/6J mouse model [
23]. Importantly, in our team’s concurrent dose-finding study conducted under identical heat stress conditions, we observed that PEO supplementation effectively suppressed inflammatory responses in beef cattle [
13]. We therefore propose that PEO mitigates heat stress-induced damage by modulating inflammatory processes and enhancing epithelial barrier integrity. Our findings demonstrate that PEO supplementation counteracts heat stress-induced barrier impairment by upregulating ZO-1 and occludin expression, which is likely through the combined effects of suppressed inflammation and enhanced rumen epithelial metabolic activity.
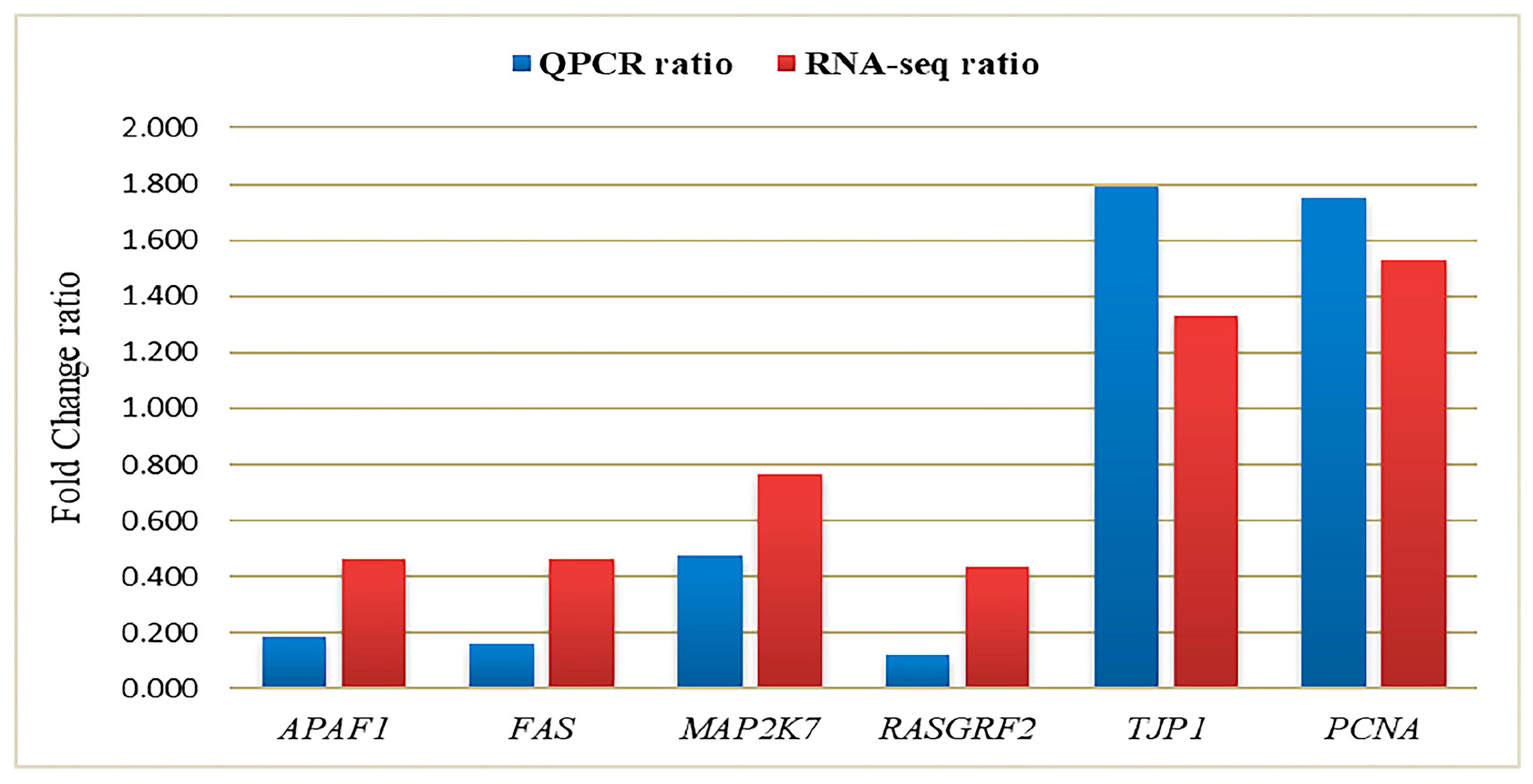
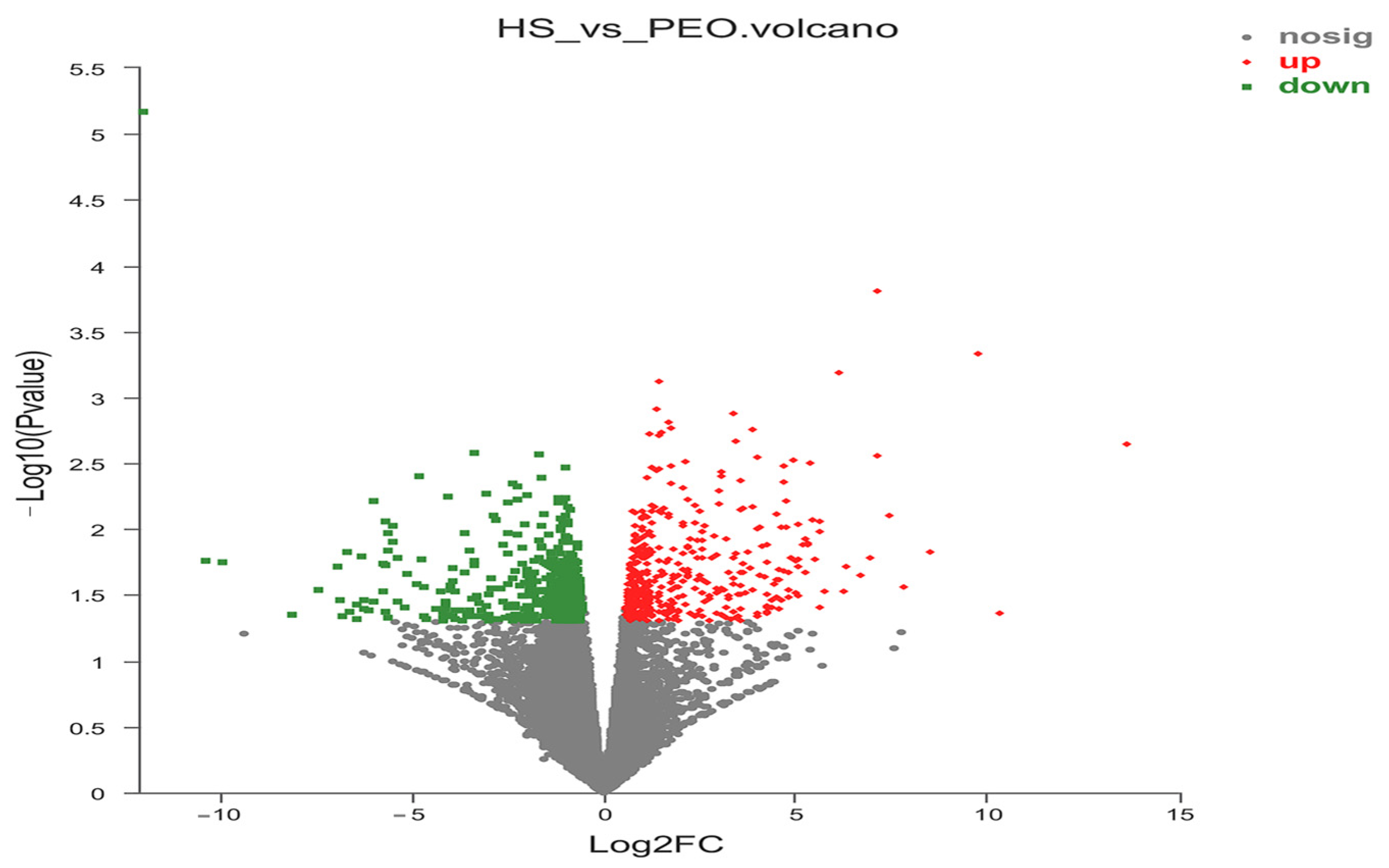
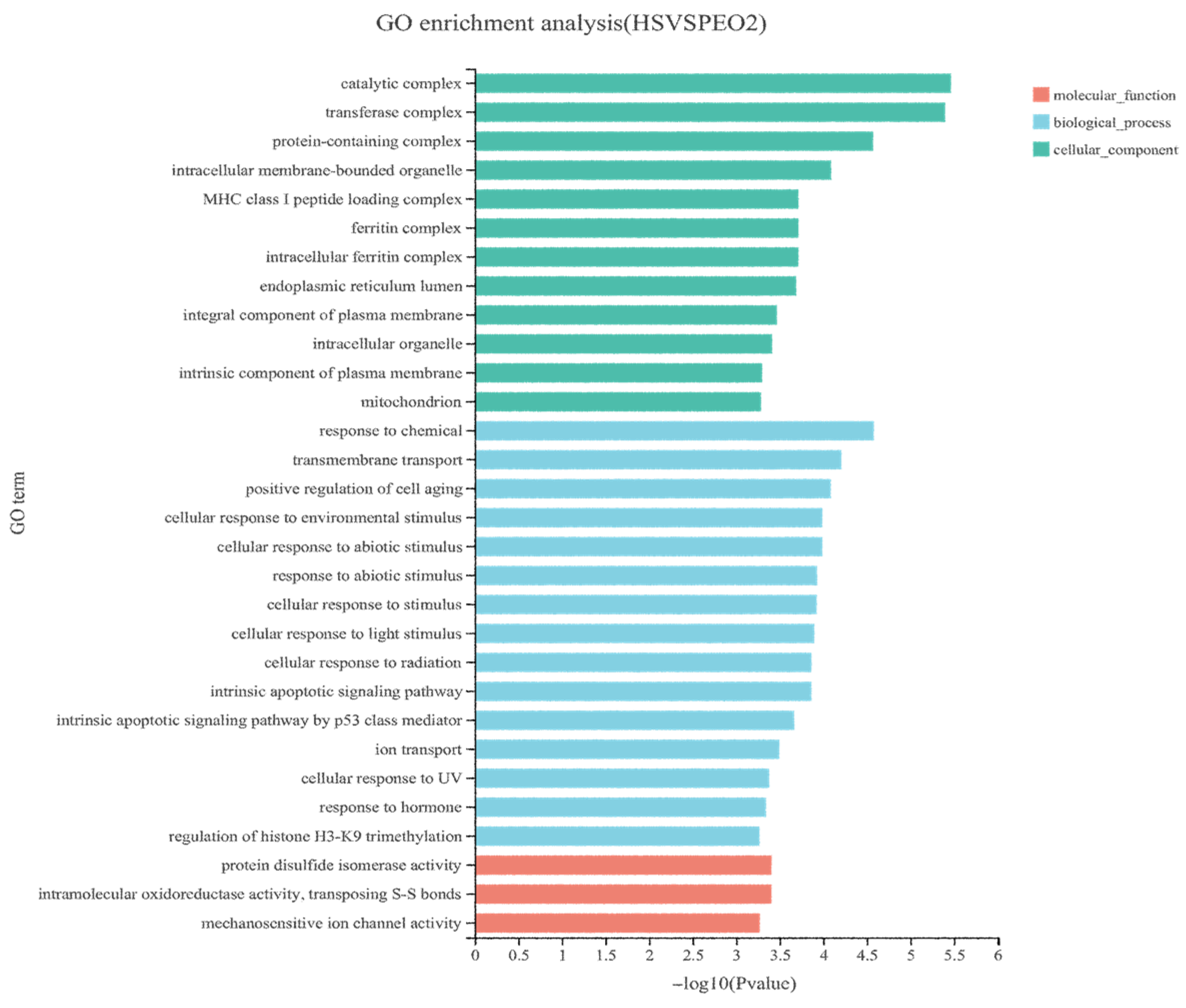
To elucidate the molecular mechanisms by which dietary PEO supplementation enhances rumen barrier function in heat-stressed beef cattle, we performed KEGG pathway enrichment analysis on DEGs. Twenty-four pathways were significantly enriched (
p < 0.05) with key pathways impacting rumen barrier integrity including: tight junction signaling, phagosome regulation, and apoptosis modulation. Integrated GO and KEGG analyses identified 10 core genes that were involved in the regulation of tight junction proteins and apoptosis. Among the upregulated genes,
BAK1 acts as an antagonist of apoptotic proteins and can inhibit the expression of apoptotic factors [
50];
TJP1 (encoding ZO-1) is a scaffold protein bridging occludin to the actin cytoskeleton [
51];
PCNA encodes a protein essential for cell replication, which serves as an auxiliary factor for DNA polymerase and plays a critical role in DNA replication and repair [
52].
TUBA1B encodes α-tubulin, which is a core subunit of microtubules—dynamic cytoskeletal polymers critical for cell division, intracellular transport, and cell shape maintenance. Among the downregulated genes, key apoptotic pathways were identified: exogenous (receptor-mediated) and intrinsic (mitochondrial). The exogenous apoptosis pathway is activated by the
FAS receptor, and
RASGRF2 enhances the RAS signal. The intrinsic apoptotic pathway begins with the assembly of the cytochrome c complex. This process involves
APAF1 [
53]. Additionally,
BBC3 is a pro-apoptotic Bcl-2 family member. Studies have indicated that the activation of the
BBC3 gene in the organism accelerates cell apoptosis [
54]. These data indicate that PEO enhances the barrier function of ruminal epithelial cells, promotes cell proliferation and repair, and inhibits apoptosis by upregulating the expression of genes such as
TJP1,
PCNA, and
BAK1. Simultaneously, by downregulating the expression of genes including
APAF1,
FAS, and
BBC3, PEO suppresses apoptosis and the stress response. This coordinated transcriptional reprogramming likely preserves rumen epithelial homeostasis under heat stress, enhancing thermotolerance. Li et al. [
55] have shown that acute heat stress can downregulate the expression of genes related to morphogenesis and cytoskeletal structure and upregulate pro-apoptotic genes, thus inhibiting the proliferation of bovine mammary epithelial cells cultured in vitro. This result indirectly supports our conclusion.