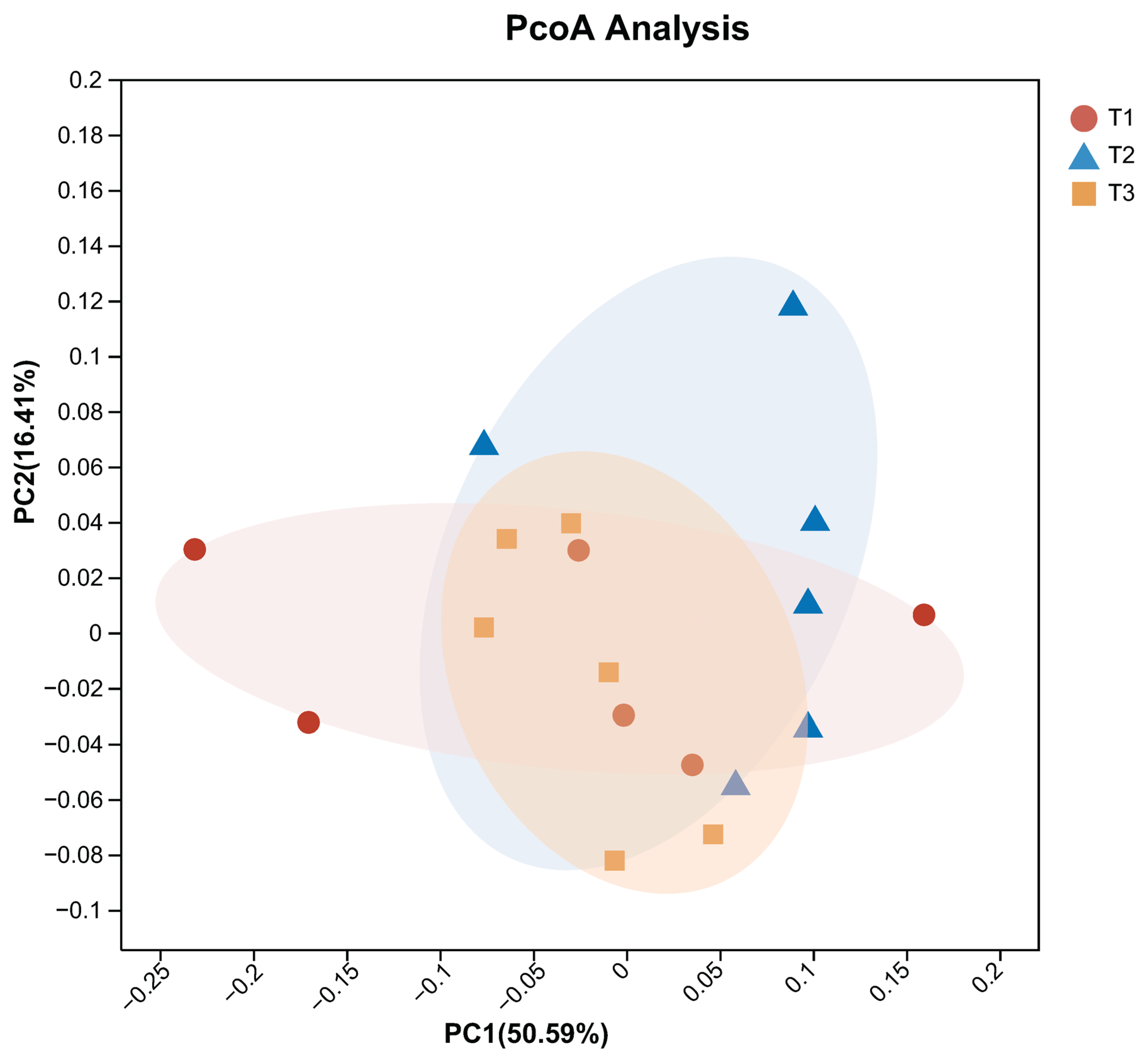
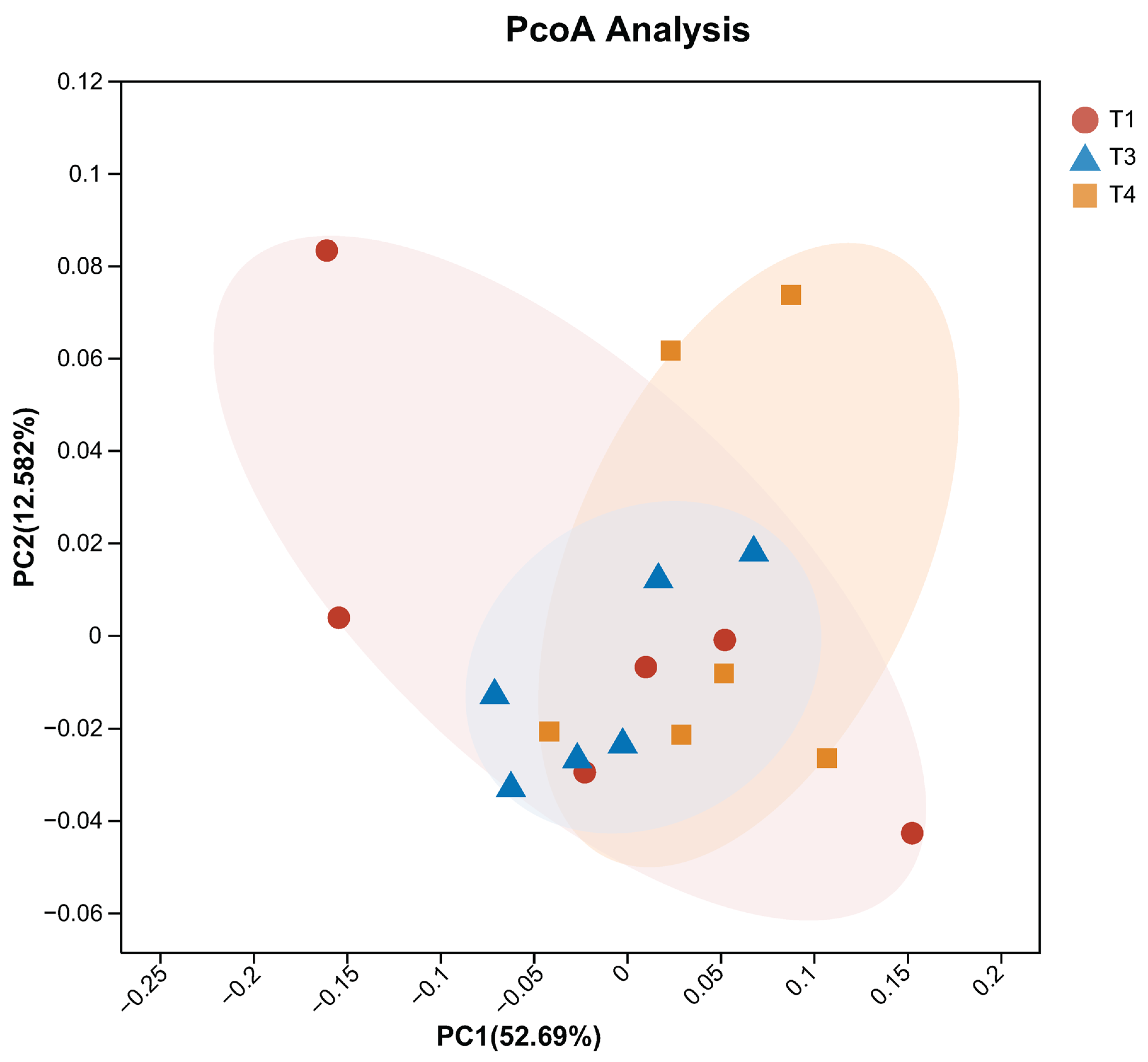
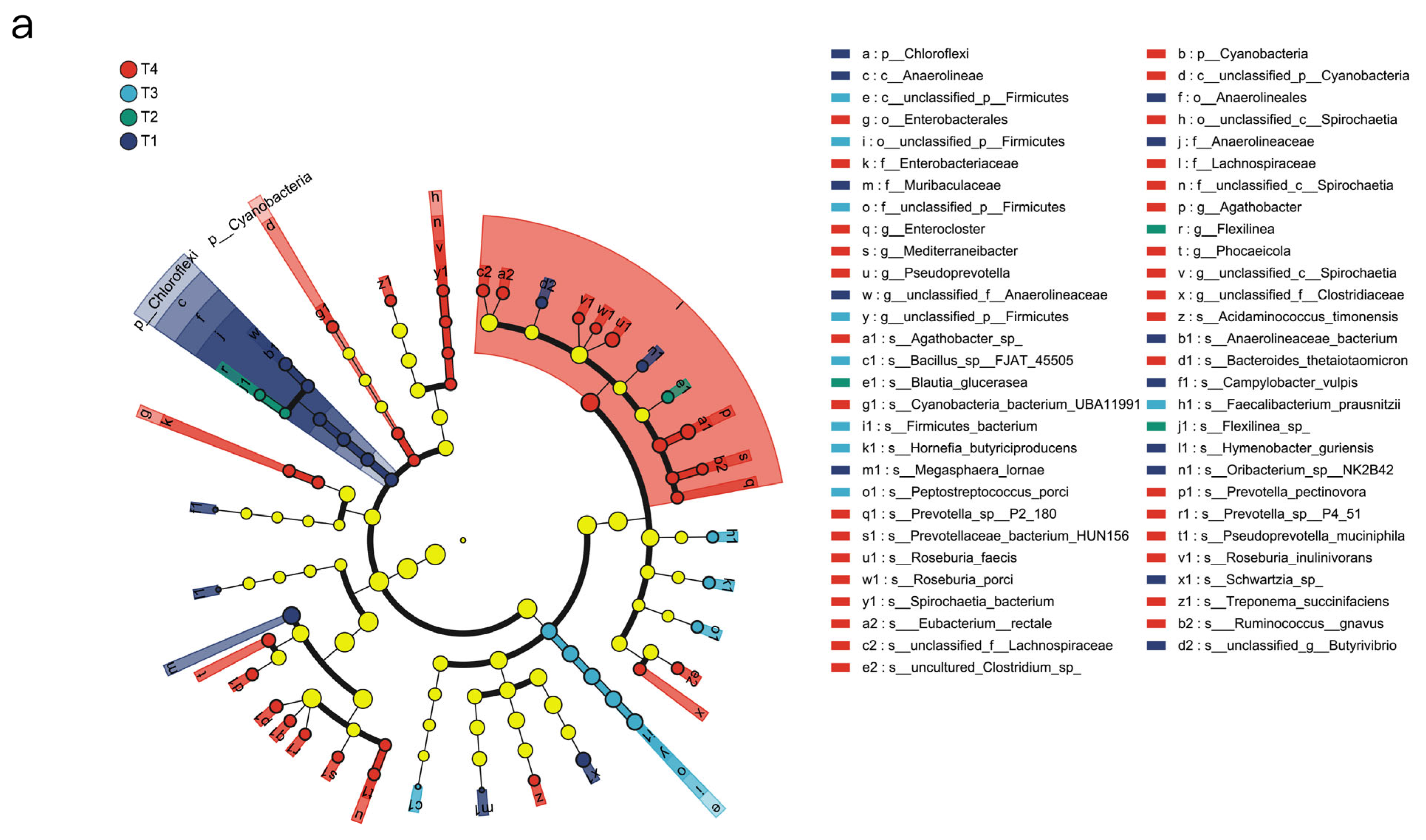
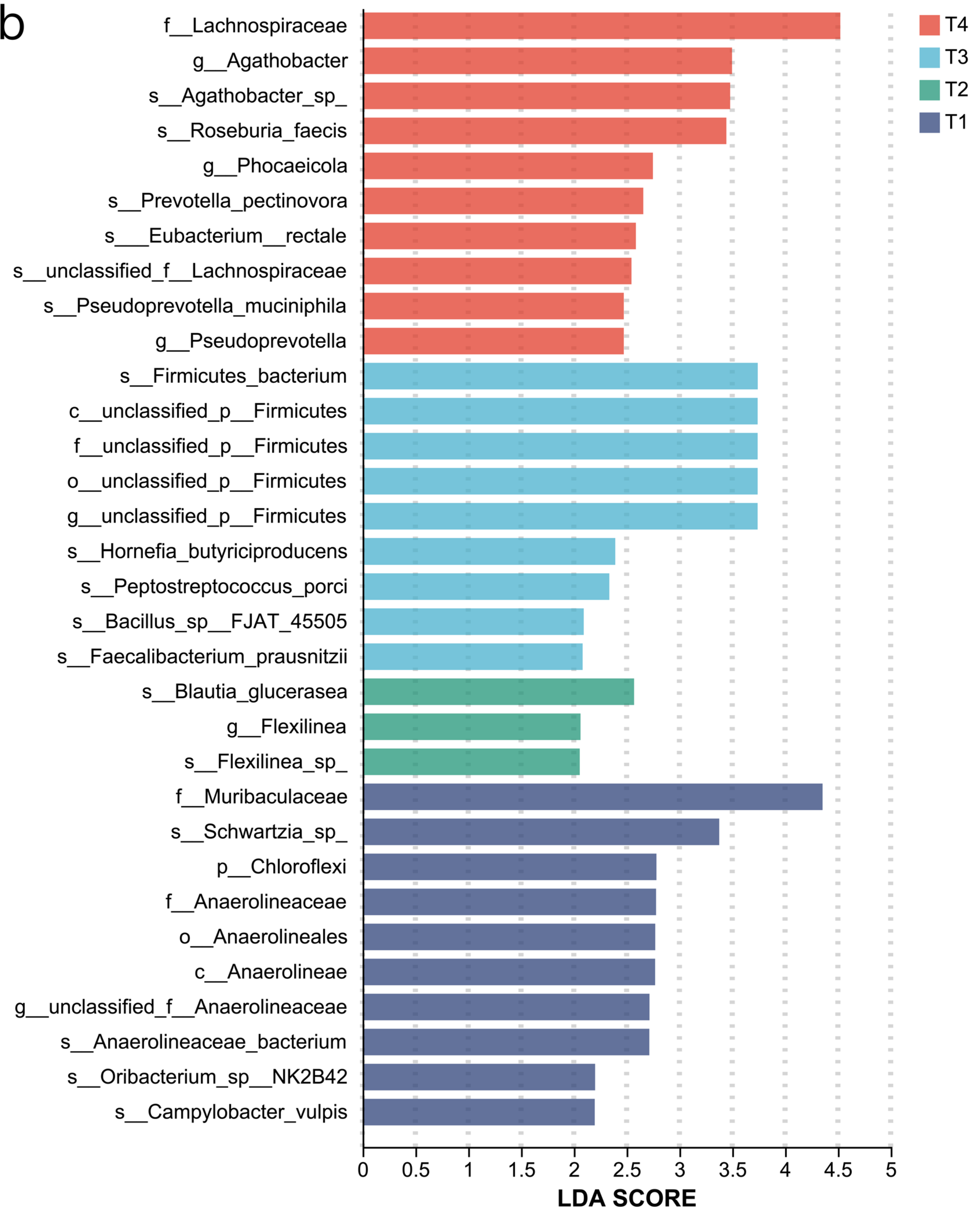
1. Introduction
Mutton is of significant importance in consumption patterns due to its exceptional nutritional value and distinctive flavour, making the sheep industry a flourishing sector in China, resulting in increased demand for feed. The supply of soybean meal, one of the most important protein feed raw materials in the feeding industry, is especially insufficient due to resource competition and excessive demand, seriously restricting the healthy development of animal husbandry in China [
1]. Therefore, the development and utilization of protein feed substitutes to reduce the utilization of soybean meal is one of the most important and difficult endeavours at present. As a new breed, the Dumont sheep is the hybrid offspring of the Dorper sheep and the Mongolian sheep. It was identified in 2022 as exhibiting a highly adaptable digestive system [
2]. To fully exploit its production performance, the research team conducted some studies on the dietary composition in the early stage and found that this breed requires more concentrated feed to meet its energy and nitrogen demands compared to Mongolian sheep. Therefore, it has a higher demand for protein feed. In view of this, exploring the substitution of soybean meal is of greater importance. As a new breed, Dumont sheep are the offspring of crossbreeding Dorper and Mongolian sheep, officially recognized in 2022. They inherit the robust meat production genes from their Dorper sire lineage [
2]. To maximize their production potential, our research team conducted early-stage feed formulation studies. We found that when fed identical diets, Dumont sheep consume an additional 2.38 MJ of feed daily and achieve an average daily gain 1.29 times that of Mongolian sheep [
3]. This indicates superior appetite and digestive capacity for nutrient uptake. Whether this elevated production performance is underpinned by unique rumen microbiome characteristics warrants further investigation.
From the mid-20th century to the present, different nitrogen sources have been used in ruminant production to replace some conventional protein raw materials to reduce cost and increase efficiency, with one of the oldest being urea. Zheng et al. [
4] conducted research showing that adding 1% urea to the feed can significantly increase the growth performance of 5-month-old fattening male Hu sheep. Slow-release urea can enhance rumen fermentation in heat-stressed dairy cows, alleviate heat stress in dairy cows, and reduce methane production [
5]. However, previous research has primarily focused on the effects of urea alone on phenotypic indicators such as lamb growth performance and changes in rumen microbial communities. Few studies have attempted to combine urea with inexpensive protein feeds to comprehensively replace soybean meal, nor have they thoroughly investigated the systemic alterations in rumen microbial communities and their metabolites. For instance, Xu et al. demonstrated that 10 g of urea could substitute 130 g of soybean meal per kilogram of feed dry matter without adversely affecting growth performance or health in fattening lambs fed a high-concentrate diet but did not investigate alterations in rumen microbiota or metabolites [
6]. Li et al. suggested that the rumen microbiome of sheep fed a low N diet with different urea supplementation are significantly different [
7]. Research by Lu et al. suggests that urea supplementation affects microorganisms that carry nickel-dependent enzyme genes [
8].
To account for the risk of ruminal acidosis, the use of urea in lambs is subject to restrictions. Moreover, there are alternative protein sources available here that can replace soybean meal. Cottonseed meal and rapeseed meal, as substitutes for soybean meal, can also alleviate the shortage of soybean meal supply due to their relatively low price and diverse sources while maintaining the same animal production performance [
1,
7,
9]. Rapeseed protein exhibits a relatively balanced amino acid profile, with a high protein content, containing virtually no limiting amino acids. Compared to other meal products, rapeseed meal possesses the highest sulphur-containing amino acid content, followed by notably elevated levels of methionine and lysine [
10]. Cottonseed meal is a byproduct of oil extraction from cottonseeds, serving as a valuable protein source for animal nutrition. Cottonseed meal contains 30–50% protein on a dry matter basis and is characterized by high amino acid concentrations, though it has relatively low lysine content [
11]. In practical applications, cottonseed meal and rapeseed meal exhibit a complementary relationship. In actual production, when cottonseed meal serves as the primary protein source, supplementary additions of lysine and methionine are required. Combining cottonseed meal with rapeseed meal not only reduces the required methionine supplementation but also lowers the arginine-to-lysine ratio in cottonseed meal towards normal levels. This compensates for the insufficient arginine content in rapeseed meal, offering greater economic viability and safety in practical production. However, China’s abundant resources of cottonseed meal and rapeseed meal remain underutilised, with utilization rates of only 30–50%. The comprehensive utilisation rate of agricultural products stands at just 40%, significantly below the 90% achieved in developed nations. Furthermore, the development and utilisation of technologies for non-conventional protein resources (such as mixed meals) lag behind, resulting in severe resource wastage [
12].
Compared to the isolated use of urea, the combined application of nitrogen sources such as urea, rapeseed meal, and cottonseed meal exhibits distinct degradation characteristics. When their ruminal degradation rates align with energy carriers (e.g., carbohydrates), they provide rumen microorganisms with synchronously available nitrogen and energy substrates, thereby promoting efficient MCP synthesis. The rational synchronised release of energy and nitrogen within the diet not only optimises the combination of various feed ingredients but also satisfies the carbon and nitrogen requirements of rumen microorganisms. This enables more complete utilisation of feed nutrients, thereby reducing waste and pollution while enhancing economic benefits [
13].
Advancements in high-throughput sequencing technologies have provided new perspectives through omics approaches for analysing how different feeds influence rumen fermentation functions and growth performance indicators. Although previous studies have utilised omics approaches to investigate the role of urea in the rumen of ruminants, this study represents the first evaluation using three-month-old Dumont crossbred lambs as a model to assess the feasibility of reducing soybean meal and replacing it with urea, rapeseed meal, and cottonseed meal in high-performance breeds. This provides a theoretical basis for developing low-cost, highly efficient finishing diets for high-yielding crossbred lambs. In this manuscript, we replaced all soybean meal with urea, rapeseed meal, and cottonseed meal to investigate the feasibility of substituting low-cost nitrogen sources for soybean meal in Dumont lambs. Combining rumen microbial metagenomics and metabolomics, we analysed rumen microbial community composition and structural diversity, functional modules, rumen metabolites, and metabolic pathways. By applying rumen microbiome and metabolomics technologies, we elucidated the mechanisms underlying phenotypic data changes, providing theoretical support for reducing soybean meal dependency in practical production.
4. Discussion
4.1. Growth Performance and Rumen Fermentation Characteristics
Body weight (BW), average daily weight gain (ADG), and average daily feed intake (ADFI) are important indicators reflecting the growth performance of livestock, which can directly reflect the growth status of the animal’s body. In this experiment, replacing part of the soybean meal with urea, cottonseed meal, and rapeseed meal did not change the BW, ADG, and ADFI of Dumont lambs, indicating that appropriate dosages of urea, cottonseed meal, and rapeseed meal do not have any negative impact on the lambs when used in conjunction with cost reduction measures. Studies [
19] have shown that in the research on lambs, using 10 g urea to replace 130 g/kg DM soybean meal has no adverse effect on the digestion, metabolism, and growth of low-protein and high-concentrate-fed fattening Hu sheep, but as the urea replacement level increases, the growth performance of Hu sheep decreases. Similarly, replacing 6.4% soybean meal with 1.5% urea in the lamb diet has no significant effect on the DM intake and digestion coefficient of the animal, but increasing the replacement level to 2.5% will reduce the DM and organic matter intake of the animal [
20]. Feed conversion ratio (FCR) is a key indicator for evaluating the economic benefits of sheep farming and directly affects feed utilization efficiency. In this experiment, replacing all soybean meal with 1% urea, cottonseed meal, and rapeseed meal significantly increased the FCR of Dumont lambs, a result that is conducive to improving the economic benefits of farming and achieving economic maximization.
The rumen is a natural anaerobic space that provides the essential environment for the rumen microbiota to reproduce and develop. During the fermentation process, the rumen microbiota synthesizes substances such as VFA and NH
3-N, which are important indicators of the physiological health of the rumen and the ability of ruminants to convert nutrients. Rumen pH is an important indicator for evaluating rumen fermentation status, which is jointly affected by multiple factors [
21]. In this experiment, the pH values of each group were not significantly different, which was consistent with the results of [
6,
22]. When there was an adequate amount of fermentable carbohydrates in the diet, the rate of NH
3-N release matched the microbial requirements.
Ammonia is an important source of nitrogen for MCP synthesis and growth in the rumen, and the rumen microorganisms can utilise the NH
3-N produced by degradation as a nitrogen source. Through amino acid deamination or NPN hydrolysis, ammonia is generated, and then ammonia is converted into microbial protein. The synthesized and transformed MCP can provide 50% to 80% of the absorbable protein for ruminant animals [
23]. Urea, which can be rapidly hydrolysed to ammonia by rumen ureolytic microbes, leads to an increase in the concentration of NH
3-N [
24]. When the energy–nitrogen synchronization degree in the rumen is high, microorganisms can more effectively utilise the nitrogen source, reduce ammonia loss, and promote microbial protein synthesis [
25]. In this experiment, different doses of urea were used to replace part of the soybean meal, and the NH
3-N content was significantly different, but the contents of MCP were significantly increased. This indicates that replacing 4.3% soybean meal with 1% urea improved the utilization efficiency of CP in the diet by rumen microorganisms, which is related to the growth of protein-degrading microorganisms in the rumen. Consistent with the research results of these individuals [
26], they also reported that 1.5% urea would increase the NH
3-N value, and 1% urea could enhance the production of MCP.
VFA is the main source of energy in ruminants, accounting for 70% to 80% of digestible energy intake in ruminants, and is the main energy source of ruminants [
27]. In this experiment, acetic acid content in each group accounted for the most TVFA, indicating that acetic acid was the main factor in the rumen fermentation of sheep fed different nitrogen sources. Compared with other groups, acetic acid and propionic acid contents in the T4 group were significantly increased. This is related to the fact that cottonseed meal contains more crude protein [
28], while rapeseed meal has a higher cellulose content. This will increase the number of fibrolytic bacteria and anaerobic fungi in the rumen and promote acetic acid fermentation in the rumen. Isobutyric acid serves as an energy source for ruminants and is an important metabolic product of cellulolytic bacteria in the rumen, such as succinic acid, fibrobacter, and rumen bacteria. The changes in its content are closely related to the activity of these microorganisms [
29]. In this experiment, the isobutyric acid content in the T3 group was significantly lower than that in the other groups. This suggests that the decrease in the pH value might have inhibited or reduced the activity of the cellulose-degrading bacteria [
30]. Studies have shown that an increase in the non-fibre carbohydrate (NFC) level of the diet leads to a decrease in the rumen ethyl-pentyl ratio. In high-concentrate diets, the NFC level is high, and the oligosaccharides and monosaccharides produced by NFC degradation can only be utilised by a portion of the microorganisms. In high-concentrate diets, many microorganisms related to NFC degradation multiply in the rumen. The single sugars or oligosaccharides produced by NFC degradation are quickly captured and utilised by these types of microorganisms, and these microorganisms are often closely related to the production of propionic acid, inlcuding starch-degrading bacteria and lactic acid-producing bacteria [
31].
4.2. Rumen Microbial Characteristics
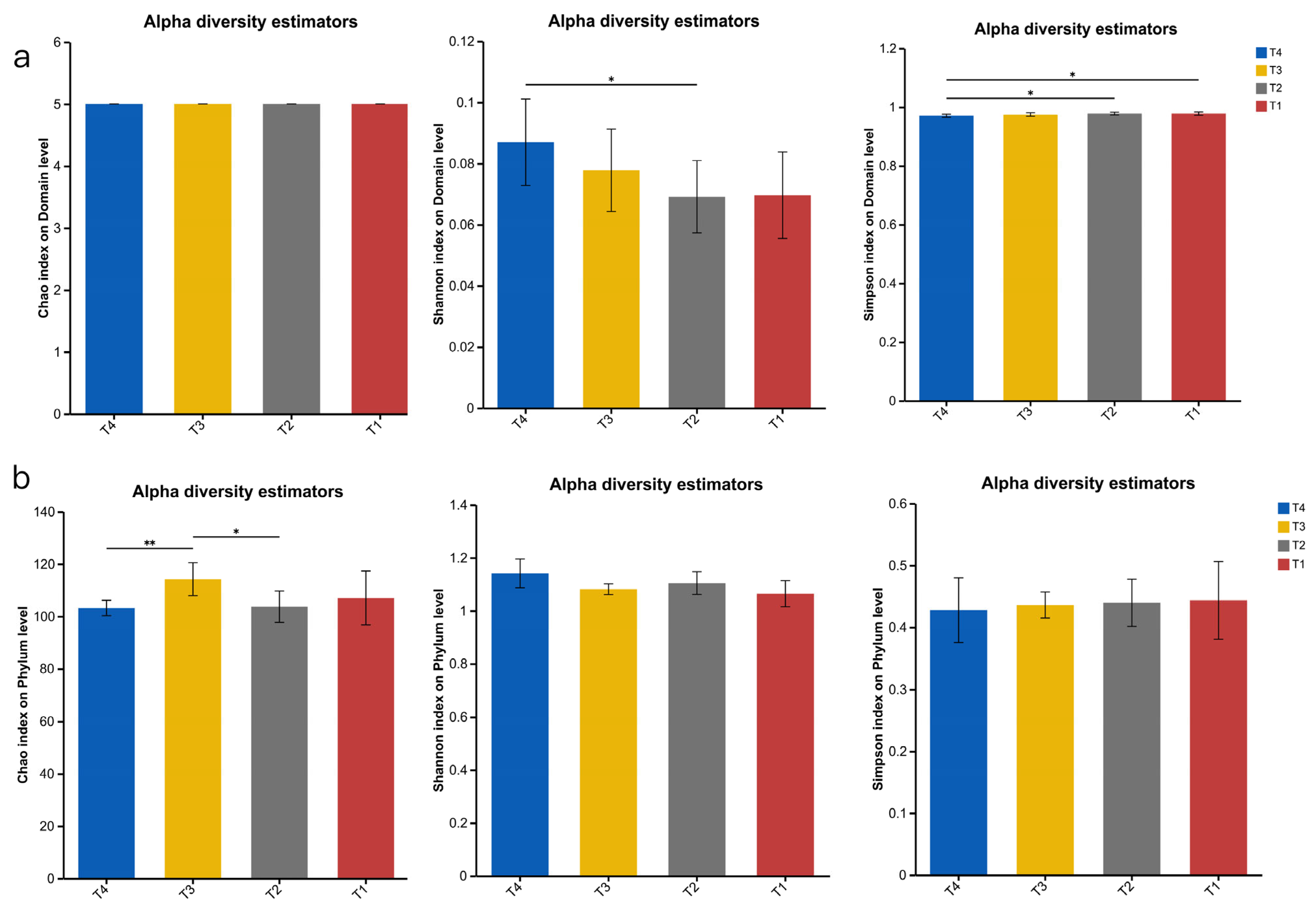
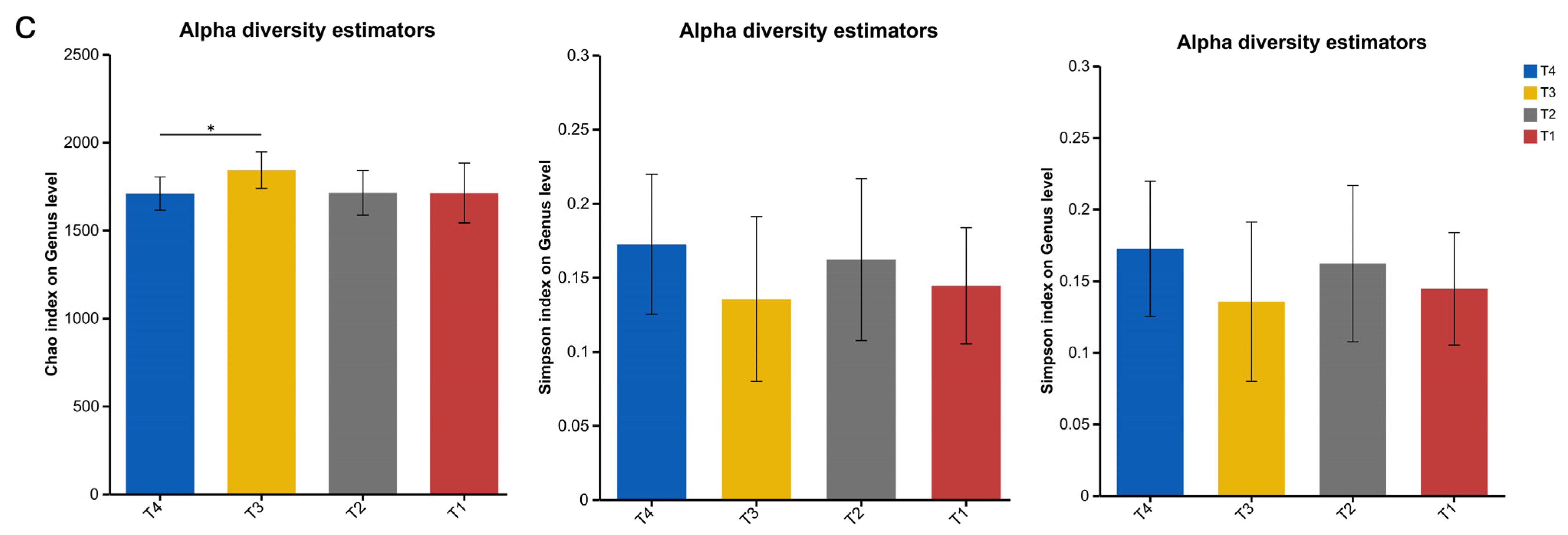
The rumen microbiota of ruminant animals is an important component of their digestive system. In this study, the urea, cottonseed meal, and rapeseed meal contained in the T4 group were rich in sulphur-containing amino acids, providing a more abundant nitrogen source for the T4 rumen microbiota, thereby enhancing microbial diversity, consistent with the findings of [
32]. Our data indicate that the rumen of Dumont lambs is dominated by the
Bacteroidetes and
Firmicutes phyla regardless of diet, with the abundance of
Firmicutes ranking second only to
Bacteroidetes at the phylum level. Firmicutes utilise free enzymes and cellulosomes for polysaccharide degradation, while Bacteroidetes employ polysaccharide utilization loci [
33]. They can convert plant fibres into low-molecular-weight carbohydrates and short-chain fatty acids (mainly butyric acid), providing energy sources for rumen microorganisms and nutrients for host animals [
25].
At the genus level,
Prevotella are key players in hemicellulose digestion and contribute significantly to the degradation of starch, cellulose, and pectin in the rumen [
34]. Their ability to produce propionate as a fermentation product may help reduce methane emissions in ruminants [
35]. Our study showed that in the T4 group,
Prevotella abundance increased but there was no significant difference. An increase in rumen
Prevotella has been linked to enhanced branched-chain amino acid synthesis, potentially contributing to improved muscle growth in ruminants [
36].
We also found that the microbial communities of certain genera underwent significant changes across the four treatment groups. In the urea-added treatment group, the abundance of
Acidaminococcus significantly increased. Research indicates that bacteria belonging to the
Acidaminococcus genus can grow using amino acids as their sole energy source and produce volatile fatty acids (VFAs) [
37,
38]. These bacteria produce metabolic products such as acetic acid and butyric acid through amino acid metabolism, thereby influencing the balance of the rumen microbial community and the host’s absorption of nutrients [
37]. The abundance of
Acidaminococcus is positively correlated with the total volatile fatty acid (TVFA) concentration in the rumen. Research indicates that the association between dominant rumen bacterial communities and methanogenic archaea is relatively weak. However, certain bacteria with comparatively low abundance exhibit strong correlations with methanogenic archaea, such as the succinate-producing Succinivibrionaceae, the succinate-utilising Dialister, and the amino-acid-fermenting Acidaminococcus, as well as methanogens belonging to the
Methanomassiliicoccaceae,
Methanosphaera sp. A4, and
Methanobrevibacter boviskoreani.
Succinivibrio spp. [
39]. Replacing part of the soybean meal with urea can effectively regulate the rumen microbial community and enhance fermentation efficiency. However, this is accompanied by a slight increase in methane emissions, and the T2 group also showed a significant increase in the abundance of
Candiatus_Themoplasmatota, accompanied by increased methane production [
40].
The
Sphaerochaeta genus is an important component of the rumen microbial community, and its metabolic characteristics are mainly reflected in fermentation and carbohydrate metabolism The genomes are highly enriched in fermentation and carbohydrate metabolism genes, many acquired from non-spirochetes, particularly
clostridia [
41]. Sphaerochaeta genus exhibit pleomorphic cell shapes including spherical, annular, curved rod, helical, and coccoid forms [
42]. They are chemoorganotrophic fermenters that utilise various carbohydrates, producing acetate, ethanol, hydrogen, and carbon dioxide as major end products, acetic acid supplies 70% of the body’s energy requirements and is utilised in the synthesis of body fat and milk fat. The hydrogen it produces is utilised by methanogenic bacteria to reduce CO
2 into methane. However, in the presence of sulphate-reducing bacteria or nitrate-reducing bacteria, hydrogen is employed to reduce sulphate or nitrate. This process competes with methanogenesis and can effectively reduce methane emissions [
41,
42]. Research has found that the
globotrichonium genus can generate acetic acid, lactic acid, and ethanol in the rumen using galacturonic acid and pectin, and these products are key intermediates in rumen methane production [
43]. Additionally, this bacterium is positively correlated with rumen nitrogen content [
44], which may also be one of the reasons for the significantly higher ammonia nitrogen content observed in the T4 group.
4.3. Rumen Microbial Functions
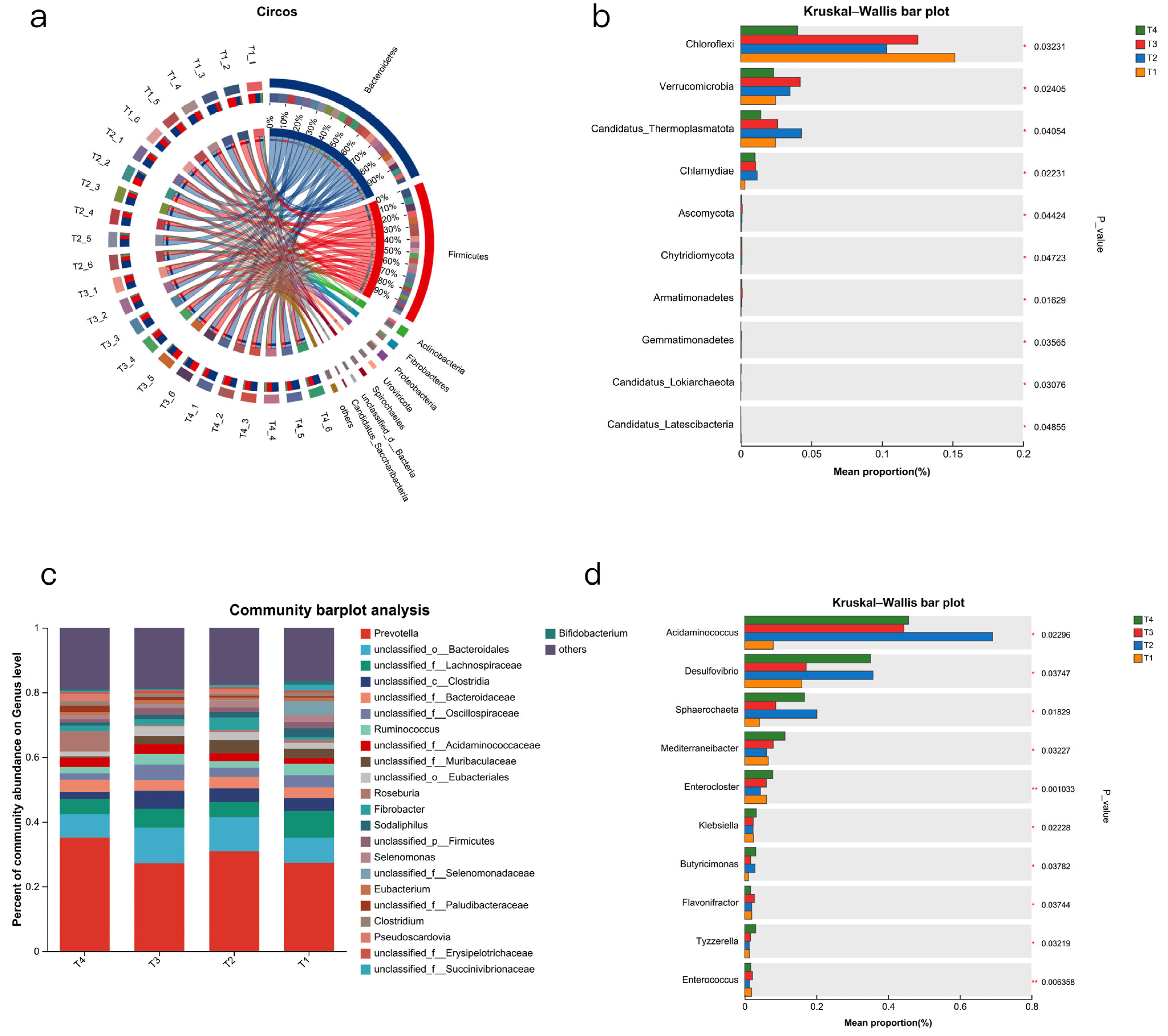
According to the KEGG function, we found that all groups had abundant “amino acid metabolism”, “carbohydrate metabolism” and “energy metabolism” functions regardless of diet. Interestingly, although all used urea, the results of its KEGG function enrichment at level 3 were different. The T2 group exhibited significantly abundant fructose and mannose metabolism ability, indicating that the rumen microorganisms in this group can effectively utilise the intermediate products of fibre degradation and avoid carbon waste [
45]. The ability of rumen microorganisms to utilise polysaccharides and succinic acid to produce acetic acid and propionic acid was enhanced, but the final VFA content was not significantly affected, while MCP was significantly increased. This indicates that replacing 6.4% the soybean meal with 1.5% urea is beneficial for the synthesis of polysaccharides, fumaric acid and other substances, and that the efficiency of microbial utilisation of VFA is enhanced [
46].
The T3 group has abundant ABC transporters functions. In rumen bacteria, ABC transporters are involved in carbohydrate uptake. These transporters use ATP hydrolysis to drive substrate translocation across membranes [
47]. Ruminal bacteria employ various transport mechanisms, including passive diffusion for hydrophobic substances and carrier-mediated transport for hydrophilic compounds. ABC transporters are membrane proteins that use ATP energy to transport various substances across biological membranes [
48]. They play crucial roles in cellular physiology, including nutrient import, toxin export, and protection against xenobiotics [
49]. This also explains the reason for the significant increase in microbial protein content in the T3 group. These proteins are also involved in mitochondrial membrane protein degradation, working alongside AAA proteases and other components to maintain membrane integrity and function [
50]. Additionally, ABC transporters participate in peptide trafficking and translocation, which is essential for cellular signalling and self-defence mechanisms [
51]. The peptides resulting from protein degradation can serve as signalling molecules or be further broken down for amino acid recycling and energy production [
51]. Ribosomes play a crucial role in protein synthesis and cellular function. Ribosomes are essential for mRNA selection, protein translation, and folding, with quality control mechanisms ensuring proper assembly and functionality [
52].
The T4 group has the richest ribosome, indicating that the rumen microorganisms are in an active proliferation state and require a large amount of synthetases (such as cellulase, amylase) and structural proteins. It is related to the host’s high feed utilization efficiency in the T4 group.
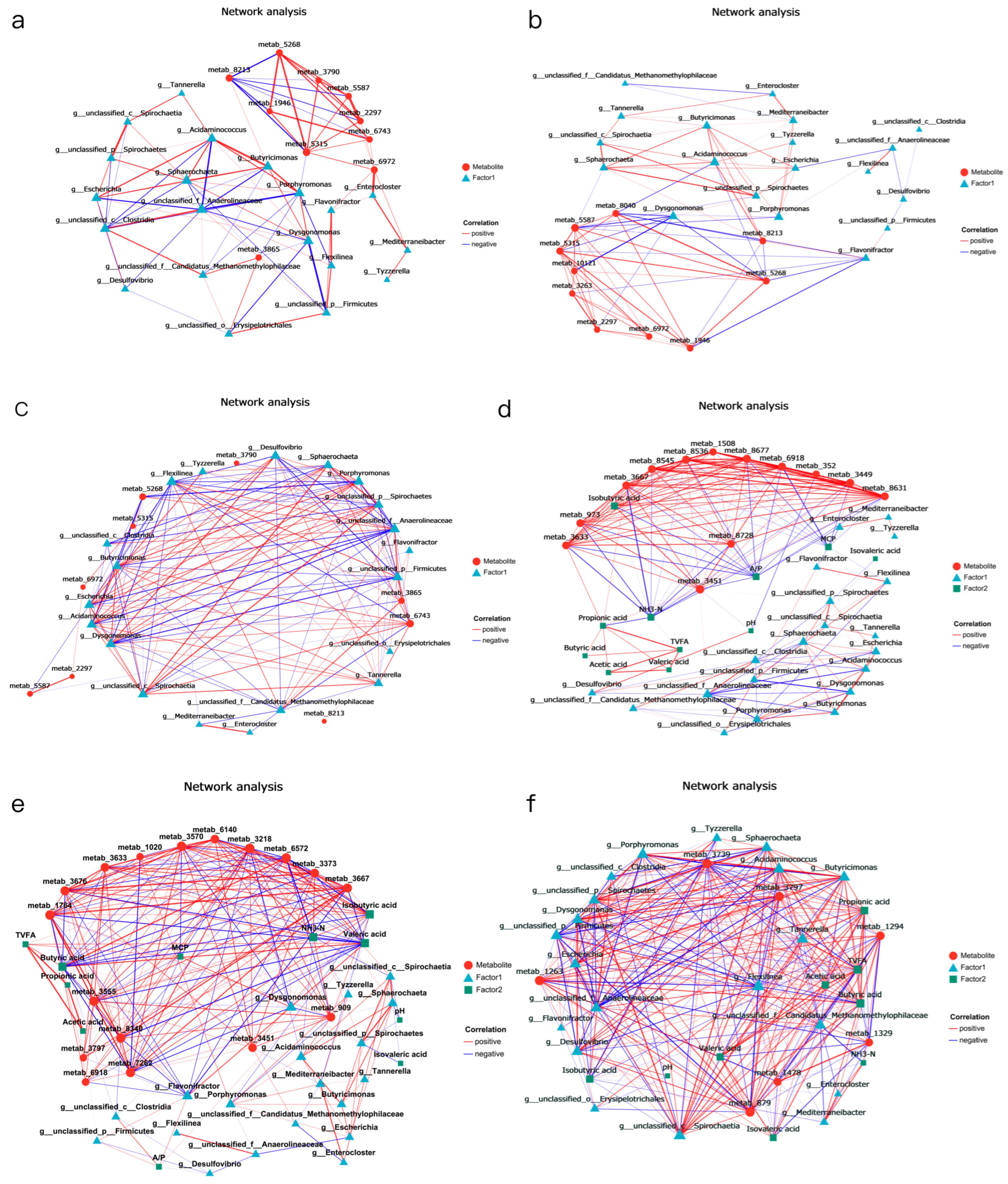
4.4. The Correlation Between Rumen Microbial Communities, Growth Performance, and Rumen Fermentation in Dumont Lambs
In this study, by replacing 4.3% the soybean meal with 1% urea, rapeseed meal, and cottonseed meal in the diet of Dumont lambs, it was found that the enrichment of arginine biosynthesis was significantly higher than in other groups, and there was a significant positive correlation between the
Firmicutes phylum and
Chloroflexi in the rumen and arginine biosynthesis. This finding is related to the regulation of microbial metabolism and amino acid metabolic pathways by changes in protein source components. Additionally, the addition of urea may promote microbial protein synthesis by providing a non-protein nitrogen (NPN) source, thereby influencing amino acid metabolic pathways. The increased arginine synthesis provides precursor substances for propionic acid production [
53], which is also one of the reasons for the improved feed conversion efficiency in the T4 group. Consistent with study [
54], this study found that cows with high feed conversion efficiency had higher propionic acid levels in the rumen. Additionally, study [
55] indicated that increased arginine metabolism in the rumen is associated with enhanced microbial community activity, particularly among microorganisms involved in the urea cycle.
In the contribution analysis of amino acid metabolism and carbohydrate metabolism, regardless of diet,
Prevotella plays a crucial role in the metabolism of carbohydrates, lipids, and amino acids in the rumen and is correlated with livestock growth performance [
56].
Prevotella’s ability to produce propionate during fermentation may reduce methane emissions in ruminants [
35]. Studies have shown a correlation between
Prevotella abundance and improved glucose metabolism in both humans and animals [
57]. The urea-added group reduced the abundance of
unclassified Lachnospiraceae. Studies indicate that its isolates exhibit metabolic flexibility when exposed to different substrates and hydrogen concentrations, producing multiple end products, including acetate, butyrate, and hydrogen [
58].
The 1% urea, rapeseed meal, and cottonseed meal group increased
Roseburia abundance.
Roseburia bacteria produce short-chain fatty acids, particularly butyrate, which play a crucial role in colon health, immune function, and anti-inflammatory properties [
59]. In energy metabolism, the abundance of Clostridium increased, capable of fermenting cellulose and other carbohydrates [
60]. However, their content in the rumen is extremely low, indicating limited contributions to rumen metabolism [
61]. This suggests that dietary regulation can influence rumen fermentation, amino acid metabolism, carbohydrate metabolism, and energy metabolism by altering the structure and function of the microbiota, primarily through the regulation of the
Prevotella genus.
4.5. Rumen Metabolites
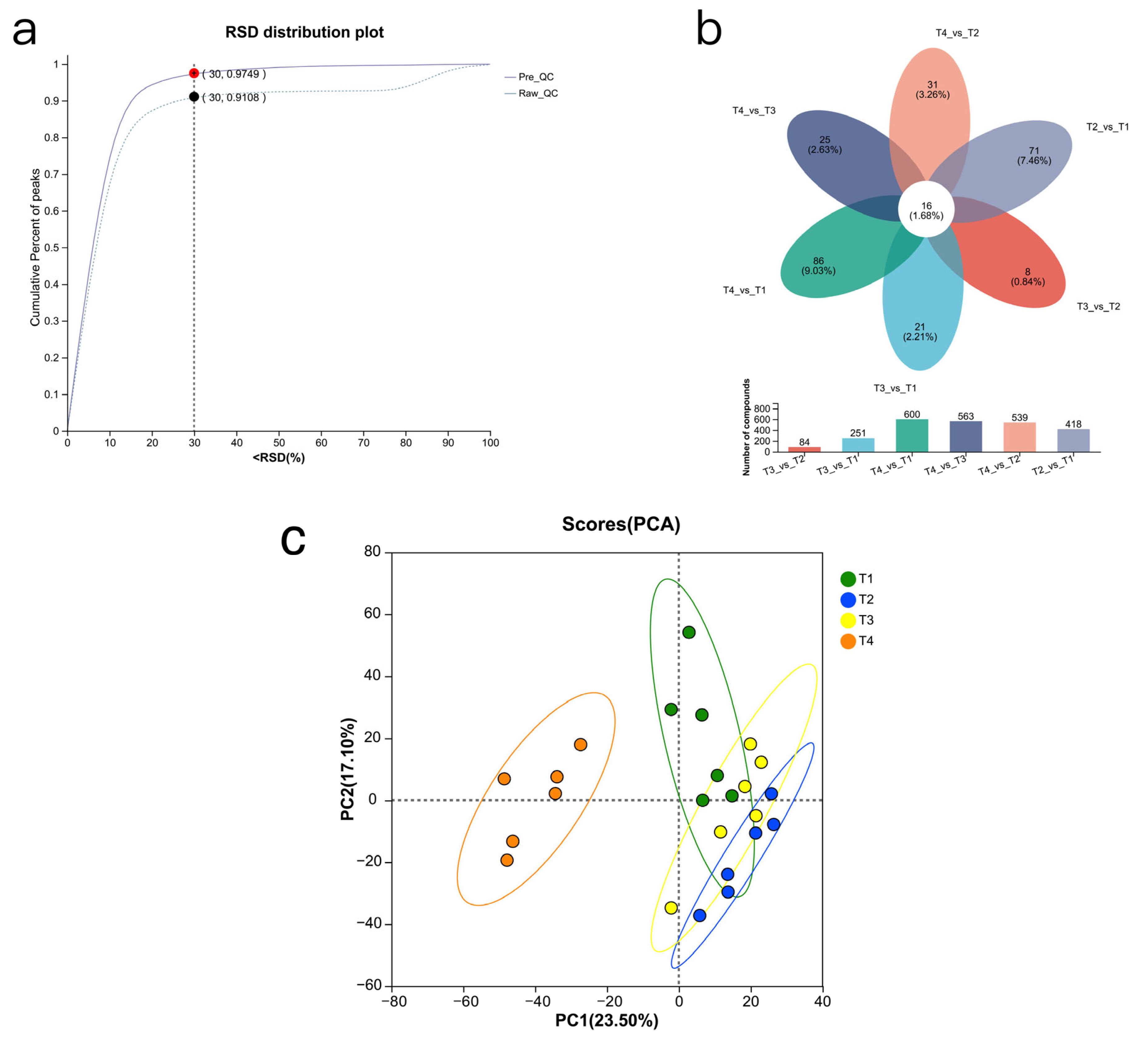
Changing the nitrogen source in the feed can affect rumen metabolites by altering the diversity or relative abundance of rumen microorganisms [
62].
In this study, Dumont lambs fed urea, rapeseed meal, and cottonseed meal had more unique metabolites. The dry matter (DM), organic matter (OM), and crude protein (CP) degradation rates and effective degradation rates of soybean meal in the rumen were higher than those of cottonseed meal and rapeseed meal, and the rumen retention time was shorter. This indicates that cottonseed meal and rapeseed meal degrade relatively slowly, prolonging microbial utilisation time and promoting the accumulation of various metabolic products. Additionally, replacing soybean meal with urea, cottonseed meal, and rapeseed meal simultaneously increased rumen volatile fatty acid (VFA) production and microbial diversity, resulting in the generation of more specific metabolites.
In the KEGG functional enrichment analysis, the functional enrichment in the T2 group primarily involved enhanced amino acid metabolism and synthesis, as well as protein digestion, absorption, and transport. This indicates that the use of rapidly degradable proteins facilitates the activation of nutrient digestion within the rumen and meets the microbial demand for protein synthesis.
In addition, the ammonia produced by urea decomposition can be used by rumen microorganisms for amino acid synthesis, further participating in the proliferation, apoptosis, absorption and transport functions of rumen epithelial cells. The synthesis of amino acids may promote the metabolism of D-amino acids in the rumen, enhance the biosynthesis of aminoacyl-tRNA, and regulate the expression and activity of functional enzymes and genes involved in phenylalanine, tyrosine, and tryptophan metabolism. These processes are important components of microbial proteins.
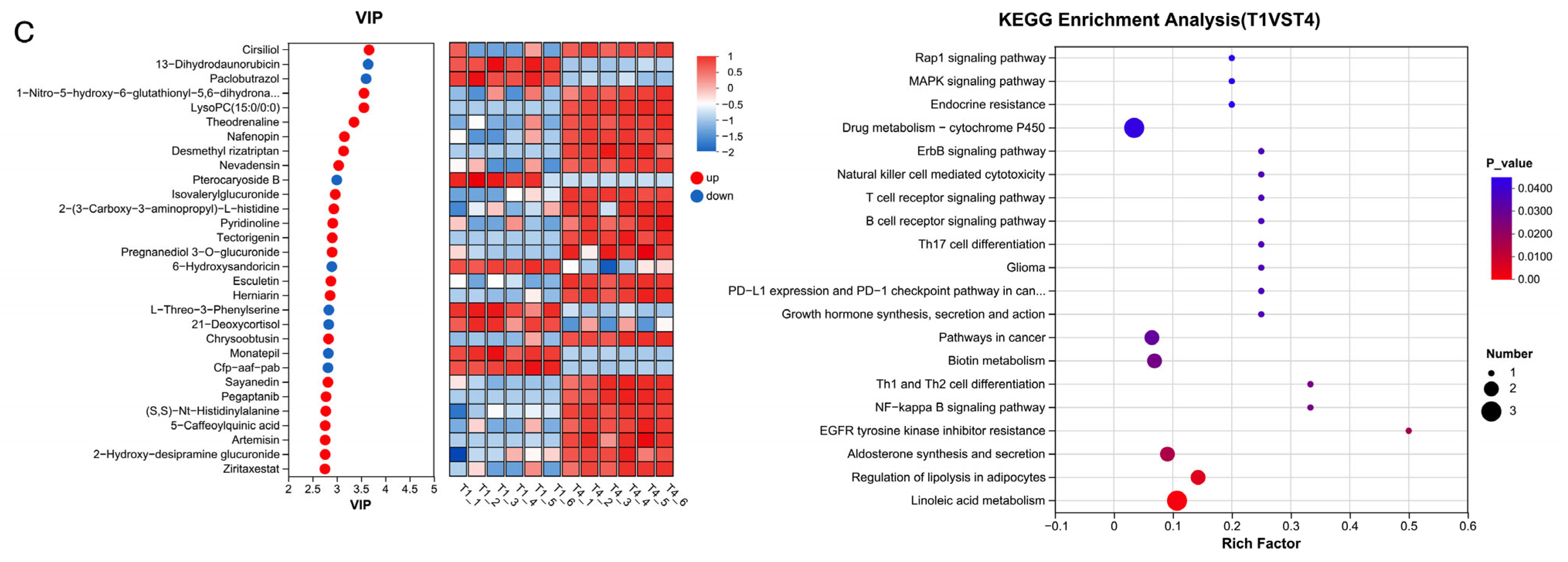
The 1% urea, rapeseed meal, cottonseed meal group had a significantly enriched “Linoleic acid metabolism” KEGG function. It indicated that cotton meal and rapeseed meal contain a high proportion of linoleic acid, and rumen microorganisms need to activate metabolic pathways to hydrogenate it into conjugated linoleic acid and stearic acid [
32]. The enrichment of metabolic pathways indicates that the hydrogenation process is active. The hydrogenation process of linoleic acid consumes H
2, which may reduce methane emissions, but also results in the loss of some dietary energy [
63]. Studies have shown that rapid hydrolysis of urea leads to a short-term spike in rumen ammonia concentration, inhibiting some fibre-degrading bacteria such as
Ruminococcus flavefaciens, while ammonia-tolerant bacteria such as
Prevotella proliferate relatively more, endowing them with stronger lipid metabolism ability [
64]. In addition, some rumen microorganisms (such as
Clostridium) can promote nitrogen assimilation through short-chain fatty acids (such as propionic acid) produced by linoleic acid metabolism, helping to maintain ammonia homeostasis [
65]. In the KEGG functional enrichment analysis of other groups, we found that the T2 group exhibited abundant ABC transporters functions, etc.
4.6. Relation Analysis
Urea is a type of rapidly degradable protein, various bacterial species have been identified as ureolytic, including
Selenomonas ruminantium,
Lactobacillus sp.,
Enterococcus sp., and
Staphylococcus sp. [
66]. Other ureolytic species include
Peptostreptococcus productus,
Ruminococcus albus, and
Succinivibrio dextrinosolvens [
67]. Recent studies using high-throughput sequencing have identified additional ureolytic genera, such as
Pseudomonas,
Haemophilus,
Neisseria, and
Bacillus [
68]. Rumen microorganisms participate in urea hydrolysis through compartment—specific activities. Several studies report that bacteria adhering to the rumen wall display the highest urease activity—with over 55% of ureC gene sequences remaining unclassified—indicating a reservoir of novel taxa [
69]. Liquid-associated bacteria (e.g.,
Prevotella and
Succinivibrionaceae) exhibit intermediate enzyme activity, while solid-associated microbes (including fibre degraders such as
Lachnospiraceae) contribute at lower, yet functionally significant, levels [
54].
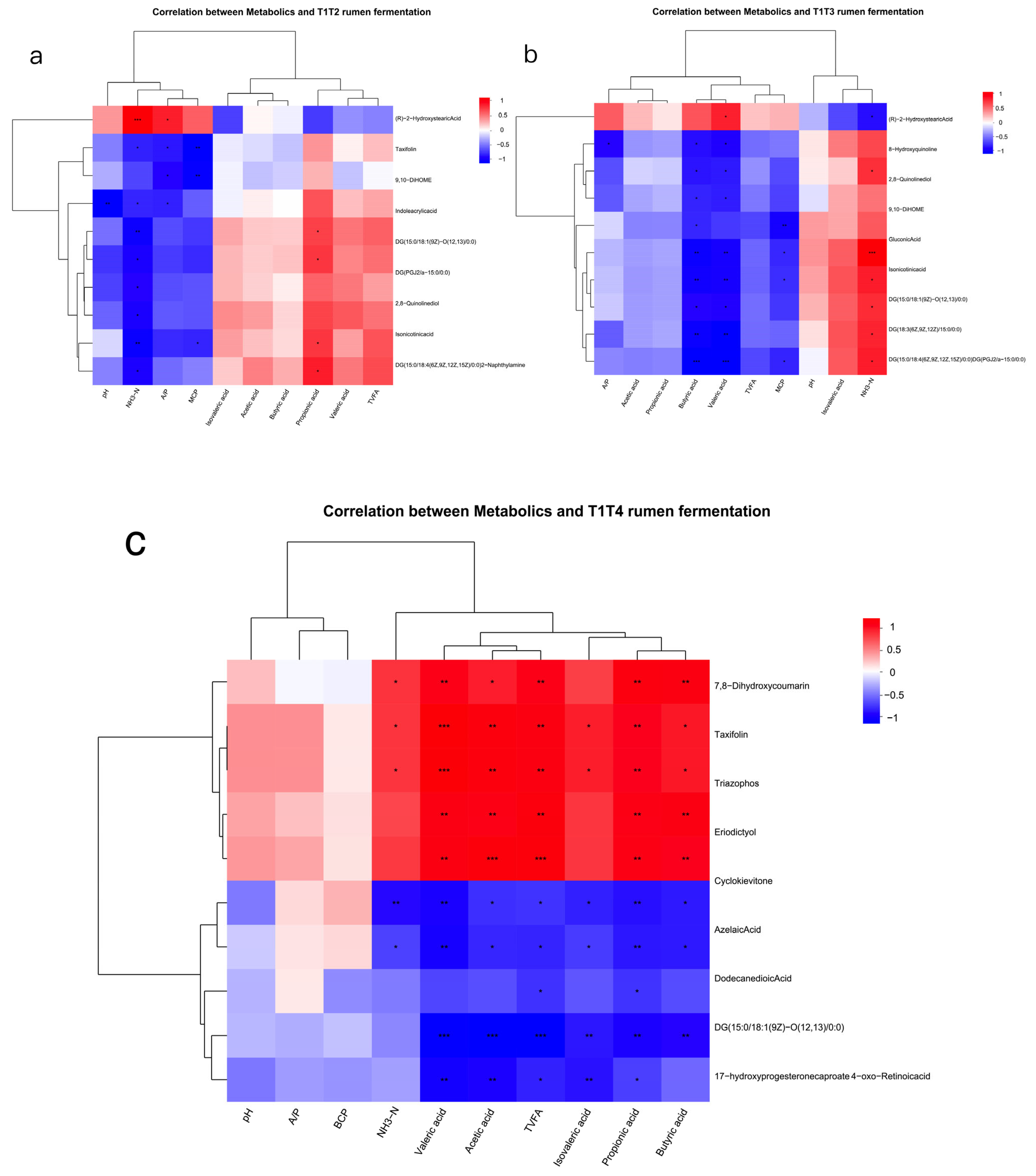
In this experiment, when 1.5% urea was used to replace 6.4% soybean meal, the rumen microbiota dominated by Enterocloster influenced the accumulation of rumen fermentation-related metabolites such as 9,10-DiHOME, DG (PGJ2/a-15:0/0:0), and isonicotinic acid. Urea is rapidly hydrolysed by urease in the rumen into ammonia (NH3-N), leading to an increase in NH3-N concentration in the rumen. Excessive NH3-N promotes the proliferation of bacteria such as Enterocloster, thereby affecting the synthesis of the aforementioned metabolites.
When 1% urea replaced 4.3% the soybean meal, the microbial community, dominated by
Acidominococcus, affected the accumulation of DG(PGJ2/a-15:0/0:0), and this metabolite showed a significant positive correlation with NH
3-N content and a negative correlation with MCP. This phenomenon is related to changes in the fermentation rate of carbohydrates in the rumen after replacing part of the soybean meal with urea [
70].
Acidaminococcus has been shown to utilise citric acid and trans-citric acid as energy sources, producing hydrogen and hydrogen sulphide. The high NH
3-N concentration generated by urea degradation inhibits
Acidominococcus activity by competing with hydrogen sulphide for H2, thereby enhancing the rumen microbial conversion efficiency of NH
3-N and increasing the MCP content. When 1% urea meal and cottonseed meal replaced soybean meal, the accumulation of
Sphaerochaeta and taxifolin showed a positive correlation, and taxifolin was also extremely significantly positively correlated with TVFA and VFA components.
Taxifolin, a flavonoid compound with potent antioxidant properties, has shown promising therapeutic potential across various biological systems. It protects retinal pigment epithelial cells against oxidative stress-induced apoptosis by activating NRF2 and enhancing phase II antioxidant enzymes [
71]. While not directly related to rumen VFA, taxifolin has been shown to alter gut microbiota and increase short-chain fatty acid production, particularly butyric acid, in mice with induced colitis [
72].
Consistent with this study, many studies have shown that [
20,
73] certain metabolites are positively correlated with the formation of specific bacterial groups, while negatively correlated with the formation of other bacterial groups, and this is related to different feed utilization efficiencies. The relationships between the rumen microbial taxa, functions, and metabolome provide new insights into the functional roles of the rumen microbiome in producing small molecule metabolites and contributing to host traits. Also, this article has certain limitations. After replacing part of the soybean meal with urea, rapeseed meal, and cottonseed meal in the diet of Dumont lambs, the research mainly focused on the rumen part. In the future, based on an expanded sample size, this study should be extended to the hindgut to clarify how urea, cottonseed meal, and rapeseed meal affect the absorption, digestion, and metabolism of nutrients in the host. The specific mechanism still needs further research.
5. Conclusions
The degradation rates of urea, rapeseed meal, and cottonseed meal in the rumen differ, stimulating carbon metabolism and arginine synthesis pathways primarily mediated by Precolleta, thereby affecting the accumulation of metabolites such as 9,10-DiHOME, DG (PGJ2/a-15:0/0:0), isonicotinic acid, and taxifolin, which in turn influence rumen fermentation. Replacing part of the soybean meal with urea in the diet of Dumont lambs can increase microbial protein (MCP) production, which aids in the absorption of subsequent nutrients. Replacing all soybean meal with 1% urea + 5% rapeseed meal + 6.6% cottonseed meal can improve rumen fermentation, enhance rumen microbial and metabolite diversity, and optimise the synergistic metabolic efficiency of carbon, nitrogen, and sulphur. However, the specific mechanisms of post-rumen digestion and metabolism require further investigation.