1. Introduction
Eggshell quality is an important index to evaluate egg quality, which directly impacts the egg qualification rate, hatchability, and market value. Especially with the continuous application of mechanized production in egg collection and packaging, an increase in cracked eggs poses a great threat to laying production. A study showed that eggshell calcium (Ca) metabolism indirectly determines eggshell quality [
1]. Eggshell Ca supply and estrogenic regulation effects are the main factors contributing to eggshell calcification and eggshell quality. Ca derived from intestinal absorption, regulated by vitamin D receptor (VDR) and re-absorbed in the kidney, is transported to the uterus and calcified to form eggshells. This process is regulated by osteopontin (OPN) in the eggshell gland [
2]. Ca transportation is regulated by Ca transporters, such as plasma membrane calcium-ATPase (PMCA), sodium-calcium exchanger (NCX), and calbindin-D
28k (CaBP-D
28k) [
3]. Moreover, several reproductive hormones, mainly including follicle-stimulating hormone (FSH), luteinizing hormone (LH), and 17-β-Estradiol (E2), also have an important regulatory effects on Ca metabolism [
4].
Egg cholesterol content in egg yolk is another critical value for evaluating egg quality. Although eggs are rich in many essential nutrients needed for human health, such as high-quality protein, minerals, and vitamins, the high concentration of cholesterol is a risk for human health, especially hypercholesteremia and cardiovascular disease. The cholesterol content is approximately 200~275 mg in one large egg yolk, which is close to the recommended daily cholesterol intake according to the American Heart Association [
5]. A study showed that 100 mg of egg cholesterol increased total cholesterol [
6]. Therefore, excessive egg consumption is generally positively correlated with atheroscle rotic cardiovascular disease [
6]. In addition to liver lipid metabolism, the cholesterol content in egg yolk is also regulated by cholesterol biosynthesis and transformation in the livers of laying hens. Cholesterol synthesized in the liver is transported to the ovaries in the form of very-low-density lipoprotein cholesterol (VLDL) via the bloodstream and participates in the formation of egg yolks [
7]. The rate-limiting enzyme of cholesterol biosynthesis is 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR). The key enzyme regulating the transformation of cholesterol to bile acids is cholesterol 7α hydroxylase (CYP7A1) in the liver [
8].
Recent studies have indicated that Chinese herbs and their extracts have the ability to improve laying rates, egg quality, and eggshell quality and to regulate lipid metabolism and estrogen levels [
9,
10,
11]. In China, several specific Chinese herbs, such as
Leonurus japonicus (Chinese motherwort),
Jujube,
Artemisia argyi (
A. argyi), and
Radix isatidis, have been used for thousands of years to regulate hormone metabolism, enhance immune effects, and improve blood circulation [
12,
13,
14,
15]. Additionally, numerous studies have shown that these Chinese herbs have exhibited regulating effects for inhibiting hepatic lipid synthesis and reducing fat deposition [
5,
16,
17,
18]. These herbs contain a variety of active ingredients, especially including alkaloids, flavonoids, and diterpenes, which have been reported to regulate estrogen metabolism and anti-oxidative stress effects [
11,
19]. Practical experience with traditional Chinese herbs has confirmed that Chinese herb compounds exhibit greater biological effects than individual herbs [
20,
21]. To date, the combination of
Leonurus japonicus,
jujube,
Artemisia argyi (
A. argyi), and
Radix isatidis has not been studied in laying hens. Therefore, based on traditional Chinese herbal theory, the present study utilizes herb compounds to modulate antioxidant properties and improve egg quality and cholesterol content.
The results show that processing technology has an important influence on Chinese herbal pharmacological activities. Because Chinese herbs are protected by a hard cell wall, their application as additives results in low utilization rates and poor palatability, which reduces their regulatory effects [
4]. Numerous studies have shown that probiotic fermentation and enzymatic methods can effectively enhance the utilization efficiency of Chinese herbs [
22,
23,
24]. Previous findings indicated that fermented Chinese herbal medicines significantly improved laying rates, egg quality, and eggshell strength, and increased plasma estradiol levels [
25].
Therefore, the purpose of the present study was to clarify the regulatory influence of fermented Chinese herb compounds on egg production, eggshell quality, yolk cholesterol, and estrogenic effects.
2. Materials and Methods
Experimental protocols were performed in accordance with the guidelines of the Ethics Committee of Yangtze University (No. DKYB202400412) in China for the humane care and use of animals in research.
2.1. Preparation of Fermented Chinese Herbs
Fermented Chinese Herbs (FCH) were supplied by Wuhan Dabeinong Science and Technology Park (Wuhan, China). Briefly, the formulation of Chinese herbs consisted of a combination of 15%
motherwort, 8%
Artemisia argyi, 11%
radix isatidis, and 8%
jujube powder, with 28% corn, 14% wheat bran, and 16% soybean meal as the fermentation carriers. The fermentation bacterial agent included
Bacillus subtilis 1.0 × 10
10 CFU/mL,
Bacillus coagulans 3.0 × 10
10 CFU/mL,
Enterococcus faecalis 2.4 × 10
10 CFU/mL,
Lactobacillus plantarum 4.0 × 10
11 CFU/mL, and
Saccharomyces cerevisiae 4.5 × 10
10 CFU/mL. The fermentation substrate was processed to 50% moisture content, then inoculated with 4% of microbial combination liquid. Subsequently, fermentation was carried out in a fermentation bag with a one-way breather valve for 7 d to prepare FCH. Then, the FCH (with water content of about 38~40%) was added to feed. The main approximate nutrient contents of the FCH, measured by AOAC [
26], are shown in
Table 1.
2.2. Experimental Animals and Design
A total of 1260 healthy Hy-Line laying hens (34-week-old) were randomly divided into three groups, with 6 replicates per group and 70 hens per replicate: the CON group, the 2% FCH group, and the 3% FCH group. The hens in the 2% FCH group and the 3% FCH group were fed the diet supplemented with 2% FCH and 3% FCH, respectively. The diet was prepared according to NRC (1994) [
27] to meet the nutrient needs of laying hens (
Table 2). The experiment lasted for 8 weeks. After one week of adaptation, all laying hens were fed the assigned experimental diets for 7 weeks. Artificial light was used throughout the house of the test laying hens. A total of 3~4 hens were housed in each metal cage (48 × 36 × 40 cm), with an adjoining cage consisting of 70 hens per replicate. The temperature was kept at 24~26 °C, and the relative humidity was kept at 50~60%. Feeds were supplied ad libitum in mash.
2.3. Sample Collection
At the end of the experiment, two hens per replicate were randomly selected and slaughtered for sample collection. The serum was separated from blood with centrifugation at 1000 g/min for 15 min and stored at −20 °C to analyze the serum biochemical indexes, serum hormone, and bile acids. Liver, duodenal tissue mucosa, kidney, and uterus samples (one hen per replicate) were removed, placed into tubes, snap-frozen in liquid nitrogen, and then stored at −80 °C until analysis. Meanwhile, 4 eggs per replicate were selected to determine egg quality, eggshell, and egg yolk indices on the 28th and 56th days of the experiment.
2.4. Performance
During the experiment, the number of eggs produced, egg weight, and feed consumption were recorded daily. At the time of egg collection, all eggs were classified as normal, cracked, or soft. Average daily egg weight (ADEW), average daily feed intake (ADFI), feed–egg ratio, egg laying rate, cracked shell egg rate, and soft shell egg rate were calculated in replicate units based on daily records.
2.5. Measurement of Egg Quality
A high-precision digital egg tester (DET-6 000, Beijing Bulad Technology Development Co., Ltd., Beijing, China) was used to measure the yolk color, albumen height, and Haugh unit score. The eggshell strength was determined by the EFG-0503 egg shell force gauge (Robotmation, Tokyo, Japan). The eggshell thickness was determined by the SD201 digital display thickness gauge (Shenzhen Yuanhengtong Technology Co., Ltd., Shenzhen, China) by calculating the average thickness of the air cell, equator, and sharp end segments. Eggshell color was measured using a TS20 color colorimeter (Shenzhen 3nh Technology Co., Ltd., Shenzhen, China).
2.6. Ca Determination in Eggshell
After peeling off the inner membrane, the eggshell was crushed into powder and oven-dried at 103 °C for 2 h. Then, the powder was ashed at 600 °C in a muffle furnace for 3 h. Finally, the content of Ca was measured by potassium permanganate titration [
28].
2.7. Assay of Serum Biochemical Indices, Serum Hormone, and Serum Bile Acids
The serum biochemical indices were determined with ELISA using a Hitachi 7600 automated biochemical analyzer (Hitachi, Tokyo, Japan). Assay kits for aspartate transaminase (AST, C010-2-1), alanine aminotransferase (ALT, C009-2-1), triglyceride (TG, F001-1-1), total cholesterol (TC, F002-1-1), high-density lipoprotein cholesterol (HDL-c, A112-1-1), low-density lipoprotein cholesterol (LDL-c, A113-2-1), very-low-density lipoprotein cholesterol (VLDL-c, H249-1-2), and total bile acid (TBA, E003-1-1) were purchased from the Nanjing Jiancheng Bioengineering Institute (Nanjing, China). The determination was carried out following the manufacturer’s instructions.
Serum hormone levels of FSH (H101-1-2) and LH (H206-1-2) were determined by ELISA kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China). Serum concentrations of E2 (TW15203) were determined by ELISA kits (Shanghai Tongwei Biotechnology Co., Ltd., Shanghai, China).
2.8. Measurement of Antioxidant Enzyme Activity in the Liver and Uterus
The total antioxidant capacity (T-AOC), superoxide dismutase (SOD) activity, and malondialdehyde (MDA) content in the liver and uterus were determined by an assay kit (Nanjing Jiancheng Bioengineering Institute, Nanjing, China).
2.9. Measurements of TC and TG in Liver and Egg Yolk and Liver TBA
The TC (F002-1-1), TG (F001-1-1) in the liver and egg yolk were determined with commercial assay kits (Nanjing Jiancheng Bioengineering Institute, Nanjing, China) and performed using a Hitachi 7600 automated biochemical analyzer (Hitachi, Tokyo, Japan). The liver TBA (MAK309) was determined with assay kits (Sigma-Aldrich Life Science, Shanghai, China).
2.10. Gene Expression of Cholesterol Metabolism in Liver and Ca Metabolism in Kidney, Duodenum and Uterus
Methods of RNA extraction and qRT-PCR of samples were followed the procedures described in our previous research [
29]. Total RNA was extracted from duodenum, liver, and uterus samples using Trizol reagent (Thermo Fisher Scientific, New York, NY, USA). First-strand cDNA synthesis was performed using the FastKing cDNA first-strand synthesis kit (Beijing Tiangen Biotechnology Co., Ltd., Beijing, China). The primers used in this study were designed using the Primer 5.0 software package (
Table 3). The
GAPDH gene was used as an internal reference to standardize the target gene levels, and the relative mRNA expression of the target genes was calculated as a ratio to the
GAPDH gene using the 2
−ΔΔCT method.
2.11. Statistical Analysis
Statistical analyses were performed using SAS V9.4. Data were analyzed with one-way ANOVA using Duncan’s test. Orthogonal polynomial contrasts were also used to evaluate the linear and quadratic effects of FCH levels. Statistical differences were considered significant at p < 0.05.
4. Discussion
In general, the present study with laying hens showed that dietary supplementation with FCH enhanced egg production and improved egg quality, mainly by enhancing eggshell quality and decreasing egg yolk cholesterol content. The results also reveal that the FCH exhibited positive effects on serum estrogen, liver lipid metabolism, and oxidative stress in laying hens.
Egg shell quality is a major concern for egg producers, as it directly affects the egg appearance and cracked rate in the process of packaging and transportation [
30]. The evaluation of eggshell quality mainly includes eggshell strength, eggshell thickness, eggshell color degree, egg breaking rate, and soft shell egg rate. Eggshells are mainly composed of calcium carbonate. Therefore, Ca metabolism determines eggshell calcification and quality during formation [
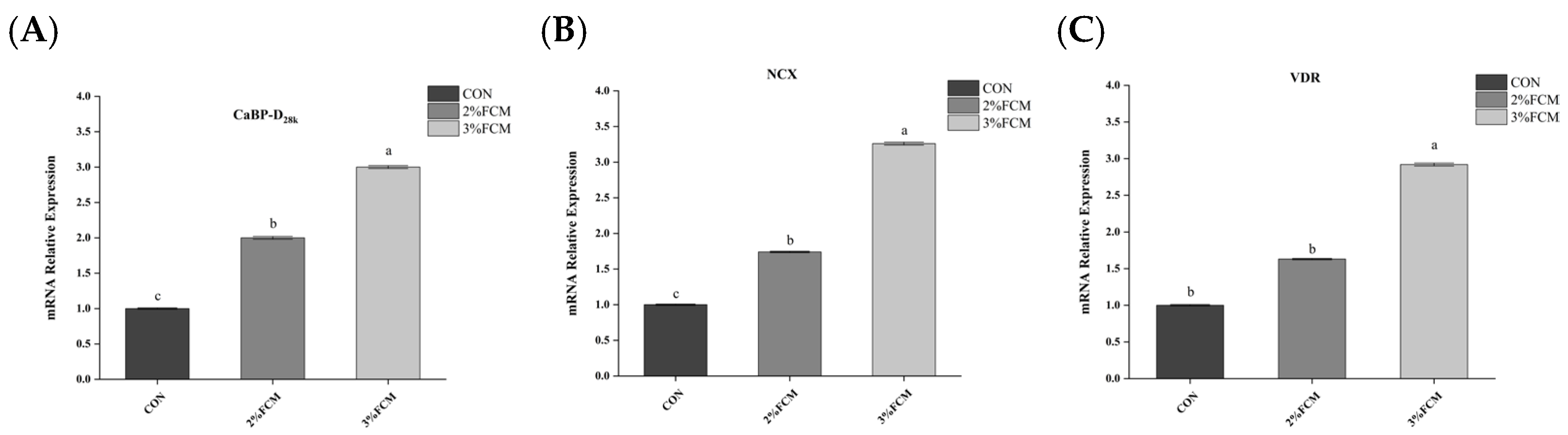
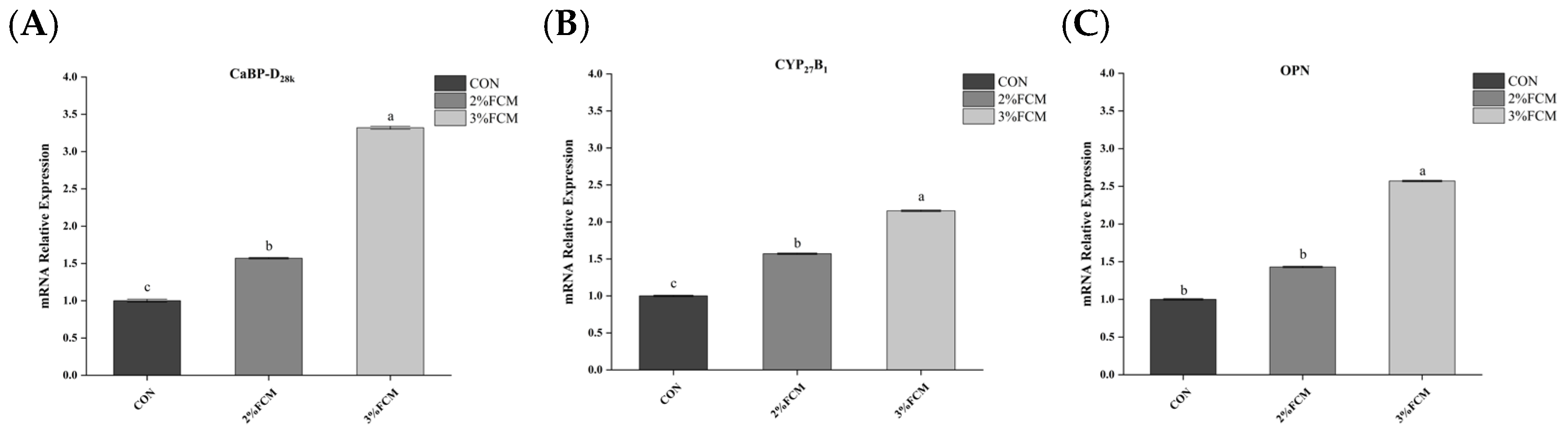
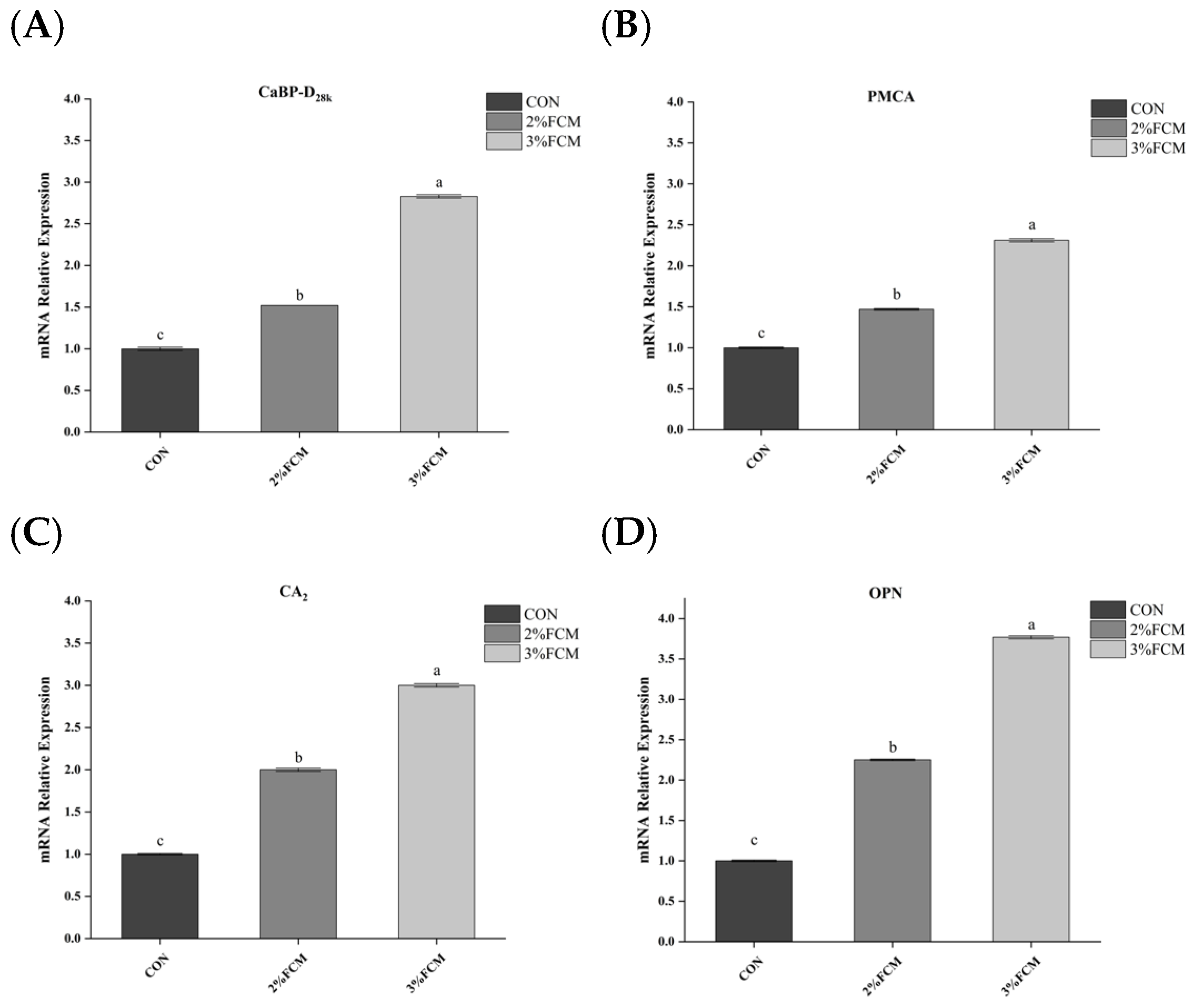
21]. Ca derived from intestinal absorption and re-absorption in the kidney is transported to the uterus and calcified to form eggshell. Ca transportation is regulated by calcium transporters, such as PMCA, NCX, and CaBP-D
28k [
31]. CaBP-D
28k is the main calcium-binding protein in laying hens, involved in calcium metabolism in the duodenum, kidney, and eggshell gland [
2]. VDR is involved in the uptake of active vitamin D3. Meanwhile, active vitamin D3 is also regulated by CYP27B1, which promotes 1,25(OH)
2D
3 activity [
32,
33]. NCX takes part in the transport of Ca
2+ from the duodenum into the blood. Eggshell calcification is regulated by the glycoprotein OPN [
2]. Therefore, the expression of genes related to eggshell calcification is an important factor affecting eggshell quality. This experimental study found that FCH significantly increased the relative mRNA expressions of
NCX, liver
CaBP-D28k, and uterine
PMCA,
CA2, and
OPN. The results indicate that FCH improved eggshell quality by regulating calcium metabolism.
A study showed that a Chinese herb mixture regulated eggshell Ca metabolism by modulating sex hormone levels [
11]. Eggshell calcification is regulated by several estrogen, such as FSH, LH, E2, and gonadotropin-releasing hormone (GnRH). Specific Chinese herbs, such as
Leonurus japonicus, exhibit the function of regulating sex hormone levels and egg quality [
34]. The main active compounds (flavonoids and alkaloids) in
Leonurus japonicus and A.
argyi were reported to regulate estrogen metabolism [
34]. The present study showed that FCH increased the laying rate and the levels of FSH, LH, and E2. FSH and LH play important roles in regulating follicular maturation and reproductive function [
28]. E2 regulates shell calcification via calcium metabolism in poultry [
35]. Extensive studies have also shown that higher estrogen concentrations in serum are closely positively related to egg production and eggshell quality [
28,
36].
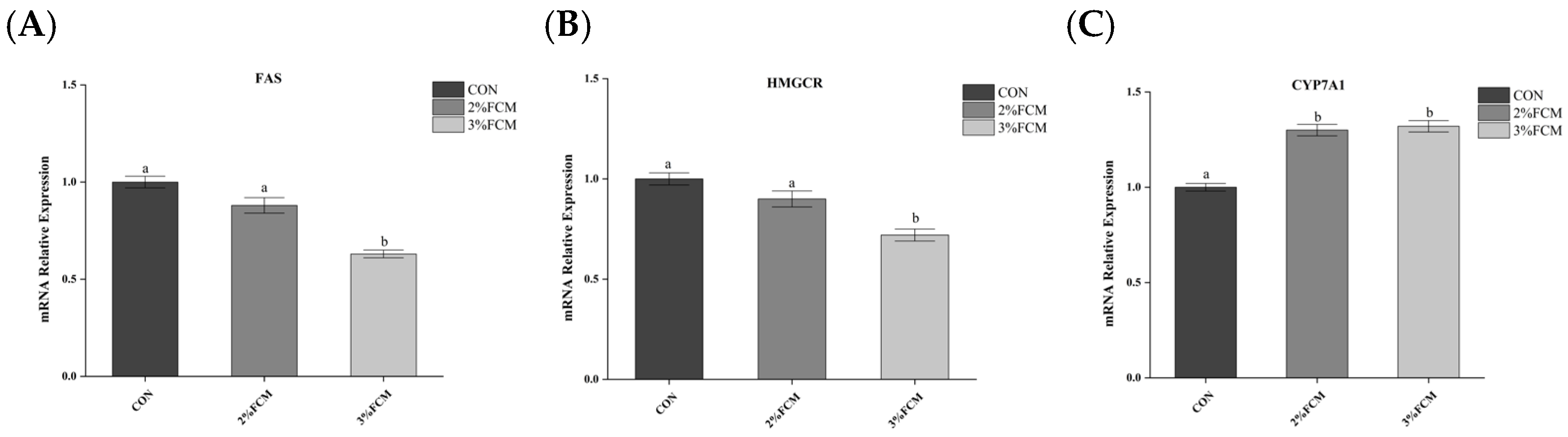
Egg cholesterol content is a major concern for consumers. Excessive egg consumption contributes to high-cholesterol-related diseases, such as atheroscle rotic cardiovascular disease [
6]. It is an important objective in laying hen production to reduce cholesterol content in eggs. Egg yolk cholesterol comes from the liver. Therefore, the egg cholesterol content is affected by cholesterol synthesis, transportation, and degradation. Cholesterol is synthesized by the critical rate-limiting enzyme HMGCR in the liver. With the action of lipoprotein VLDL, the cholesterol is transported to the ovaries and takes part in the formation of egg yolks [
7]. Cholesterol content is also reduced by breaking down to bile acid with the action of
CYP7A1 mRNA expression. Therefore, egg cholesterol content is affected by the expressions of
HMGCR and
CYP7A1 and the VLDL level. The present study showed that FCH decreased the serum VLDL-C level and
HMGCR mRNA expression and increased the
CYP7A1 mRNA expression, which resulted in a decrease in egg cholesterol.
Numerous studies have shown that Chinese herbs have regulatory effects on hepatic lipid metabolism in laying hens [
5,
16,
17,
18].
Leonurus japonicus exhibited inhibiting effects on hepatic lipid synthesis [
17].
Artemisia argyi exerted depression effects on lipid metabolism [
16].
Jujube and
Radix isatidis also repressed fat deposition and down-regulated the expressions of the main adipogenic transcription factors (such as
PPARγ) via the AMPK signaling pathway [
5,
18]. The present research also showed that the Chinese herb compounds inhibited
FAS and
HMGCR expression and decreased the TC and TG contents in the liver and egg yolk. Additionally, a previous study showed that estrogen plays an important role in lipid metabolism in the liver of hens. Estrogen decreased the expressions of genes involved in lipid metabolism and reduced lipid deposition [
37]. This study also indicated that the Chinese herb compounds decreased the liver lipid contents by enhancing the estrogen FSH, LH, and E2 levels.
Oxidative stress is a key factor affecting egg production, egg quality, and lipid metabolism in laying hens [
38,
39]. Multiple studies have shown that excess ROS can induce follicular atresia and repress follicular growth and ovulation, which leads to drops in egg production [
38]. Moreover, oxidative stress increases the occurrence of liver impairment, resulting in fatty liver syndrome [
38]. The present study showed that FCH increased the activities of SOD and T-AOC and decreased MDA levels in the liver and uterus. Meanwhile, FCH also increased the liver total bile acid (TBA) contents and decreased the activities of AST and ALT. AST and TBA are critical indices for evaluating liver health status [
39]. Multiple studies have indicated that the natural activity components (flavonoids, terpenoids, polysaccharides, and polyphenols) of the present Chinese herb compounds exhibit important roles in antioxidant properties [
13,
14,
40]. The present study also indicated that CFM increased egg production and decreased egg cholesterol content by enhancing antioxidant properties in the liver and uterus.