Chasing Pinna nobilis Survivors: Current Status in Spanish Open Coastal Waters
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
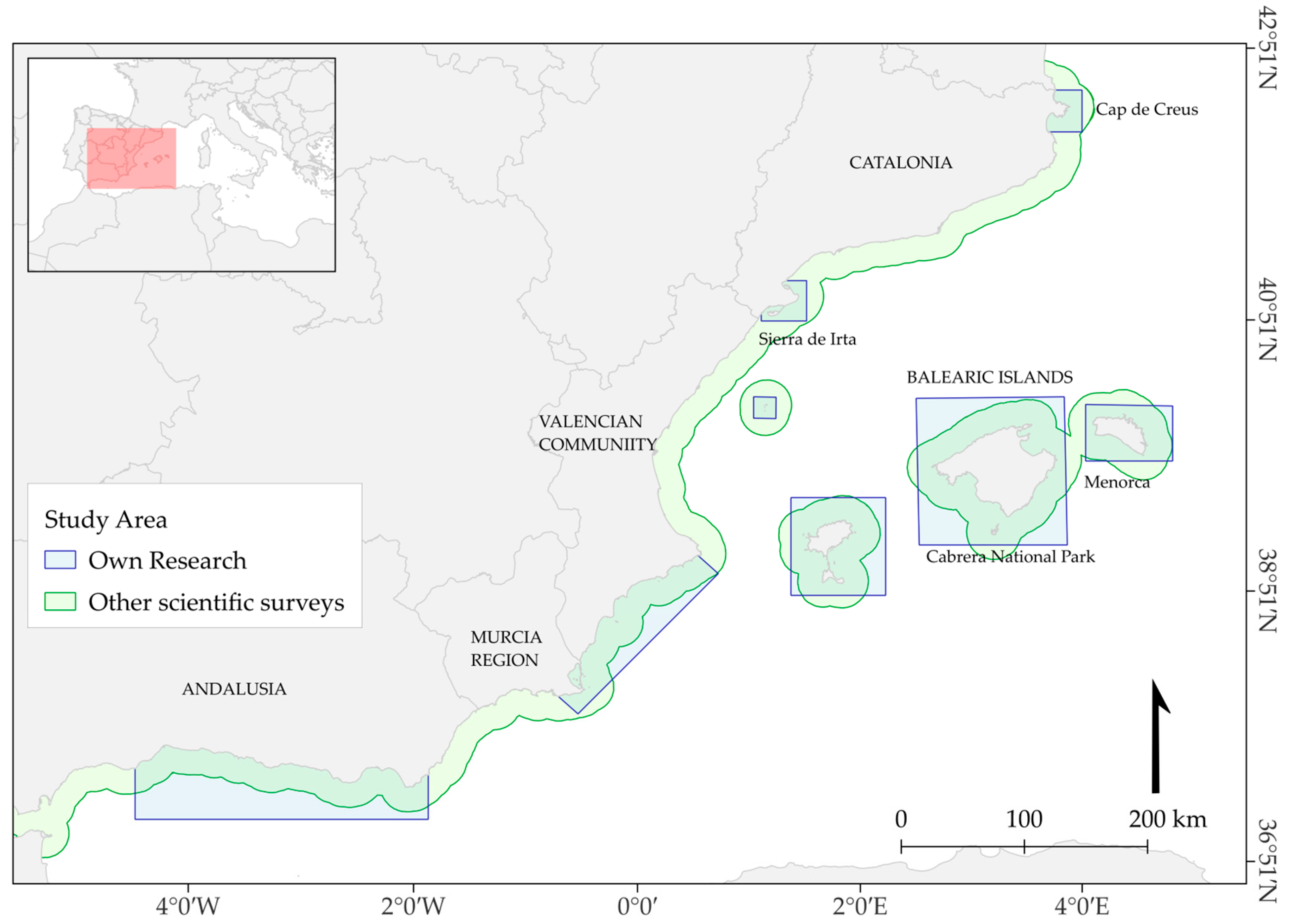
2.1. Study Area and Data Collection
2.2. Molecular Analyses
2.3. Data Structure and Analyses
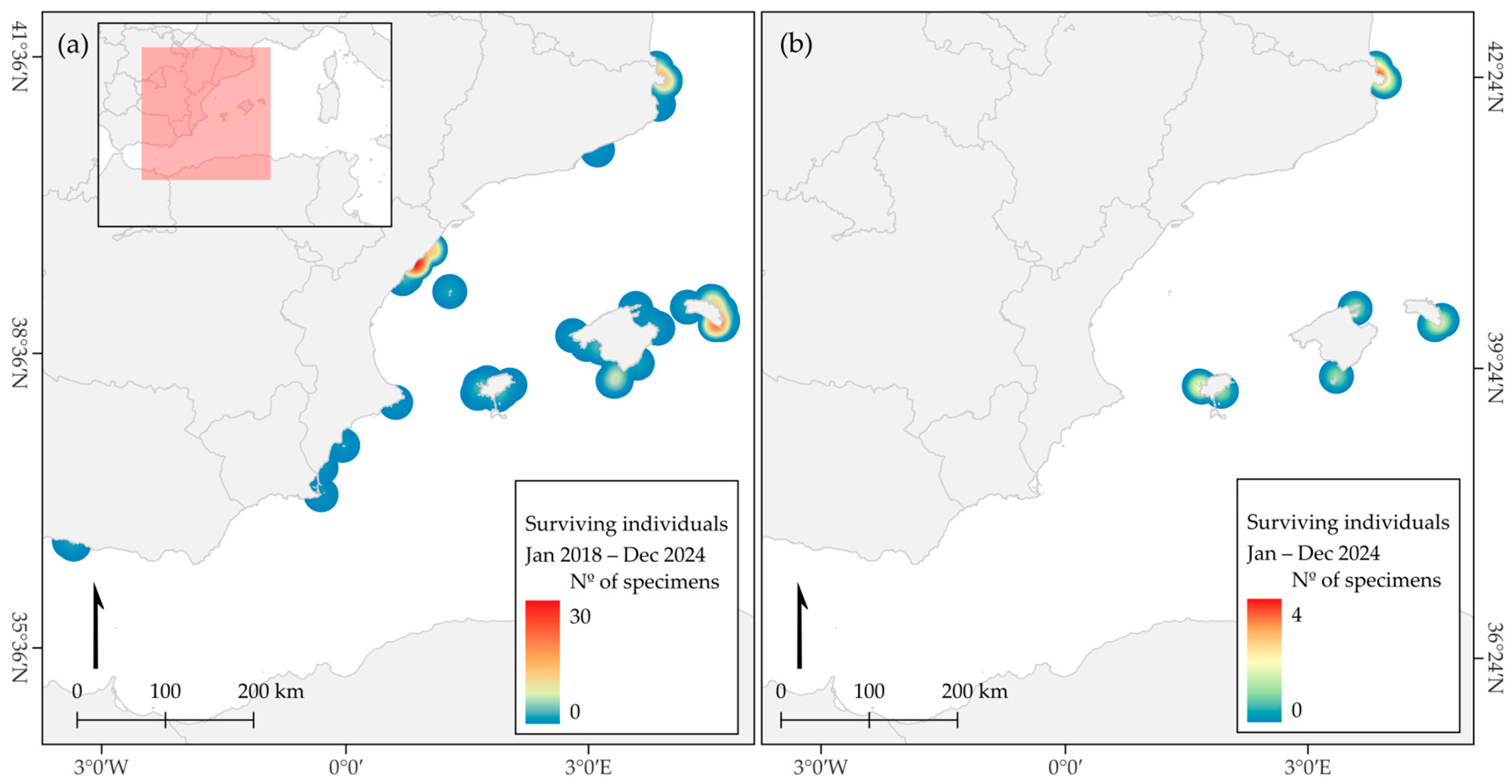
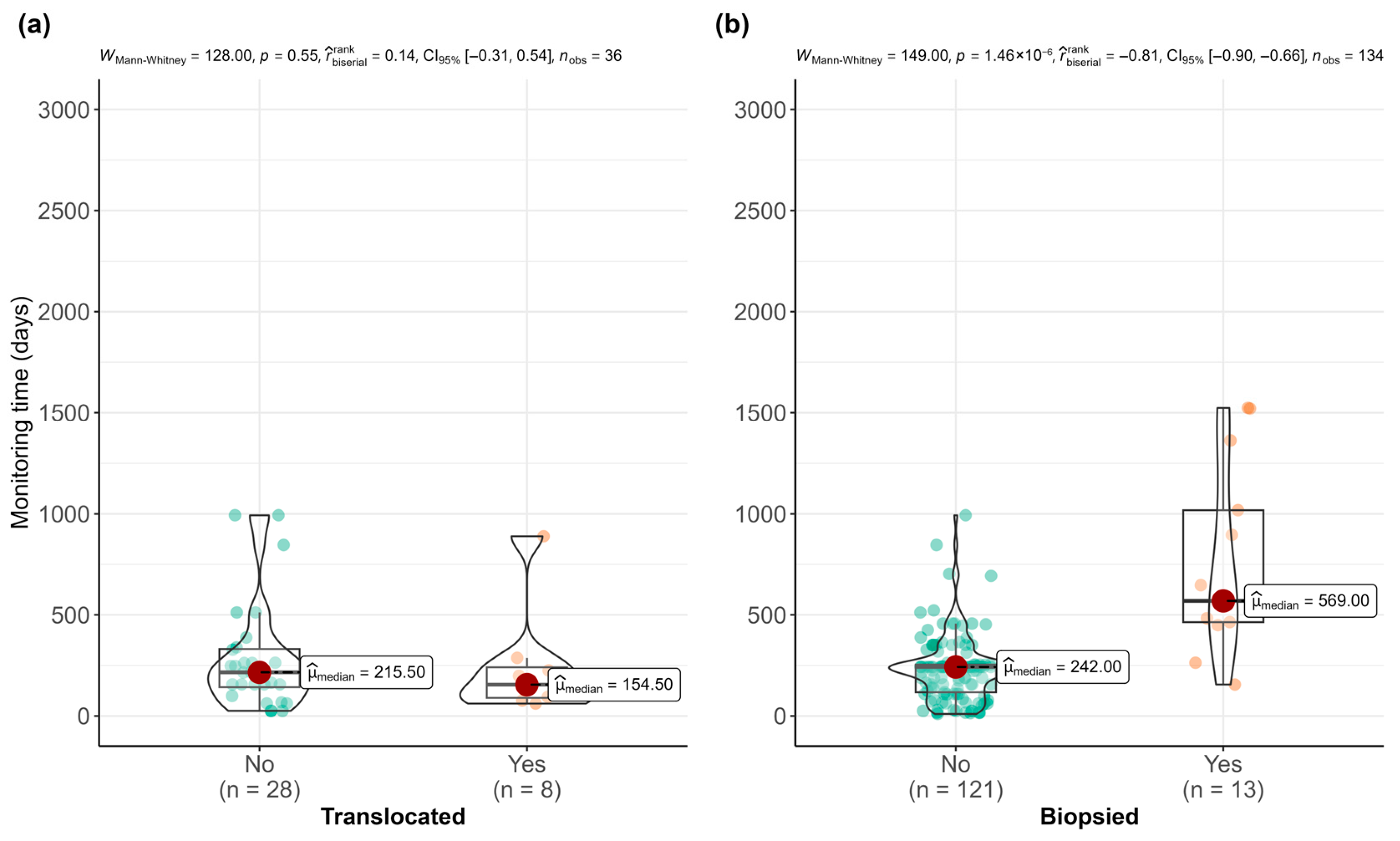
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SCI | Site of Conservation Interest (European Union Nature 2000 Network) |
| SAC | Special Area of Conservation |
| DPV | Diver Propulsion Vehicle |
| SW | Sea Watchers (citizen science platform) |
| PAMS | Posterior Abductor Muscles |
| TMA | Time Monitored Alive |
| SPS | Species Protection Service of Regional government of the Balearic Islands (Spain) |
| DUI | Doubly Uniparental Inheritance |
Appendix A
Appendix A.1
Appendix A.2

References
- Fey, S.B.; Siepielski, A.M.; Nusslé, S.; Cervantes-Yoshida, K.; Hwan, J.L.; Huber, E.R.; Fey, M.J.; Catenazzi, A.; Carlson, S.M. Recent Shifts in the Occurrence, Cause, and Magnitude of Animal Mass Mortality Events. Proc. Natl. Acad. Sci. USA 2015, 112, 1083–1088. [Google Scholar] [CrossRef]
- Lande, R. Risks of Population Extinction from Demographic and Environmental Stochasticity and Random Catastrophes. Am. Nat. 1993, 142, 911–927. [Google Scholar] [CrossRef]
- Vázquez-Luis, M.; Álvarez, E.; Barrajón, A.; García-March, J.R.; Grau, A.; Hendriks, I.E.; Jiménez, S.; Kersting, D.; Moreno, D.; Pérez, M.; et al. S.O.S. Pinna nobilis: A Mass Mortality Event in Western Mediterranean Sea. Front. Mar. Sci. 2017, 4, 220. [Google Scholar] [CrossRef]
- Darriba, S. First Haplosporidan Parasite Reported Infecting a Member of the Superfamily Pinnoidea (Pinna nobilis) during a Mortality Event in Alicante (Spain, Western Mediterranean). J. Invertebr. Pathol. 2017, 148, 14–19. [Google Scholar] [CrossRef]
- Catanese, G.; Grau, A.; Valencia, J.M.; García-March, J.R.; Vázquez-Luis, M.; Alvarez, E.; Deudero, S.; Darriba, S.; Carballal, M.J.; Villalba, A. Haplosporidium pinnae Sp. Nov., a Haplosporidan Parasite Associated with Mass Mortalities of the Fan Mussel, Pinna nobilis, in the Western Mediterranean Sea. J. Invertebr. Pathol. 2018, 157, 9–24. [Google Scholar] [CrossRef]
- Scarpa, F.; Sanna, D.; Azzena, I.; Mugetti, D.; Cerruti, F.; Hosseini, S.; Cossu, P.; Pinna, S.; Grech, D.; Cabana, D.; et al. Multiple Non-species-specific Pathogens Possibly Triggered the Mass Mortality in Pinna nobilis. Life 2020, 10, 238. [Google Scholar] [CrossRef]
- Grau, A.; Villalba, A.; Navas, J.I.; Hansjosten, B.; Valencia, J.M.; García-March, J.R.; Prado, P.; Follana-Berná, G.; Morage, T.; Vázquez-Luis, M.; et al. Wide-Geographic and Long-Term Analysis of the Role of Pathogens in the Decline of Pinna nobilis to Critically Endangered Species. Front. Mar. Sci. 2022, 9. [Google Scholar] [CrossRef]
- Hamelin, F.M.; Allen, L.J.S.; Bokil, V.A.; Gross, L.J.; Hilker, F.M.; Jeger, M.J.; Manore, C.A.; Power, A.G.; Rúa, M.A.; Cunniffe, N.J. Coinfections by Noninteracting Pathogens Are Not Independent and Require New Tests of Interaction. PLoS Biol. 2019, 17, e3000551. [Google Scholar] [CrossRef]
- Lattos, A.; Giantsis, I.A.; Karagiannis, D.; Michaelidis, B. First Detection of the Invasive Haplosporidian and Mycobacteria Parasites Hosting the Endangered Bivalve Pinna nobilis in Thermaikos Gulf, North Greece. Mar. Environ. Res. 2020, 155, 104889. [Google Scholar] [CrossRef]
- Künili, İ.E.; Ertürk Gürkan, S.; Aksu, A.; Turgay, E.; Çakir, F.; Gürkan, M.; Altinağaç, U. Mass Mortality in Endangered Fan Mussels Pinna nobilis (Linnaeus 1758) Caused by Co-Infection of Haplosporidium pinnae and Multiple Vibrio Infection in Çanakkale Strait, Turkey. Biomarkers 2021, 26, 450–461. [Google Scholar] [CrossRef]
- Carella, F.; Palić, D.; Šarić, T.; Župan, I.; Gorgoglione, B.; Prado, P.; Andree, K.B.; Giantsis, I.A.; Michaelidis, B.; Lattos, A.; et al. Multipathogen Infections and Multifactorial Pathogenesis Involved in Noble Pen Shell (Pinna nobilis) Mass Mortality Events: Background and Current Pathologic Approaches. Vet. Pathol. 2023, 60, 560–577. [Google Scholar] [CrossRef]
- Azzena, I.; Locci, C.; Pascale, N.; Deplano, I.; Senigaglia, R.; Scarpa, F.; Casu, M.; Sanna, D. Pinna nobilis, the Vanishing Giant: A Comprehensive Review on the Decline of a Mediterranean Icon. Animals 2025, 15, 2044. [Google Scholar] [CrossRef]
- Iaria, C.; Piro, M.G.; Natale, S.; Capparucci, F.; Villari, G.; De Vico, G.; Carella, F. Cytopathology and Phylogeny of Pinna nobilis Picornavirus (PnPV) Infecting Haemocytes of the Noble Pen Shell Population from Lake Faro (Southern Italy). J. Invertebr. Pathol. 2025, 212, 108381. [Google Scholar] [CrossRef]
- Katsanevakis, S. The Cryptogenic Parasite Haplosporidium pinnae Invades the Aegean Sea and Causes the Collapse of Pinna nobilis Populations. Aquat. Invasions 2019, 14, 150–164. [Google Scholar] [CrossRef]
- Vicente, N. Estudio Ecológico y Protección Del Molusco Lamelibranquio Pinna nobilis L. 1758 En La Costa Mediterránea. Iberus 1990, 9, 269–279. [Google Scholar]
- Rouanet, E.; Trigos, S.; Vicente, N. From Youth to Death of Old Age: The 50-Year Story of a Pinna nobilis Fan Mussel Population at Port-Cros Island (Port-Cros National Park, Provence, Mediterranean Sea). Sci. Rep. Port-Cros natl. Park. 2015, 29, 209–222. [Google Scholar]
- García-March, J.R.; Hernandis, S.; Vázquez-Luis, M.; Prado, P.; Deudero, S.; Vicente, N.; Tena-Medialdea, J. Age and Growth of the Endangered Fan Mussel Pinna nobilis in the Western Mediterranean Sea. Mar. Environ. Res. 2020, 153, 104795. [Google Scholar] [CrossRef]
- Zavodnik, D.; Hrs-Brenko, M.; Legac, M. Synopsis on the Fan Shell Pinna nobilis L. in the Eastern Adriatic Sea. In Les Espèces Marines a Proteger en Mèditerranée; Boudouresque, C.F., Avon, M., Gravez, V., Eds.; GIS Posidonie: Marseille, France, 1991; pp. 169–178. [Google Scholar]
- García-March, J.R.; García-Carrascosa, A.M.; Pena, Á.L. In Situ Measurement of Pinna nobilis Shells for Age and Growth Studies: A New Device. Mar. Ecol. 2002, 23, 207–217. [Google Scholar] [CrossRef]
- Vázquez-Luis, M.; March, D.; Alvarez, E.; Alvarez-Berastegui, D.; Deudero, S. Spatial Distribution Modelling of the Endangered Bivalve Pinna nobilis in a Marine Protected Area. Mar. Sci. 2014, 15, 626–634. [Google Scholar] [CrossRef]
- Katsanevakis, S. Growth and Mortality Rates of the Fan Mussel Pinna nobilis in Lake Vouliagmeni (Korinthiakos Gulf, Greece): A Generalized Additive Modelling Approach. Mar. Biol. 2007, 152, 1319–1331. [Google Scholar] [CrossRef]
- Acarli, S. Population, Aquaculture and Transplantation Applications of Critically Endangered Species Pinna nobilis (Linnaeus 1758) in the Mediterranean Sea. Mar Sci Technol Bull 2021, 10, 350–369. [Google Scholar] [CrossRef]
- Hernandis, S.; Prado, P.; García-March, J.R.; Gairin, I.; Vázquez-Luis, M.; Tena-Medialdea, J. Reproduction of the Endangered Fan Mussel Pinna nobilis under Natural and Captivity Conditions. Aquat. Conserv. 2023, 33, 1501–1513. [Google Scholar] [CrossRef]
- García-March, J.R. Aportaciones al Conocimiento de la Biología de Pinna nobilis Linneo, 1758 (Mollusca bivalvia) en el Litoral Mediterráneo Ibérico; University of Valencia: Valencia, Spain, 2005; Volume 1758. [Google Scholar]
- Guallart, J.; Templado, J. Pinna nobilis. In Bases ecológicas preliminares para la conservación de las especies de interés comunitario en España: Invertebrados; Ministerio de Agricultura, Alimentación y Medio Ambiente: Madrid, Spain, 2012; p. 81. [Google Scholar]
- Banach-Esteve, G.; Vázquez-Luis, M.; Deudero, S. Temporal Trends in Sessile Epibionts of the Endemic Bivalve Pinna nobilis: Variability in Lophocladia lallemandii Colonization. Thalassas 2015, 31, 19–29. [Google Scholar]
- Giacobbe, S. Epibiontic Mollusc Communities on Pinna nobilis L. (Bivalvia, Mollusca). J. Nat. Hist. 2002, 36, 1385–1396. [Google Scholar] [CrossRef]
- Maeder, F.; Hänggi, A.; Wunderlin, D. Muschelseide—Goldene Fäden Vom Meeresgrund / Bisso Marino—Fili d’oro Dal Fondo Del Mare; Naturhistorisches Museum und Museum der Kulturen, Basel, & 5 Continents Editions: Milan, Italy, 2004; p. 127. ISBN 88-7439-114-5. [Google Scholar]
- Basso, L.; Vázquez-Luis, M.; García-March, J.R.; Deudero, S.; Alvarez, E.; Vicente, N.; Duarte, C.M.; Hendriks, I.E. The Pen Shell, Pinna nobilis: A Review of Population Status and Recommended Research Priorities in the Mediterranean Sea. In Advances in Marine Biology; Elsevier: Orlando, FL, USA, 2015; Volume 71, pp. 109–160. [Google Scholar] [CrossRef]
- Katsanevakis, S.; Poursanidis, D.; Issaris, Y.; Panou, A.; Petza, D.; Vassilopoulou, V.; Chaldaiou, I.; Sini, M. “Protected” Marine Shelled Molluscs: Thriving in Greek Seafood Restaurants. Mediterr. Mar. Sci. 2011, 12, 429–438. [Google Scholar] [CrossRef]
- Marbà, N.; Díaz-Almela, E.; Duarte, C.M. Mediterranean Seagrass (Posidonia oceanica) Loss between 1842 and 2009. Biol. Conserv. 2014, 176, 183–190. [Google Scholar] [CrossRef]
- Hendriks, I.E.; Tenan, S.; Tavecchia, G.; Marbà, N.; Jordà, G.; Deudero, S.; Álvarez, E.; Duarte, C.M. Boat Anchoring Impacts Coastal Populations of the Pen Shell, the Largest Bivalve in the Mediterranean. Biol. Conserv. 2013, 160, 105–113. [Google Scholar] [CrossRef]
- Deudero, S.; Vázquez-Luis, M.; Álvarez, E. Human Stressors Are Driving Coastal Benthic Long-Lived Sessile Fan Mussel Pinna nobilis Population Structure More than Environmental Stressors. PLoS ONE 2015, 10, e0134530. [Google Scholar] [CrossRef]
- Vázquez-Luis, M.; Borg, J.A.; Morell, C.; Banach-Esteve, G.; Deudero, S. Influence of Boat Anchoring on Pinna nobilis: A Field Experiment Using Mimic Units. Mar. Freshw. Res. 2015, 66, 786–794. [Google Scholar] [CrossRef]
- Vázquez-Luis, M.; Morató, M.; Campillo, J.A.; Guitart, C.; Deudero, S. High Metal Contents in the Fan Mussel Pinna nobilis in the Balearic Archipelago (Western Mediterranean Sea) and a Review of Concentrations in Marine Bivalves (Pinnidae). Sci. Mar. 2016, 80, 11–22. [Google Scholar] [CrossRef]
- Ministry for the Ecological Transition (Spain). Order TEC/1078/2018, of 28 September, Declaring the Critical Situation of Cistus Heterophyllus Carthaginensis, Lanius Minor, Margaritifera Auricularia, Marmaronetta Angustirostris, Mustela Lutreola, Pinna nobilis and Tetrao Urogallus Cantabricus in Spain, and Declaring of General Interest the Works and Projects Aimed at the Recovery of These Taxa; Ministry for the Ecological Transition: Madrid, Spain, 2018; pp. 100677–100679. [Google Scholar]
- Ministry for the Ecological Transition (Spain). Order TEC/596/2019, of 8 April, Amending the Annex to Royal Decree 139/2011, of 4 February, for the Development of the List of Wild Species under Special Protection Regime and the Spanish Catalogue of Endangered SpeciesOrder TEC/596/2019, of 8 April, Amending the Annex to Royal Decree 139/2011, of 4 February, for the Development of the List of Wild Species under Special Protection Regime and the Spanish Catalogue of Endangered Species; Ministry for the Ecological Transition: Madrid, Spain, 2019. [Google Scholar]
- Ministry for the Ecological Transition and the Demographic Challenge (Spain). Disposition 344. Resolution of 20 December 2022, Approving the Conservation Strategy for the Fan Mussel (Pinna nobilis) in Spain. Off. State Gaz. (BOE) 2023, 4, 2092–2093. [Google Scholar]
- UNEP/MAP-SPA/RAC. Restoration Programme of Pinna nobilis; UNEP/MAP-SPA/RAC: Tunis, Tunisia, 2023. [Google Scholar]
- Kersting, D.; Čižmek, H.; Benabdi, M.; Jimenez, C.; Grau, A.; Katsanevakis, S.; Öztürk, B.; Tuncer, S.; Tunesi, L.; Vázquez-Luis, M.; et al. Pinna nobilis. In The IUCN Red List of Threatened Species 2019; IUCN: Cambridge, UK, 2019. [Google Scholar]
- Katsanevakis, S.; Carella, F.; Çinar, M.E.; Čižmek, H.; Jimenez, C.; Kersting, D.K.; Moreno, D.; Rabaoui, L.; Vicente, N. The Fan Mussel Pinna nobilis on the Brink of Extinction in the Mediterranean. In Imperiled: The Encyclopedia of Conservation; Elsevier: Amsterdam, The Netherlands, 2022; Volume 1–3, pp. 700–709. [Google Scholar]
- Prado, P.; Andree, K.B.; Trigos, S.; Carrasco, N.; Caiola, N.; García-March, J.R.; Tena, J.; Fernández-Tejedor, M.; Carella, F. Breeding, Planktonic and Settlement Factors Shape Recruitment Patterns of One of the Last Remaining Major Population of Pinna nobilis within Spanish Waters. Hydrobiologia 2020, 847, 771–786. [Google Scholar] [CrossRef]
- Nebot-Colomer, E.; Hernandis, S.; Mourre, B.; Fraile-Nuez, E.; Álvarez, E.; Deudero, S.; Albentosa, M.; Vázquez-Luis, M. No Recruits for an Ageing Population: First Signs of Probable Population Extinction in One of the Last Reservoirs of the Critically Endangered Species Pinna nobilis. J. Nat. Conserv. 2024, 79, 126600. [Google Scholar] [CrossRef]
- Katsanevakis, S. Transplantation as a Conservation Action to Protect the Mediterranean Fan Mussel Pinna nobilis. Mar. Ecol. Prog. Ser. 2016, 546, 113–122. [Google Scholar] [CrossRef]
- Bottari, T.; Spinelli, A.; Busalacchi, B.; Rinelli, P.; Giacobbe, S. Transplantation Trials of the Fan Mussel Pinna nobilis inside the Coastal Lagoon of Capo Peloro (Central Mediterranean, Italy). J. Shellfish. Res. 2017, 36, 3–8. [Google Scholar] [CrossRef]
- Tursi, A.; Corbelli, V.; Cipriano, G.; Capasso, G.; Velardo, R.; Chimienti, G. Mega-Litter and Remediation: The Case of Mar Piccolo of Taranto (Ionian Sea). Rend. Lincei Sci. Fis. Nat. 2018, 29, 817–824. [Google Scholar] [CrossRef]
- García-March, J.R.; Tena, J.; Hernandis, S.; Vázquez-Luis, M.; López, D.; Téllez, C.; Prado, P.; Navas, J.I.; Bernal, J.; Catanese, G.; et al. Can We Save a Marine Species Affected by a Highly Infective, Highly Lethal, Waterborne Disease from Extinction? Biol. Conserv. 2020, 243, 108498. [Google Scholar] [CrossRef]
- Vázquez-Luis, M.; Nebot-Colomer, E.; Deudero, S.; Planes, S.; Boissin, E.; Deudero, S.; Planes, S.; Boissin, E.; Deudero, S.; Planes, S.; et al. Natural Hybridization between Pen Shell Species: Pinna rudis and the Critically Endangered Pinna nobilis May Explain Parasite Resistance in P. nobilis. Mol. Biol. Rep. 2021, 48, 997–1004. [Google Scholar] [CrossRef]
- Lichten, C.; Ioppolo, R.; D’Angelo, C.; Simmons, R.K.; Jones, M.M. Citizen Science: Crowdsourcing for Research; THIS Institute: Cambridge, UK, 2018; ISBN 9781999653903. [Google Scholar]
- Krželj, M.; Cerrano, C.; Gioia, C.; Camillo, D. Enhancing Diversity Knowledge through Marine Citizen Science and Social Platforms: The Case of Hermodice carunculata (Annelida, Polychaeta). Diversity 2020, 12, 23. [Google Scholar] [CrossRef]
- Kelly, R.; Fleming, A.; Pecl, G.T.; Von Gönner, J.; Bonn, A. Citizen Science and Marine Conservation: A Global Review. Philos. Trans. R. Soc. B 2020, 375, 20190461. [Google Scholar] [CrossRef]
- Fontaine, A.; Simard, A.; Brunet, N.; Elliott, K.H. Scientific Contributions of Citizen Science Applied to Rare or Threatened Animals. Conserv. Biol. 2022, 36, e13976. [Google Scholar] [CrossRef]
- Goffredo, S.; Pensa, F.; Neri, P.; Orlandi, A.; Gagliardi, M.S.; Velardi, A.; Piccinetti, C.; Zaccanti, F. Unite Research with What Citizens Do for Fun: “Recreational Monitoring” of Marine Biodiversity. Ecol. Appl. 2010, 20, 2170–2187. [Google Scholar] [CrossRef]
- Dickinson, J.L.; Shirk, J.; Bonter, D.; Bonney, R.; Crain, R.L.; Martin, J.; Phillips, T.; Purcell, K. The Current State of Citizen Science as a Tool for Ecological Research and Public Engagement. Front. Ecol. Environ. 2012, 10, 291–297. [Google Scholar] [CrossRef]
- Branchini, S.; Meschini, M.; Covi, C.; Piccinetti, C.; Zaccanti, F.; Goffredo, S. Participating in a Citizen Science Monitoring Program: Implications for Environmental Education. PLoS ONE 2015, 10, e0131812. [Google Scholar] [CrossRef]
- Conrad, C.C.; Hilchey, K.G. A Review of Citizen Science and Community-Based Environmental Monitoring: Issues and Opportunities. Environ. Monit. Assess. 2011, 176, 273–291. [Google Scholar] [CrossRef]
- Vann-Sander, S.; Clifton, J.; Harvey, E. Can Citizen Science Work? Perceptions of the Role and Utility of Citizen Science in a Marine Policy and Management Context. Mar. Policy 2016, 72, 82–93. [Google Scholar] [CrossRef]
- Malpica-Cruz, L.; Chaves, L.C.T.; Côté, I.M. Managing Marine Invasive Species through Public Participation: Lionfish Derbies as a Case Study. Mar. Policy 2016, 74, 158–164. [Google Scholar] [CrossRef]
- Loerzel, J.L.; Goedeke, T.L.; Dillard, M.K.; Brown, G. SCUBA Divers above the Waterline: Using Participatory Mapping of Coral Reef Conditions to Inform Reef Management. Mar. Policy 2017, 76, 79–89. [Google Scholar] [CrossRef]
- Burgess, H.K.; DeBey, L.B.; Froehlich, H.E.; Schmidt, N.; Theobald, E.J.; Ettinger, A.K.; HilleRisLambers, J.; Tewksbury, J.; Parrish, J.K. The Science of Citizen Science: Exploring Barriers to Use as a Primary Research Tool. Biol. Conserv. 2017, 208, 113–120. [Google Scholar] [CrossRef]
- Tiago, P.; Ceia-Hasse, A.; Marques, T.A.; Capinha, C.; Pereira, H.M. Spatial Distribution of Citizen Science Casuistic Observations for Different Taxonomic Groups. Sci. Rep. 2017, 7, 12832. [Google Scholar] [CrossRef]
- Young, B.E.; Dodge, N.; Hunt, P.D.; Ormes, M.; Schlesinger, M.D.; Shaw, H.Y. Using Citizen Science Data to Support Conservation in Environmental Regulatory Contexts. Biol. Conserv. 2019, 237, 57–62. [Google Scholar] [CrossRef]
- Trigos, S.; Vicente, N. Protocole Pour La Transplantation Des Nacres Pinna nobilis Dans Divers Substrats. Mar. Life 2016, 18, 55–61. [Google Scholar]
- García-March, J.R.; Marquez-Aliaga, A.; Wang, Y.G.; Surge, D.; Kersting, D.K. Study of Pinna nobilis Growth from Inner Record: How Biased Are Posterior Adductor Muscle Scars Estimates? J. Exp. Mar. Biol. Ecol. 2011, 407, 337–344. [Google Scholar] [CrossRef]
- Nebot-Colomer, E.; Alvarez, E.; Belando, M.D.; Deudero, S.; Catanese, G.; Bernardeau-Esteller, J.; García-Muñoz, R.; Ramos-Segura, A.; Ruiz, J.M.; Vázquez-Luis, M.; et al. Living under Threat: Will One of the Last Pinna nobilis Populations Be Able to Survive? Aquat. Conserv. 2021, 32, 1–13. [Google Scholar] [CrossRef]
- Nebot-Colomer, E.; Peyran, C.; Deudero, S.; Boissin, E.; Vázquez-Luis, M.; Planes, S. The Mass Mortality of Pinna nobilis throughout the Mediterranean Sea Has Not yet Affected the Genetic Diversity of the Species in One of the Last Genetic Reservoirs. Estuar. Coast. Shelf Sci. 2025, 317, 109202. [Google Scholar] [CrossRef]
- Catanese, G.; Coupé, S.; Bunet, R. Mitogenome Sequence Comparison in the Endangered Congeneric Pinna nobilis and Pinna rudis Bivalves. Mol. Biol. Rep. 2022, 49, 3627–3635. [Google Scholar] [CrossRef] [PubMed]
- Catanese, G.; Tena-Medialdea, J.; Dajković, M.A.B.; Mičić, M.; García-March, J.R. An Incubation Water EDNA Method for a Non-Destructive Rapid Molecular Identification of Pinna nobilis and Pinna rudis Bivalve Juveniles. MethodsX 2022, 9, 101708. [Google Scholar] [CrossRef] [PubMed]
- Catanese, G.; Vázquez-Luis, M.; Giacobbe, S.; García-March, J.R.; Zotou, M.; Patricia, P.; Papadakis, O.; Tena-Medialdea, J.; Katsanevakis, S.; Grau, A. Internal Transcribed Spacer as Effective Molecular Marker for the Detection of Natural Hybridization between the Bivalves Pinna nobilis and Pinna rudis. Ecol. Evol. 2024, 14, e70227. [Google Scholar] [CrossRef]
- López-Sanmartín, M.; Catanese, G.; Grau, A.; Valencia, J.M.; García-March, J.R.; Navas, J.I. Real-Time PCR Based Test for the Early Diagnosis of Haplosporidium pinnae Affecting Fan Mussel Pinna nobilis. PLoS ONE 2019, 14, e0212028. [Google Scholar] [CrossRef]
- Böddinghaus, B.; Rogall, T.; Flohr, T.; Blöcker, H.; Böttger, E.C. Detection and Identification of Mycobacteria by Amplification of RRNA. J. Clin. Microbiol. 1990, 28, 1751–1759. [Google Scholar] [CrossRef]
- Moreno, D. Noticiario De La Sociedad Española De Malacología: No. 67; Rolán, E., Troncoso, J.S., Eds.; Sociedad Española de Malacología: Madrid, Spain, 2017; 96p, ISSN 1131-527 X. De. Legal: M-17441-2014. [Google Scholar]
- Cabanellas-Reboredo, M.; Vázquez-Luis, M.; Mourre, B.; Álvarez, E.; Deudero, S.; Amores, Á.; Addis, P.; Ballesteros, E.; Barrajón, A.; Coppa, S.; et al. Tracking a Mass Mortality Outbreak of Pen Shell Pinna nobilis Populations: A Collaborative Effort of Scientists and Citizens. Sci. Rep. 2019, 9, 13355. [Google Scholar] [CrossRef]
- Wolf, C.M.; Griffith, B.; Reed, C.; Temple, S.A. Avian and Mammalian Translocations: Update and Reanalysis of 1987 Survey Data. Conserv. Biol. 1996, 10, 1142–1154. [Google Scholar] [CrossRef]
- Fischer, J.; Lindenmayer, D.B. An Assessment of the Published Results of Animal Relocations. Biol. Conserv. 2000, 96, 1–11. [Google Scholar] [CrossRef]
- Bubac, C.M.; Johnson, A.C.; Fox, J.A.; Cullingham, C.I. Conservation Translocations and Post-Release Monitoring: Identifying Trends in Failures, Biases, and Challenges from around the World. Biol. Conserv. 2019, 238, 108239. [Google Scholar] [CrossRef]
- IUCN/SSC. Guidelines for Reintroductions and Other Conservation; IUCN/SSC: Gland, Switzerland, 2013. [Google Scholar]
- Armstrong, D.P.; Seddon, P.J. Directions in Reintroduction Biology. Trends Ecol. Evol. 2008, 23, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Groombridge, J.J.; Raisin, C.; Bristol, R.; Richardson, D.S. Genetic Consequences of Reintroductions and Insights from Population History. In Reintroduction Biology; Wiley: Hoboken, NJ, USA, 2012; pp. 395–440. [Google Scholar]
- Claramonte, L.; Álvarez, E.; Hidalgo, M.; Deudero, S.; Vázquez-Luis, M. Demographic Regulation Processes in Pinna nobilis Population Subunits: Implications for Restocking. Estuar. Coast. Shelf Sci. 2024, 306, 108894. [Google Scholar] [CrossRef]
- Feria-Rodríguez, A.; March, D.; Mourre, B.; Hendriks, I.E.; Vázquez-Luis, M. Sink-Source Connectivity for Restocking of Pinna nobilis in the Western Mediterranean Sea. Mar. Environ. Res. 2024, 197, 106428. [Google Scholar] [CrossRef]
- Reynolds, J.H.; Knutson, M.G.; Newman, K.B.; Silverman, E.D.; Thompson, W.L. A Road Map for Designing and Implementing a Biological Monitoring Program. Environ. Monit. Assess. 2016, 188, 399. [Google Scholar] [CrossRef]
- Legge, S.; Lindenmayer, D.B.; Robinson, N.M.; Scheele, B.C.; Southwell, D.M.; Wintle, B.A. Monitoring Threatened Species and Ecological Communities; CSIRO Publishing: Clayton South, Australia, 2018. [Google Scholar]
- Lindenmayer, D.; Likens, G. Effective Ecological Monitoring; CSIRO Publishing: Clayton South, Australia, 2018; ISBN 9781486308934. [Google Scholar]
- Lindenmayer, D.; Woinarski, J.; Legge, S.; Southwell, D.; Lavery, T.; Robinson, N.; Scheele, B.; Wintle, B. A Checklist of Attributes for Effective Monitoring of Threatened Species and Threatened Ecosystems. J. Environ. Manag. 2020, 262, 110312. [Google Scholar] [CrossRef]
- Ludvigsen, M.; Sortland, B.; Johnsen, G.; Singh, H. Applications of Geo-Referenced Underwater Photo Mosaics in Marine Biology and Archaeology. Oceanography 2007, 20, 140–149. [Google Scholar] [CrossRef]
- Crates, R.; Terauds, A.; Rayner, L.; Stojanovic, D.; Heinsohn, R.; Ingwersen, D.; Webb, M. An Occupancy Approach to Monitoring Regent Honeyeaters. J. Wildl. Manag. 2017, 81, 669–677. [Google Scholar] [CrossRef]
- Garrard, G.E.; Bekessy, S.A.; McCarthy, M.A.; Wintle, B.A. Incorporating Detectability of Threatened Species into Environmental Impact Assessment. Conserv. Biol. 2015, 29, 216–225. [Google Scholar] [CrossRef] [PubMed]
- Zotou, M.; Gkrantounis, P.; Karadimou, E.; Tsirintanis, K.; Sini, M.; Poursanidis, D.; Azzolin, M.; Dailianis, T.; Kytinou, E.; Issaris, Y.; et al. Pinna nobilis in the Greek Seas (NE Mediterranean): On the Brink of Extinction? Mediterr. Mar. Sci. 2020, 21, 558–574. [Google Scholar] [CrossRef]
- Donato, G.; Vázquez-Luis, M.; Nebot-Colomer, E.; Lunetta, A.; Giacobbe, S. Noble Fan-Shell, Pinna nobilis, in Lake Faro (Sicily, Italy): Ineluctable Decline or Extreme Opportunity? Estuar. Coast. Shelf Sci. 2021, 261, 107536. [Google Scholar] [CrossRef]
- Foulquié, M.; Coupé, S.; Vicente, N.; Bunet, R. Population Status of the Critically Endangered Fan Mussel Pinna nobilis in Thau Lagoon (France). Endanger. Species Res. 2024, 55, 21–36. [Google Scholar] [CrossRef]
- Lunetta, A.; Spinelli, A.; Donato, G.; Gatì, I.A.; Giacobbe, S. Lake Faro (Central Mediterranean): A Potential Short-Term Reservoir for Pinna nobilis. J. Nat. Conserv. 2024, 81, 126690. [Google Scholar] [CrossRef]
- Prado, P.; Grau, A.; Catanese, G.; Cabanes, P.; Carella, F.; Fernández-Tejedor, M.; Andree, K.B.; Añón, T.; Hernandis, S.; Tena, J.; et al. Pinna nobilis in Suboptimal Environments Are More Tolerant to Disease but More Vulnerable to Severe Weather Phenomena. Mar. Environ. Res. 2021, 163, 105220. [Google Scholar] [CrossRef]
- Donato, G.; Lunetta, A.; Spinelli, A.; Catanese, G.; Giacobbe, S. Sanctuaries Are Not Inviolable: Haplosporidium pinnae as Responsible for the Collapse of the Pinna nobilis Population in Lake Faro (Central Mediterranean). J. Invertebr. Pathol. 2023, 201, 108014. [Google Scholar] [CrossRef]
- Coupé, S.; Giantsis, I.A.; Vázquez Luis, M.; Scarpa, F.; Foulquié, M.; Prévot, J.M.; Casu, M.; Lattos, A.; Michaelidis, B.; Sanna, D.; et al. The Characterization of Toll-like Receptor Repertoire in Pinna nobilis after Mass Mortality Events Suggests Adaptive Introgression. Ecol. Evol. 2023, 13, e10383. [Google Scholar] [CrossRef]
- Moro-Martínez, I.; Vázquez-Luis, M.; García-March, J.R.; Prado, P.; Mičić, M.; Catanese, G. Haplosporidium pinnae Parasite Detection in Seawater Samples. Microorganisms 2023, 11, 1146. [Google Scholar] [CrossRef]
- Papadakis, O.; Mamoutos, I.; Ramfos, A.; Catanese, G.; Papadimitriou, E.; Theodorou, A.J.; Batargias, C.; Papaioannou, C.; Kamilari, M.; Tragou, E.; et al. Status, Distribution, and Threats of the Last Surviving Fan Mussel Populations in Greece. Mediterr. Mar. Sci. 2023, 24, 679–708. [Google Scholar] [CrossRef]
- Labidi, S.; Vázquez-Luis, M.; Catanese, G.; Grau, A.; Khammassi, M.; Ben Youssef, S.; Achouri, M.S. First Detection of the Invasive Protozoan Haplosporidium pinnae in the Critically Endangered Bivalve Pinna nobilis in the Southern Mediterranean Sea (Bizerte Lagoon, Tunis) and Update of Its Current Status. Mediterr. Mar. Sci. 2023, 24, 470–481. [Google Scholar] [CrossRef]
- Trigos, S.; Vicente, N.; Prado, P.; Espinós, F.J. Adult Spawning and Early Larval Development of the Endangered Bivalve Pinna nobilis. Aquaculture 2018, 483, 102–110. [Google Scholar] [CrossRef]
- Lotterhos, K.E.; Levitan, D.R. Gamete Release and Spawning Behavior in Broadcast Spawning Marine Invertebrates. In The Evolution of Primary Sexual Characters; Leonard, J.L., Cordoba-Aguilar, A., Eds.; Oxford University Press: New York, NY, USA, 2010; pp. 99–120. [Google Scholar]
- Boero, F.; Putti, M.; Trainito, E.; Prontera, E.; Piraino, S.; Shiganova, T. First Records of Mnemiopsis leidyi (Ctenophora) from the Ligurian, Thyrrhenian and Ionian Seas (Western Mediterranean) and First Record of Phyllorhiza punctata (Cnidaria) from the Western Mediterranean. Aquat. Invasions 2009, 4, 675–680. [Google Scholar] [CrossRef]
- Mominó, J.M.; Piera, J.; Jurado, E. Citizen Observatories as Advanced Learning Environments. In Analyzing the Role of Citizen Science in Modern Research; IGI Global: Hershey, PA, USA, 2017; pp. 192–212. [Google Scholar]
- Pecl, G.T.; Stuart-Smith, J.; Walsh, P.; Bray, D.J.; Kusetic, M.; Burgess, M.; Frusher, S.D.; Gledhill, D.C.; George, O.; Jackson, G.; et al. Redmap Australia: Challenges and Successes with a Large-Scale Citizen Science-Based Approach to Ecological Monitoring and Community Engagement on Climate Change. Front. Mar. Sci. 2019, 6, 349. [Google Scholar] [CrossRef]
- Izquierdo-Gómez, D. Synergistic Use of Facebook, Online Questionnaires and Local Ecological Knowledge to Detect and Reconstruct the Bioinvasion of the Iberian Peninsula by Callinectes sapidus Rathbun, 1896. Biol. Invasions 2022, 24, 1059–1082. [Google Scholar] [CrossRef]
- Azzurro, E.; Sbragaglia, V.; Cerri, J.; Bariche, M.; Bolognini, L.; Ben Souissi, J.; Busoni, G.; Coco, S.; Chryssanthi, A.; Fanelli, E.; et al. Climate Change, Biological Invasions, and the Shifting Distribution of Mediterranean Fishes: A Large-Scale Survey Based on Local Ecological Knowledge. Glob. Change Biol. 2019, 25, 2779–2792. [Google Scholar] [CrossRef]
- Tiralongo, F.; Oscar, A.; Tibullo, D.; Tondo, E.; Martire, L.; Agnese, R.D.; Macali, A.; Mancini, E.; Giovos, I.; Coco, S.; et al. Monitoring Uncommon and Non-Indigenous Fishes in Italian Waters: One Year of Results for the AlienFish Project. Reg. Stud. Mar. Sci. 2019, 28, 100606. [Google Scholar] [CrossRef]
- Encarnação, J.; Teodósio, M.A.; Morais, P. Citizen Science and Biological Invasions: A Review. Front. Environ. Sci. 2021, 8, 602980. [Google Scholar] [CrossRef]
- Edwards, T.; Jones, C.B.; Perkins, S.E.; Corcoran, P. Passive Citizen Science: The Role of Social Media in Wildlife Observations. PLoS ONE 2021, 16, e0255416. [Google Scholar] [CrossRef]
- de la Ballina, N.R.; Maresca, F.; Real, E.; Baena-Vega, I.; Díez, S.; Martín-Arjona, A.; Mallol, S.; Díaz, D. Signs of Northward Expansion of the Fireworm Hermodice carunculata in the Spanish Western Mediterranean Sea. Mediterr. Mar. Sci. 2025, 26, 515–532. [Google Scholar] [CrossRef]
- Perazzo, R.; Compagnone, F.; Varricchione, M.; Innangi, M.; Di Febbraro, M.; Loy, A.; Stanisci, A.; Carla De Francesco, M.; Matteucci, G.; Carranza, M.L. Coastal Biodiversity Assessment Aided by Citizen Science Volunteers: A Look at the Italian Central Adriatic. Land 2023, 12, 2023. [Google Scholar] [CrossRef]
- Castejón-Silvo, I.; Terrados, J.; Morales-Nin, B. Citizen Science in the Study of Marine Biodiversity: The Case of Iconic and Cryptic Syngnathids. Thalass. Int. J. Mar. Sci. 2023, 39, 679–686. [Google Scholar] [CrossRef]
- Figuerola-Ferrando, L.; Linares, C.; Zentner, Y.; López-Sendino, P.; Garrabou, J. Marine Citizen Science and the Conservation of Mediterranean Corals: The Relevance of Training, Expert Validation, and Robust Sampling Protocols. Environ. Manag. 2024, 73, 646–656. [Google Scholar] [CrossRef] [PubMed]
- Bunet, R.; Prévot, J.-M.; Vicente, N.; García-March, J.R.; Martinović, R.; Tena-Medialdea, J.; Joksimovic, D.; Bonnefont, J.-L.; Coupé, S. First Insight into the Whole Genome Shotgun Sequence of the Endangered Noble Pen Shell Pinna nobilis: A Giant Bivalve Undergoing a Mass Mortality Event. J. Molluscan Stud. 2021, 87, eyaa041. [Google Scholar] [CrossRef]
- Richardson, C.A.; Kennedy, H.; Duarte, C.M.; Kennedy, D.P.; Proud, S.V. Age and Growth of the Fan Mussel Pinna nobilis from South-East Spanish Mediterranean Seagrass (Posidonia Oceanica) Meadows. Mar. Biol. 1999, 133, 205–212. [Google Scholar] [CrossRef]
- Siletic, T.; Peharda, M. Population Study of the Fan Shell Pinna nobilis L. in Malo and Veliko Jezero of the Mljet National Park (Adriatic Sea). Sci. Mar. 2003, 67, 91–98. [Google Scholar] [CrossRef]
- García-March, J.R.; Pérez-Rojas, L.; García-Carrascosa, A.M. Influence of Hydrodynamic Forces on Population Structure of Pinna nobilis L., 1758 (Mollusca: Bivalvia): The Critical Combination of Drag Force, Water Depth, Shell Size and Orientation. J. Exp. Mar. Biol. Ecol. 2007, 342, 202–212. [Google Scholar] [CrossRef]
- Hendriks, I.E.; Cabanellas-Reboredo, M.; Bouma, T.J.; Deudero, S.; Duarte, C.M. Seagrass Meadows Modify Drag Forces on the Shell of the Fan Mussel Pinna nobilis. Estuaries Coasts 2011, 34, 60–67. [Google Scholar] [CrossRef]
- Hendriks, I.E.; Sintes, T.; Bouma, T.J.; Duarte, C.M. Experimental Assessment and Modeling Evaluation of the Effects of the Seagrass Posidonia Oceanica on Flow and Particle Trapping. Mar. Ecol. Prog. Ser. 2008, 356, 163–173. [Google Scholar] [CrossRef]
- Cerrano, C.; Bavestrello, G.; Bianchi, C.N.; Cattaneo-vietti, R.; Bava, S.; Morganti, C.; Morri, C.; Picco, P.; Sara, G.; Schiaparelli, S.; et al. A Catastrophic Mass-mortality Episode of Gorgonians and Other Organisms in the Ligurian Sea (North-western Mediterranean), Summer 1999. Ecol. Lett. 2000, 3, 284–293. [Google Scholar] [CrossRef]
- Garrabou, J.; Coma, R.; Bensoussan, N.; Bally, M.; Chevaldonné, P.; Cigliano, M.; Diaz, D.; Harmelin, J.-G.; Gambi, M.C.; Kersting, D.K.; et al. Mass Mortality in Northwestern Mediterranean Rocky Benthic Communities: Effects of the 2003 Heat Wave. Glob. Change Biol. 2009, 15, 1090–1103. [Google Scholar] [CrossRef]
- Trigos, S.; García-March, J.R.; Vicente, N.; Tena, J.; Torres, J. Respiration Rates of the Fan Mussel Pinna nobilis at Different Temperatures. J. Molluscan Stud. 2015, 81, 217–222. [Google Scholar] [CrossRef]
- Carella, F.; Prado, P.; García-March, J.R.; Tena-Medialdea, J.; Melendreras, E.C.; Porcellini, A.; Feola, A. Measuring Immunocompetence in the Natural Population and Captive Individuals of Noble Pen Shell Pinna nobilis Affected by Pinna nobilis Picornavirus (PnPV). Fish. Shellfish. Immunol. 2024, 151, 109664. [Google Scholar] [CrossRef] [PubMed]
- Kersting, D.K.; Vázquez-Luis, M.; Mourre, B.; Belkhamssa, F.Z.; Álvarez, E.; Bakran-Petricioli, T.; Barberá, C.; Barrajón, A.; Cortés, E.; Deudero, S.; et al. Recruitment Disruption and the Role of Unaffected Populations for Potential Recovery After the Pinna nobilis Mass Mortality Event. Front. Mar. Sci. 2020, 7, 594378. [Google Scholar] [CrossRef]
- Oprandi, A.; Aicardi, S.; Azzola, A.; Benelli, F.; Bertolino, M.; Bianchi, C.N.; Chiantore, M.; Ferranti, M.P.; Mancini, I.; Molinari, A.; et al. A Tale of Two Sisters: The Southerner Pinna rudis Is Getting North after the Regional Extinction of the Congeneric P. Nobilis (Mollusca: Bivalvia). Diversity 2024, 16, 120. [Google Scholar] [CrossRef]
- Petović, S.; Djordjević, N. Is Pinna rudis Linnaeus, 1758 Spreading Towards the North (Adriatic Sea, Montenegro). Thalass. Int. J. Mar. Sci. 2025, 41, 128. [Google Scholar] [CrossRef]
- Evans, M.J.; Gordon, I.J.; Pierson, J.C.; Neaves, L.E.; Wilson, B.A.; Brockett, B.; Ross, C.E.; Smith, K.J.; Rapley, S.; Andrewartha, T.A.; et al. Reintroduction Biology and the IUCN Red List: The Dominance of Species of Least Concern in the Peer-Reviewed Literature. Glob. Ecol. Conserv. 2022, 38, e02242. [Google Scholar] [CrossRef]
- Batson, W.G.; Gordon, I.J.; Fletcher, D.B.; Manning, A.D. Translocation Tactics: A Framework to Support the IUCN Guidelines for Wildlife Translocations and Improve the Quality of Applied Methods. J. Appl. Ecol. 2015, 52, 1598–1607. [Google Scholar] [CrossRef]
- Taylor, G.; Canessa, S.; Clarke, R.H.; Ingwersen, D.; Armstrong, D.P.; Seddon, P.J.; Ewen, J.G. Is Reintroduction Biology an Effective Applied Science? Trends Ecol. Evol. 2017, 32, 873–880. [Google Scholar] [CrossRef]
- Caronni, S.; Cristo, B.; Torelli, A. Osservazioni Sul Reimpianto Di Esemplari Di Pinna nobilis Ritrovati Staccati e Privi Di Bisso. Biol. Mar. Mediterr. 2008, 15, 300–301. [Google Scholar]
- Martin, T.G.; Nally, S.; Burbidge, A.A.; Arnall, S.; Garnett, S.T.; Hayward, M.W.; Lumsden, L.F.; Menkhorst, P.; McDonald-Madden, E.; Possingham, H.P. Acting Fast Helps Avoid Extinction. Conserv. Lett. 2012, 5, 274–280. [Google Scholar] [CrossRef]
- Lindenmayer, D.B.; Piggott, M.P.; Wintle, B.A. Counting the Books While the Library Burns: Why Conservation Monitoring Programs Need a Plan for Action. Front. Ecol. Environ. 2013, 11, 549–555. [Google Scholar] [CrossRef]
- Theissinger, K.; Fernandes, C.; Formenti, G.; Bista, I.; Berg, P.R.; Bleidorn, C.; Bombarely, A.; Crottini, A.; Gallo, G.R.; Godoy, J.A.; et al. How Genomics Can Help Biodiversity Conservation. Trends Genet. 2023, 39, 545–559. [Google Scholar] [CrossRef]
- Government of the Balearic Islands. Decree 25/2018, of 27 July, on the Conservation of Posidonia Oceanica in the Balearic Islands; Government of the Balearic Islands: Mallorca, Spain, 2018; pp. 1–17. [Google Scholar]




| Year | P. nobilis | Hybrids | % Hybrids |
|---|---|---|---|
| 2017 | 60 | 1 | 1.6 |
| 2018 | 36 [6] | 1 [0] | 2.9 |
| 2019 | 14 [8] | 3 [2] | 17.6 |
| 2020 | 21 [14] | 7 [4] | 25.0 |
| 2021 | 10 [6] | 5 [0] | 33.3 |
| 2022 | 18 [11] | 8 [4] | 30.8 |
| 2023 | 13 [2] | 13 [6] | 50.0 |
| 2024 | 8 [0] | 9 [1] | 52.9 |
| Collection Date | Location | Site | Species | Width | Length | Age Range (Years) |
|---|---|---|---|---|---|---|
| 01/11/2020 | Cabrera | Conejera | Hybrid | 16 | 36 | 10–15 |
| 24/01/2018 | Cabrera | Santa María | P. nobilis | 17 | 49 | 7–9 |
| 01/08/2019 | Cabrera | Cala Blava | P. nobilis | 12 | 26 | 3–4 |
| 17/08/2020 | Menorca | Cala Moli | P. nobilis | 15 | 32 | 3–5 |
| 17/01/2023 | Gerona | Cadaqués | P. nobilis | 18 | 45 | 8–10 |
| 25/09/2023 | Gerona | Arenella | P. nobilis | 19 | 46 | 7–10 |
| 21/03/2023 | Menorca | Es Grau | P. nobilis | 16 | 37 | 5–7 |
| 08/11/2023 | Menorca | Binisafuller | Hybrid | 16 | 34 | 5–6 |
| Original Location | Destination | 1st Observation | Species | Translocation | Death |
|---|---|---|---|---|---|
| Es Castell (C) | Santa María (C) | 26/06/2017 | P. nobilis | 07/08/2018 | 22/10/2018 |
| Rodona (C) a | Santa María (C) | 13/06/2017 | P. nobilis | 13/06/2017 | 24/01/2018 |
| Cala Blava (M) | Santa María (C) | 29/01/2018 | P. nobilis | 07/06/2018 | 12/11/2020 |
| Cala Blava (M) | Santa María (C) | 18/12/2018 | P. nobilis | 29/04/2019 | 01/08/2019 |
| Cala Blava (M) b | Santa María (C) | 14/02/2018 | P. nobilis | 07/06/2018 | 07/08/2018 |
| Caló des Llamp (M) | Santa María (C) | 23/12/2017 | P. nobilis | 07/08/2018 | 21/02/2019 |
| Cala Llumenera (G) c | Arenella (G) | 01/09/2021 | P. nobilis | 25/09/2023 | 14/02/2024 |
| Clot de la Mola (M) e | Santa María (C) | 10/11/2022 | Hybrid | 19/03/2024 | - |
| C. San Jordi (M) d | Santa María (C) | 27/11/2023 | Hybrid | 19/03/2024 | 08/07/2024 |
| Location | Site | Taxon | Multiplex PCR (nDNA) |
|---|---|---|---|
| Ibiza | Cala Espart | Hybrid | Hybrid |
| Cabrera | Es Castell | P. nobilis | P. nobilis |
| Cabrera | Cala Emboixar | Hybrid | Hybrid |
| Cabrera | Conejera | Hybrid | Hybrid |
| Cabrera | Na Pobra | P. nobilis | P. nobilis |
| Cabrera | Sifón Foradada | Hybrid | Hybrid |
| Mallorca | Cala Blava | P. nobilis | P. nobilis |
| Menorca | Clot de la Mola | Hybrid | Hybrid |
| Mallorca | Illa de Formentor | P. nobilis | P. nobilis |
| Menorca | Es Grau | P. nobilis | P. nobilis |
| Barcelona | Mataró | P. nobilis | P. nobilis |
| Girona | Cala Llumenera | P. nobilis | P. nobilis |
| Girona | Illa de S’Arenella | P. nobilis | P. nobilis |
| Girona | Portlligat | P. nobilis | P. nobilis |
| Girona | Port de la Selva | P. nobilis | P. nobilis |
| Girona | Illa de S’Arenella | P. nobilis | P. nobilis |
| Girona | Platja Can Perefet | P. nobilis | P. nobilis |
| Girona | Platja de Garvet | P. nobilis | P. nobilis |
| Girona | Badia de Colera | P. nobilis | P. nobilis |
| Girona | Badia de Colera | P. nobilis | P. nobilis |
| Alicante | Orihuela | Hybrid | Hybrid |
| Alicante | Moraira | Hybrid | Hybrid |
| Menorca | Binissafuller | Hybrid | Hybrid |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maresca, F.; Álvarez, E.; Zafra, L.; Hendriks, I.E.; Catanese, G.; González, R.; García-March, J.R.; Vázquez-Luis, M. Chasing Pinna nobilis Survivors: Current Status in Spanish Open Coastal Waters. Animals 2025, 15, 3075. https://doi.org/10.3390/ani15213075
Maresca F, Álvarez E, Zafra L, Hendriks IE, Catanese G, González R, García-March JR, Vázquez-Luis M. Chasing Pinna nobilis Survivors: Current Status in Spanish Open Coastal Waters. Animals. 2025; 15(21):3075. https://doi.org/10.3390/ani15213075
Chicago/Turabian StyleMaresca, Francesco, Elvira Álvarez, Lara Zafra, Iris E. Hendriks, Gaetano Catanese, Raul González, José Rafael García-March, and Maite Vázquez-Luis. 2025. "Chasing Pinna nobilis Survivors: Current Status in Spanish Open Coastal Waters" Animals 15, no. 21: 3075. https://doi.org/10.3390/ani15213075
APA StyleMaresca, F., Álvarez, E., Zafra, L., Hendriks, I. E., Catanese, G., González, R., García-March, J. R., & Vázquez-Luis, M. (2025). Chasing Pinna nobilis Survivors: Current Status in Spanish Open Coastal Waters. Animals, 15(21), 3075. https://doi.org/10.3390/ani15213075







