Appraisal of the Use of Proteomics Methodological Approaches and Technologies on Sheep and Goat Research and Clinical Work
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Search Procedure
2.2. Paper Evaluation
- Type of published paper: original article or review.
- Animal species referred to in the paper: sheep, goats, or both species.
- Year of publication of paper and country—scientific establishment of origin of the paper (as indicated in the affiliation(s) of the author(s)).
- For original articles only: (i) the type of work ((a) experimental work with animals, (b) field work, (c) computational work, (d) in vitro work), (ii) the topic of study ((a) sheep/goat production, (b) sheep/goat reproduction, (c) physiology, (d) animal diseases, (e) study that involved small ruminants as models for the study of various conditions in humans), (iii) the tissue analyzed by proteomics examinations, (iv) the methodological approaches for proteomics analyses employed, (v) the additional use of quantification analysis of the proteomics findings and (vi) the application of additional -omics technologies.
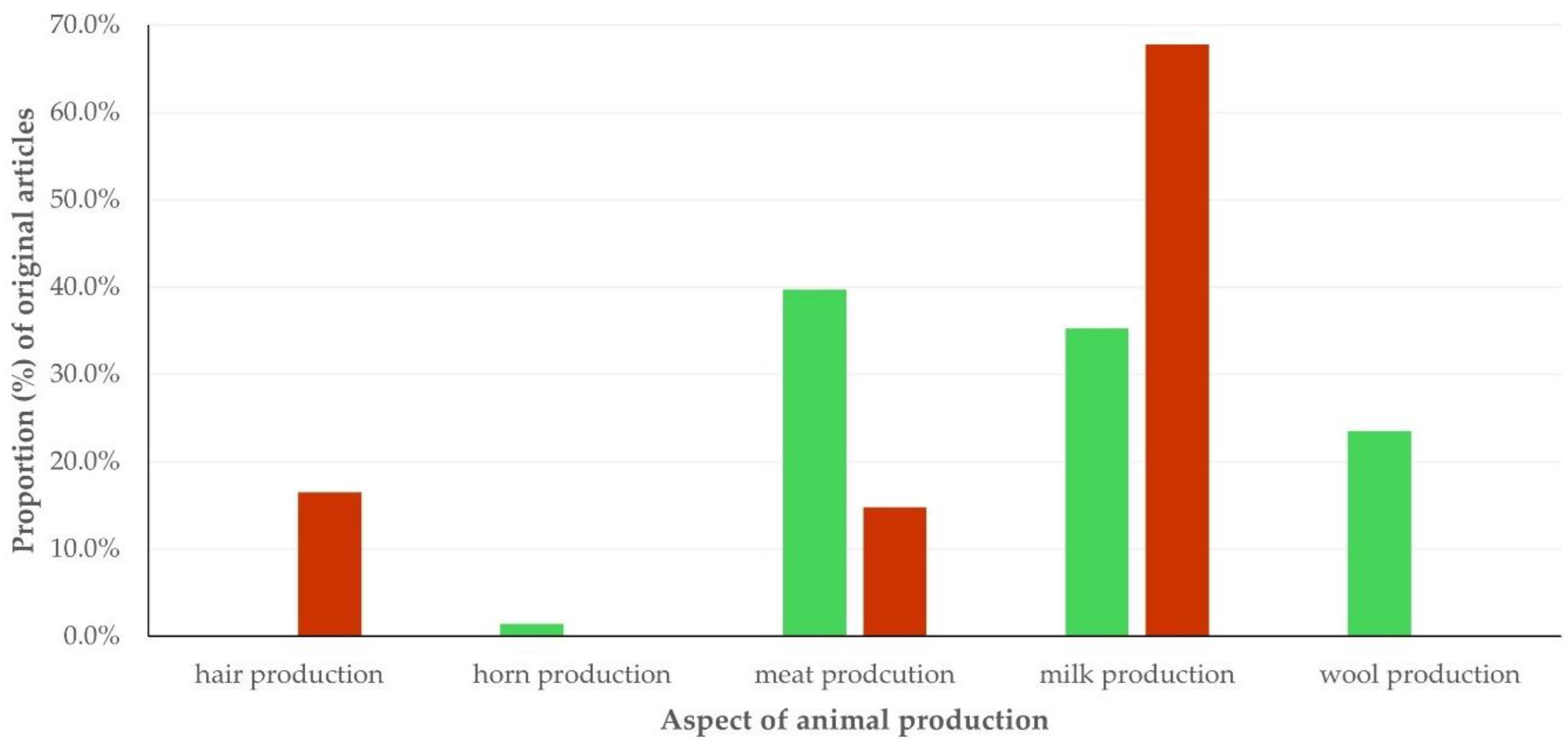
- For original articles on sheep/goat production, the specific field (i.e., milk, meat, wool, hair, or horn production); for original articles on animal diseases, the specific disease studied; for original articles referring to small ruminants as models, the specific conditions studied.
- Keywords listed in the published papers, journals in which the papers were published, authors of the papers (names and total numbers), references cited therein, and total number of citations received until the end of 2024 [8]. The number of citations received by the papers was normalized by calculating the average citations received annually per paper since the year of publication of each paper.
2.3. Data Management and Analysis
3. Results and Discussion
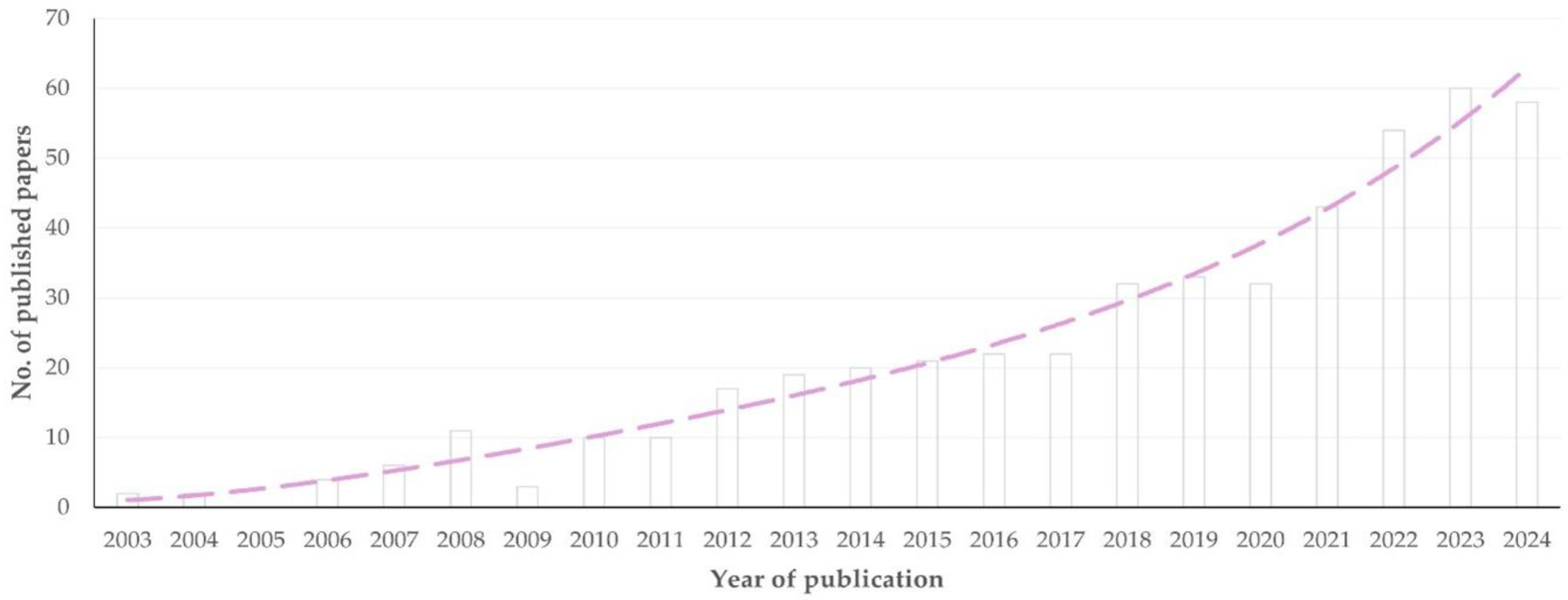
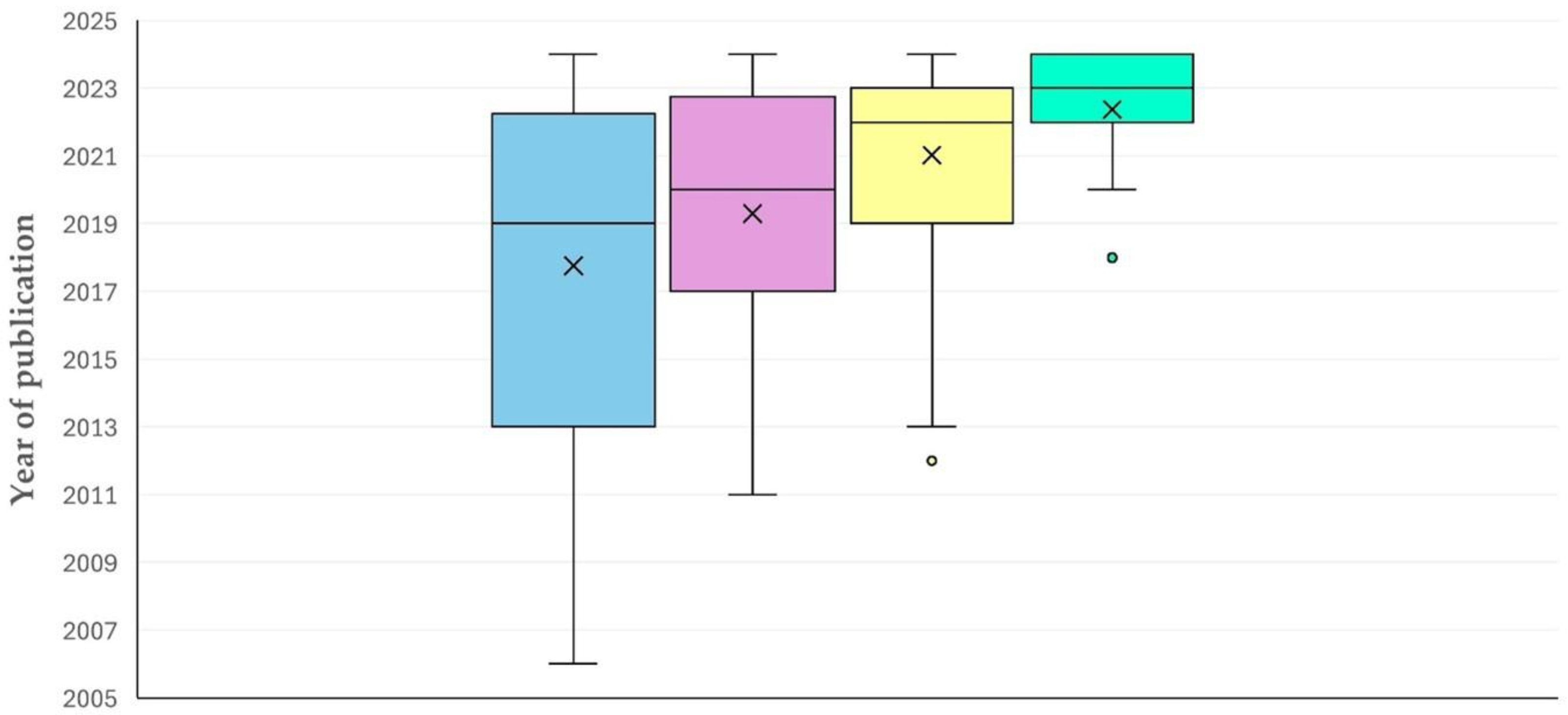
3.1. Year of Publication
3.1.1. Findings
3.1.2. Comments
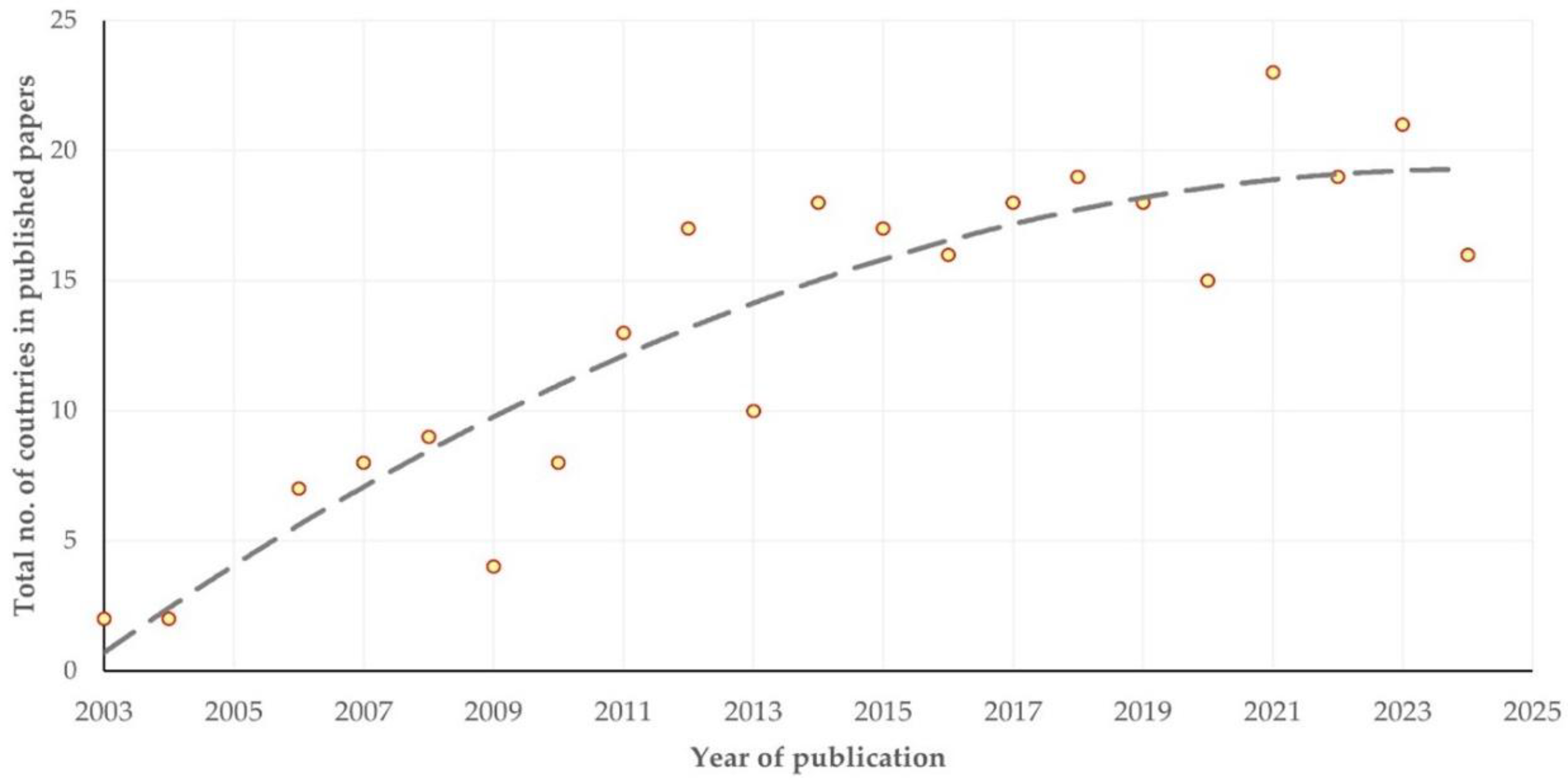
3.2. Countries and Scientific Establishments of Origin
3.2.1. Findings
3.2.2. Comments
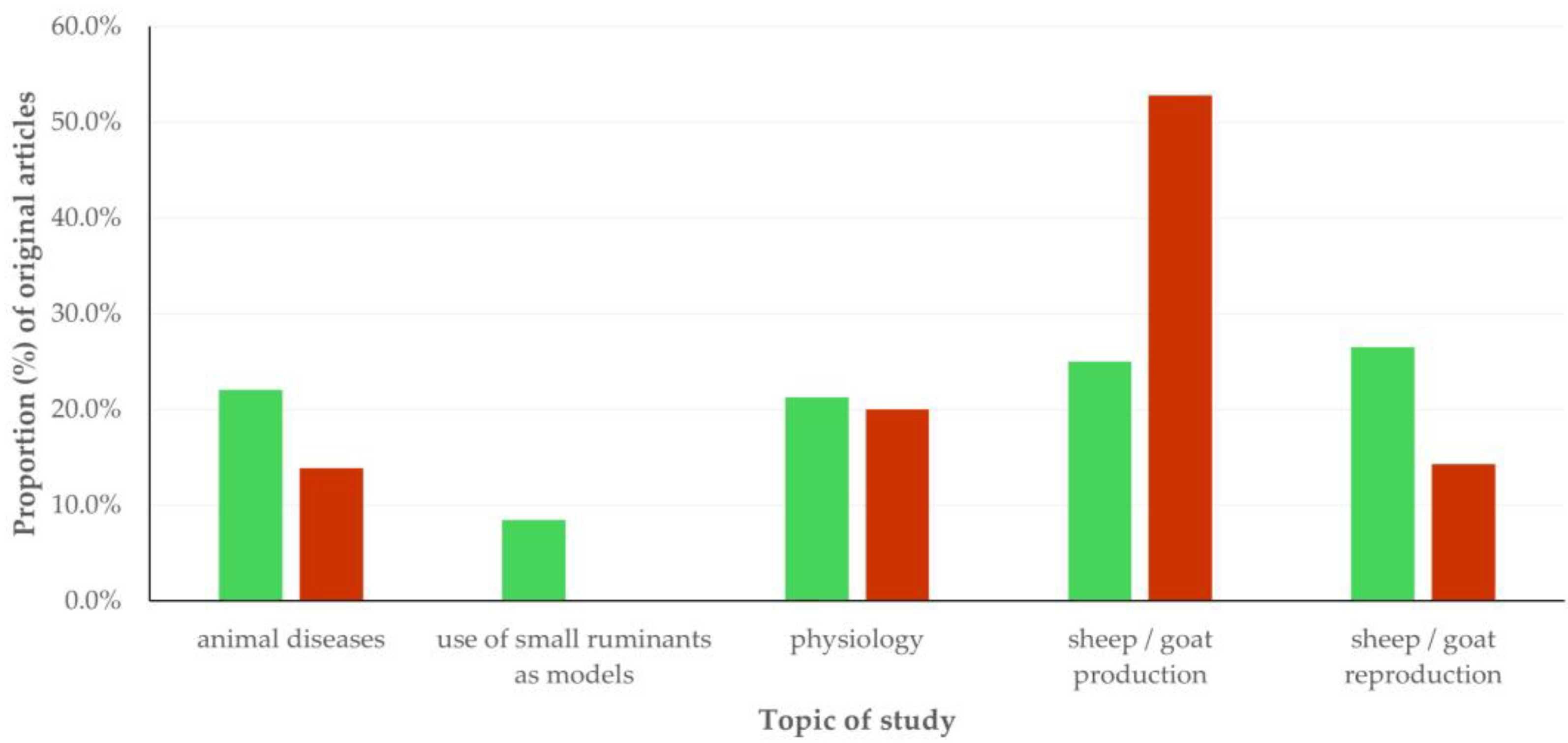
3.3. Type of Work and Topic of Study in Original Articles
3.3.1. Findings
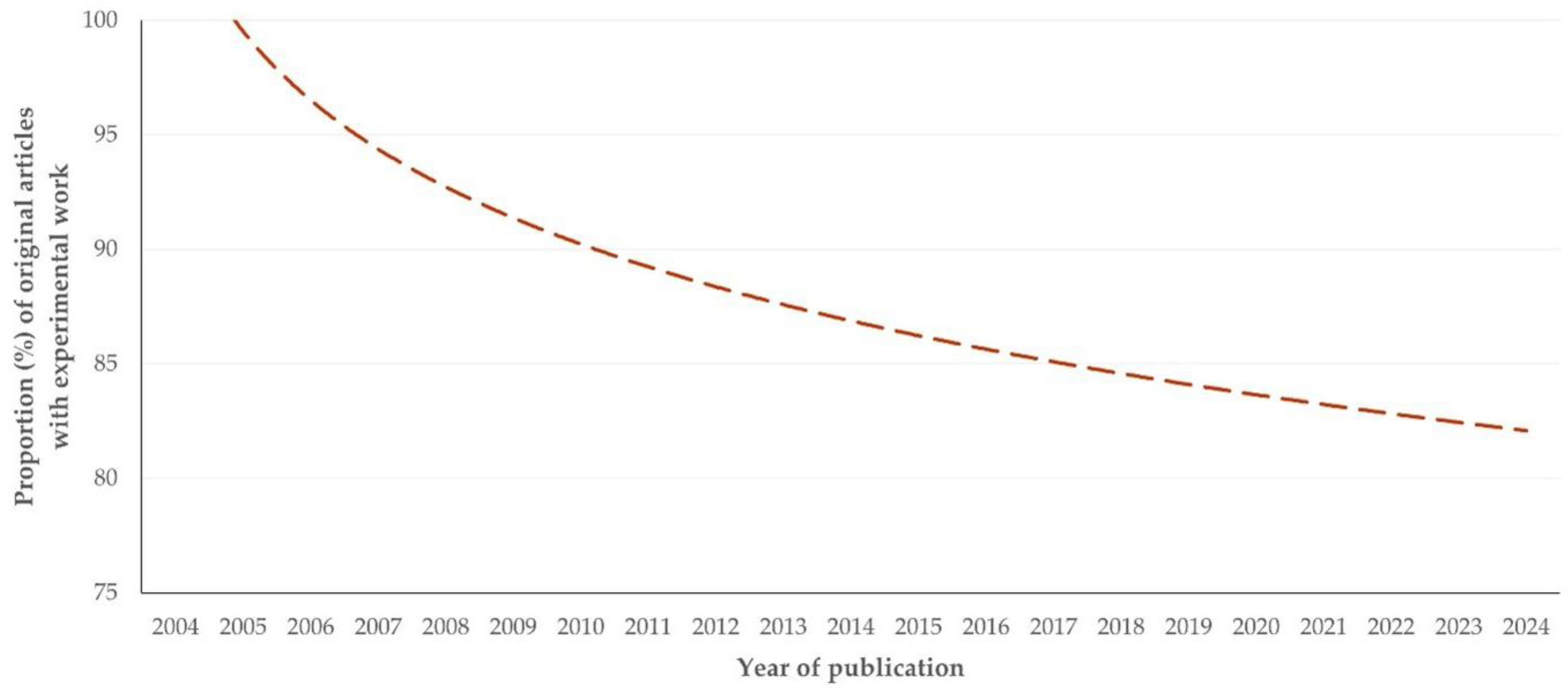
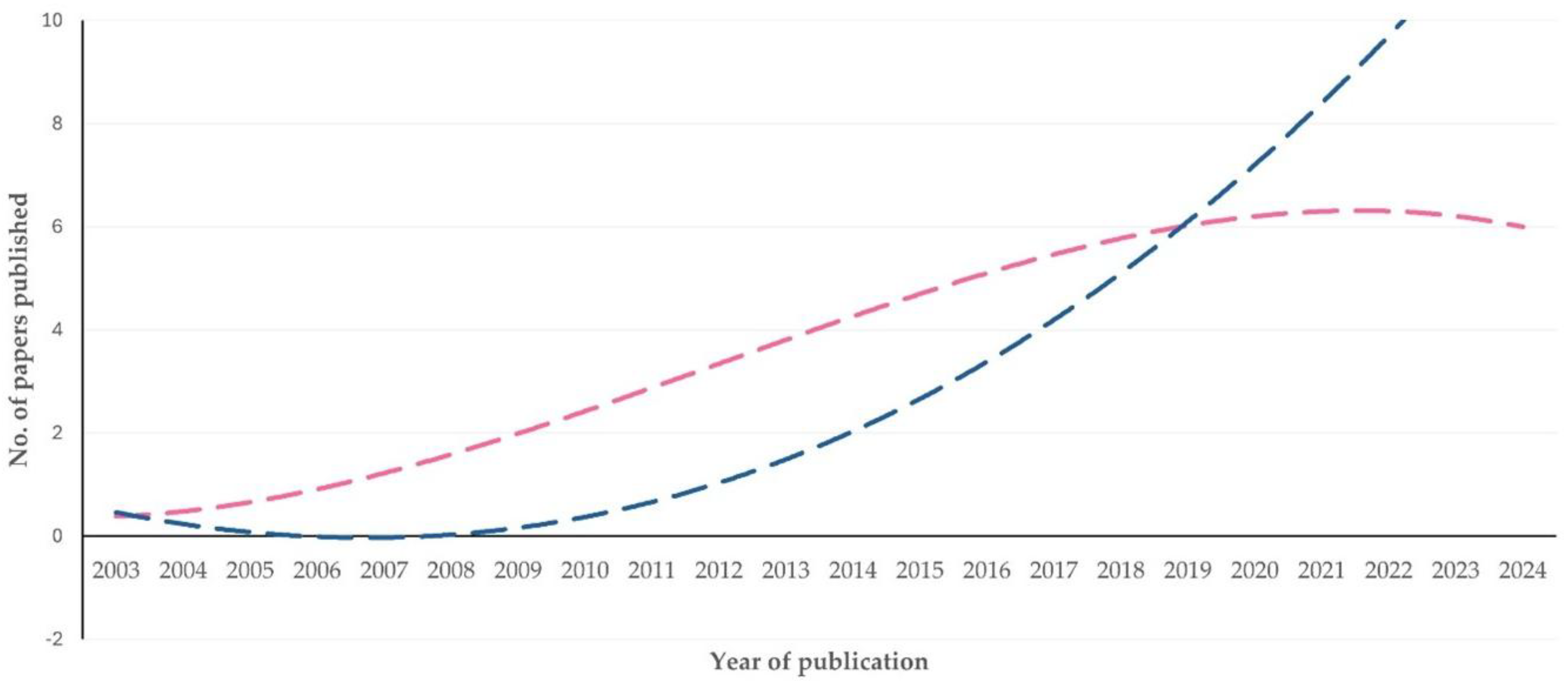
Type of Work
Topics of Studies
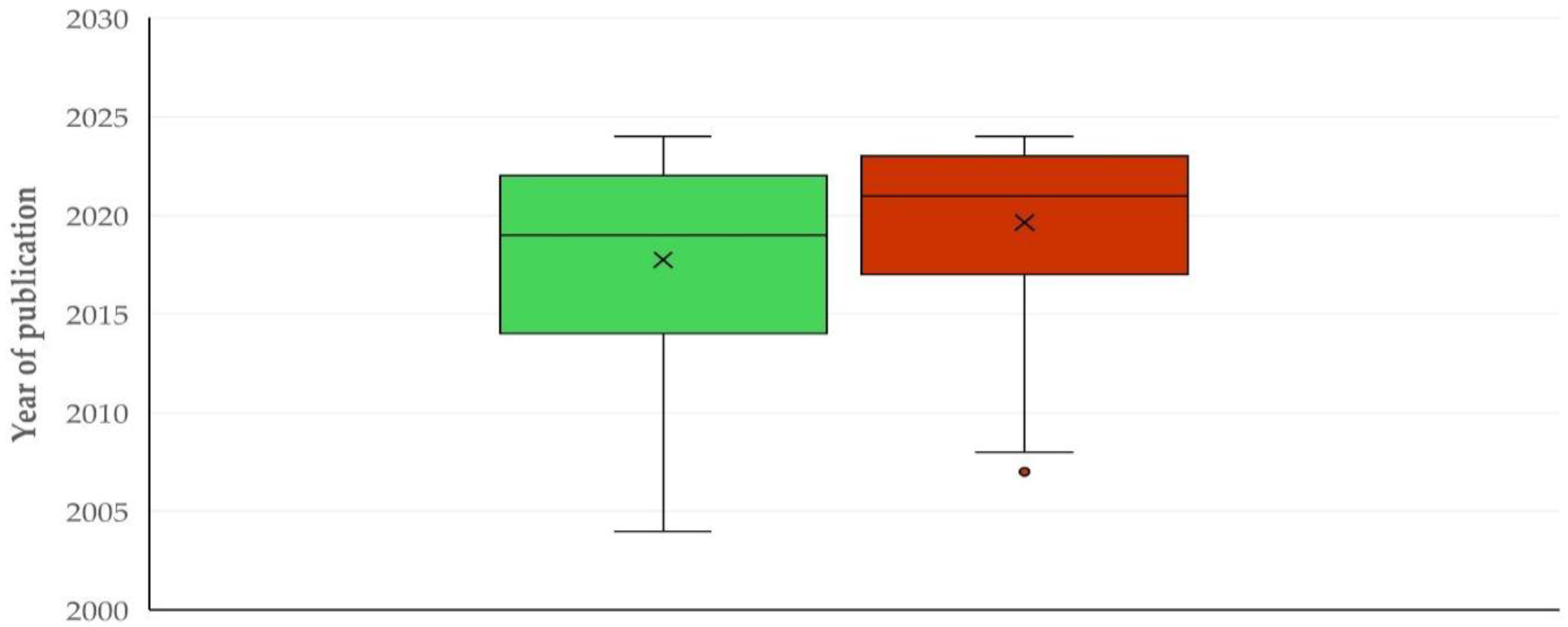
Thematic Priorities Between Sheep and Goat Work
3.3.2. Comments
3.4. Tissues Analyzed
3.4.1. Findings
3.4.2. Comments
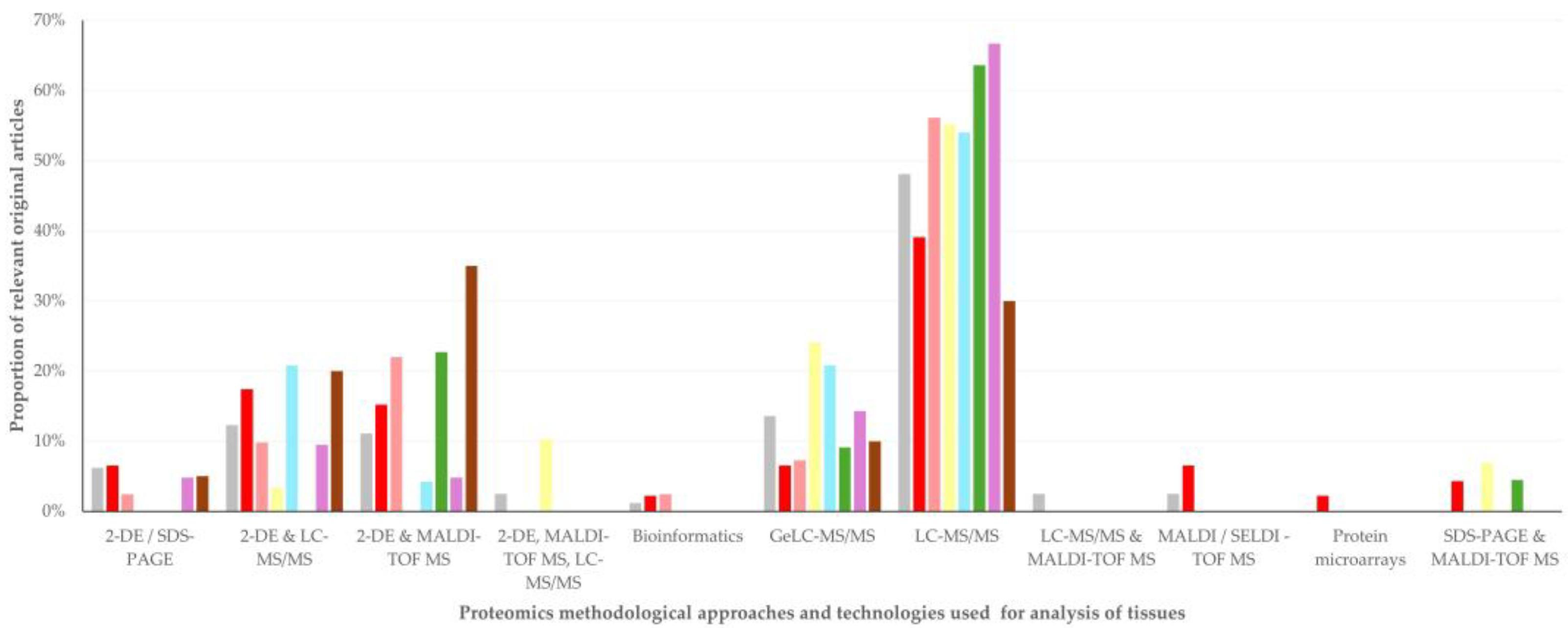
3.5. Proteomics Methodological Approaches and Technologies Used
3.5.1. Findings
3.5.2. Comments
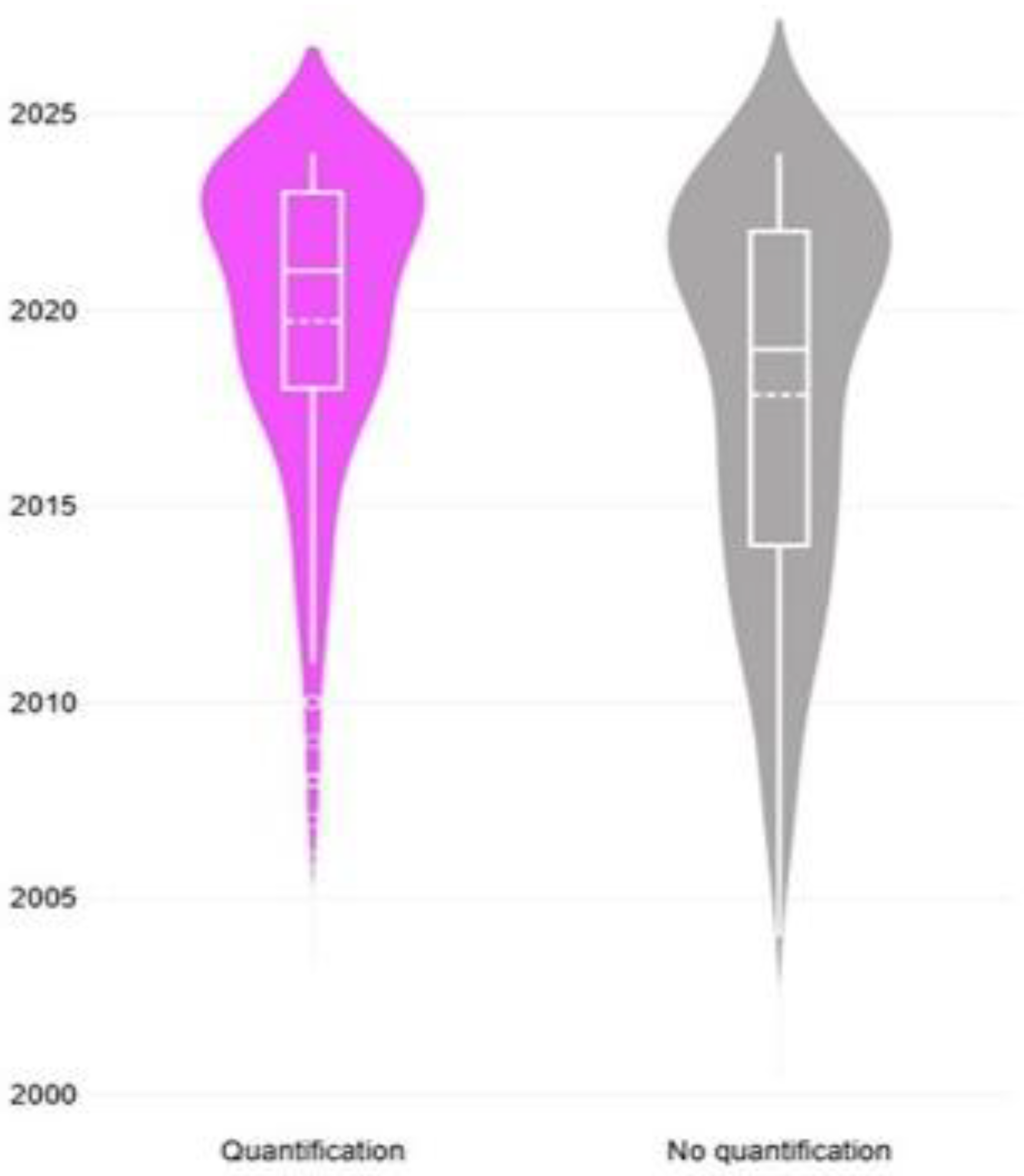
3.6. Quantification Analysis of Proteomics Findings
3.6.1. Findings
3.6.2. Comments
3.7. Concurrent Use of Additional -Omics Technologies
3.7.1. Findings
3.7.2. Comments
3.8. Keywords
3.8.1. Findings
3.8.2. Comments
3.9. Journals
3.9.1. Findings
3.9.2. Comments
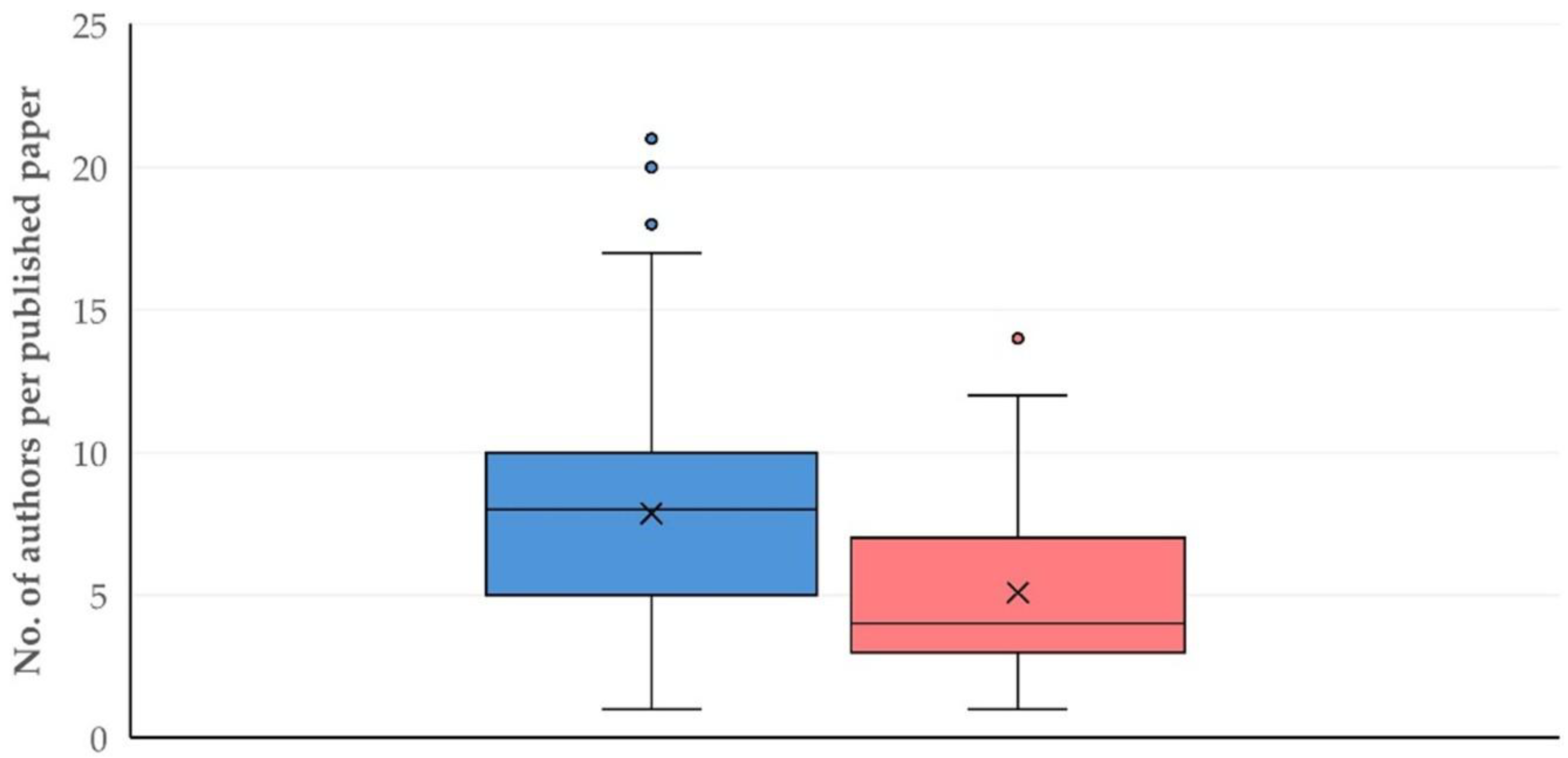
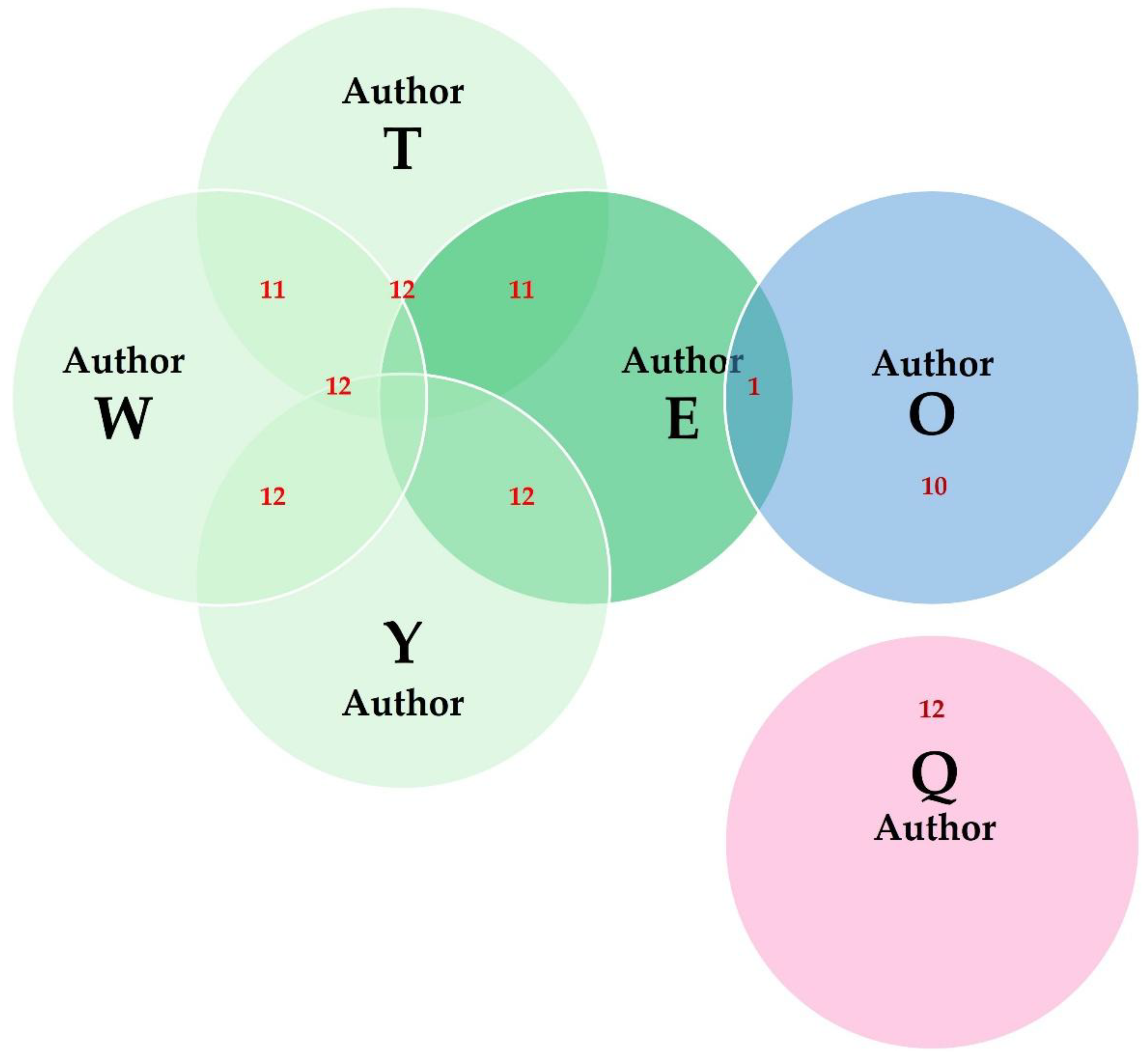
3.10. Authors
3.10.1. Findings
3.10.2. Comments
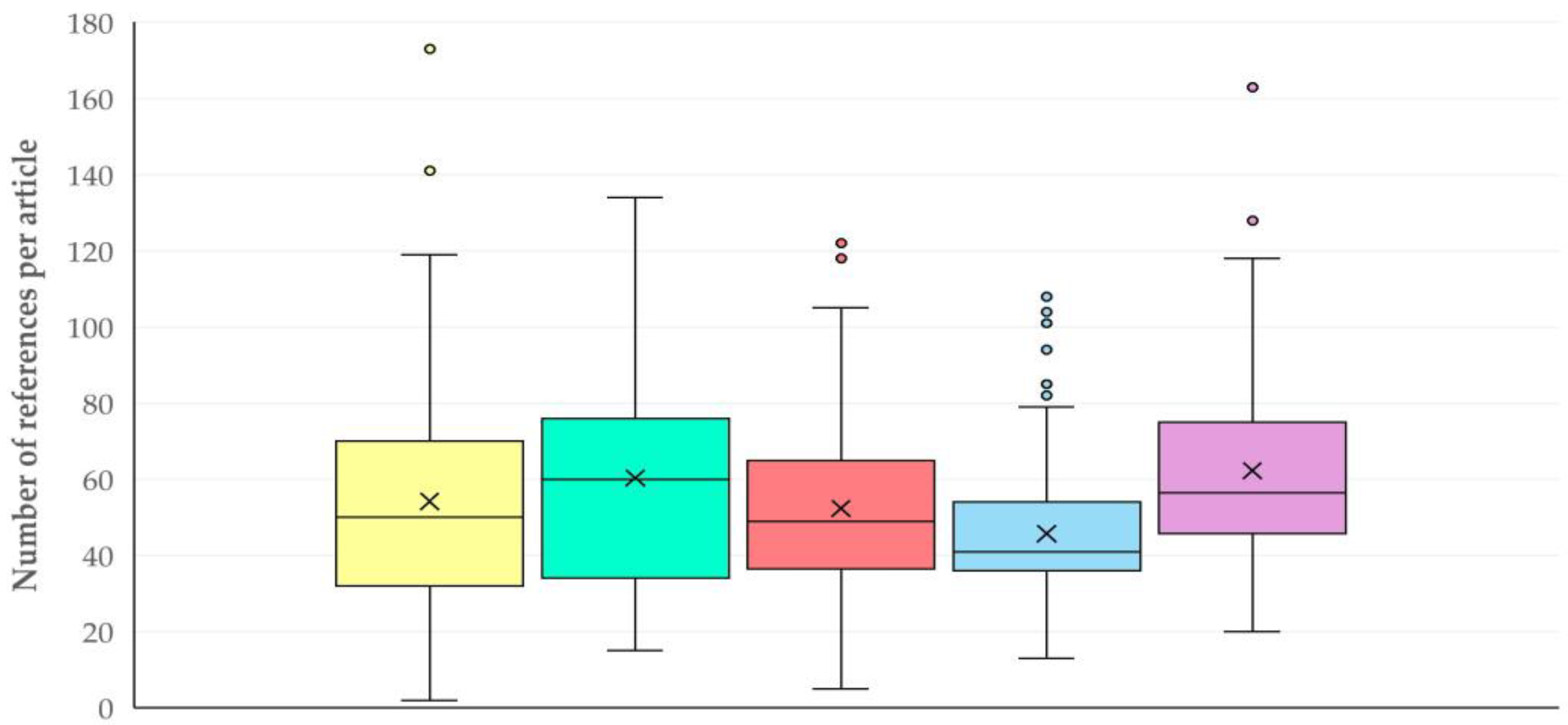
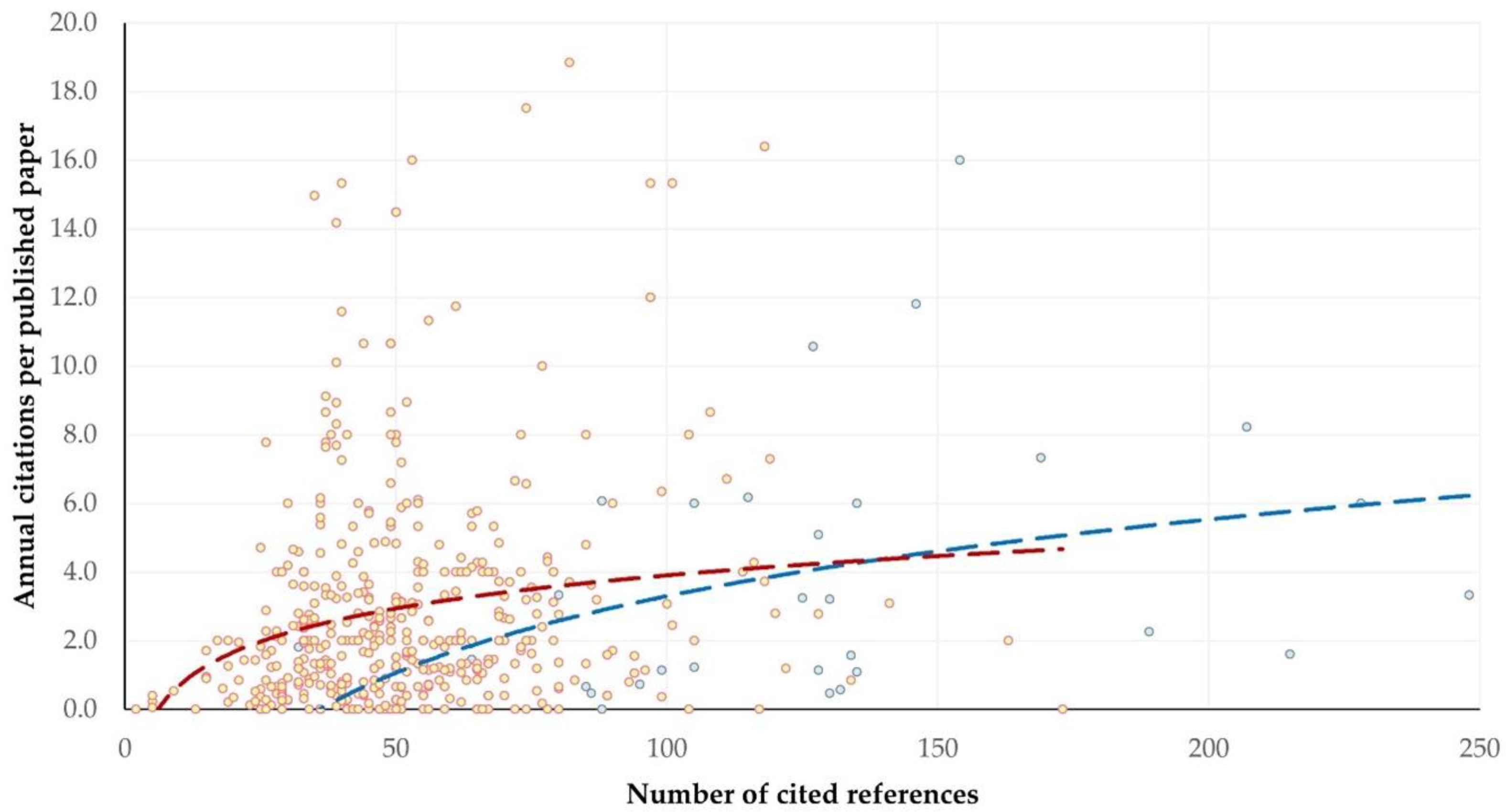
3.11. References and Citations
3.11.1. Findings
References
Citations
3.11.2. Comments
4. Epimeter
4.1. Milk Production
4.2. Meat Production
4.3. Mastitis
4.4. Gastrointestinal Helminth Infections
4.5. Zoonotic Relevance of Findings
4.6. Reproductive Management
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jiang, Y.; Rex, D.A.B.; Schuster, D.; Neely, B.A.; Rosano, G.L.; Volkmar, N.; Momenzadeh, A.; Peters-Clarke, T.M.; Egbert, S.B.; Kreimer, S.; et al. Comprehensive overview of bottom-up proteomics using mass spectrometry. ACS Meas. Sci. Au 2024, 4, 338–417. [Google Scholar] [CrossRef] [PubMed]
- Conti, A.; Alessio, M. Comparative proteomics for the evaluation of protein expression and modifications in neurodegenerative diseases. Int. Rev. Neurobiol. 2015, 121, 117–152. [Google Scholar] [CrossRef]
- Katsafadou, A.I.; Tsangaris, G.T.; Billinis, C.; Fthenakis, G.C. Use of proteomics in the study of microbial diseases of small ruminants. Vet. Microbiol. 2015, 181, 27–33. [Google Scholar] [CrossRef]
- Katsarou, E.I.; Billinis, C.; Galamatis, D.; Fthenakis, G.C.; Tsangaris, G.T.; Katsafadou, A.I. Applied proteomics in ‘One Health’. Proteomes 2021, 9, 31. [Google Scholar] [CrossRef]
- Cho, W.C. Proteomics technologies and challenges. Genom. Proteom. Bioinform. 2007, 5, 77–85. [Google Scholar] [CrossRef]
- Jordan, B.A.; Fernholz, B.D.; Neubert, T.A.; Ziff, E.B. New tricks for an old dog: Proteomics of the PSD. In The Dynamic Synapse: Molecular Methods in Ionotropic Receptor Biology; Kittler, J.T., Moss, S.J., Eds.; Chapter 3; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Oxfordshire, UK, 2006. Available online: https://www.ncbi.nlm.nih.gov/books/NBK1844/ (accessed on 18 July 2025).
- Adnane, M.; de Almeida, A.M.; Chapwanya, A. Unveiling the power of proteomics in advancing tropical animal health and production. Trop. Anim. Health Prod. 2024, 56, 182. [Google Scholar] [CrossRef] [PubMed]
- Vaitsi, G.A.; Bourganou, M.V.; Lianou, D.T.; Kiouvrekis, Y.; Michael, C.C.; Gougoulis, D.A.; Fthenakis, G.C. Scientometric analysis: An emerging tool in veterinary and animal scientific research. Animals 2024, 14, 3132. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Dubeuf, J.P.; Genis, J.C.; Morand-Fehr, P.; Morales, F.D.R. The contribution of goats in the future redesigning of livestock activities and value chains. Small Rumin. Res. 2023, 227, 107065. [Google Scholar] [CrossRef]
- Webb, E.C.; Casey, N.H.; Simela, L. Goat meat quality. Small Rum. Res. 2005, 60, 153–166. [Google Scholar] [CrossRef]
- Jankiewicz, M.; van Lee, L.; Biesheuvel, M.; Brouwer-Brolsma, E.M.; van der Zee, L.; Szajewska, H. The effect of goat-milk-based infant formulas on growth and safety parameters: A systematic review and meta-analysis. Nutrients 2023, 15, 2110. [Google Scholar] [CrossRef]
- World Population Review (Agriculture). Available online: https://worldpopulationreview.com/categories/agriculture?place=country%2Cstate&subcat= (accessed on 12 August 2025).
- Messner, C.B.; Demichev, V.; Wang, Z.; Hartl, J.; Kustatscher, G.; Mülleder, M.; Ralser, M. Mass spectrometry-based high-throughput proteomics and its role in biomedical studies and systems biology. Proteomics 2023, 23, e2200013. [Google Scholar] [CrossRef]
- Vitorino, R. Transforming clinical research: The power of high-throughput omics integration. Proteomes 2024, 12, 25. [Google Scholar] [CrossRef]
- Lamond, A.I.; Uhlen, M.; Horning, S.; Makarov, A.; Robinson, C.V.; Serrano, L.; Hartl, F.U.; Baumeister, W.; Werenskiold, A.K.; Andersen, J.S.; et al. Advancing cell biology through proteomics in space and time (PROSPECTS). Mol. Cell. Proteom. 2012, 11, O112.017731. [Google Scholar] [CrossRef]
- Richter, S.H. Challenging current scientific practice: How a shift in research methodology could reduce animal use. Lab. Anim. 2024, 53, 9–12. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K. Trends in the use of animals and non-animal methods over the last 20 years. ALTEX 2024, 41, 503–524. [Google Scholar] [CrossRef]
- Marinou, K.A.; Dontas, I.A. European Union legislation for the welfare of animals used for scientific purposes: Areas identified for further discussion. Animals 2023, 13, 2367. [Google Scholar] [CrossRef]
- Robinson, T.P.; Thornton, P.K.; Franceschini, G.; Kruska, R.L.; Chiozza, F.; Notenbaert, A.; Cecchi, G.; Herrero, M.; Epprecht, M.; Fritz, S.; et al. Global Livestock Production Systems; Food and Agriculture Organization of the United Nations (FAO) and International Livestock Research Institute (ILRI): Rome, Italy, 2011; 152p. [Google Scholar]
- Hadjigeorgiou, I.; Vallerand, F.; Tsimpoukas, K.; Zervas, G. The socio-economics of sheep and goat farming in Greece and the implications for future rural development. Options Méditerr. Sér. B Etudes Rec. 2002, 39, 83–93. [Google Scholar]
- Taylor, M.A.; Coop, R.L.; Wall, R.L. Veterinary Parasitology, 3rd ed.; Blackwell Publishing: London, UK, 2007. [Google Scholar]
- Sargison, N. Sheep Flock Health A Planned Approach; Blackwell Science: Oxford, UK, 2008. [Google Scholar]
- Taylor, M.A. Emerging parasitic diseases of sheep. Vet. Parasitol. 2012, 189, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Fthenakis, G.C.; Mavrogianni, V.S.; Gallidis, E.; Papadopoulos, E. Interactions between parasitic infections and reproductive efficiency in sheep. Vet. Parasitol. 2015, 208, 56–66. [Google Scholar] [CrossRef]
- Fthenakis, G.C.; Papadopoulos, E. Impact of parasitism in goat production. Small Rum. Res. 2018, 163, 21–23. [Google Scholar] [CrossRef]
- Lianou, D.T. Mapping the Small Ruminant Industry in Greece: Health Management and Diseases of Animals, Preventive Veterinary Medicine and Therapeutics, Reproductive Performance, Production Outcomes, Veterinary Public Health, Socio-Demographic Characteristics of the Farmers. Ph.D. Thesis, University of Thessaly, Volos, Greece, 2023. [Google Scholar]
- Tsiokos, D.; Perucho, L.; Bouzalas, I.; Fança, B.; Grisot, P.G.; de Heredia, I.B.; Tsiligianni, T. Insights on the health challenges of the dairy sheep farming in the Mediterranean countries of Europe. Small Rumin. Res. 2024, 238, 107332. [Google Scholar] [CrossRef]
- Lawrence, K.E.; Leathwick, D.M.; Rhodes, A.P.; Jackson, R.; Heuer, C.; Pomroy, W.E.; West, D.M.; Waghorn, T.S.; Moffat, J.R. Management of gastrointestinal nematode parasites on sheep farms in New Zealand. N. Z. Vet. J. 2007, 55, 28–234. [Google Scholar] [CrossRef]
- Jack, C.; Hotchkiss, E.; Sargison, N.D.; Toma, L.; Milne, C.; Bartley, D.J. A quantitative analysis of attitudes and behaviours concerning sustainable parasite control practices from Scottish sheep farmers. Prev. Vet. Med. 2017, 139, 134–145. [Google Scholar] [CrossRef] [PubMed]
- Ali, E.A.; Abbas, G.; Beveridge, I.; Baxendell, S.; Squire, B.; Stevenson, M.A.; Ghafar, A.; Jabbar, A. Knowledge, attitudes and practices of Australian dairy goat farmers towards the control of gastrointestinal parasites. Paras. Vectors 2025, 18, 25. [Google Scholar] [CrossRef]
- Schubert, O.T.; Röst, H.L.; Collins, B.C.; Rosenberger, G.; Aebersold, R. Quantitative proteomics: Challenges and opportunities in basic and applied research. Nat. Protoc. 2017, 12, 1289–1294. [Google Scholar] [CrossRef] [PubMed]
- Bian, Y.; Zheng, R.; Bayer, F.P.; Wong, C.; Chang, Y.C.; Meng, C.; Zolg, D.P.; Reinecke, M.; Zecha, J.; Wiechmann, S.; et al. Robust, reproducible and quantitative analysis of thousands of proteomes by micro-flow LC–MS/MS. Nat. Commun. 2017, 11, 157. [Google Scholar] [CrossRef]
- Carrillo-Rodriguez, P.; Selheim, F.; Hernandez-Valladares, M. Mass spectrometry-based proteomics workflows in cancer research: The relevance of choosing the right steps. Cancers 2023, 15, 555. [Google Scholar] [CrossRef] [PubMed]
- Dasari, S.; Pereira, L.; Reddy, A.P.; Michaels, J.E.; Lu, X.; Jacob, T.; Thomas, A.; Rodland, M.; Roberts, C.T., Jr.; Gravett, M.G.; et al. Comprehensive proteomic analysis of human cervical-vaginal fluid. J. Proteome Res. 2007, 6, 1258–1268. [Google Scholar] [CrossRef]
- Pereira, L.; Reddy, A.P.; Jacob, T.; Thomas, A.; Schneider, K.A.; Dasari, S.; Lapidus, J.A.; Lu, X.; Rodland, M.; Roberts, C.T., Jr.; et al. Identification of novel protein biomarkers of preterm birth in human cervical-vaginal fluid. J. Proteome Res. 2007, 6, 1269–1276. [Google Scholar] [CrossRef]
- Chen, X.; Sun, Y.; Zhang, T.; Shu, L.; Roepstorff, P.; Yang, F. Quantitative proteomics using isobaric labeling: A practical guide. Genom. Proteom. Bioinform. 2021, 19, 689–706. [Google Scholar] [CrossRef]
- Aebersold, R.; Bensimon, A.; Collins, B.C.; Ludwig, C.; Sabido, E. Applications and developments in targeted proteomics: From SRM to DIA/SWATH. Proteomics 2016, 16, 2065–2067. [Google Scholar] [CrossRef] [PubMed]
- Borràs, E.; Sabidó, E. What is targeted proteomics? A concise revision of targeted acquisition and targeted data analysis in mass spectrometry. Proteomics 2017, 17, 1700180. [Google Scholar] [CrossRef]
- Birhanu, A.G. Mass spectrometry-based proteomics as an emerging tool in clinical laboratories. Clin. Proteom. 2023, 20, 32. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Zhao, C.; Chen, F. Multiomics research: Principles and challenges in integrated analysis. Biodes. Res. 2024, 6, 0059. [Google Scholar] [CrossRef] [PubMed]
- Wadood, A.A.; Bordbar, F.; Zhang, X. Integrating omics approaches in livestock biotechnology: Innovations in production and reproductive efficiency. Front. Anim. Sci. 2025, 6, 1551244. [Google Scholar] [CrossRef]
- Armengaud, J. Journal of Proteomics—«Renaissance»! J. Proteom. 2024, 307, 105295. [Google Scholar] [CrossRef]
- Bourganou, M.V.; Chatzopoulos, D.C.; Lianou, D.T.; Tsangaris, G.T.; Fthenakis, G.C.; Katsafadou, A.I. Scientometrics evaluation of published scientific papers on the use of proteomics technologies in mastitis research in ruminants. Pathogens 2024, 13, 324. [Google Scholar] [CrossRef]
- Gleasner, R.M.; Sood, A. Special issues: The roles of special issues in scholarly communication in a changing publishing landscape. Learned Publ. 2025, 38, e1635. [Google Scholar] [CrossRef]
- Liebman, M.N.; Franchini, M.; Molinaro, S. Bridging the gap between translational medicine and unmet clinical needs. Technol. Health Care 2015, 23, 109–118. [Google Scholar] [CrossRef]
- Filosto, M. Bridging the gap: Translational medicine and novel therapies in neuromuscular diseases. J. Integr. Neurosci. 2024, 23, 94. [Google Scholar] [CrossRef]
- Fanelli, D.; Larivière, V. Researchers’ individual publication rate has not increased in a century. PLoS ONE 2016, 11, e0149504. [Google Scholar] [CrossRef]
- Houfani, A.A.; Foster, L.J. Review of the real and sometimes hidden costs in proteomics experimental workflows. Methods Mol. Biol. 2022, 2456, 1–14. [Google Scholar]
- Onodera, N.; Yoshikane, F. Factors affecting citation rates of research articles. large-scale study based on Web of Science data. J. Assoc. Inform. Sci. Technol. 2015, 66, 739–764. [Google Scholar] [CrossRef]
- Fox, C.W.; Paine, C.E.T.; Sauterey, B. Citations increase with manuscript length, author number, and references cited in ecology journals. Ecol. Evol. 2016, 6, 7717–7726. [Google Scholar] [CrossRef] [PubMed]
- Mammola, S.; Fontaneto, D.; Martínez, A.; Chichorro, F. Impact of the reference list features on the number of citations. Scientometrics 2021, 126, 785–799. [Google Scholar] [CrossRef]
- Didegah, F.; Thelwall, M. Determinants of research citation impact in nanoscience and nanotechnology. J. Am. Soc. Inf. Sci. Technol. 2013, 64, 1055–1064. [Google Scholar] [CrossRef]
- Athanasiadou, S.; Huntley, J.F. Emerging technologies and their applications in interactions between nutrition and immunity to gastrointestinal parasites in sheep. Paras. Immunol. 2008, 30, 101–111. [Google Scholar] [CrossRef]
- Anagnostopoulos, A.K.; Katsafadou, A.I.; Pierros, V.; Kontopodis, E.; Fthenakis, G.C.; Arsenos, G.; Karkabounas, S.C.; Tzora, A.; Skoufos, I.; Tsangaris, G.T. Milk of Greek sheep and goat breeds; characterization by means of proteomics. J. Proteom. 2016, 147, 76–84. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Almeida, A.M.; Renaut, J.; Argüello, A.; Castro, N. A proteomics study of colostrum and milk from the two major small ruminant dairy breeds from the Canary Islands: A bovine milk comparison perspective. J. Dairy Res. 2016, 83, 366–374. [Google Scholar] [CrossRef]
- Di Gerlando, R.; Tolone, M.; Sutera, A.M.; Monteleone, G.; Portolano, B.; Sardina, M.T.; Mastrangelo, S. Variation of proteomic profile during lactation in Girgentana goat milk: A preliminary study. It. J. Anim. Sci. 2019, 18, 88–97. [Google Scholar] [CrossRef]
- Rout, P.K.; Verma, M. Post translational modifications of milk proteins in geographically diverse goat breeds. Sci. Rep. 2021, 11, 5619. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Sun, X.; Guo, M. Proteomic analysis of whey proteins in the colostrum and mature milk of Xinong Saanen goats. J. Dairy Sci. 2020, 103, 1164–1174. [Google Scholar] [CrossRef]
- Zhang, X.; Li, F.; Qin, F.; Li, W.; Yue, X. Exploration of ovine milk whey proteome during postnatal development using an iTRAQ approach. PeerJ 2020, 8, e10105. [Google Scholar] [CrossRef]
- Paramasivam, S.; Ramkumar, B.; Chinnaiyan, U.; Polaki, S.; Vegulada, D.R.; Ranganathan, P.; Ramasamy, R. Bioactive peptides from goat colostrum: Isolation, identification and in-silico characterization. J. Food Measure. Character. 2023, 17, 5247–5255. [Google Scholar] [CrossRef]
- Raimondo, R.F.S.; Miyashiro, S.I.; Birgel Junior, E.H. Whey protein dynamics in goat mammary secretions during colostrum and early lactation periods. J. Dairy Res. 2024, 91, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Bu, D.; Zhao, X.; Sun, P.; Wang, J.; Zhou, L. Proteomic analysis of cow, yak, buffalo, goat and camel milk whey proteins: Quantitative differential expression patterns. J. Proteome Res. 2013, 12, 1660–1667. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Zheng, N.; Yang, J.; Bu, D.; Wang, J.; Ma, L.; Sun, P. Animal species milk identification by comparison of two-dimensional gel map profile and mass spectrometry approach. Int. Dairy J. 2014, 35, 15–20. [Google Scholar] [CrossRef]
- El-Hatmi, H.; Jrad, Z.; Salhi, I.; Aguibi, A.; Nadri, A.; Khorchani, T. Comparison of composition and whey protein fractions of human, camel, donkey, goat and cow milk. Mljekarstvo 2015, 65, 159–167. [Google Scholar] [CrossRef]
- Pisanu, S.; Marogna, G.; Pagnozzi, D.; Piccinini, M.; Leo, G.; Tanca, A.; Roggio, A.M.; Roggio, T.; Uzzau, S.; Addis, M.F. Characterization of size and composition of milk fat globules from Sarda and Saanen dairy goats. Small Rumin. Res. 2013, 109, 141–151. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, C.; Sun, X.; Guo, M. Comparative proteomics of whey and milk fat globule membrane proteins of Guanzhong goat and Holstein cow mature milk. J. Food Sci. 2019, 84, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Zhang, R.; Zhu, Z.; Shi, L. A high-throughput comparative proteomics of milk fat globule membrane reveals breed and lactation stages specific variation in protein abundance and functional differences between milk of Saanen dairy goat and Holstein bovine. Front. Nutr. 2021, 8, 680683. [Google Scholar] [CrossRef]
- Signorelli, F.; Cifuni, G.F.; Miarelli, M. Differentially expressed mammary proteins during lactation in dairy sheep. Liv. Sci. 2012, 149, 224–231. [Google Scholar] [CrossRef]
- Zhang, L.; Yan, D.; Roy, M.C.; Huang, J.; Zhou, P. Variation in both proteome and N-glycoproteome of goat MFGM over lactation. J. Food Comp. Analys. 2022, 111, 104635. [Google Scholar] [CrossRef]
- Sun, X.; Yu, Z.; Liang, C.; Xie, S.; Wen, J.; Wang, H.; Wang, J.; Yang, U.; Han, R. Developmental changes in proteins of casein micelles in goat milk using data-independent acquisition-based proteomics methods during the lactation cycle. J. Dairy Sci. 2023, 106, 47–60. [Google Scholar] [CrossRef]
- He, M.; Nie, X.; Wang, H.; Yan, S.; Zhang, Y. Effects of a high-grain diet with a buffering agent on milk protein synthesis in lactating goats. Front. Vet. Sci. 2021, 8, 696703. [Google Scholar] [CrossRef]
- Oliveira Marques, I.T.; Vasconcelos, F.R.; Martins Alves, J.P.; Montenegro, A.R.; Linhares Fernandes, C.C.; Bezerra Oliveira, F.B.; Pessoa Silva, C.; Shiniti Nagano, C.; Figueiredo, F.C.; Beserra, F.J.; et al. Proteome of milk fat globule membrane and mammary gland tissue in goat fed different lipid supplementation. Small Rumin. Res. 2021, 199, 106378. [Google Scholar] [CrossRef]
- Hernández-Castellano, L.E.; Ferreira, A.M.; Nanni, P.; Grossmann, J.; Argüello, A.; Capote, J.; Cai, G.; Lippolis, J.; Castro, N.; de Almeida, A.M. The goat (Capra hircus) mammary gland secretory tissue proteome as influenced by weight loss: A study using label free proteomics. J. Proteom. 2016, 145, 60–69. [Google Scholar] [CrossRef]
- Qu, Y.; Chen, L.; Ren, X.; Shari, A.; Yuan, Y.; Yu, M.; Xiao, H.; Li, G. Milk proteomic analysis reveals differentially expressed proteins in high-yielding and low-yielding Guanzhong dairy goats at peak lactation. J. Dairy Res. 2024, 91, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Medeiros, G.K.; Queiroga, R.C.; Costa, W.K.; Gadelha, C.A.; e Lacerda, R.R.; Lacerda, J.T.; Pinto, L.S.; Braganhol, E.; Teixeira, F.C.; Barbosa, P.P.D.S.; et al. Proteomic of goat milk whey and its bacteriostatic and antitumour potential. Int. J. Biol. Macromol. 2018, 113, 116–123. [Google Scholar] [CrossRef]
- Petre, M.L.; Kontouli Pertesi, A.N.; Boulioglou, O.E.; Sarantidi, E.; Korovesi, A.G.; Kozei, A.; Katsafadou, A.I.; Tsangaris, G.T.; Trichopoulou, A.; Anagnostopoulos, A.K. Bioactive peptides in Greek goat colostrum: Relevance to human metabolism. Foods 2024, 13, 3949. [Google Scholar] [CrossRef]
- McDonagh, M.B.; Ferguson, K.L.; Bacic, A.; Gardner, G.E.; Hegarty, R.S. Variation in protein abundance profiles in the M. semitendinosus of lambs bred from sires selected on the basis of growth and muscling potential. Aus. J. Agric. Res. 2006, 57, 671–682. [Google Scholar] [CrossRef]
- Zhu, M.J.; Ford, S.P.; Means, W.J.; Hess, B.W.; Nathanielsz, P.W.; Du, M. Maternal nutrient restriction affects properties of skeletal muscle in offspring. J. Physiol. 2006, 575 Pt 1, 241–250. [Google Scholar] [CrossRef] [PubMed]
- Gulyas, G.; Pohoczky, K.; Csosz, E.; Simon, A.; Javor, A.; Czegledi, L. Comparative proteome analysis of skeletal muscle between Merino and Tsigai lambs. Small Rumin. Res. 2018, 158, 35–41. [Google Scholar] [CrossRef]
- Wang, Z.; He, F.; Rao, W.; Ni, N.; Shen, Q.; Zhang, D. Proteomic analysis of goat Longissimus dorsi muscles with different drip loss values related to meat quality traits. Food Sci. Biotechnol. 2016, 25, 425–431. [Google Scholar] [CrossRef]
- Gu, M.; Wei, Y.; Zhang, D.; Liu, Y. iTRAQ based proteomic profile analysis for goat Longissimus thoracis under repeated freeze-thaw treatments. LWT 2020, 134, 109934. [Google Scholar] [CrossRef]
- della Malva, A.; Lamri, M.; Albenzio, M.; Gagaoua, M. First comparison of early post-mortem proteomes in two goat muscle types: M. Longissimus thoracis and M. semitendinosus. Food Biosci. 2023, 56, 103234. [Google Scholar] [CrossRef]
- do Prado Paim, T.; Viana, P.; van Tilburg, M.F.; Moura, A.A.; de Souza, J.R.; McManus, C.; Abdalla, A.L.; Louvandin, H. Feeding effects of cottonseed and its co-products on the meat proteome from ram lambs. Sci. Agric. 2019, 76, 463–472. [Google Scholar] [CrossRef]
- Marques, I.T.O.; Fernandes, C.C.L.; Vasconcelos, F.R.; Alves, J.P.M.; Montenegro, A.R.; da Silva, C.P.; Oliveira, F.B.B.; Figueiredo, F.C.; Moura, A.A.; Rondina, D. Meat quality of culled adult goats finished with increased feeding plans. Food Sci. Technol. 2022, 42, e37721. [Google Scholar] [CrossRef]
- Gao, X.; Zhao, D.; Wang, L.; Cui, Y.; Wang, S.; Lv, M.; Zang, F.; Dai, R. Proteomic changes in sarcoplasmic and myofibrillar proteins associated with color stability of ovine muscle during post-mortem storage. Foods 2021, 10, 2989. [Google Scholar] [CrossRef]
- Lamri, M.; Della Malva, A.; Djenane, D.; Albenzio, M.; Gagaoua, M. First insights into the dynamic protein changes in goat Semitendinosus muscle during the post-mortem period using high-throughput proteomics. Meat Sci. 2023, 202, 109207. [Google Scholar] [CrossRef]
- Kiran, M.; Naveena, B.M.; Smrutirekha, M.; Baswa Reddy, P.; Rituparna, B.; Praveen Kumar, Y.; Venkatesh, C.; Rapole, S. Traditional halal slaughter without stunning versus slaughter with electrical stunning of sheep (Ovis aries). Meat Sci. 2019, 148, 127–136. [Google Scholar] [CrossRef]
- Martínez, T.F.; Alcalde, M.J.; Sáez, M.I.; Suárez, M.D. Effects of farm management practices and transport time on post-mortem changes of Longissimus lumborum muscle proteins in suckling goat kids. Foods 2020, 9, 934. [Google Scholar] [CrossRef]
- Namratha, K.B.; Naveena, B.M.; Fairoze, N.; Banerjee, R.; Mohan, K.; Muthupalani, M. Deciphering proteome changes and meat texture of traditional halal slaughtered spent sheep subjected to low-voltage electrical stimulation and ageing. Int. J. Anim. Sci. 2024, 94, 375–380. [Google Scholar] [CrossRef]
- Addis, M.F.; Pisanu, S.; Ghisaura, S.; Pagnozzi, D.; Marogna, G.; Tanca, A.; Biosa, G.; Cacciotto, C.; Alberti, A.; Pittau, M.; et al. Proteomics and pathway analyses of the milk fat globule in sheep naturally infected by Mycoplasma agalactiae provide indications of the in vivo response of the mammary epithelium to bacterial infection. Infect. Immun. 2011, 79, 3833–3845. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Addis, M.F.; Pisanu, S.; Marogna, G.; Cubeddu, T.; Pagnozzi, D.; Cacciotto, C.; Campesi, F.; Schianchi, G.; Rocca, S.; Uzzau, S. Production and release of antimicrobial and immune defense proteins by mammary epithelial cells following Streptococcus uberis infection of sheep. Infect. Immun. 2013, 81, 3182–3197. [Google Scholar] [CrossRef]
- Pisanu, S.; Cubeddu, T.; Pagnozzi, D.; Rocca, S.; Cacciotto, C.; Alberti, A.; Marogna, G.; Uzzau, S.; Addis, M.F. Neutrophil extracellular traps in sheep mastitis. Vet. Res. 2015, 46, 59. [Google Scholar] [CrossRef] [PubMed]
- Dore, S.; Liciardi, M.; Amatiste, S.; Bergagna, S.; Bolzoni, G.; Caligiuri, V.; Cerrone, A.; Farina, G.; Montagna, C.; Saletti, M.; et al. Survey on small ruminant bacterial mastitis in Italy, 2013–2014. Small Rumin. Res. 2016, 141, 91–96. [Google Scholar] [CrossRef][Green Version]
- Katsafadou, A.I.; Tsangaris, G.T.; Anagnostopoulos, A.K.; Billinis, C.; Barbagianni, M.S.; Vasileiou, N.G.C.; Spanos, S.A.; Mavrogianni, V.S.; Fthenakis, G.C. Differential quantitative proteomics study of experimental Mannheimia haemolytica mastitis in sheep. J. Proteom. 2019, 205, 103393. [Google Scholar] [CrossRef]
- Gelasakis, A.I.; Mavrogianni, V.S.; Petridis, I.G.; Vasileiou, N.G.; Fthenakis, G.C. Mastitis in sheep—The last 10 years and the future of research. Vet. Microbiol. 2015, 181, 136–146. [Google Scholar] [CrossRef]
- Addis, M.F.; Tedde, V.; Dore, S.; Pisanu, S.; Puggioni, G.M.G.; Roggio, A.M.; Pagnozzi, D.; Lollai, S.; Cannas, E.A.; Uzzau, S. Evaluation of milk cathelicidin for detection of dairy sheep mastitis. J. Dairy Sci. 2016, 99, 6446–6456. [Google Scholar] [CrossRef]
- Katsafadou, A.I.; Tsangaris, G.T.; Vasileiou, N.G.C.; Ioannidi, K.S.; Anagnostopoulos, A.K.; Billinis, C.; Fragkou, I.A.; Papadopoulos, E.; Mavrogianni, V.S.; Michael, C.K.; et al. Detection of cathelicidin-1 in the milk as an early indicator of mastitis in ewes. Pathogens 2019, 8, 270. [Google Scholar] [CrossRef] [PubMed]
- Katsafadou, A.I.; Vasileiou, N.G.C.; Tsangaris, G.T.; Ioannidi, K.S.; Anagnostopoulos, A.K.; Billinis, C.; Fragkou, I.A.; Papadopoulos, E.; Mavrogianni, V.S.; Lianou, D.T.; et al. Presence of cathelicidin-1 in milk as an indicator of the severity of mammary infection in ewes. Curr. Proteom. 2021, 18, 162–168. [Google Scholar] [CrossRef]
- Bourganou, M.V.; Liagka, D.V.; Vougas, K.; Lianou, D.T.; Vasileiou, N.G.C.; Dimoveli, K.S.; Politis, A.P.; Kordalis, N.G.; Petinaki, E.; Mavrogianni, V.S.; et al. Detection of cathelicidin-1 and cathelicidin-2 biomolecules in the milk of goats and their use as biomarkers for the diagnosis of mastitis. Animals 2025, 15, 2301. [Google Scholar] [CrossRef] [PubMed]
- Cubeddu, T.; Cacciotto, C.; Pisanu, S.; Tedde, V.; Alberti, A.; Pittau, M.; Dore, S.; Cannas, A.; Uzzau, S.; Rocca, S.; et al. Cathelicidin production and release by mammary epithelial cells during infectious mastitis. Vet. Immunol. Immunopathol. 2017, 189, 66–70. [Google Scholar] [CrossRef]
- Chiaradia, E.; Valiani, A.; Tartaglia, M.; Scoppetta, F.; Renzone, G.; Arena, S.; Avellini, L.; Benda, S.; Gaiti, A.; Scaloni, A. Ovine subclinical mastitis: Proteomic analysis of whey and milk fat globules unveils putative diagnostic biomarkers in milk. J. Proteom. 2013, 83, 144–159. [Google Scholar] [CrossRef] [PubMed]
- Miglio, A.; Moscati, L.; Fruganti, G.; Pela, M.; Scoccia, E.; Valiani, A.; Maresca, C. Use of milk amyloid A in the diagnosis of subclinical mastitis in dairy ewes. J. Dairy Res. 2013, 80, 496–502. [Google Scholar] [CrossRef]
- Vasileiou, N.G.C.; Fthenakis, G.C.; Mavrogianni, V.S. Comparison of the efficacy of intramammary or injectable antibiotic administration against staphylococcal mastitis in ewes. Pathogens 2022, 11, 1164. [Google Scholar] [CrossRef]
- Belecke, A.; Kupčinskas, T.; Stadalienė, I.; Höglund, J.; Thamsborg, S.M.; Stuen, S.; Petkevičiu, S. Anthelmintic resistance in small ruminants in the Nordic-Baltic region. Acta Vet. Scand. 2021, 63, 18. [Google Scholar] [CrossRef]
- Alkadir, G.; Kumsa, B.I.; Terefe, G. Review on anthelminthic resistance in domestic ruminants. J. Vet. Med. Res. 2023, 10, 1237. [Google Scholar]
- Macedo, L.O.; Silva, S.S.; Alves, L.C.; Carvalho, G.A.; Ramos, R.A.N. An overview of anthelmintic resistance in domestic ruminants in Brazil. Ruminants 2023, 3, 214–232. [Google Scholar] [CrossRef]
- Rebuma, T.; Regassa, M.; Tariku, F. Review on anthelmintic drug resistance of nematodes in ruminants and methods of detection. Med. Parasitol. Epidemiol. Sci. 2023, 4, 50–54. [Google Scholar] [CrossRef]
- Kapo, N.; Softić, A.; Goletić, T.; Goletić, Š.; Cvetkovikj, A.; Omeragić, J. Anthelmintic resistance in livestock farming: Challenges and perceptions of farmers and veterinarians. Pathogens 2025, 14, 649. [Google Scholar] [CrossRef] [PubMed]
- Hoste, H. Alternative methods for the sustainable control of gastrointestinal nematodes in small ruminants. Options Méditerr. Sér. A Sémin. Méditerr. 2005, 67, 431–436. [Google Scholar]
- Stear, M.J.; Doligalska, M.; Donskow-Schmelter, K. Alternatives to anthelmintics for the control of nematodes in livestock. Parasitology 2007, 134, 139–151. [Google Scholar] [CrossRef]
- Šimpraga, M.; Ljubičić, I.; Hlede, J.P.; Vugrovečki, A.S.; Marinculić, A.; Tkalčić, S. Alternative approaches for the control of gastrointestinal nematodes in sheep farming: A review. Berl. Munch. Tierarztl. Wochenschr. 2015, 128, 257. [Google Scholar]
- Mukherjee, A.; Kar, I.; Patra, A.K. Understanding anthelmintic resistance in livestock using “omics” approaches. Environ. Sci. Pollut. Res. 2023, 30, 125439–125463. [Google Scholar] [CrossRef]
- Diez-Tascon, C.; Keane, O.M.; Wilson, T.; Zadissa, A.; Hyndman, D.L.; Baird, D.B.; McEwan, J.C.; Crawford, A.M. Microarray analysis of selection lines from outbred populations to identify genes involved with nematode parasite resistance in sheep. Physiol. Genom. 2005, 21, 59–69. [Google Scholar] [CrossRef]
- Liu, J.; Tan, M.; Xu, X.D.; Shen, T.B.; Zhou, Z.H.; Hunt, P.W.; Zhang, R.F. From innate to adaptive immunity: Abomasal transcriptomic responses of merino sheep to Haemonchus contortus infection. Molec. Biochem. Parasitol. 2021, 246, 111424. [Google Scholar] [CrossRef]
- Chagas, A.C.S.; Ribeiro, D.M.; Osório, H.; Abreu, A.A.P.; Okino, C.H.; Niciura, S.C.M.; Amarante, A.F.T.; Bello, H.J.S.; Melito, G.R.; Esteves, S.N.; et al. Molecular signatures of Haemonchus contortus infection in sheep: A comparative serum proteomic study on susceptible and resistant sheep breeds. Vet. Parasitol. 2024, 331, 110280. [Google Scholar] [CrossRef] [PubMed]
- Donskow-Lysoniewska, K.; Maruszewska-Cheruiyot, M.; Stear, M. The interaction of host and nematode galectins influences the outcome of gastrointestinal nematode infections. Parasitology 2021, 148, 648–654. [Google Scholar] [CrossRef] [PubMed]
- Laderach, D.J.; Compagno, D.; Toscano, M.A.; Croci, D.O.; Dergan-Dylon, S.; Salatino, M.; Rabinovich, G.A. Dissecting the signal transduction pathways triggered by galectin-glycan interactions in physiological and pathological settings. IUBMB Life 2010, 62, 1–13. [Google Scholar] [CrossRef]
- Wang, W.; Yuan, C.; Wang, S.; Song, X.K.; Xu, L.X.; Yan, R.F.; Hasson, I.A.; Li, X.R. Transcriptional and proteomic analysis reveal recombinant galectins of Haemonchus contortus down-regulated functions of goat PBMC and modulation of several signaling cascades in vitro. J. Proteom. 2014, 98, 123–137. [Google Scholar] [CrossRef]
- Jackson, F.; Greer, A.W.; Huntley, J.; McAnulty, R.W.; Bartley, D.J.; Stanley, A.; Stenhouse, L.; Stankiewicz, M.; Sykes, A.R. Studies using Teladorsagia circumcincta in an in vitro direct challenge method using abomasal tissue explants. Vet. Parasitol. 2004, 124, 73–89. [Google Scholar] [CrossRef]
- Ruiz-Campillo, M.T.; Molina Hernandez, V.; Escamilla, A.; Stevenson, M.; Perez, J.; Martinez-Moreno, A.; Donnelly, S.; Dalton, J.P.; Cwiklinsk, K. Immune signatures of pathogenesis in the peritoneal compartment during early infection of sheep with Fasciola hepatica. Sci. Rep. 2017, 7, 2782. [Google Scholar] [CrossRef]
- Lan, Z.; Liu, X.-L.; Lv, Q.-B.; Zeng, M.-H.; Gao, J.-F.; Chang, Q.-C.; Chen, Y.-Y.; Wang, C.-R. Proteomic analysis of Fasciola hepatica excretory and secretory products co-immunoprecipitated using time course infection sera. Pathogens 2021, 10, 749. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Lin, J.; Han, B.; Wang, L.; Chen, Y.; Liu, M.; Huang, J. Proteomic profiling of follicle fluids after superstimulation in one-month-old lambs. Reprod. Dom. Anim. 2018, 53, 186–194. [Google Scholar] [CrossRef]
- Wareth, G.; Eravci, M.; Weise, C.; Roesler, U.; Melzer, F.; Sprague, L.D.; Neubauer, H.; Murugaiyan, J. Comprehensive identification of immunodominant proteins of Brucella abortus and Brucella melitensis using antibodies in the sera from naturally infected hosts. Int. J. Mol. Sci. 2016, 17, 659. [Google Scholar] [CrossRef]
- Becerro-Recio, D.; González-Miguel, J.; Ucero, A.; Sotillo, J.; Martínez-Moreno, Á.; Pérez-Arévalo, J.; Cwiklinski, K.; Dalton, J.P.; Siles-Lucas, M. Recognition pattern of the Fasciola hepatica excretome/secretome during the course of an experimental infection in sheep by 2D immunoproteomics. Pathogens 2021, 10, 725. [Google Scholar] [CrossRef] [PubMed]
- Qin, P.; Ye, J.; Gong, X.; Yan, X.; Lin, M.; Lin, T.; Liu, T.; Li, H.; Wang, X.; Zhu, Y.; et al. Quantitative proteomics analysis to assess protein expression levels in the ovaries of pubescent goats. BMC Genom. 2022, 23, 507. [Google Scholar] [CrossRef]
- Paula Junior, A.R.; van Tilburg, M.F.; Lobo, M.D.P.; Monteiro-Moreira, A.C.O.; Moreira, R.A.; Melo, C.H.S.; Souza-Fabjan, J.M.G.; Araújo, A.A.; Melo, L.M.; Teixeira, D.I.A.; et al. Proteomic analysis of follicular fluid from tropically-adapted goats. Anim. Reprod. Sci. 2018, 188, 35–44. [Google Scholar] [CrossRef]
- Otávio, K.S.; Passos, J.R.; Silva, R.F.; Lima, L.F.; Cadenas, J.; Paes, V.M.; Correia, H.H.; Ferreira, A.C.A.; Canafístula, F.G.; Bezerra, M.J.B.; et al. Comprehensive proteomic profiling of early antral follicles from sheep. Anim. Reprod. Sci. 2023, 248, 107153. [Google Scholar] [CrossRef]
- Koch, J.M.; Ramadoss, J.; Magness, R.R. Proteomic profile of uterine luminal fluid from early pregnant ewes. J. Proteome Res. 2010, 9, 3878–3885. [Google Scholar] [CrossRef] [PubMed]
- La, Y.; Tang, J.; Guo, X.; Zhang, L.; Gan, S.; Zhang, X.; Zhang, J.; Hu, W.; Chu, M. Proteomic analysis of sheep uterus reveals its role in prolificacy. J. Proteom. 2020, 210, 103526. [Google Scholar] [CrossRef]
- Al-Gubory, K.H.; Arianmanesh, M.; Garrel, C.; Bhattacharya, S.; Cash, P.; Fowler, P.A. Proteomic analysis of the sheep caruncular and intercaruncular endometrium reveals changes in functional proteins crucial for the establishment of pregnancy. Reproduction 2014, 147, 599–614. [Google Scholar] [CrossRef][Green Version]
- El-Samahy, M.A.; Yao, X.; Zhang, G.; Zhang, Y.; Wang, F. A proposed sample handling of ovine cotyledon for proteomic studies. Analyt. Biochem. 2020, 593, 113585. [Google Scholar] [CrossRef]
- Oliveira, C.H.; Silva, A.M.; Silva, L.M.; van Tilburg, M.F.; Fernandes, C.C.; Velho, A.L.; Moura, A.A.; Moreno, F.B.; Monteiro-Moreira, A.C.; Moreira, R.A.; et al. Growth, testis size, spermatogenesis, semen parameters and seminal plasma and sperm membrane protein profile during the reproductive development of male goats supplemented with de-oiled castor cake. Reprod. Toxicol. 2015, 53, 152–161. [Google Scholar] [CrossRef]
- Lu, Z.; Ma, Y.; Zhang, Q.; Zhao, X.; Zhang, Y.; Zhang, L. Proteomic analyses of ram (Ovis aries) testis during different developmental stages. Anim. Reprod. Sci. 2018, 189, 93–102. [Google Scholar] [CrossRef] [PubMed]
- Bezerra, M.J.B.; Silva, M.B.; Lobo, C.H.; Vasconcelos, F.R.; Lobo, M.D.; Monteiro-Moreira, A.C.O.; Moreira, R.A.; Machado-Neves, M.; Figueiredo, J.R.; Moura, A.A. Gene and protein expression in the reproductive tract of Brazilian Somalis rams. Reprod. Dom. Anim. 2019, 54, 939–948. [Google Scholar] [CrossRef] [PubMed]
- Druart, X.; Rickard, J.P.; Mactier, S.; Kohnke, P.L.; Kershaw-Young, C.M.; Bathgate, R.; Gibb, Z.; Crossett, B.; Tsikis, G.; Labas, V.; et al. Proteomic characterization and cross species comparison of mammalian seminal plasma. J. Proteom. 2013, 91, 13–22. [Google Scholar] [CrossRef]
- Zhu, W.; Cheng, X.; Ren, C.; Chen, J.; Zhang, Y.; Chen, Y.; Zhang, Y.; Chen, Y.; Jia, X.; Wang, S.; et al. Proteomic characterization and comparison of ram (Ovis aries) and buck (Capra hircus) spermatozoa proteome using a data independent acquisition mass spectometry (DIA-MS) approach. PLoS ONE 2020, 15, e0228656. [Google Scholar] [CrossRef]
- Liang, J.; Lv, C.; Xiang, D.; Zhang, Y.; Zhang, B.; Raza, S.H.A.; Wu, G.; Quan, G. The establishment of goat semen protein profile using a tandem mass tag-based proteomics approach. Res. Vet. Sci. 2022, 150, 22–32. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, J.; Di, R.; Liu, Q.; Wang, X.; Gan, S.; Zhang, X.; Zhang, J.; Chen, W.; Hu, W.; et al. Identification of prolificacy-related differentially expressed proteins from sheep (Ovis aries) hypothalamus by comparative proteomics. Proteomics 2019, 19, e1900118. [Google Scholar] [CrossRef]
- Wang, C.; Zhao, Y.; Yuan, Z.; Wu, Y.; Zhao, Z.; Wu, C.; Hou, J.; Zhang, M. Genome-wide identification of mRNAs, lncRNAs, and proteins, and their relationship with sheep fecundity. Front. Genet. 2022, 12, 750947. [Google Scholar] [CrossRef]
- Li, C.; Zhou, M.; He, X.; Di, R.; Zhang, Z.; Ren, C.; Liu, Q.; Chu, M. Comparative proteomics of ovaries elucidated the potential targets related to ovine prolificacy. Front. Vet. Sci. 2023, 10, 1096762. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, X.; He, X.; Di, R.; Zhang, X.; Zhang, J.; Chu, M. Integration analysis of pituitary proteome and transcriptome reveals fertility–related biomarkers in FecB mutant Small Tail Han sheep. Front. Endocrinol. 2024, 15, 1417530. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Guo, X.; He, X.; Di, R.; Zhang, X.; Zhang, J.; Chu, M. Proteomic analysis identifies distinct protein patterns for high ovulation in FecB mutant Small Tail Han sheep granulosa cells. Animals 2024, 14, 11. [Google Scholar] [CrossRef]
- Hitit, M.; Özbek, M.; Ayaz-Guner, S.; Guner, H.; Oztug, M.; Bodu, M.; Kirbas, M.; Bulbul, B.; Bucak, M.N.; Ataman, M.B.; et al. Proteomic fertility markers in ram sperm. Anim. Reprod. Sci. 2021, 235, 106882. [Google Scholar] [CrossRef]
- Costa, T.C.; Dutra, L.L.; Mendes, T.A.d.O.; dos Santos, M.M.; Veroneze, R.; Gionbelli, M.P.; Duarte, M.d.S. Impact of maternal feed restriction at different stages of gestation on the proteomic profile of the newborn skeletal muscle. Animals 2022, 12, 1011. [Google Scholar] [CrossRef] [PubMed]
- Reed, S.A.; Balsbaugh, J.; Li, X.; Moore, T.E.; Jones, A.K.; Pillai, S.M.; Hoffman, M.L.; Govoni, K.E.; Zinn, S.A. Poor maternal diet during gestation alters offspring muscle proteome in sheep. J. Anim. Sci. 2022, 100, skac061. [Google Scholar] [CrossRef]
- Gao, Y.; Jian, L.; Lu, W.; Xue, Y.; Machaty, Z.; Luo, H. Vitamin E can promote spermatogenesis by regulating the expression of proteins associated with the plasma membranes and protamine biosynthesis. Gene 2021, 773, 145364. [Google Scholar] [CrossRef] [PubMed]
- Pini, T.; Rickard, J.P.; Leahy, T.; Crossett, B.; Druart, X.; de Graaf, S.P. Cryopreservation and egg yolk medium alter the proteome of ram spermatozoa. J. Proteom. 2018, 181, 73–82. [Google Scholar] [CrossRef]
- Lv, C.; Liang, J.; Yang, H.; Ni, X.; Raza, S.H.A.; Shah, M.A.; Wu, G.; Quan, G. The proteomic modification of buck ejaculated sperm induced by the cryopreservation process. Biopreserv. Biobank. 2023, 21, 255–266. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.; Sun, Z.; Chen, Y.; Chen, J.; Wang, S.; Liu, Q.; Wang, P.; Cheng, X.; Zhang, Z.; Wang, Q. Identification of biomarkers affecting cryopreservation recovery ratio in ram spermatozoa using tandem mass tags (TMT)-based quantitative proteomics approach. Animals 2023, 13, 2368. [Google Scholar] [CrossRef]
- Passos, J.R.S.; Guerreiro, D.D.; Otávio, K.S.; Dos Santos-Neto, P.C.; Souza-Neves, M.; Cuadro, F.; Nuñez-Olivera, R.; Crispo, M.; Vasconcelos, F.R.; Bezerra, M.J.B.; et al. How in vitro maturation changes the proteome of ovine cumulus-oocyte complexes? Mol. Reprod. Dev. 2022, 89, 459–470. [Google Scholar] [CrossRef]
- Zhao, H.; Sui, L.; Miao, K.; An, L.; Wang, D.; Hou, Z.; Wang, R.; Guo, M.; Wang, Z.; Xu, J.; et al. Comparative analysis between endometrial proteomes of pregnant and non-pregnant ewes during the peri-implantation period. J. Anim. Sci. Biotechnol. 2015, 6, 18. [Google Scholar] [CrossRef] [PubMed]
- Arianmanesh, M.; Fowler, P.A.; Al-Gubory, K.H. The sheep conceptus modulates proteome profiles in caruncular endometrium during early pregnancy. Anim. Reprod. Sci. 2016, 175, 48–56. [Google Scholar] [CrossRef]

| Country 1 | Total | with Sheep Work | with Goat Work | Total with First Author |
|---|---|---|---|---|
| CHN | 211 | 98 (46.4% 2) | 125 (59.2% 2) | 206 (97.6% 2) |
| USA | 50 | 31 (62.0%) | 24 (48.0%) | 22 (44.0%) |
| ITA | 49 | 29 (59.2%) | 23 (46.9%) | 39 (79.6%) |
| FRA | 39 | 31 (79.5%) | 15 (38.5%) | 22 (56.4%) |
| AUS | 38 | 37 (97.4%) | 6 (15.8%) | 24 (63.2%) |
| GBR | 32 | 32 (100.0%) | 5 (15.6%) | 22 (68.8%) |
| BRA | 26 | 12 (46.2%) | 15 (57.7%) | 24 (92.3%) |
| ESP | 22 | 17 (77.3%) | 7 (31.8%) | 13 (59.1%) |
| IND | 20 | 8 (40.0%) | 15 (75.0%) | 18 (90.0%) |
| PRT | 19 | 16 (84.2%) | 9 (47.4%) | 10 (52.6%) |
| NZL | 13 | 12 (92.3%) | 2 (15.4%) | 11 (84.6%) |
| GRC | 12 | 9 (75.0%) | 4 (33.3%) | 12 (100.0%) |
| DEU | 12 | 8 (66.7%) | 6 (50.0%) | 8 (66.7%) |
| Scientific Establishment | Country | Total No. of Published Papers |
|---|---|---|
| Chinese Academy of Agricultural Sciences | China | 44 |
| Ministry of Agriculture and Rural Affairs | China | 40 |
| National Institute for Research in Agriculture, Food, and Environment | France | 37 |
| Northwest A&F University—China | China | 22 |
| China Agricultural University | China | 20 |
| Federal University of Ceara | Brazil | 15 |
| Nanjing Agricultural University | China | 14 |
| Porto Conte Research | Italy | 14 |
| University of Sassari | Italy | 14 |
| Inner Mongolia Agricultural University | China | 13 |
| Anhui Academy of Agricultural Sciences | China | 12 |
| Gansu Agricultural University | China | 12 |
| University of Paris Saclay | France | 12 |
| Anhui Agricultural University | China | 11 |
| Chinese Academy of Sciences | China | 11 |
| National Centre for Scientific Research | France | 11 |
| University of Milan | Italy | 11 |
| Methodological Approach and Technology 1 | No. of Original Articles | Median Year of Publication |
|---|---|---|
| 2-DE/SDS-PAGE | 13 (2.9%) | 2018 (IQR 2: 5.5 years) |
| 2-DE and LC-MS/MS | 50 (11.2%) | 2018 (IQR: 6 years) |
| 2-DE and MALDI-TOF MS | 57 (12.7%) | 2015 (IQR: 8 years) |
| 2-DE and MALDI-TOF MS and LC-MS/MS | 9 (2.0%) | 2011 (IQR: 4 years) |
| in silico analysis | 7 (1.6%) | 2021 (IQR: 7.5 years) |
| GeLC-MS/MS | 53 (11.8%) | 2020 (IQR: 7 years) |
| LC-MS/MS | 237 (52.9%) | 2022 (IQR: 4 years) |
| LC-MS/MS and MALDI-TOF MS | 5 (1.1%) | 2019 (IQR: 7 years) |
| MALDI/SELDI-TOF MS | 8 (1.8%) | 2010 (IQR: 5 years) |
| Protein microarrays | 2 (0.4%) | 2021 (IQR: 2.5 years) |
| SDS-PAGE and MALDI-TOF MS | 7 (1.6%) | 2012 (IQR: 7 years) |
| Keyword | No. of Published Papers |
|---|---|
| proteomics | 135 |
| sheep | 80 |
| proteome | 46 |
| goat | 39 |
| mass spectrometry | 29 |
| 2-DE | 28 |
| ovine | 20 |
| isobaric tag for relative and absolute quantitation (iTRAQ) | 19 |
| biomarker | 18 |
| proteomic | 18 |
| goat milk | 16 |
| milk | 15 |
| mastitis | 14 |
| colostrum | 13 |
| protein | 13 |
| metabolomics | 12 |
| whey proteins | 11 |
| Country | Journal(s) | No. of Published Papers |
|---|---|---|
| Australia | Journal of Proteomics | 7 |
| Brazil | Reproduction in Domestic Animals | 4 |
| China | Journal of Proteomics | 16 |
| France | Journal of Dairy Science, Journal of Proteomics, Molecular Biosystems, Proteomics | 3 |
| Germany | Analytical and Bioanalytical Chemistry | 2 |
| Greece | Animals, Journal of Proteomics, Pathogens | 2 |
| India | no journal with >1 published papers | n/a |
| Italy | Journal of Proteomics | 7 |
| New Zealand | Journal of Proteomics | 3 |
| Portugal | Journal of Proteomics, Plos One | 3 |
| Spain | Scientific Reports | 2 |
| United Kingdom | Proteomics | 4 |
| United States of America | Biology of Reproduction, Journal of Dairy Science | 3 |
| Topic of Study | Journal(s) | No. of Published Papers |
|---|---|---|
| animal disease | Journal of Proteomics | 10 |
| models for the study of conditions in humans | International Journal of Molecular Sciences | 2 |
| physiology | International Journal of Molecular Sciences, Journal of Dairy Science, Journal of Proteomics, Plos One, Proteomics | 4 |
| sheep/goat production | Journal of Proteomics | 14 |
| sheep/goat reproduction | Journal of Proteomics | 9 |
| Variables | Relative Risk (±s.e. 1) | p |
|---|---|---|
| Number of References Cited in the Paper | <0.0001 | |
| per unit increase | 1.027 ± 1.006 | <0.0001 |
| Topic of Study | 0.015 | |
| animal diseases (1.5 (2.0) 2) | reference | -- |
| small ruminants as models for the study of various conditions in humans (1.7 (2.2)) | 1.495 ± 1.784 | 0.12 |
| physiology (1.7 (3.1)) | 1.560 ± 1.250 | 0.048 |
| sheep/goat production (2.5 (3.0)) | 1.638 ± 1.137 | 0.0002 |
| sheep/goat reproduction (1.8 (2.9)) | 1.222 ± 1.113 | 0.06 |
| Animal Species Involved in the Study | 0.044 | |
| sheep (1.6 (2.7)) | reference | - |
| goats (2.0 (3.1)) | 1.708 ± 1.371 | 0.09 |
| both species (2.6 (2.0)) | 1.422 ± 1.340 | 0.23 |
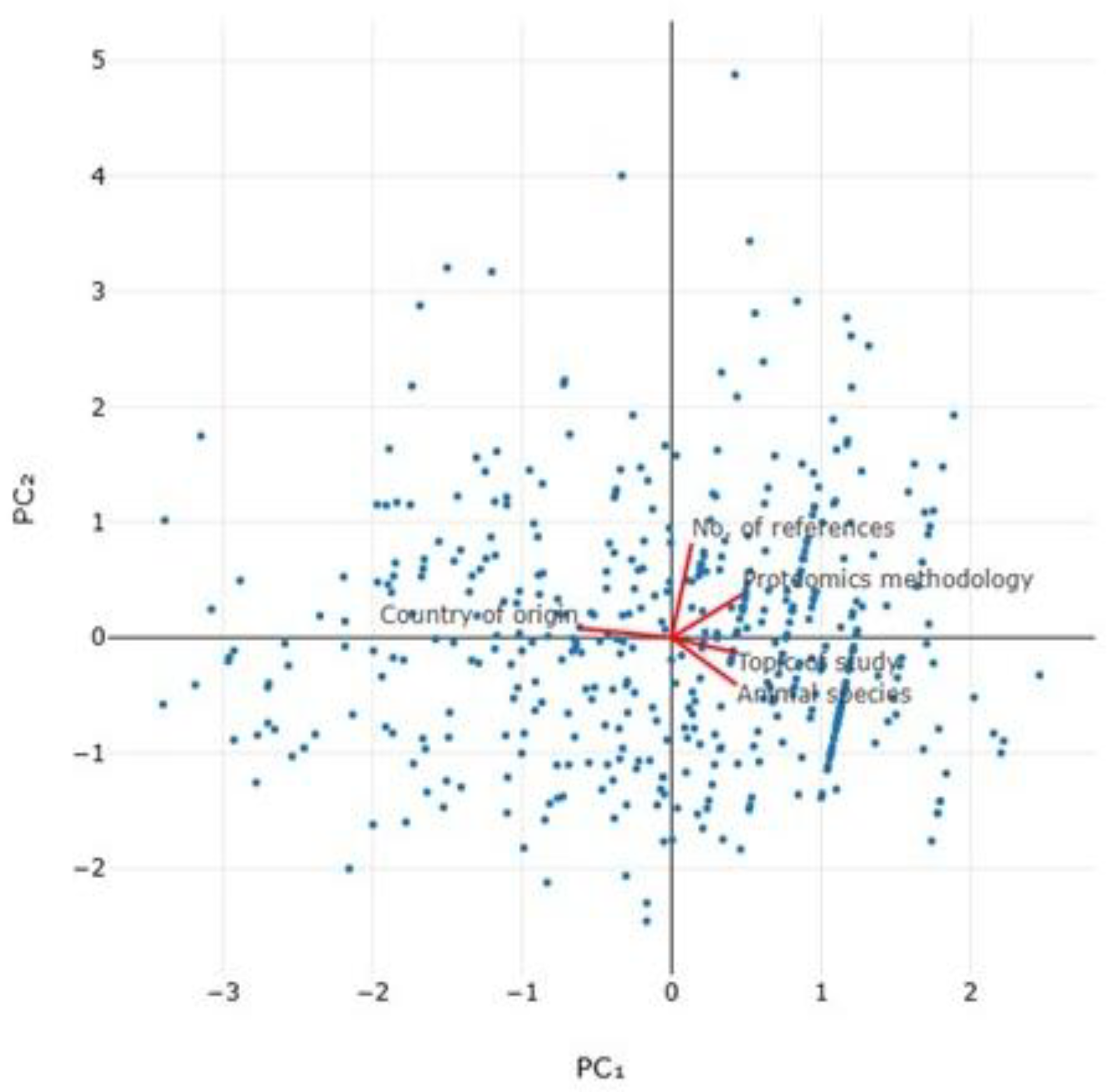
| Parameter | PC1 | PC2 | PC3 | PC4 | PC5 |
|---|---|---|---|---|---|
| Eigenvalue | 1.39 | 1.08 | 0.96 | 0.81 | 0.76 |
| % of Variance | 27.9 | 21.5 | 19.2 | 16.2 | 15.1 |
| Cumulative variance (%) | 27.9 | 49.4 | 68.6 | 84.9 | 100.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bourganou, M.V.; Vaitsi, G.A.; Liagka, D.V.; Michael, C.K.; Katsarou, E.I.; Chatzopoulos, D.C.; Vasileiou, N.G.C.; Papadopoulos, E.; Tsangaris, G.T.; Lianou, D.T.; et al. Appraisal of the Use of Proteomics Methodological Approaches and Technologies on Sheep and Goat Research and Clinical Work. Animals 2025, 15, 3050. https://doi.org/10.3390/ani15203050
Bourganou MV, Vaitsi GA, Liagka DV, Michael CK, Katsarou EI, Chatzopoulos DC, Vasileiou NGC, Papadopoulos E, Tsangaris GT, Lianou DT, et al. Appraisal of the Use of Proteomics Methodological Approaches and Technologies on Sheep and Goat Research and Clinical Work. Animals. 2025; 15(20):3050. https://doi.org/10.3390/ani15203050
Chicago/Turabian StyleBourganou, Maria V., Georgia A. Vaitsi, Dimitra V. Liagka, Charalambia K. Michael, Eleni I. Katsarou, Dimitris C. Chatzopoulos, Natalia G. C. Vasileiou, Elias Papadopoulos, George Th. Tsangaris, Daphne T. Lianou, and et al. 2025. "Appraisal of the Use of Proteomics Methodological Approaches and Technologies on Sheep and Goat Research and Clinical Work" Animals 15, no. 20: 3050. https://doi.org/10.3390/ani15203050
APA StyleBourganou, M. V., Vaitsi, G. A., Liagka, D. V., Michael, C. K., Katsarou, E. I., Chatzopoulos, D. C., Vasileiou, N. G. C., Papadopoulos, E., Tsangaris, G. T., Lianou, D. T., Mavrogianni, V. S., Fthenakis, G. C., & Katsafadou, A. I. (2025). Appraisal of the Use of Proteomics Methodological Approaches and Technologies on Sheep and Goat Research and Clinical Work. Animals, 15(20), 3050. https://doi.org/10.3390/ani15203050













