1. Introduction
In the livestock industry’s transition to efficiency and sustainability, how to enhance breeding efficiency while cutting antibiotics and other chemicals is critical for its sustainable development [
1]. The Muscovy duck, as an important specialty waterfowl variety in southern China, is in high demand in the market due to its firm meat, unique flavor, and high nutritional value [
2]. However, during its breeding process, it often faces problems such as poor intestinal health and low immunity, which restrict further improvement of production performance [
3,
4]. Therefore, the development of safe and efficient natural feed additives has become a crucial breakthrough for improving the current situation of Muscovy duck farming.
Marine algae, as one of the oldest photosynthetic organisms on Earth, have emerged as an ideal source of natural feed additives due to their rich bioactive components and renewable characteristics [
5]. Among them, seaweed polysaccharide (SP) serve as the core functional component of algae, which have been demonstrated to possess various biological activities such as antioxidant, immune regulation, and improvement of gut microecology, showcasing promising application potential in livestock and poultry farming [
6,
7]. Moreover, seaweed enzymatic hydrolysates (SEH) are prepared through enzymatic hydrolysis technology. This technology not only retains the active substances of algae but also converts the macromolecular components in algae into smaller molecular fragments [
8]. These smaller molecular fragments are more easily absorbed and utilized by animals, which further enhances the bioavailability of SEH and provides new insights for overcoming the application bottlenecks of natural extracts in waterfowl [
9].
Currently, the application of SP in livestock and poultry farming has been extensively studied, with their positive effects such as promoting growth and enhancing disease resistance being thoroughly validated. However, research related to SEH is relatively scarce [
10,
11,
12]. Notably, compared to common livestock and poultry such as chickens and pigs, systematic studies on SP and SEH concerning the Muscovy duck, are even more limited. Given the unique physiological and metabolic characteristics and breeding needs of Muscovy ducks, exploring the effects of SP and SEH on their growth performance, immune function, and intestinal health can not only enrich the application theory of marine bioactive substances in waterfowl breeding, but also provide scientific basis for promoting the development of green and healthy breeding models for Muscovy ducks.
4. Discussion
In the context of pursuing green and sustainable development in the livestock farming industry, the innovation of feed additives has become a key breakthrough for enhancing farming efficiency and product quality [
13]. Although the Muscovy duck possesses good growth potential, its growth rate, feed conversion rate, and disease resistance still face bottlenecks in high-density farming environments, necessitating improvements through scientific feed nutritional regulation methods [
14]. Seaweeds, as a significant renewable resource in the ocean, have garnered considerable attention in research focused on the development and utilization of their extracts [
15]. Seaweed polysaccharides, a type of natural macromolecular carbohydrate derived from seaweeds, demonstrate multiple beneficial effects in animal organisms due to their unique molecular structure, which contains numerous active groups, such as hydroxyl and carboxyl, and exhibits rich biological activity [
16,
17]. Pradhan et al. reviewed the immunomodulatory, antioxidant, anticancer, and pharmacokinetic activities of polysaccharides extracted from algae, confirming their substantial nutritional value, antioxidant, and anti-inflammatory capabilities, as well as their potential to enhance immune function [
18]. Similarly, Shannon et al. analyzed the components of algae as potential modulators of the gut microbiome, positing their potential as prebiotics and their ability to actively regulate the gut microbiota [
19]. Another product derived from seaweed is seaweed enzymatic hydrolysate, which is obtained through specific biological enzymatic treatment of seaweed. This technology can decompose the macromolecular substances in seaweed, which are originally difficult for animals to directly absorb, into small molecular active components [
9]. This transformation not only retains the various nutritional essences inherent in seaweed but also significantly enhances the absorption and utilization efficiency of these components within the animal body [
9]. Currently, there is limited research on SP and SEH in monogastric animals, particularly in ducks. In this experiment, feeding 20 mL/kg of SP and SEH did not significantly affect the ADG of ducks, but it significantly reduced the ADFI and F/G. The reduction in these two indicators typically suggests an improvement in feed conversion efficiency. This positive effect can be explained by the fact that SP and SEH accelerate the absorption and metabolic processes of nutrients in animals, facilitating a more efficient conversion of the energy and nutrients in the feed into animal body tissues, thereby enhancing the economic benefits of farming and resource utilization efficiency.
Serum biochemical indicators, antioxidant indicators, immunoglobulin indicators, and inflammatory factor indicators in blood metrics are important biological markers reflecting the physiological state, metabolic level, and health status of animals [
20,
21]. ALT and AST are primarily found within hepatocytes; when these cells are damaged, these enzymes are released into the bloodstream in large quantities, leading to increased serum activity [
22]. Therefore, they serve as sensitive indicators of liver function. Compared to the CON group, the serum activities of ALT and AST in the SP and SEH groups were significantly reduced, indicating that SP and SEH have a protective effect on the liver. GLU is a core substance in energy metabolism, and its serum level reflects the balance of glucose metabolism in the body, while TG and TCHO are important indicators of lipid metabolism [
23]. Zheng et al. studied the effects of polysaccharides from Bangia fusco-purpurea on obesity induced by a high-fat diet (HFD) in C57BL/6 mice, they found that these polysaccharides can enhance energy metabolism, promote lipolysis, increase fatty acid oxidation, and inhibit lipogenesis [
24]. The research conducted by Hyun et al. indicates that the L-fucose-rich sulfated polysaccharides derived from edible brown algae can exert potent anti-lipogenic properties by downregulating key regulators of lipogenesis [
25]. This result is similar to the regulatory effects of SP on animal lipid metabolism reported in previous studies, suggesting that the supplementation of 20 mL/kg of SP in the diet may exert a positive physiological regulatory effect by improving the glucose and lipid metabolism processes in ducks. The decrease in GLU levels may indicate that SP promotes the utilization or storage of glucose in ducks, maintaining blood sugar levels within a more reasonable range and preventing energy wastage. The reduction in TG and TCHO levels suggests that SP may inhibit fat synthesis or promote its catabolism, thereby reducing fat deposition in the body. The decrease in both LDL-C and HDL-C may be related to the reduction in TCHO.
This experiment also found that SP and SEH improved the antioxidant capacity of the Muscovy duck, specifically reflected in the increased T-AOC and SOD activity in the SEH group, as well as the decreased MDA content in the serum of the SP and SEH groups. Long et al. discovered that the polysaccharide from Gracilaria lemaneiformis can alleviate H
2O
2-induced oxidative stress in HepG2 cells through the
Nrf-2/Keap-1 signaling pathway [
26]. Matin et al. reviewed the bioactive potential of algae and their derivatives, indicating that algal extracts can inhibit inflammatory signaling pathways such as
NF-κB and
MAPK, thereby reducing oxidative damage by activating Nrf2 [
27]. Adalbjörnsson et al. found that enzymatic hydrolysis enhances the antioxidant components extracted from seaweeds [
28]. This indicates that SP and SEH may enhance the antioxidant capacity of the Muscovy duck by regulating specific signaling pathways or augmenting the activity of antioxidant components.
The immunoglobulin indicators and inflammatory factor indicators are important markers reflecting their immune function and inflammatory status [
29]. Immunoglobulins are the core effector molecules of the humoral immunity, and an increase in their levels indicates an enhanced ability of the body to resist pathogen invasion [
30]. Inflammatory factors, as signaling molecules of the body’s inflammatory response, when secreted in excess, can trigger chronic inflammation, deplete a large amount of nutrients, and hinder the expression of growth performance [
31]. In this experiment, the levels of IgA and IgG in the serum of the SP group and SEH group were significantly increased. Additionally, pro-inflammatory factors such as TNF-α, IL-1β, and IL-6 were significantly reduced, while anti-inflammatory factors IL-4 and IL-10 were significantly elevated. The significant increase in serum levels of IgA and IgG suggests that SP and SEH can enhance the humoral immune response of ducks. IgA, as a core antibody of mucosal immunity, can form a defensive barrier on the mucosal surfaces of the digestive and respiratory tracts, reducing pathogen invasion [
32]. IgG, on the other hand, is the most abundant immunoglobulin in body fluids, capable of eliminating pathogens through neutralizing toxins and activating the complement system [
33]. The elevation of both antibody levels indicates an enhanced resistance of ducks to diseases, providing an immune guarantee for their healthy growth. The significant decrease in pro-inflammatory factors (TNF-α, IL-1β, IL-6) and the notable increase in anti-inflammatory factors (IL-4, IL-10) reflect that SP and SEH can effectively alleviate excessive inflammatory responses in the body. The excessive secretion of pro-inflammatory factors can lead to tissue damage and the consumption of a large amount of nutrients, while the upregulation of anti-inflammatory factors can inhibit the cascading amplification of inflammation and maintain immune homeostasis [
34].
Intestinal morphology is an important indicator reflecting the digestive and absorption functions, intestinal health status, and nutrient utilization efficiency of animals [
35]. The structural integrity and morphological characteristics play a crucial role in the growth and development, immune function, and overall health of animals [
35]. Currently, there are few reports on the effects of SP and SEH on the intestinal morphology of ducks. However, for monogastric animals, dietary supplementation of seaweed-derived polysaccharides in sows has been shown to enhance the immune response of suckling piglets and improve intestinal morphology [
36]. Walsh et al. found that the supplementation of 300 mg/kg of alginate increased both the VH and the V/C in piglets [
37]. Additionally, enzymatic hydrolysates of nori can enhance the intestinal mucosal function in obese mice, improve the morphological structure of the small intestine, increase the growth of goblet cells and mucus, elevate the expression level of lysozyme, and stimulate the secretion of sIgA [
38]. In this experiment, the supplementation of SP and SEH in the diet significantly increased the VH and the V/C of the jejunum in ducks, while reducing the CD. This indicates that the addition of SP and SEH to the diet can effectively improve the morphological structure of the jejunum in ducks, thereby enhancing their intestinal digestive and absorption functions.
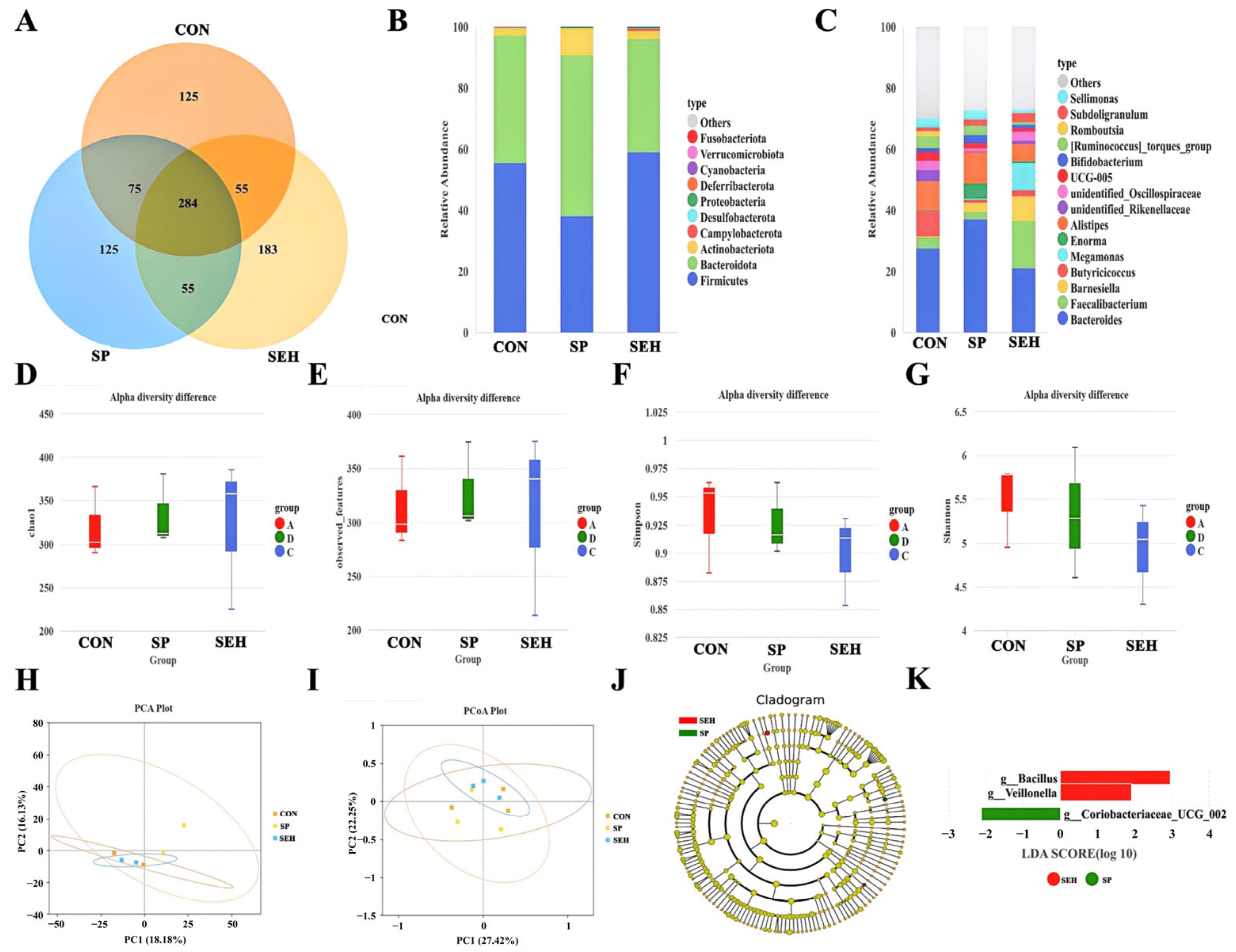
The community structure and functional balance of gut microbiota have a broad and profound impact on the host’s digestion and absorption, immune regulation, metabolic balance, and overall health [
39]. Liu et al. found that polysaccharides from
Laminaria Japonica can enhance the production performance and systemic health of ducks by mediating the gut microbiota [
40]. However, there are few reports on the improvement of the gut microbiota structure in Muscovy ducks by SEH. In this experiment, the Alpha and Beta diversity of gut microbiota in ducks supplemented with SP and SEH showed no significant effects. However, in both the SP and SEH groups, a significant increase in the abundance of
Barnesiella was observed, while the abundances of
UCG-005 and
Romboutsia significantly decreased. This indicates that SP and SEH may improve the intestinal environment by selectively regulating the core gut microbiota.
Barnesiella is a beneficial bacterium strongly associated with the synthesis of short-chain fatty acids and anti-inflammatory immune regulation in the gut [
41], whereas
UCG-005 and
Romboutsia are often linked to excessive energy absorption, inflammation risk, and dysbiosis in the intestine [
42,
43]. LEfSe analysis revealed differences in the dominant bacteria between the SP and SEH groups, with
g__Veillonella in the SP group primarily involved in the metabolism of carbohydrates and proteins, which helps maintain the normal function and structural integrity of intestinal epithelial cells and regulates intestinal immune function, inhibiting inflammatory responses [
44]. Additionally,
g__Coriobacteriaceae_UCG_002 may be related to the metabolic conversion processes of bile acids in the gut. These findings help to explain the different mechanisms by which SP and SEH affect the physiological functions of ducks [
45].