1. Introduction
In recent years, the growth rate of the domestic cat (Felis catus) population in China has exceeded that of domestic dogs (Canis lupus familiaris), with the total number of cats approaching that of dogs, particularly in urbanized areas. The expanding cat population has led to an increase in lost, abandoned, and unregulated breeding cases, resulting in a yearly surge in the number of stray cats (Felis vaga). Stray cats can carry infectious pathogens, and due to their mobile nature, they can easily transmit diseases to other healthy stray cats and even household cats, which could potentially trigger large-scale outbreaks. This situation also poses a risk of zoonotic disease transmission. Therefore, the prevention and control of major diseases in urban stray animals are of critical importance.
Common pathogens affecting cats include feline coronavirus (FCoV), feline calicivirus (FCV), feline herpesvirus (FHV), feline panleukopenia virus (FPV), and rabies virus (RABV). FCoV, which belongs to the
Coronaviridae family, possesses a positive-sense single-stranded RNA genome. It is divided into two genotypes: type I and type II. Type II feline coronavirus (FCoV-II) originated from recombination between type I feline enteric coronavirus (FECV) and canine coronavirus (CCoV) [
1]. In natural infections, type I feline coronavirus (FCoV-I) is the predominant form [
2,
3]. Based on pathogenicity, FCoVs are categorized into two biotypes: the mildly pathogenic strains causing mild or subclinical gastrointestinal symptoms (Feline enteric coronavirus, FECV) and the highly lethal strains (Feline infectious peritonitis virus, FIPV), which arise from spontaneous mutations of FECV [
3,
4]. FCV, a member of the
Caliciviridae family, possesses a positive-sense single-stranded RNA genome. It is highly contagious and primarily causes oral ulcers, mild upper respiratory symptoms, and can lead to severe pneumonia in kittens. While kittens exhibit high morbidity and mortality rates, adult cats often remain asymptomatic; however, they can become virus carriers [
5]. FHV-I, an α-herpesvirus with a double-stranded DNA genome, is the primary etiological agent of feline viral rhinotracheitis, accounting for approximately half of all feline upper respiratory infections. Clinical signs include conjunctivitis, epiphora, nasal discharge, coughing, sneezing, and anorexia. Although cats of all ages are susceptible, kittens between 2 and 4 months of age are most vulnerable, exhibiting an incidence rate of 100% and a mortality rate of up to 50%. The virus is transmitted through direct contact, fomites, and aerosol droplets, with documented potential for cross-species transmission to chinchillas
(Chinchilla lanigera) [
6]. A hallmark of FHV-1 infection is the establishment of latency and subsequent intermittent viral shedding, during which infected cats can periodically excrete infectious virus [
7]. FPV, a member of the
Parvoviridae family, possesses a single-stranded DNA genome. It is the causative agent of feline panleukopenia, also known as feline infectious enteritis (FIE). Intrauterine or neonatal infection can result in cerebellar hypoplasia, with reported mortality rates ranging from 25% to 100% [
8]. FPV exhibits high resistance to common physical and chemical factors. The virus is transmitted via the fecal-oral route, either through direct contact or indirect environmental contamination, facilitating outbreaks in feral cat populations. Consequently, effective vaccination and rigorous disinfection are essential for controlling FPV spread in animal shelters. RABV, the agent of a lethal zoonosis, can infect almost all warm-blooded animals and is responsible for approximately 60,000 human deaths annually [
9]. The predatory and nocturnal behavior of cats increases their exposure to bats (Chiroptera), a key reservoir, while their close contact with humans as companion animals heightens the risk of zoonotic transmission.
In this study, for the first time, we tested 126 stray cats sampled from six urban districts of Shenzhen (Bao’an, Nanshan, Luohu, Longgang, Longhua, and Futian) for major feline infectious diseases and zoonotic pathogens between June and August 2024. We conducted a comprehensive analysis of the pathogen carriage and transmission risks within this stray cat population. Our findings provide critical data for the prevention and early warning of feline infectious diseases in Shenzhen, as well as a scientific basis for assessing rabies transmission risks and formulating effective control strategies.
4. Discussion
Shenzhen, as a densely populated and economically developed city in China, hosts a large population of pet owners. The frequent contact opportunities between humans, pets, and stray cats may elevate the risk of disease transmission, posing a major threat to the health of domestic cats and urban public health. FCoV-I, FCV, FHV-I, and FPV are important pathogens that endanger cats. Given that Shenzhen is located in South China, a region with high rabies incidence, the detection of RABV nucleic acid and neutralizing antibodies in stray cats is highly relevant for rabies prevention and control efforts.
Currently, there are limited surveillance reports on important infectious diseases and zoonotic pathogens in stray cats. In this study, nucleic acids of FCoV-I, FCV, FHV-I, and FPV were detected in the stray cats, while no positive results for RABV nucleic acid were found, and the positive rate of neutralizing antibodies was extremely low. A notable strength of this study is the integration of molecular detection with clinical observations across all 126 cats, which provides valuable insights into the prevalence and clinical significance of these infections among stray cats in Shenzhen.
However, several limitations must be considered when interpreting these findings. First, our sampling strategy did not constitute a random sample of the stray cat population in Shenzhen. Sampling sites were limited to veterinary clinics with stray cat treatment programs, TNR shelters, and community feeding points. Therefore, our cohort may not be fully representative of the overall prevalence rates. Additionally, during sample collection, the high wariness and aggression of some stray cats permitted only brief physical restraint, resulting in the inability to collect all intended samples from every individual. This limitation may introduce selection bias.
Second, a key methodological limitation of the viral detection component is its reliance on molecular methods (qPCR) alone. While highly sensitive for identifying pathogen nucleic acid, a positive qPCR result does not necessarily indicate the presence of live, infectious virus, which limits our assessment of the actual transmission risk. For future studies aiming to characterize active shedding and transmission dynamics, integrating virus isolation with molecular methods, as demonstrated by Acar et al. for FCV [
12], would be a crucial advancement. It should be noted that this limitation applies only to the molecular detection of the five viral pathogens; the assessment of rabies virus neutralizing antibodies was conducted using the fluorescent antibody virus neutralization (FAVN) test, which is considered the gold standard.
Despite these limitations, our analysis revealed complex and insightful pathogen-symptom relationships in this population. We confirmed a strong correlation between FCV infection and stomatitis, consistent with the well-documented tropism of calicivirus for oral tissues [
13]. Other symptoms, such as diarrhea and dermatopathy, were often associated with mixed infections. Interestingly, contrary to classical expectations, ocular discharge was not definitively associated with FHV-I; some affected cats were FHV-I-negative but positive for other pathogens such as FPV. These findings suggest that while classic patterns (e.g., FCV-stomatitis) remain evident, certain clinical signs like ocular discharge may be caused by other pathogens or non-infectious factors. Collectively, these correlations indicate that a substantial proportion of detected infections were clinically active. The absence of hematological parameters further limited a comprehensive assessment of disease status for some conditions.
FPV is associated with high mortality rate and is taken seriously by countries globally. In this survey, FPV was the most prevalent pathogen, with a positivity rate of 61.90% (78/126) among the 126 stray cats tested. This is significantly higher than the 37.1% (53/143) reported in the Northeastern China between 2016 and 2017 [
14], and the 19.2% reported in China from 2016 to 2019 [
15]. It is, however, consistent with the very high prevalence of 73.5% found in a multi-pathogen survey in Southern Italy [
16], as well as a recent nationwide investigation in China [
17], collectively reinforcing that FPV remains a severe threat to feline populations. Nevertheless, considerable regional variation is evident across studies. For instance, in Changchun, China, the prevalence of FPV positivity in domestic cats with diarrhea was as high as 55.7% (39/70) [
18], while in Henan provinces, the positivity rate among suspected cases was only 30.49% (25/89) [
19]. Similarly, in 2021, the positivity rate of FPV DNA in domestic cats within and around the Xining Wildlife Park in China reached 54.55% and 47.73%, respectively [
20]. These discrepancies suggest that prevalence can vary significantly due to factors such as geographical location, sampling time, and sample size. In summary, the situation of FPV carriage in stray cats in Shenzhen, China, is relatively severe. The likelihood of unvaccinated cats suffering from feline panleukopenia is 8.83 times higher than that of vaccinated cats [
21]. Moreover, the presence of FPV has been confirmed in the environment [
20]. Vaccination is of great significance in the prevention and control of feline panleukopenia in stray cats. The carriage of FPV in wild animals is relatively common [
22]. In this study, Bao’an and Nanshan District of Shenzhen had the highest FPV detection rates. These adjacent districts are characterized by extensive wooded areas and a relatively dense human population density. These environmental and demographic factors might facilitate transmission and could contribute to the high FPV positivity rate observed in stray cats in these areas. Our findings highlight the urgent need for enhanced immunoprophylaxis against FPV in both stray and domestic cats in Shenzhen.
FCV is a highly mutable virus. Its high mutagenicity and genetic plasticity enable the virus to survive successfully within cat populations [
23]. In the present study, FCV was the second most prevalent pathogen after FPV, with a positivity rate of 57.14%. Most FCV-positive cats exhibited oral symptoms, consistent with typical clinical manifestations, though a number of asymptomatic carriers were also identified. The prevalence observed here is higher than that reported in several other regions: 31.3% in Beijing, China [
24], 26.0% in Kunshan, China [
25], 43.0% in Hangzhou, China [
26], and substantially higher than the 7.04% in Southern Italy [
16], 25.7% in Moscow [
27], and 45.0% among suspected cases in Switzerland [
28]. Although these samples were collected from clinically diseased cats, all the cats were domestic cats. The cats in this study were all strays. Not only are they unmanaged, but their vaccination coverage is likely to be even lower. This lower vaccination coverage is a plausible contributing factor to the higher FCV positivity rate observed in this population. Furthermore, while vaccination or natural immunity can to some extent prevent FCV infection in cats, those that have been vaccinated or naturally infected may still become carriers after subclinical infection [
25,
29]. Both infected cats and asymptomatic carriers can continuously shed the virus [
30]. What is more, in addition to direct contact transmission, FCV can also be spread through contaminated objects, humans, aerosols, and even flea feces [
31]. Therefore, stray cats have a stronger ability to spread FCV. Strict implementation of disinfection, isolation, and quarantine measures is crucial for managing FCV spread and reducing associated mortality.
FCoV-I is primarily transmitted via the fecal-oral route, such as through contact with fecally contaminated items [
32]. Stray cats are at high risk of infection through behaviors like competing for food provided by the public and sharing communal defecation sites [
33]. Furthermore, natural transmission of FCoV-I between domestic and stray cats has been documented [
34,
35]. Therefore, there are many factors that influence the transmission of FCoV-I, especially in multi-cat environments where stray cats congregate. In this study, the overall FCoV-I positivity rate was 46.83%, with significant geographical variation across Shenzhen. The highest rate was observed in Longgang District (73.33%), while the lowest was in Futian District (17.39%). Similar disparities in FCoV-I positivity rates have been reported in other studies. For instance, a survey conducted in the Central China region from 2018 to 2020 revealed an overall FCoV-I positivity rate of 46.6% in cats from veterinary clinics, with a positivity rate of 59.3% in samples suspected of FIP [
36]. However, the FCoV-I positivity rate was as high as 90% in 120 suspected FIP samples from North and South China [
37]. The high risk for stray cats stems not only from frequent contact with other cats but also from potential interactions with wildlife. Additionally, unneutered male cats have been reported to have a higher likelihood of developing FIP [
38]. Interestingly, one study in Japan reported a lower FCoV-I positivity rate in stray cats (15.9%) than in domestic cats (35.5%) [
2], highlighting that epidemiological patterns can be context-dependent. In conclusion, the high prevalence and distinct spatial variation in FCoV-I observed in this study, coupled with reported transmission routes, indicate that the circulation of this virus among stray cats and at the interface with domestic cats poses a significant challenge for disease control.
In this survey, 29 of 126 stray cats were positive for FHV-I (23.02%), the lowest among all pathogens tested. Previous studies have found that the FHV-I positivity rate in domestic cats in Kunshan, China, was 21.5%. Among these positive cases, the positivity rate was 27.90% for vaccinated cats and 72.09% for unvaccinated cats [
39]. Therefore, vaccination is of great significance in controlling FHV-I. In Guangxi, China, the positivity rate of FHV-I in domestic cats was 5.56% [
40]. The positivity rate of FHV-I in symptomatic cats from 13 cities in northern and southern China was 16.3% [
14]. Adding to this geographical variation, a 2024 study in Yanji City, China, reported an FHV-I prevalence of 13.8% [
41], further illustrating the widespread circulation of this pathogen. The samples tested in this study were from stray cats, and the high positivity rate of FHV-I is closely related to the vaccination coverage and management practices. Therefore, it is important to focus on the prevention and control of FHV-I in stray cats.
Rabies is a major global public health issue. This zoonotic disease causes 590,000 deaths annually and affects more than 100 countries worldwide [
42]. For live cats, we opted saliva swabs that intermittently shed the rabies virus instead of brain tissue, which is complies with China’s national standards of Nucleic Acid Detection Of Animal Rabies Virus [
43,
44]. Although recent studies have shown that RABV can be detected in feces, the detection results are related to the degree of sample dilution and exposure time [
45]. In this survey, all fresh fecal swab samples were processed promptly after collection to minimize RNA degradation, and no rabies virus nucleic acid was detected. Moreover, the positivity rate of RABV antibodies in the 100 stray cat serum samples was only 6.00%, indicating that the low immunization coverage of rabies vaccines for stray cats in Shenzhen. The overlap between cat and bat habitats creates opportunities for contact and potential rabies transmission. Reports of predation incidents indicate a spillover risk of bats transmitting rabies to cats. [
46,
47]. Given their wide roaming range, stray cats with negative rabies antibodies may become infected with RABV through wounds if attacked by wild animals or other stray animals. A survey showed that when vaccination coverage exceeded 70%, there were no rabies outbreaks in villages (defined as at least two cases not interrupted by an interval of more than one month). Small-scale outbreaks occurred in villages with lower vaccination coverage, while the largest and longest-lasting outbreak occurred only in villages with vaccination coverage below 20% [
48]. This low antibody level suggests that the population is likely susceptible to infection. Large-scale vaccination programs have effectively controlled rabies in dogs, but cats were less vaccinated, mainly due to difficulties in transporting and controlling cats, as well as underestimating of stray cats [
49,
50]. Given its high fatality rate, rabies control should be prioritized, and vaccination efforts for stray cats intensified to prevent cat-to-human transmission.
Stray cats primarily acquire rabies antibodies through two sources: first, via rabies vaccination administered after rescue; and second, from previously vaccinated domestic cats that have strayed due to accidental loss or abandonment, thereby retaining rabies virus antibodies. According to the results of this study, the antibody positivity rate in stray cats was higher in the central urban area than in the peripheral areas. This may be because pet owners or rescue volunteers in the central urban area have a higher willingness and awareness to vaccinate cats compared to those in the peripheral areas.
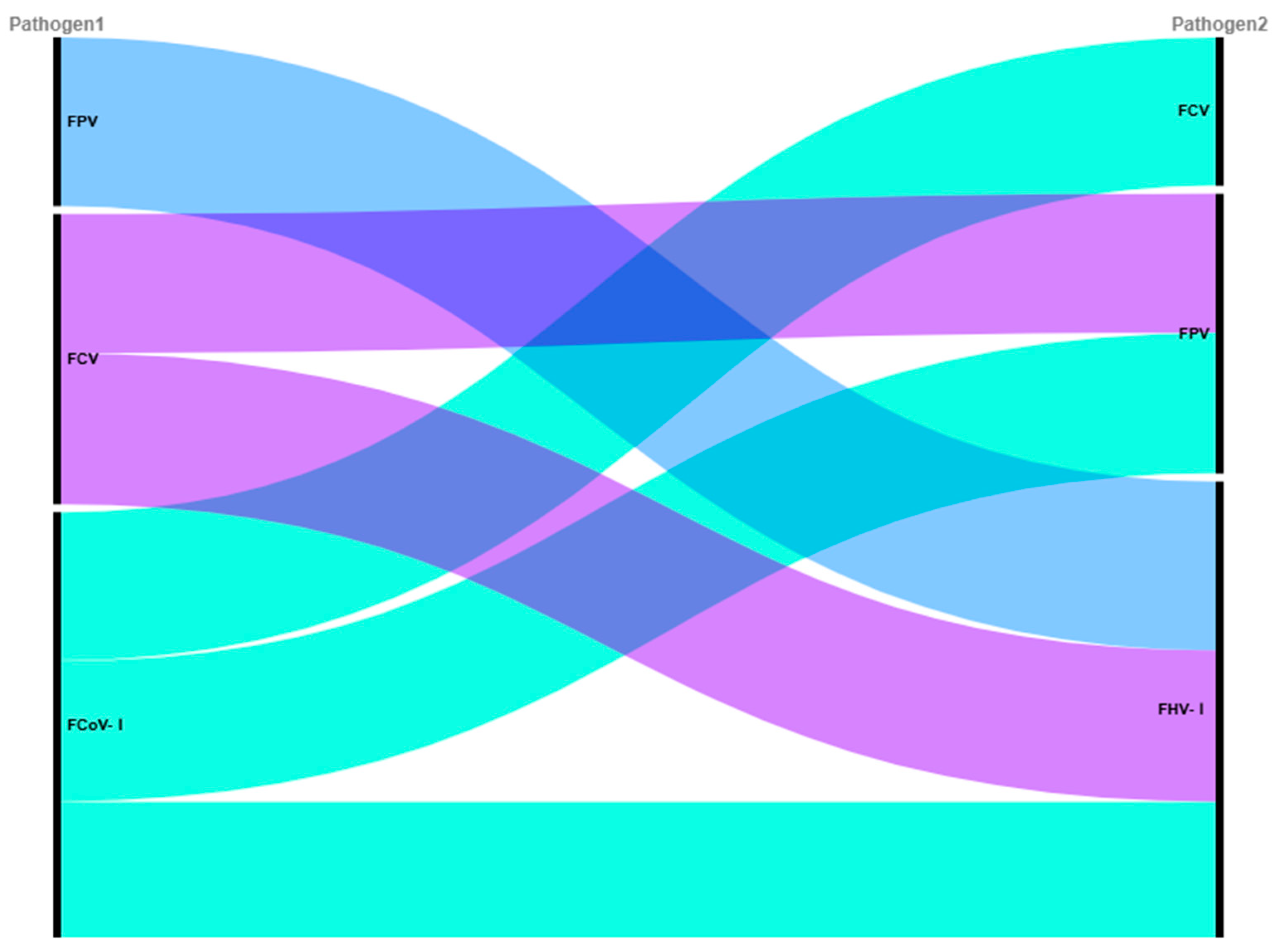
Mixed infections with different feline viral pathogens were notably prevalent in the stray cat population, with an overall rate of 62.70% for the four pathogens investigated. This finding is consistent with recent reports, such as the 60.2% mixed infection rate in feline upper respiratory tract disease cases [
51]. The most frequent triple co-infection combination involved FCV, FPV, and FCoV-I (12.70%), likely driven by their high individual prevalence and facilitated by the high mobility of stray cats, which amplifies exposure opportunities.
Pairwise association analysis revealed specific patterns: the high Jaccard index between FCV and FPV (0.456) suggests frequent co-detection, possibly due to shared fecal-oral transmission and environmental stability, which are well-documented for both viruses [
52,
53]. Furthermore, the high odds ratio for FHV–FPV co-occurrence (OR = 2.93, nominal
p < 0.05) is consistent with the hypothesis that FPV-induced immunosuppression might facilitate FHV reactivation; a mechanism supported by previous studies on the immunosuppressive nature of FPV [
8,
54]. Although not significant after multiple-testing correction, these associations highlight complex interactions that may exacerbate disease burden and complicate management.
Geospatial analysis showed significant heterogeneity in infection rates across districts. For instance, FCoV-I prevalence was significantly higher in Longgang than in Futian, even after multiple-testing correction. This spatial clustering suggests that localized environmental, ecological, or anthropogenic factors drive transmission dynamics, a phenomenon observed in other urban animal disease systems [
55]. We hypothesize that socioecological heterogeneity—such as varying cat densities, which is a known risk factor for directly transmitted pathogens [
56], human feeding practices, and access to veterinary services—may underlie these disparities. Higher population density and congregating around feeding sites likely form transmission hotspots, as predicted by core group theory [
57], suggesting that targeted interventions in these locations could be most effective.
Based on these findings, we recommend implementing integrated control strategies including: geographically targeted TNR and vaccination programs prioritizing high-prevalence districts such as Longgang for FCoV-I; regular syndromic surveillance at feeding hotspots and shelters; public education for community feeders on hygiene practices; and enhanced veterinary capacity in high-risk areas for improved diagnosis and management of co-infections, particularly those with immunosuppressive interactions.